Abstract
Background
Canine test results generated by veterinarians throughout Canada from 2013–2014 were evaluated to assess the geographical distribution of canine infection with Borrelia burgdorferi, Dirofilaria immitis, Ehrlichia spp., and Anaplasma spp.
Methods
The percent positive test results of 115,636 SNAP® 4Dx® Plus tests from dogs tested were collated by province and municipality to determine the distribution of these vector-borne infections in Canada.
Results
A total of 2,844/115,636 (2.5%) dogs tested positive for antibody to B. burgdorferi. In contrast, positive test results for D. immitis antigen and antibodies to Ehrlichia spp. and Anaplasma spp. were low, with less than 0.5% of dogs testing positive for any one of these three agents nationwide. Provincial seroprevalence for antibodies to B. burgdorferi ranged from 0.5% (Saskatchewan)–15.7% (Nova Scotia); the areas of highest percent positive test results were in proximity to regions in the USA considered endemic for Lyme borreliosis, including Nova Scotia (15.7%) and Eastern Ontario (5.1%). These high endemic foci, which had significantly higher percent positive test results than the rest of the nation (P < 0.0001), were surrounded by areas of moderate to low seroprevalence in New Brunswick (3.7%), Quebec (2.8%), and the rest of Ontario (0.9%), as well as northward and westward through Manitoba (2.4%) and Saskatchewan (0.5%). Insufficient results were available from the westernmost provinces, including Alberta and British Columbia, to allow analysis.
Conclusion
Increased surveillance of these vector-borne disease agents, especially B. burgdorferi, is important as climate, vector range, and habitat continues to change throughout Canada. Using dogs as sentinels for these pathogens can aid in recognition of the public and veterinary health threat that each pose.
Keywords: Borrelia burgdorferi, Dirofilaria immitis, Ehrlichia, Anaplasma, Canada, Canine
Background
Vector-borne diseases are an emerging concern for veterinarians and physicians in much of Canada. The prevalence of vector-borne infections, including Lyme borreliosis (LB), is increasing, apparently due to changing environmental and climatic conditions [1–3]. Lyme borreliosis, heartworm, anaplasmosis, and ehrlichiosis are four common vector-borne diseases that are regularly diagnosed in dogs in the USA [4]. Determining the range and prevalence of the agents that cause these diseases throughout Canada may enhance awareness of their importance, encouraging preventive measures and leading to prompt, accurate diagnosis and appropriate treatment.
Canine LB in North America is caused by infection with the spirochete Borrelia burgdorferi (sensu stricto); other LB agents reported from people have not been identified in dogs. Disease in dogs is characterised by fever, lethargy, anorexia, and lymphadenopathy, but can progress to more severe manifestations such as arthritis and glomerulonephritis [5]. Transmission to humans and dogs is by Ixodes sp. ticks; I. scapularis is the vector for the eastern half of Canada and I. pacificus the most important vector in British Columbia [6]. Ticks harbouring B. burgdorferi have been identified throughout central and eastern Canada, including parts of Manitoba, Ontario, Quebec, Nova Scotia, and New Brunswick [7]. LB-endemic areas of Canada are defined as locations where all three life stages of the tick (larva, nymph, adult) have been collected for two consecutive years and B. burgdorferi infection has been confirmed in ticks or vertebrate hosts [8]. LB is the most commonly reported vector-borne disease in people in the USA [9]; approximately 25,000 cases are reported each year in the USA, while in Canada, approximately 900 new cases were reported in 2015, growing from only 140 cases in 2009 [10, 11]. This higher risk of infection in the US is also seen in pet dogs. Between 2012 and 2014, 7.2% of dogs tested had antibodies to B. burgdorferi in the USA [12]. In contrast, only 0.7% and 2.1% of dogs were reported to test positive in Canada in 2008–2010, respectively [13, 14].
Dirofilaria immitis, the causative agent of canine heartworm disease, is considered the most important helminth infection of dogs in the United States [4]. Mosquito vectors acquire D. immitis microfilariae when feeding on infected dogs and transmit the third-stage larvae, which then migrate and develop within dogs [15, 16]. The presence of adult heartworms in the pulmonary vasculature is a potential source of significant pathology [17–19]. Heartworm infection has been reported in dogs in Canada since 1977, but the prevalence has remained relatively low at around 0.2% [13, 20]. Because heartworm has historically been relatively uncommon in the region, most Canadian veterinary parasitologists recommend a seasonal preventive strategy. In addition, yearly testing is recommended for patients in high-risk groups, including dogs who travel to endemic areas or those not receiving any preventive, or those on a preventive with poor compliance [21]. Interestingly, over 77% of dogs that tested positive for infection with D. immitis in one report had no travel history outside the region, supporting autochthonous infection, albeit at a low level [13].
