Abstract
Background
Colorectal cancer is the third most commonly diagnosed malignancy and the fourth leading cause of cancer-related death globally. It is the second most common cancer among both males and females in Malaysia. The economic burden of colorectal cancer is likely to increase over time owing to its current trend and aging population. Cancer survival analysis is an essential indicator for early detection and improvement in cancer treatment. However, there was a scarcity of studies concerning survival of colorectal cancer patients as well as its predictors. Therefore, we aimed to determine the 1-, 3- and 5-year survival rates, compare survival rates among ethnic groups and determine the predictors of survival among colorectal cancer patients.
Methods
This was an ambidirectional cohort study conducted at the University Malaya Medical Centre (UMMC) in Kuala Lumpur, Malaysia. All Malaysian citizens or permanent residents with histologically confirmed diagnosis of colorectal cancer seen at UMMC from 1 January 2001 to 31 December 2010 were included in the study. Demographic and clinical characteristics were extracted from the medical records. Patients were followed-up until death or censored at the end of the study (31st December 2010). Censored patients’ vital status (whether alive or dead) were cross checked with the National Registration Department. Survival analyses at 1-, 3- and 5-year intervals were performed using the Kaplan-Meier method. Log-rank test was used to compare the survival rates, while Cox proportional hazard regression analysis was carried out to determine the predictors of 5-year colorectal cancer survival.
Results
Among 1212 patients, the median survival for colorectal, colon and rectal cancers were 42.0, 42.0 and 41.0 months respectively; while the 1-, 3-, and 5-year relative survival rates ranged from 73.8 to 76.0%, 52.1 to 53.7% and 40.4 to 45.4% respectively. The Chinese patients had the lowest 5-year survival compared to Malay and Indian patients. Based on the 814 patients with data on their Duke’s staging, independent predictors of poor colorectal cancer (5-year) survival were male sex (Hazard Ratio [HR]: 1.41; 95% CI: 1.12, 1.76), Chinese ethnicity (HR: 1.41; 95% CI: 1.07,1.85), elevated (≥ 5.1 ng/ml) pre-operative carcino-embryonic antigen (CEA) level (HR: 2.13; 95% CI: 1.60, 2.83), Duke’s stage C (HR: 1.68; 95% CI: 1.28, 2.21), Duke’s stage D (HR: 4.61; 95% CI: 3.39, 6.28) and emergency surgery (HR: 1.52; 95% CI: 1.07, 2.15).
Conclusions
The survival rates of colorectal cancer among our patients were comparable with those of some Asian countries but lower than those found in more developed countries. Males and patients from the Chinese ethnic group had lower survival rates compared to their counterparts. More advanced staging and late presentation were important predictors of colorectal cancer survival. Health education programs targeting high risk groups and emphasizing the importance of screening and early diagnosis, as well as the recognition of symptoms and risk factors should be implemented. A nationwide colorectal cancer screening program should be designed and implemented to increase early detection and improve survival outcomes.
Keywords: Colorectal cancer, Survival rates, Ethnic disparities, Malaysia
Background
Colorectal cancer is the third most commonly diagnosed malignancy and the fourth leading cause of cancer-related death in the world. Its burden is expected to increase to more than 2.2 million new cases and 1.1 million cancer deaths by 2030 [1]. Rapid increases in both colorectal cancer incidence and mortality are now being observed in the Asian countries.
Malaysia is an upper middle income country with a multi-ethnic population of 31.7 million. The major ethnic groups are Malays (68.6%), Chinese (23.4%) and Indians (7%) [2]. In Malaysia, colorectal cancer is the second most common cancer in both males and females [3]. According to the National Cancer Patient Registry - Colorectal Cancer data from 2008 to 2013, the overall incidence and mortality rates of colorectal cancers among the Malaysian population were 21.32 and 9.79 per 100,000 respectively [4]. Those of Chinese ethnicity had the highest incidence of colorectal cancer (27.35 per 100,000), followed by the Malays (18.95 per 100,000), and Indians (17.55 per 10,000) [4]. The economic burden of colorectal cancer is substantial and is likely to increase over time owing to the current trend and aging population.
Mortality due to colorectal cancer can be effectively reduced with early diagnosis and treatment. However, previous studies showed that colorectal cancer patients in Malaysia usually presented late compared to developed countries [5, 6]. This could be due to the lack of a national screening program, poor appreciation of its common symptoms, risk factors and available measures for its early detection [6–8].