The rickettsial agents Anaplasma phagocytophilum, A. platys, Ehrlichia canis, and E. ewingii are all tick-borne bacterial pathogens infecting leukocytes or platelets of their host [22]. These agents induce similar clinical signs and laboratory findings ranging from fever, anorexia, myalgia, and thrombocytopenia to severe manifestations such as epistaxis and death [22].
Anaplasma phagocytophilum is transmitted through the bite of an Ixodes spp. tick, and is the causative agent of human granulocytic anaplasmosis (HGA) [23]. Previous canine serologic surveys in Canada have reported that the prevalence of dogs with antibodies to A. phagocytophilum is rising, with no dogs testing positive in 2006 but a prevalence ranging between 0.2–1.1% just five years later [13, 14, 24]. Anaplasma platys, causative agent of canine cyclic thrombocytopenia, is transmitted by Rhipicephalus sanguineus and infects platelets of dogs [25, 26]. In a previous study, 1.8% of dogs tested in Canada were reported to have antibodies to A. platys [14].
Ehrlichia canis is the causative agent of canine monocytic ehrlichiosis, and is also transmitted by R. sanguineus; infection causes anaemia, thrombocytopenia, and, in severe cases, potentially fatal bleeding diathesis [27, 28]. Ehrlichia ewingii is the causative agent of canine granulocytic ehrlichiosis and is transmitted by Amblyomma americanum. The range of A. americanum has dramatically expanded northward and eastward in recent decades [29]. While A. americanum populations are not yet considered established in Canada, the tick is occasionally reported from domestic dogs in Ontario with no travel history out of the region (Peregrine unpublished). Of the two, only E. canis has been reported in Canada previously, with 3.2% of dogs tested having antibodies to the pathogen, while 0/285 dogs tested positive for Ehrlichia chaffeensis or E. ewingii [14].
Evidence of past or current infection with all of these pathogens can be identified with assays commonly used for annual heartworm testing and as a screening tool for tick-borne infections, and the composite results can be evaluated on both a local and national level. For example, by reviewing the changing prevalence of antibody-positive dogs over time, previously undocumented areas of expansion of LB were detected [4, 12]. The present paper seeks to build on previous publications [13, 14], potentially identifying areas of recent expansion of LB as well as monitoring the overall distribution of these vector-borne infections in Canada.
Methods
Source of data
The data collected were obtained from the SNAP® 4Dx® Plus Test kit (IDEXX Laboratories, Inc., Westbrook, Maine, USA), an in-clinic ELISA for the simultaneous detection of canine antibodies to B. burgdorferi, A. phagocytophilum, A. platys, E. canis, and E. ewingii, and antigen of D. immitis. The results were generated from January 2013 through December 2014 by veterinarians testing patients in-clinic and recording the data manually or by IDEXX SnapShot Dx® instrumentation. For privacy, results were provided with no patient or owner identification; therefore, travel history, confirmatory diagnostics, and clinical outcome for each result is not known.
Borrelia burgdorferi assay
The analyte utilised for the B. burgdorferi assay is the C6 peptide, which detects antibodies to a surface lipoprotein of B. burgdorferi (sensu stricto). The sensitivity and specificity of the analyte are reported in the package insert to be 94.1 and 96.2% (IDEXX Laboratories, Inc., Westbrook, ME, USA), respectively, but published studies with different populations report different values. For example, in comparison to a two-tiered, gold standard diagnostic process utilising immunofluorescence (IFA) and Western blot (WB), the test sensitivity was 94.4% [30, 31], and the test specificity has been reported to be 99.5% when used on field samples from dogs [30, 32]. The C6 analyte has also been shown to not cross-react with other Borrelia spp. found in the USA or react to antibodies produced through vaccination [30].
Heartworm assay
The assay utilised detects D. immitis antigen primarily produced from the uterus of female heartworms. The sensitivity and specificity reported for the heartworm portion of the assay are 99.0 and 99.3%, respectively (IDEXX Laboratories, Inc.). Other studies have reported the sensitivity of this analyte as 84%, but that value varies with the intensity of infection, with a sensitivity of 64% when only one adult, female heartworm is present and 98% when 4 or more adult heartworms are present [31, 33].
Anaplasma assay
A single analyte used that detect antibodies to a peptide from the MSP2/p44 major surface protein of two distinct Anaplasma spp.: A. phagocytophilum and A. platys. Detection of A. platys was added after recognising significant cross-reactivity (SNAP® 4Dx® Test kit insert, IDEXX Laboratories, Inc.). The reported sensitivity and specificity of the test are 90.3% and 94.3%, respectively (IDEXX Laboratories, Inc.). The sensitivity of the assay is 99.1% for A. phagocytophilum and 89.1% for A. platys when compared to IFA, while the specificities are reported as 100 and 99.8%, respectively, although sensitivity and specificity against field samples may vary [34, 35].
Ehrlichia assay
Analytes were used that detect antibodies to the p30 and p30-1 proteins of E. canis and the p28 protein of E. ewingii. The reported sensitivity and specificity of this assay is 97.1 and 95.3%, respectively (IDEXX Laboratories, Inc.). In other studies, when compared to IFA or WB, the sensitivity was 95.7% for E. canis and 96.5% for E. ewingii [35, 36]. The test specificity for E. canis has been shown to be 100% [30, 37], while specificity for the detection of antibodies to E. ewingii is 93.9% [35]. Infection with other Ehrlichia spp. may induce cross-reactive antibodies leading to a positive test result on the Ehrlichia spp. analyte [30, 38].
Data and statistical analysis
Data were collated by a three-digit postal code of the veterinary practice where the test was performed and then assembled into municipalities or major metropolitan areas and provinces. Only municipalities reporting more than 30 test results were included in the study. Percent positive test results were calculated by dividing the number of dogs with a positive test result by the total number of test results reported for each agent of interest. For all samples, 95% confidence intervals were calculated using the modified Wald method (GraphPad Software, La Jolla, CA, USA). Maps were assembled using the Canada base map and the Hatch Map function on MapViewer 7 (Golden Software, Golden, CO, USA), which provides base maps of Canada, and then modified using the hatch map function in the software.
Differences in reported frequency of positive test results between municipalities and provinces were evaluated using a Chi-square test in StatPlus (Windows 7, Redmond, WA; AnalystSoft, Alexandria, VA, USA) with significance assigned at P < 0.0001 as previously described [4].
Results
Test results were available from a total of 225 practices in 2013 and 198 practices in 2014, representing 115,636 data points from 84 different municipalities across Canada. Ontario reported the highest number of test results (77,143) followed by Quebec (23,701), Manitoba (12,765), New Brunswick (1,631), Nova Scotia (210), and Saskatchewan (186). All other provinces and territories had fewer than 30 test results reported in a single municipality.
Borrelia burgdorferi
The prevalence of antibody positive dogs nationwide was 2.5% (2,844/115,636) with provincial prevalence ranging from 0.5–15.7% (Table 1). Over half (44/84) of the municipalities reported 2% or greater positive test results, while 7 reported less than 0.5% positive test results (Fig. 1). Positive test results for antibodies to B. burgdorferi were most common in Nova Scotia, with 15.7% of samples from this province testing positive, which was higher than the national average (χ 2 = 148.591, P < 0.0001). Other provinces had percent positive test results higher than the national average, including New Brunswick (3.7%; χ 2 = 9.743, P = 0.0018), and Quebec (2.8%; χ 2 = 15.456, P < 0.0001) (Fig. 1). Ontario had a lower overall seroprevalence than the national average (2.3%; χ 2 = 22.140, P < 0.0001), but in a cluster of 11 municipalities in eastern Ontario more than 5.1% (1,335/26,081; χ 2 = 991.266, P < 0.0001) of dogs tested positive.
Table 1.
Vector-borne infections in dogs in Canada, 2013–2014. Percent positive test results (95% confidence intervals, CI), and total number positive by province for dogs tested from 2013–2014 for antigen of Dirofilaria immitis and antibody to Borrelia burgdorferi, Ehrlichia spp. and Anaplasma spp.