There was also a scarcity of studies on the treatment outcomes as well as the overall survival of colorectal cancer patients in our country [6]. Only three local studies on colorectal cancer survival outcomes with small sample sizes [9–11] were published. Cancer survival analysis is an essential indicator for early detection and improvement in cancer treatment. Therefore, we aimed to (1) determine the 1-, 3- and 5-year relative survival rates of colorectal cancer patients; (2) compare the survival rates of colorectal cancer patients among the three major ethnic groups; and (3) evaluate the roles of selected demographic, clinical and treatment factors in the prediction of survival for colorectal cancer patients treated at the University Malaya Medical Centre (UMMC) from January 2001 to December 2010.
Methods
This was an ambidirectional cohort study, which comprised of retrospective and prospective components. All Malaysian citizens or permanent residents with histologically confirmed diagnosis of colorectal cancer seen at UMMC from 1 January 2001 to 31 December 2010 were included. The patients’ unique National Registration Identity Card (NRIC) numbers and their hospital registration numbers were used to identify and link their medical records. Data were retrieved from the patients’ medical records retrospectively from January 2001 to December 2008, and prospectively from January 2009 to December 2010.
Data extracted from the medical records included demographic characteristics such as age, sex and ethnicity (Malays, Chinese, Indians and Others) and clinical characteristics i.e.: comorbidities (such as hypertension and diabetes mellitus), anatomic site of the tumour, Duke’s stages (A to D), tumour grades and pre-operative serum carcino-embryonic antigen (CEA) levels. Treatment modalities such as surgery, chemotherapy and radiotherapy or best supportive treatment were also collected. The date of surgery or commencement of treatment in UMMC was considered as the zero date. Patients were followed-up until death or censored at the end of the study (31st December 2010). Censored patients’ NRIC numbers were used to cross check with the data of the National Registration Department (NRD) for their vital status (whether still alive or dead).
Data were analysed using SPSS, version 21.0 for Windows, (SPSS Inc., and Chicago, Illinois, USA). The level of significance was pre-set at 0.05. Quantitative variables were screened for normality. Normally distributed variables were summarized using means and standard deviations (SD), while skewed data were reported as median and inter-quartile range. Categorical variables were presented using proportions and percentages.
Survival analyses at 1-, 3- and 5-year intervals were performed using the Kaplan-Meier method. The log-rank test was used to compare the survival rates. The Cox proportional hazard regression analysis was carried out to determine the hazard ratios of predictors on survival. Variables with p < 0.25 in the univariate analyses were entered into the multivariate model. An automatic stepwise backward elimination method was performed. Confidence intervals of 95% were reported where appropriate.
Results
Patients’ characteristics, follow-up and relative survival rates
A total of 1212 eligible patients were included in the analysis. Details of the demographics, clinical and treatment characteristics of the patients are reported in Table 1. Slightly more than half of the patients were males and 67% were of Chinese ethnicity. The majority were aged more than 50 years with a mean (SD) age of 61 (13) years. There was an equal proportion of patients with colon or rectal cancers. Almost half of the patients had non-elevated pre-operative CEA levels, and two thirds had low-grade tumours. About one third of the patients’ tumours were diagnosed at Duke’s stage C, while only 5% were in Dukes’ stage A. Surgery was the most common treatment modality (82.3%), while 8.3% were on chemo- and/or radiotherapy alone; and 9.4% on best supportive care. A total of 13.4% of the surgeries were performed as emergencies. More details of these patients are published elsewhere [12].
Table 1.
Demographic and clinical characteristics of patients (n = 1212) in UMMC
| Characteristics | n | % | |
|---|---|---|---|
| Sex | Male | 668 | 55.1 |
| Female | 544 | 44.9 | |
| Ethnic groups | Chinese | 808 | 66.7 |
| Malays | 225 | 18.6 | |
| Indians | 157 | 13.0 | |
| Others | 22 | 1.7 | |
| Age groups (years) | ≤ 39 | 83 | 6.8 |
| 40–49 | 121 | 10.0 | |
| 50–59 | 291 | 24.0 | |
| 60–69 | 410 | 34.0 | |
| ≥ 70 | 307 | 25.3 | |
| Co-morbidities | Hypertension | 318 | 26.2 |
| Diabetes | 196 | 16.2 | |
| Site | Colon | 596 | 49.2 |
| Rectum | 552 | 45.5 | |
| Unknown | 64 | 5.3 | |
| Pre-operative carcino-embryonic antigen (CEA) level | Not elevated (≤ 5.0 ng/ml) | 543 | 44.8 |
| Elevated (> 5.0 ng/ml) | 256 | 21.1 | |
| Unknown | 413 | 34.1 | |
| Tumour Grades | Low | 802 | 66.2 |
| High | 60 | 5.0 | |
| Unknown | 350 | 28.8 | |
| Duke’s Stages | A&B | 297 | 24.5 |
| C | 366 | 30.2 | |
| D | 151 | 12.5 | |
| Unknown | 398 | 32.8 | |
| Treatment modalities | Surgery alone | 488 | 40.3 |
| Surgery plus Chemo- or Radiotherapy | 393 | 32.4 | |
| Surgery plus Chemo- and Radiotherapy | 116 | 9.6 | |
| Chemo- and Radiotherapy alone | 101 | 8.3 | |
| Best supportive care | 114 | 9.4 | |
| Urgency of surgerya | Elective | 481 | 48.2 |
| (n = 997) | Emergency | 134 | 13.4 |
| Unknown | 382 | 38.2 |
a n = 215 patients on chemo- and radiotherapy alone or on best supportive care
Relative and median survival rates
The median follow-up was 28 months (inter quartile range 11, 59 months). The median survival for colorectal cancer was 42.00 (95% CI: 35.42–48.58) months and the 5-year survival rate was 43% (Table 2). There was no significant difference in median survival among patients with colon (42 months) or rectal (41 months) cancers. However, patients with colon cancer had higher 1-, 3- and 5-year survival rates compared to rectal cancer patients.