| Province (Number of tests) | Borrelia burgdorferi % (95% CI) [No. positive] | Dirofilaria immitis % (95% CI) [No. positive] | Anaplasma spp. % (95% CI) [No. positive] | Ehrlichia spp. % (95% CI) [No. positive] |
|---|---|---|---|---|
| Manitoba (n = 12,765) | 2.4 (2.1–2.7) [303] | 0.20 (0.12–0.28) [26] | 0.86% (0.70–1.0) [110] | 0.24% (0.16–0.32) [31] |
| New Brunswick (n = 1,631) | 3.7 (2.9–4.7) [60] | 0.12 (0.01–0.48) [2] | 0.43 (0.19–0.90) [1] | 0.12 (0.01–0.48) [2] |
| Nova Scotia (n = 210) | 15.7 (11.4–21.3) [33] | 0.48 (0.01–2.9) [1] | 0.95 (0.04–3.6) [2] | 0 (0–2.2) [0] |
| Ontario (n = 77,143) | 2.3 (2.2–2.4) [1,780] | 0.50 (0.45–0.55) [385] | 0.22 (0.19–0.25) [166] | 0.19 (0.16–0.22) [146] |
| Quebec (n = 23,701) | 2.8 (2.6–3.0) [667] | 0.30 (0.23–0.37) [71] | 0.19 (0.13–0.25) [46] | 0.16 (0.11–0.21) [37] |
| Saskatchewan (n = 186) | 0.54 (0.01–3.3) [1] | 0 (0–2.4) [0] | 0 (0–2.4) [0] | 1.6 (0.33–4.9) [3] |
| National (n = 115,636) | 2.5 (2.4–2.6) [1,844] | 0.42 (0.38–0.46) [485] | 0.29 (0.26–0.32) [331] | 0.19 (0.16–0.22) [219] |
Fig. 1.
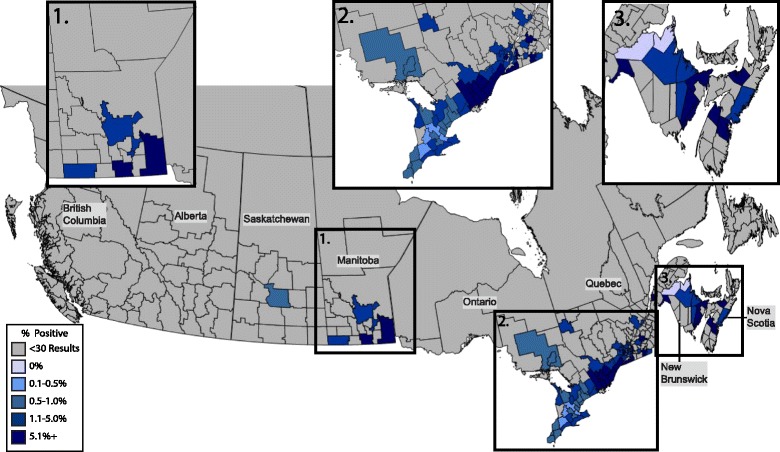
Percent positive antibody tests to Borrelia burgdorferi in dogs by municipality. Evidence of antibody to Borrelia burdorferi in dogs by municipality throughout Canada, 2013–2014, grouped according to percent positive tests
Dirofilaria immitis
Nationwide, 0.42% (485/115,636) of dogs tested positive for heartworm antigen, and no province had percent positive test results greater than 0.5% (0–0.5%) (Table 1). Ontario had the highest percent positive tests (0.50%). Two municipalities had percent positive test results higher than 2%: Mirabel, just west of Montreal, Quebec (5.0%; 2/40; 95% CI: 0.50–17.4%) and Caledonia, in southern Ontario near Toronto (4.1%; 207/5,111; 95% CI: 3.5–4.6%) (Fig. 2). Both municipalities had a higher prevalence than the national average and the rest of the respective province (χ 2 = 10.627, P = 0.0011; χ 2 = 1678.59, P < 0.0011).
Fig. 2.
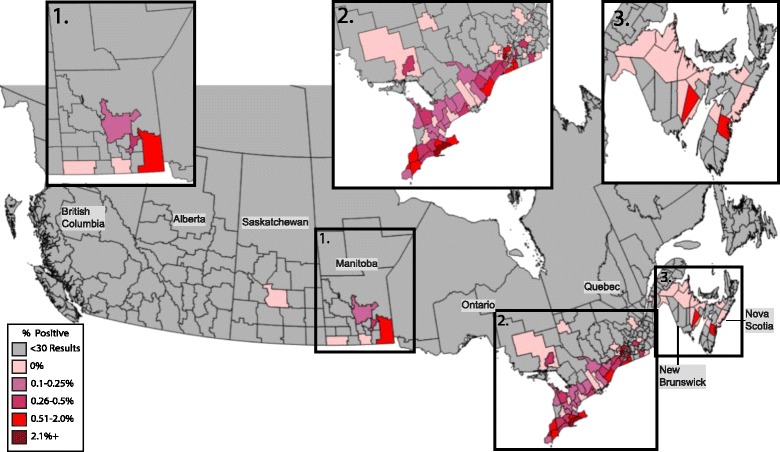
Percent positive antigen tests of Dirofilaria immitis in dogs by municipality. Evidence of antigen of Dirofilaria immitis in dogs by municipality throughout Canada, 2013–2014, grouped according to percent positive tests
Anaplasma spp
Antibody to Anaplasma spp. was detected in 0.29% (331/115,636) of dogs, with a provincial seroprevalence ranging from 0.0–0.95% (Table 1). Nova Scotia and Manitoba were the only provinces that had a higher prevalence than the national average with 0.95%, and 0.86% of all tests reported positive, respectively; the total number of positive tests in municipalities within these provinces that had a seroprevalence above 1.0% ranged between 2 and 12 positive tests (Fig. 3). Percent positive test results in Ontario were significantly lower than the national average at 0.22% (χ 2 = 40.252, P < 0.0001); no municipalities in Ontario had percent positive test results over 1.0%.
Fig. 3.
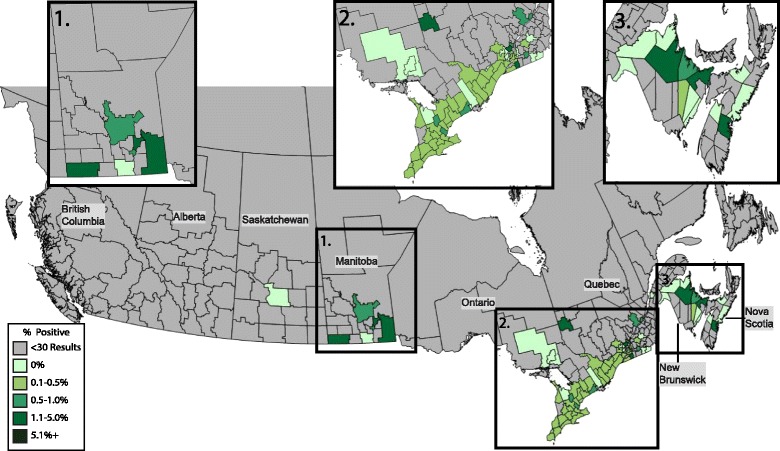
Percent positive antibody tests to Anaplasma spp. in dogs by municipality. Evidence of antibody to Anaplasma spp. in dogs by municipality throughout Canada, 2013–2014, grouped according to percent positive tests
Ehrlichia spp
Antibody to Ehrlichia spp. was identified in 0.19% of tests with a range among the provinces of 0–1.6% (Table 1). Saskatchewan had the highest seroprevalence of any province and was significantly higher than the national average (1.6%; χ 2 = 13.141, P = 0.0003). A total of 4 municipalities across Canada had a reported seroprevalence higher than 1%; Saskatoon, in central Saskatchewan (1.6%; 3/186; 95% CI: 0.33–4.9%), Hampton, in southern New Brunswick (1.3%; 2/152; 95% CI: 0.06–5.0%), and Bruce and Port Hope, in southwestern and southeastern Ontario, respectively (1.2%; 3/250; 95% CI: 0.24–3.6% and 1.0%; 6/590; 95% CI: 0.41–2.2%, respectively) (Fig. 4).
Fig. 4.
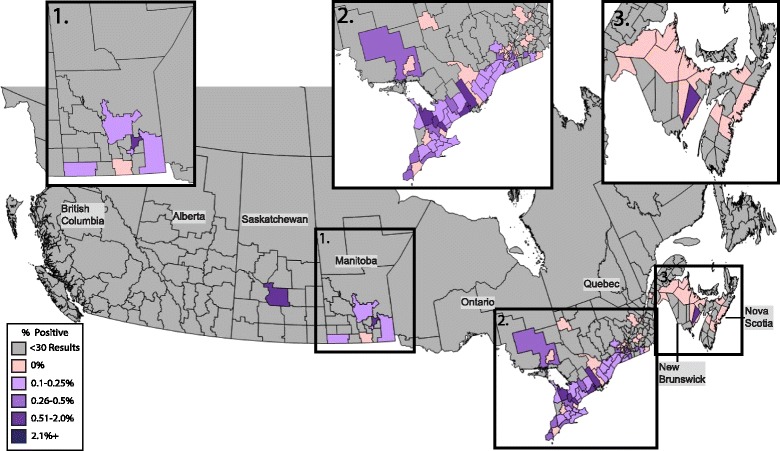
Percent positive antibody tests to Ehrlichia spp. in dogs by municipality. Evidence of antibody to Ehrlichia spp. in dogs by municipality throughout Canada, 2013–2014, grouped according to percent positive tests
Discussion
The dataset in the present paper was obtained from veterinarians in practice and allowed us to determine the prevalence of four vector-borne infections throughout Canada. As reported in previous studies, the data are biased towards major population centres where most dogs and dog owners reside [12]. While the prevalence of positive tests for heartworm antigen and antibody to Ehrlichia spp. and Anaplasma spp. were low in all provinces; there was evidence of past or current infection with at least one of these agents in every province reporting data (Table 1 and Figs. 1–4).