Table 2.
One, three and five-year relative survival (%) and median survival (months) for colorectal cancer patients
| Cancer location | Relative survival rates (%) | Median survival (months) |
|---|---|---|
| (95% CI) | (95% CI) | |
| Colorectal cancer (n = 1212) | 42.00 (35.42–48.58) | |
| 1 year | 74.92 (72.38–77.26) | |
| 3 years | 53.13 (50.20–55.97) | |
| 5 years | 42.85 (39.81, 45.84) | |
| Colon cancer (n = 596)a | 42.00 (31.11–52.89) | |
| 1 year | 77.52 (73.95–80.66) | |
| 3 years | 55.04 (50.81–59.07) | |
| 5 years | 45.00 (40.59–49.31) | |
| Rectal cancer (n = 552)a | 41.00 (32.34–49.66) | |
| 1 year | 74.09 (70.23–77.54) | |
| 3 years | 52.47 (48.13–56.62) | |
| 5 years | 41.26 (36.82–45.64) | |
a Total did not add up to 1212 as there were 64 unknown cases
In the stratified analysis by ethnic groups (Table 3), patients from the Chinese ethnic group had significantly lower 5-year survival compared to the Indian and Malay patients in colon cancers (p = 0.039), and both colon and rectal cancers combined (p = 0.005). However, there was no significant difference in the 5-year survival rates for rectal cancer between ethnic groups. The 5-year survival rates for colorectal cancer patients with Duke’s stages A & B were higher (65.8%) compared to patients with advanced stages (Duke’s stages C, 49.4% and D, 12.6%) (p < 0.001) (Fig. 1). However, there was no significant difference in Duke’s stage by ethnic groups in the 5-year survival rates.
Table 3.
Five-year survival of colorectal cancer patients by anatomic sites and Duke’s stage, stratified by ethnic groups
| Clinical characteristics | Survival rate, % (95% CI) | |||
|---|---|---|---|---|
| aMalay (n = 225) | Chinese (n = 808) | Indians (n = 157) | p | |
| Colorectal | 48.50 (41.29–55.32) | 39.68 (36.01–43.33) | 47.49 (38.84–55.63) | 0.039 |
| Site: | ||||
| Colon | 56.51 (44.75–66.69) | 40.07 (34.86–45.22) | 54.52 (42.18–65.29) | 0.005 |
| Rectum | 43.87 (34.42–52.91) | 39.81 (34.23–45.33) | 36.91 (24.56–49.28) | 0.674 |
| Duke's stageb | Malay (n = 163) | Chinese (n = 546) | Indians (n = 105) | p |
| A & B | 68.68 (51.43–80.88) | 63.79 (55.88–70.66) | 69.95 (51.73–82.39) | 0.714 |
| C | 56.18 (42.43–67.86) | 45.01 (37.96–51.80) | 58.85 (41.56–72.61) | 0.136 |
| D | 17.98 (6.27–34.53) | 13.05 (7.03–20.96) | NA | - |
CI confidence interval, NA not available
a 3 ethnic groups ≠ 1212, as ethnic group, Others (n = 22) was excluded
b Total sample with data on Duke’s stage, n = 814
Fig. 1.