Percent positive tests for antibodies to B. burgdorferi were higher in the present study than reported in 2011 (0.72%; P < 0.0001), but not significantly different than more recent reports (2.1%; P = 0.70) [13, 14]. Moderate (> 1%) or high (> 5%) percent positive tests in dogs were identified in areas with frequent reports of human LB and where surveillance of ticks has confirmed the presence of B. burgdorferi [11, 39–41]. These areas are also near the northeastern or upper midwestern regions of the United States where LB is endemic or hyperendemic [12]. While the prevalence of B. burgdorferi-specific antibodies ranged from 0.5–15% for different provinces, there were also four municipalities with percent positive test results above 20%, the highest of which was Pictou County, in northern Nova Scotia at 40.6% (13/32). Areas such as Pictou County, southern Quebec, and eastern Ontario appear to constitute hyperendemic foci (> 5% positive tests) with a declining prevalence radiating outward (Fig. 1). This effect is likely exaggerated by human population clusters in southern Ontario but can also represent true foci of increased infection risk including the 11 municipalities in eastern Ontario where the seroprevalence is 5.1% versus the rest of the province with a seroprevalence of 0.87% (P < 0.0001).
Positive test results for heartworm antigen were most commonly seen near major population centres like Montreal and Toronto, with the rest of the municipalities reporting a prevalence of < 2% (Fig. 2). This urban-centered phenomenon is common in heartworm ecology in the US as domestic dogs serve as the major reservoir for infection of mosquitoes and large cities may harbour “heat islands” that create more favourable biologic conditions for the mosquitoes as compared to the surrounding rural areas [42]. While the total prevalence across Canada was quite low (0.42%) in the present paper, it was significantly higher than the previously described prevalence of 0.22% (P < 0.0001) [13]. Other studies have shown that heartworm prevalence in dogs in Canada has remained stable at approximately 0.2% over the last 30 years [21]. This apparent doubling in prevalence over the last five years may indicate increased testing of dogs in which infection is suspected, including dogs who have been adopted from areas where heartworm infections are endemic [43]. Alternatively, it could reflect a northward expansion of mosquito vectors due to changes in climate patterns in the region [44].
The analyte for Anaplasma spp. detects antibodies to both A. phagocytophilum and A. platys. Anaplasma phagocytophilum is transmitted by I. scapularis, like B. burgdorferi, and thus when mapped these two tick-borne infections often co-localize [12]. Some correlation between the two test results can be seen in this dataset, but it was not as strong as expected (Pearson’s correlation coefficient ρ = 0.34). While the municipalities with the highest Anaplasma spp. seroprevalence (> 2.0%) were associated with B. burgdorferi seroprevalence over 4.8% (ρ = 0.6), the municipalities with the highest prevalence of antibodies to B. burgdorferi (> 10%) did not correspond to high Anaplasma spp. seroprevalence (> 1%) (ρ = 0.17). Anaplasma phagocytophilum appears to circulate in nature at a lower level than B. burgdorferi, and detection of this pathogen in newly endemic areas may be difficult [4, 12, 39, 45]. The assays used in the present paper also detect antibody to A. platys, and it is not possible to differentiate that response from antibody to A. phagocytophilum. Reports of R. sanguineus, the vector for A. platys, are rare in Canada with less than 20 ticks reported per year in Ontario, in comparison to I. scapularis, which averages over 1,000 submissions each year [46]. Nonetheless, confirmed cases of A. platys in Canada have been reported as co-infections with E. canis and explained by travel to areas where R. sanguineus are more common [47].
Antibodies to Ehrlichia spp. were least commonly detected in the present study, likely due to a dearth of vector ticks in the region. As for A. platys, the risk for autochthonous transmission of E. canis by R. sanguineus in Canada is low, although travel cases may be diagnosed and reported [47]. Similarly, A. americanum, the vector of E. ewingii and E. chaffeensis, is still considered rare in this area of North America [29, 46]. Interestingly, the majority of positive tests for antibodies to Ehrlichia spp. were in southwestern Ontario, directly adjacent to the Midwest region of the United States that has now described Ehrlichia muris-like agent (EMLA) as a new I. scapularis-transmitted pathogen [48]. While more research is needed, existing data suggest antibodies to EMLA may be cross-reactive with existing assays for Ehrlichia spp. antibodies including that used in the present paper [38]. Although the natural maintenance cycle is not fully defined, EMLA has been identified in I. scapularis and white-footed mice (Peromyscus leucopus) [49, 50].