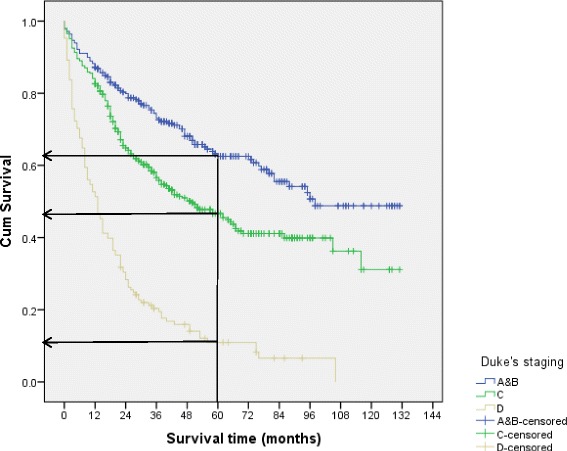
Survival curves of colorectal cancer by Duke’s staging
Predictors of colorectal cancer survival
The total sample size used in the following analyses was 814 as cases with missing information on diagnosis of Duke’s staging (n = 398) were excluded. From the Cox proportional hazard regression model, predictors of 5-year survival for colorectal cancer patients were sex, ethnicity, pre-operative CEA levels, Duke’s stages, tumour grades and urgency of surgery (all p < 0.05); after adjusting for age, sex, comorbidities (diabetes mellitus and hypertension), tumour grades, treatment modalities and urgency of surgery (Table 4). The risk of colorectal cancer death was higher among male patients (HR: 1.41; 95% CI: 1.12, 1.76), Chinese ethnicity (HR:1.41; 95% CI: 1.07, 1.85), elevated pre-operative CEA levels (HR: 2.13; 95% CI: 1.60, 2.83), Duke’s stage C (HR: 1.68; 95% CI: 1.28, 2.21) and D (HR: 4.61; 95% CI: 3.39, 6.28); and patients with emergency surgery (HR: 1.52; 95% CI: 1.07, 2.15).
Table 4.
Predictors of 5-year survival in colorectal cancer patients
| Variables | aCrude HR (95% CI) | bAdjusted HR (95% CI) |
|---|---|---|
| Sex | ||
| Female | 1.00 | 1.00 |
| Male | 1.13 (0.96–1.31) | 1.41 (1.12–1.76) |
| Ethnicity | ||
| Malays | 1.00 | 1.00 |
| Chinese | 1.26 (1.03–1.56) | 1.41 (1.07–1.85) |
| Indians | 1.06 (0.79–1.42) | 1.32 (0.78–1.71) |
| Preoperative carcino-embryonic antigen (CEA) level | ||
| Not elevated | 1.00 | 1.00 |
| Elevated | 2.31 (1.91–2.79) | 2.13 (1.60–2.83) |
| Duke’s stages | ||
| A&B | 1.00 | 1.00 |
| C | 1.66 (1.29–2.12) | 1.68 (1.28–2.21) |
| D | 4.94 (3.78–6.45) | 4.61 (3.39–6.28) |
| Urgency of surgery | ||
| Elective | 1.00 | 1.00 |
| Emergency | 1.42 (1.11–1.82) | 1.52 (1.07–2.15) |
HR hazard ratio, CI confidence interval
aUnivariate Cox Proportional Hazard Regression analysis
bMultivariate Cox Proportional Hazard Regression analysis, adjusted for age, gender, co-morbidities (diabetes mellitus, hypertension), tumour grades, and treatment modalities
Site-specific analyses for colon (n = 440) and rectal (n = 374) cancers after adjusting for confounders were also performed (Tables 5 and 6). Significant predictors for both colon and rectal cancer survivals were pre-operative CEA levels and Duke’s stages. The risks of colon and rectal cancer deaths for pre-operative CEA levels were 2.00 (95% CI: 1.30, 3.03) and 2.09 (95% CI: 1.39, 3.15) respectively. The hazard ratios for the diagnosis of tumour at Duke’s stage C and D were double in colon cancer (2.33 and 5.87) compared to rectal cancer (1.16 and 3.97). Ethnicity was only found to be significant in colon cancer survival, where Chinese (HR: 1.74; 95% CI: 1.07, 2.84) and Indian patients (HR: 1.88; 95% CI: 1.01, 3.51) had higher hazard ratios compared to Malays. On the other hand, males (HR: 1.68; 95% CI: 1.19, 2.37) and higher tumour grades (HR: 2.91; 95% CI: 1.65, 5.14) significantly predicted rectal cancer death.
Table 5.
Predictors of 5-year survival in colon cancer patients
| Predictors | aCrude HR (95% CI) | bAdjusted HR (95% CI) |
|---|---|---|
| Ethnicity | ||
| Malays | 1.00 | 1.00 |
| Chinese | 1.55 (1.10–2.17) | 1.74 (1.07–2.84) |
| Indians | 1.13 (0.71–1.78) | 1.88 (1.01–3.51) |
| Preoperative CEA levels | ||
| Not elevated | 1.00 | 1.00 |
| Elevated | 2.58 (1.94–3.44) | 2.00 (1.30–3.03) |
| Duke’s stages | ||
| A&B | 1.00 | 1.00 |
| C | 2.13 (1.49–3.05) | 2.33 (1.56–3.49) |
| D | 5.51 (3.73–8.16) | 5.87 (3.73–9.24) |
HR hazard ratio, CI confidence interval
aUnivariate Cox Proportional Hazard Regression analysis
bMultivariate Cox Proportional Hazard Regression analysis, adjusted for age, gender, co- morbidities (diabetes mellitus, hypertension), tumour grades and treatment modalities
Table 6.