When nationwide data are collected, as in this study, there are limitations to the utility and interpretation of the data. Reporting bias, travel history, and detection method all factor into the prevalences presented [4]. In regions where low numbers of total tests are being reported, veterinarians may be using the SNAP® 4Dx® Plus Test Kit as a targeted diagnostic test rather than an annual wellness screening tool, a factor which may explain the high seroprevalence to B. burgdorferi reported from Nova Scotia (Table 1). Unfortunately, the current lack of data in western Canada prevents analysis in that region despite confirmation that B. burgdorferi is endemic in the northwestern United States and British Columbia [51]. It should also be noted that the low number of test results available in some areas and the low positive predictive values in low prevalence populations complicate interpretation [52, 53].
This nationwide data can aid veterinarians in making informed decisions on annual canine wellness procedures that would be most beneficial, including acaricide use, heartworm prevention, and vaccination for B. burgdorferi, and when evaluated over time, the results can help document the changing distribution of vector-borne infections [4, 12]. Finally, these vector-borne pathogens have been documented to cause disease in humans, and mapping the risk of canine infection also describes the areas where humans are most likely to be infected [32, 54, 55]. The species-specific nature of the B. burgdorferi analyte used in the SNAP® 4Dx® Plus Test kit may also allow for the differentiation of areas endemic for B. burgdorferi (sensu stricto) and those regions where other, or emerging, Borrelia spp. may be the main pathogen, allowing for more accurate diagnosis and specific treatments [30]. Further prevalence studies are warranted to investigate regions with no data at present and to provide updates on the changing distribution of these infections, particularly as they become newly endemic.
Conclusions
This study serves as an update on the positive test results for common vector-borne infections in dogs, in Canada. Antibodies to B. burgdorferi were most commonly identified; the prevalence of infection in many provinces and the national average was higher than previously reported. While still low, percent positive D. immitis antigen tests were twice that reported 20 years ago, suggesting an increase in the prevalence of mosquito-borne heartworm. Infections with Anaplasma spp. and Ehrlichia spp. appear to remain fairly uncommon throughout Canada. While the work described here did not control for travel or false positives, canine serology may be a tool for monitoring vector-borne infections on a large scale and can be used to track the geographic spread of these agents and assess public health risks over time. Collectively, the data support efforts by veterinarians and physicians to protect pets and people from an increasing threat of vector-borne infections.
Acknowledgements
We are grateful to the veterinary practitioners throughout Canada who are annually monitoring the health of their patients through routine screening for evidence of exposure to vector-borne pathogens.
Funding
Funding to support the data analyses and the creation of the maps was provided by the Krull-Ewing Endowment at Oklahoma State University. Salary support for BH comes from Boehringer Ingelheim.
Availability of data and materials
IDEXX Laboratories, Inc. (Westbrook, ME) maintains the proprietary rights to the dataset. The corresponding author can be directly contacted for examination or potential use of the dataset.
Author’s contributions
SL and MB conceived of the study, SL, BH and MB coordinated its design and execution and drafted the manuscript, and JG and AP reviewed and validated the data and the manuscript. All authors read and approved the final manuscript.
Competing interests
In the past five years, SL has received reimbursement, speaking fees, or research support from IDEXX Laboratories, a manufacturer of diagnostic tests for the heartworm and tick-borne disease agents. In addition, JG and MB are employees of IDEXX Laboratories. BH and AP have no competing interests to disclose.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Testing reported was conducted during routine, annual examination. The data were reported to IDEXX by the veterinary clinic with no identifying information regarding client or patient.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ELISA
Enzyme-linked immunosorbent assay
- EMLA
Ehrlichia muris-like agent
- IFA
Immunofluorescent assay
- LB
Lyme borreliosis
- WB
Western blot
Contributor Information
Brian H. Herrin, Email: brian.h.herrin@okstate.edu
Andrew S. Peregrine, Email: aperegri@ovc.uoguelph.ca
Jonas Goring, Email: jonasgoring@idexx.com.