Predictors of 5-year survival in rectal cancer patients
| Predictors | aCrude HR (95% CI) | bAdjusted HR (95% CI) |
|---|---|---|
| Sex | ||
| Female | 1.00 | 1.00 |
| Male | 1.27 (1.02–1.60) | 1.68 (1.19–2.37) |
| Preoperative carcino-embryonic antigen (CEA) level | ||
| Not elevated | 1.00 | 1.00 |
| Elevated | 2.03 (1.55–2.65) | 2.09 (1.39–2.37) |
| Duke’s stages | ||
| A&B | 1.00 | 1.00 |
| C | 1.27 (0.89–1.80) | 1.16 (0.79–1.72) |
| D | 3.98 (2.37–5.80) | 3.97 (2.59–6.10) |
| Tumour grades | ||
| Low | 1.00 | 1.00 |
| High | 2.99 (1.92–4.67) | 2.91 (1.65–5.14) |
HR hazard ratio, CI confidence interval
aUnivariate Cox Proportional Hazard Regression analysis
bMultivariate Cox Proportional Hazard Regression analysis, adjusted for age, gender, co- morbidities (diabetes mellitus, hypertension), tumour grade and treatment modalities
Discussion
The detailed characteristics of our colorectal cancer patients had been published elsewhere [12]. In brief, the demographic, clinical and treatment characteristics of our patients were comparable to other patients in Malaysia and some South East Asian countries [9, 11, 13, 14]. Our patients’ 1-, 3- and 5-year relative survival rates of colorectal cancer were also comparable to some countries in Asia [14–16]. Reports across Asia indicated that the 5-year survival rates ranged from 31.2% in India [17], 57.0 to 58.9% in Singapore [18], 41 to 61% in Korea [16] and 77% in China (highest) [19]. Although developed countries such as Australia, New Zealand, Canada, the United States and parts of Europe had the highest incidence rates of colorectal cancer, however their survival rates were better [20] than ours. Disparities in survival rates may be due to the different management practices and the effectiveness of implemented colorectal cancer screening programs among these countries.
On the other hand, there are not many local studies available for the comparison of colorectal cancer survival rates. Our 5-year colorectal cancer survival rate was slightly lower than the survival rate reported on patients recruited in 2008–2009 from the National Cancer Patient Registry-Colorectal Cancer data, which was 48.7% [21]. As a tertiary centre, patients referred to our hospital are usually of more advanced stage, as evidenced by nearly two-thirds of those with retrievable stage being Stage III or IV; this would skew the overall survival rates. When corrected for stage, a comparative study between hospitals from Kuala Lumpur and Kuching [10] showed that the 5-year colorectal cancer survival was lower for patients in Kuching compared to Kuala Lumpur. Another hospital from Kelantan, situated at the east coast of Peninsular Malaysia reported a lower 5-year survival rate for colorectal cancer (34.3%) [11] compared to our hospital. These discrepancies could be due to different stages at diagnosis or presentation as well as availability of expertise and facilities in different hospitals. Socio-economic status of patients from Kuala Lumpur or nearby location may be better than those from Kuching and Kelantan, as lower socio-economic status was a significant factor for late and more advanced stage at diagnosis, as well as poorer 3- and 5-year survival rates for colorectal cancers [10].
We did not find age, treatment modalities, diabetes mellitus and hypertension to be significant predictors of colorectal cancer survival. However, males had higher risks of death for colorectal cancer (Table 4). Contradicting results have been reported where some studies reported males to have poorer survival for colorectal cancer. [22, 23] while others did not find any difference in survival between sex [11, 21, 24, 25]. This may be related to the different proportions of colon and rectal cancer patients included in the different studies. In addition, different levels of health awareness and risk behaviours (smoking, physical inactivity etc.) as well as health seeking behaviours between genders [22] may be the contributing factors. Further research on these aspects should be conducted.
About two thirds of our patients were from the Chinese ethnic group. According to the findings from Abu Hassan et al. [4] on patients diagnosed from 2008 to 2013, those of Chinese ethnicity had the highest incidence for colorectal cancer (27.35 per 100,000), followed by the Malays (18.95 per 100,000), and Indians (17.55 per 100,000). The observed ethnic disparities may be due to the fact that our hospital is located in the Klang valley with more residents from the Chinese ethnic group compared to others. The ethnic distribution among patients in the hospital was also predominantly Chinese, thus giving rise to the observed results. The incidence of colorectal cancer among Chinese in our country was also higher than the Chinese from China [26]. This may be due to different lifestyle practices especially higher animal fat and red meat in their diet and physical inactivity. However, information on diet and physical activity levels were not available in our study.
Lowest survival rate was also observed among the Chinese compared to the Malay patients irrespective of anatomic site and Duke’s stages. These findings are in contrast with earlier reports in Malaysia and Singapore where non-Malays (including Chinese) were reported to have better survival rates [9, 11, 27]. Chinese were reported to have lower level of awareness in colorectal cancer compared to Malays [7] while another study reported that Malays had better recognition of symptoms [28] compared to the Chinese ethnic group. These could contribute to the late diagnosis or presentation at advance stage resulting poorer survival.
More advance Duke’s staging and elevated pre-operative CEA levels significantly predicted both colon and rectal cancers survival, similarly reported by other studies [11, 24, 29, 30]. However, given similar staging in comparison with patients from the developed countries, our patients’ survival was poorer [15, 27, 30], probably due to the different management practices and different patients’ compliance to treatment.
Emergency surgery was another predictor of poor colorectal cancer survival, as reported elsewhere [31]. Poor awareness on colorectal cancer [7, 28, 32] resulted in diagnosis at advanced stage with obstructive symptoms that needed emergency surgery. Effective health education campaigns need to be implemented to increase the awareness of our population. The programs should focus on increasing the knowledge on symptoms and risk factors of colorectal cancer, awareness on benefits of screening, and promotion of healthy life styles to prevent this disease. Primary care physicians should be given a bigger role in educating the public, as well as assist in identifying and recommending high-risk patients for early colorectal cancer screening and further expert management. Availability of programs for colorectal screening nationwide should also be provided.
Patients from the Malay ethnic group had better colon cancer survival than other ethnic groups as the Malay patients were younger [12] and hence had better survival. On the other hand, males had poorer survival for rectal cancer, as the narrow pelvis in males makes surgery more difficult technically and this may cause inadequate clearance in males compared to females. As expected, higher tumour grades significantly predicted rectal cancer survival.
Limitations and strengths
Our study has a few limitations, which need to be considered while interpreting the results. As the data were extracted from the medical records, important information such as family history of colorectal cancer, socio-economic status, lifestyle practices such as smoking, diet and physical activity, adherence to treatment and follow-up/check-ups were unavailable. Future studies should be planned to collect these data. These data should also be routinely collected and recorded in the medical records. There were substantial missing data on clinical characteristics (such as Duke’s staging, pre-operative CEA levels, tumour grades etc.) in the medical records as these data were recorded in hard copies and had higher chance of being misplaced. However, documentation of these data should be improved with the implementation of electronic medical records. Future research addressing the above limitations should be conduted.
On the other hand, our study may have the largest number of patients from a single centre with a follow up duration of 10 years. Our study explored and provided multivariate analyses on a population substantially understudied and provided important ethnic comparisons.
Conclusions
The survival rates of colorectal cancer among our patients were comparable with some Asian countries but lower than the developed countries. As expected, more advanced stage and late presentation were important predictors for colorectal cancer survival. A less expected finding was that males and patients from the Chinese ethnic group had lower survival rates compared to their counterparts. Therefore, health education programs targeting males and high risk ethnic groups, emphasizing the importance of screening and early diagnosis, recognition of symptoms and risk factors, should be implemented. Nationwide colorectal cancer screening programs should be made available, to increase early detection of colorectal cancer and improve the survival rates in the future.
Acknowledgements
The authors would like to thank the UMMC Medical Records Department for providing access to the data. We would also like to acknowledge the support from the surgical clinics from UMMC, Julius Centre University of Malaya and Department of Social & Preventive Medicine, Faculty of Medicine, University of Malaya.
Funding
This study was funded by the University of Malaya postgraduate fund (UM-IPPP PS212/2010A and PV015-2011B) and partially supported by the STeMM Programme, the University of Malaya/Ministry of Higher Education (UM/MOHE) High Impact Research Grant (No: E000010–20001). The funding agencies had no roles in the design of the study; collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available as the data set belong to the UMMC Medical Record Department but are available from the corresponding author on reasonable request.
Authors’ contributions
BAM, FMM, CWL, and ACR participated in the design and coordination of the study. FMM, BAM provided the statistical support. CWL and ACR provided clinical input. All authors read, revised and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Ethics clearance which included the use of data extracted from the medical records was obtained from the ethics committee of the University Malaya Medical Centre (PPUM/UPP/300/02/02, MEC 770.2). Written informed consent was not required as all data were extracted from the medical records and all personal identifiers were removed from the working database.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- CEA
Carcino-embryonic antigen
- NRD
National Registration Department
- NRIC
National Registration Identity Cards
- UMMC
University Malaya Medical Centre
Contributor Information
Bello Arkilla Magaji, Email: mbarkilla@gmail.com.
Foong Ming Moy, Phone: +60-3-7967-6657, Email: moyfm@ummc.edu.my.
April Camilla Roslani, Email: april@ummc.edu.my, Email: aprilroslani@um.edu.my.
Chee Wei Law, Email: drcwlaw@gmail.com.
References
- 1.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2016;66(4):683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 2.Department of Statistics Malaysia: Current Population Estimates, Malaysia, 2014–2016. In.http://www.statistics.gov.my/dosm/index.php?r=column/cthemeByCat&cat=155&bul_id=OWlxdEVoYlJCS0hUZzJyRUcvZEYxZz09&menu_id=L0pheU43NWJwRWVSZklWdzQ4TlhUUT09; 2016.
- 3.Zainal Ariffin O, Nor Saleha IT. National cancer Registry report 2007 Ministry of Health, Malaysia. 2011. [Google Scholar]
- 4.Abu Hassan MR, Ismail I, Mohd Suan MA, Ahmad F, Wan Khazim WK, Othman Z, Mat Said R, Tan WL, Mohammed SR, Soelar SA, et al. Incidence and mortality rates of colorectal cancer in Malaysia. Epidemiol Health. 2016;9(38):e2016007. doi: 10.4178/epih.e2016007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Law CW, Roslani AC, Ng LL. Treatment delay in rectal cancer. Med J Malaysia. 2009;64(2):163–165. [PubMed] [Google Scholar]
- 6.Lim KG: A review of colorectal cancer research in malaysia. Med J Malaysia 2014, 69 Suppl A:23-32. [PubMed]
- 7.Su TT, Goh JY, Tan J, Muhaimah AR, Pigeneswaren Y, Khairun NS, Normazidah AW, Tharisini DK, Majid HA. Level of colorectal cancer awareness: a cross sectional exploratory study among multi-ethnic rural population in Malaysia. BMC Cancer. 2013;13:376. doi: 10.1186/1471-2407-13-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yusoff HM, Daud N, Noor NM, Rahim AA. Participation and barriers to colorectal cancer screening in Malaysia. Asian Pac J Cancer Prev. 2012;13(8):3983–3987. doi: 10.7314/APJCP.2012.13.8.3983. [DOI] [PubMed] [Google Scholar]
- 9.Rashid MR, Aziz AF, Ahmad S, Shah SA, Sagap I. Colorectal cancer patients in a tertiary referral centre in Malaysia: a five year follow-up review. Asian Pac J Cancer Prev. 2009;10(6):1163–1166. [PubMed] [Google Scholar]
- 10.Kong CK, Roslani AC, Law CW, Law SC, Arumugam K. Impact of socio-economic class on colorectal cancer patient outcomes in Kuala Lumpur and Kuching, Malaysia. Asian Pac J Cancer Prev. 2010;11(4):969–974. [PubMed] [Google Scholar]
- 11.Ghazali AK, Musa KI, Naing NN, Mahmood Z. Prognostic factors in patients with colorectal cancer at Hospital Universiti Sains Malaysia. Asian J Surg. 2010;33(3):127–133. doi: 10.1016/S1015-9584(10)60022-X. [DOI] [PubMed] [Google Scholar]
- 12.Magaji BA, Moy FM, Roslani AC, Law CW. Descriptive epidemiology of colorectal cancer in University Malaya medical Centre, 2001 to 2010. Asian Pac J Cancer Prev. 2014;15(15):6059–6064. doi: 10.7314/APJCP.2014.15.15.6059. [DOI] [PubMed] [Google Scholar]
- 13.Chong VH, Abdullah MS, Telisinghe PU, Jalihal A. Colorectal cancer: incidence and trend in Brunei Darussalam. Singap Med J. 2009;50(11):1085–1089. [PubMed] [Google Scholar]
- 14.Pourhoseingholi MA. Increased burden of colorectal cancer in Asia. World J Gastrointest Oncol. 2012;4(4):68–70. doi: 10.4251/wjgo.v4.i4.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moghimi-Dehkordi B, Safaee A: An overview of colorectal cancer survival rates and prognosis in Asia. World J Gastrointest Oncol 2012, 4(4):71-75. [DOI] [PMC free article] [PubMed]
- 16.Park HM, Woo H, Jung SJ, Jung KW, Shin HR, Shin A. Colorectal cancer incidence in 5 Asian countries by subsite: an analysis of cancer incidence in five continents (1998-2007) Cancer Epidemiol. 2016;45:65–70. doi: 10.1016/j.canep.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Yeole BB, Sunny L, Swaminathan R, Sankaranarayanan R, Parkin DM. Population-based survival from colorectal cancer in Mumbai, (Bombay) India. Eur J Cancer. 2001;37(11):1402–1408. doi: 10.1016/S0959-8049(01)00108-3. [DOI] [PubMed] [Google Scholar]
- 18.Teo MC, Soo KC. Cancer trends and incidences in Singapore. Jpn J Clin Oncol. 2013;43(3):219–224. doi: 10.1093/jjco/hys230. [DOI] [PubMed] [Google Scholar]
- 19.Fang YJ, Wu XJ, Zhao Q, Li LR, Lu ZH, Ding PR, Zhang RX, Kong LH, Wang FL, Lin JZ, et al. Hospital-based colorectal cancer survival trend of different tumor locations from 1960s to 2000s. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0073528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Estimated Cancer Incidence, Mortality and Prevalence Worldwide (available at http://globocan.iarc.fr/Pages/fact_sheets_population.aspx).
- 21.Hassan MR, Suan MA, Soelar SA, Mohammed NS, Ismail I, Ahmad F. Survival analysis and Prognostic factors for colorectal cancer patients in Malaysia. Asian Pac J Cancer Prev. 2016;17(7):3575–3581. [PubMed] [Google Scholar]
- 22.Oberoi DV, Jiwa M, McManus A, Hodder R. Colorectal cancer--applying a gender lens. Qual Prim Care. 2014;22(2):71–79. [PubMed] [Google Scholar]
- 23.Al-Ahwal MS, Shafik YH, Al-Ahwal HM. First national survival data for colorectal cancer among Saudis between 1994 and 2004: what's next? BMC Public Health. 2013;13(73):1471–2458. doi: 10.1186/1471-2458-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang M, Zhao QC, Liu YP, Yang L, Zhu HM, Chhetri JK. Prognostic analysis and comparison of colon cancer in Han and Hui patients. World J Gastroenterol. 2014;20(17):5082–5086. doi: 10.3748/wjg.v20.i17.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyle T, Fritschi L, Platell C, Heyworth J. Lifestyle factors associated with survival after colorectal cancer diagnosis. Br J Cancer. 2013;109(3):814–822. doi: 10.1038/bjc.2013.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Virk R, Gill S, Yoshida E, Radley S, Salh B. Racial differences in the incidence of colorectal cancer. Can J Gastroenterol. 2010;24(1):47–51. doi: 10.1155/2010/565613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du WB, Chia KS, Sankaranarayanan R, Sankila R, Seow A, Lee HP. Population-based survival analysis of colorectal cancer patients in Singapore, 1968-1992. Int J Cancer. 2002;99(3):460–465. doi: 10.1002/ijc.10333. [DOI] [PubMed] [Google Scholar]
- 28.Loh KW, Majid HA, Dahlui M, Roslani AC, Su TT. Sociodemographic predictors of recall and recognition of colorectal cancer symptoms and anticipated delay in help- seeking in a multiethnic Asian population. Asian Pac J C Prev. 2013;14(6):3799–3804. doi: 10.7314/APJCP.2013.14.6.3799. [DOI] [PubMed] [Google Scholar]
- 29.Zacharakis M, Xynos ID, Lazaris A, Smaro T, Kosmas C, Dokou A, Felekouras E, Antoniou E, Polyzos A, Sarantonis J, et al. Predictors of survival in stage IV metastatic colorectal cancer. Anticancer Res. 2010;30(2):653–660. [PubMed] [Google Scholar]
- 30.Mehrkhani F, Nasiri S, Donboli K, Meysamie A, Hedayat A. Prognostic factors in survival of colorectal cancer patients after surgery. Colorectal Dis. 2009;11(2):157–161. doi: 10.1111/j.1463-1318.2008.01556.x. [DOI] [PubMed] [Google Scholar]
- 31.Stillwell AP, Buettner PG, Siu SK, Stitz RW, Stevenson AR, Ho YH. Predictors of postoperative mortality, morbidity, and long-term survival after palliative resection in patients with colorectal cancer. Dis Colon rectum. 2011;54(5):535–544. doi: 10.1007/DCR.0b013e3182083d9d. [DOI] [PubMed] [Google Scholar]
- 32.Veettil SK, Lim KG, Chaiyakunapruk N, Ching SM, Abu Hassan MR. Colorectal cancer in Malaysia: its burden and implications for a multiethnic country. Asian J Surg. 2016;1(16):30177–30174. doi: 10.1016/j.asjsur.2016.07.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available as the data set belong to the UMMC Medical Record Department but are available from the corresponding author on reasonable request.