Melissa J. Beall, Email: Melissa-beall@idexx.com
Susan E. Little, Email: susan.little@okstate.edu
References
- 1.Simon JA, Marrotte RR, Desorsiers N, Fiset J, Gaitan J, et al. Climate change and habitat fragmentation drive the occurrence of Borrelia burgdorferi, the agent of Lyme disease, at the northeastern limit of its distribution. Evolut Appl. 2014;7(7):750–764. doi: 10.1111/eva.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden NH, Radojevic M, Wu X, Duvvuri VR, Leighton PA, Wu J. Estimated effects of projected climate change on the basic reproductive number of the Lyme disease vector Ixodes scapularis. Environ Health Perspect. 2014;122(6):631–638. doi: 10.1289/ehp.1307799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisen RJ, Eisen L, Ogden NH, Beard CB. Linkages of weather and climate with Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae), enzootic transmission of Borrelia burgdorferi, and Lyme disease in North America. J Med Entomol. 2016;53(2):250–261. doi: 10.1093/jme/tjv199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowman DD, Little SE, Lorentzen L, Shields J, Sulivan MP, Carlin EP. Prevalence and geographic distribution of Dirofilaria immitis, Borrelia burgdorferi, Ehrlichia canis, and Anaplasma phagocytophilum in dogs in the United States: results of a national clinic-based serologic survey. Vet Parasitol. 2009;160(1–2):138–148. doi: 10.1016/j.vetpar.2008.10.093. [DOI] [PubMed] [Google Scholar]
- 5.Krupka I, Straubinger RK. Lyme borreliosis in dogs and cats: background, diagnosis, treatment, and prevention of infections with Borrelia burgdorferi sensu stricto. Vet Clin North Am Small Anim Prac. 2010;40(6):1103–1119. doi: 10.1016/j.cvsm.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Ogden NH, Lindsay LR, Morshed M, Sockett M, Artsob H. The rising challenge of Lyme borreliosis in Canada. Canada Comm Dis Report. 2008;34(1). http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/08vol34/dr-rm3401a-eng.php. Accessed Nov 2015. [PubMed]
- 7.Ogden NC, Trudel L, Artsob H, Barker IK, Beauchamp G, et al. Ixodes scapularis ticks collected by passive surveillance in Canada: analysis of geographic distribution and infection with the Lyme borreliosis agent Borrelia burgdorferi sensu lato. J Med Entomol. 2006;43(3):600–609. doi: 10.1093/jmedent/43.3.600. [DOI] [PubMed] [Google Scholar]
- 8.Laboratory Centre for Disease Control Consensus conference on Lyme disease. CDWR. 1991;17(13):63–70. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) National Center for Emerging and Zoonotic Infectious Diseases (NCEZID) Division of Vector-Borne Diseases (DVBD). Reported Cases of Lyme Disease- United States, 2013. Updated March 4, 2015. http://www.cdc.gov/lyme/stats/maps/map2013.html
- 10.Public Heath Agency of Canada (PHAC) National Lyme disease surveillance in Canada 2013. 2015. [Google Scholar]
- 11.Hatchette TF, Johnston BL, Schleihauf E, Mask A, Haldan D, et al. Epidemiology of Lyme disease, nova Scotia, Canada, 2002–2013. Emerg Infect Dis. 2015;21(10):1751–1758. doi: 10.3201/eid2110.141640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Little SE, Beall MJ, Bowman DD, Chandrashekar R, Stamaris J. Canine infection with Dirofilaria immitis, Borrelia burgdorferi, Anaplasma spp., and Ehrlichia spp. in the United States, 2010–2012. Parasit Vectors. 2014;7:257–265. doi: 10.1186/1756-3305-7-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villeneuve A, Goring J, Marcotte L, Overvelde S. Seroprevalence of Borrelia burgdorferi, Anaplasma phagocytophilum, Ehrlichia canis and Dirofilaria immitis among dogs in Canada. Can Vet J. 2011;42(5):527–530. [PMC free article] [PubMed] [Google Scholar]
- 14.Quorollo BA, Chandrashekar R, Hegarty BC, Beall MJ, Stillman BA, Liu J, et al. A serological survey of tick-borne pathogens in dogs in North America and the Caribbean as assessed by Anaplasma phagocytophilum, A. platys, Ehrlichia canis, E. chaffeensis, E. ewingii and Borrelia burgdorferi species-specific peptides. Infect Ecol Epidemiol. 2014 Oct;4:24699. http://dx.doi.org/10.3402/iee.v4.24699. [DOI] [PMC free article] [PubMed]
- 15.Kotani T, Powers KG. Developmental stages of Dirofilaria immitis in the dog. Am J Vet Res. 1982;43:2199–2206. [PubMed] [Google Scholar]
- 16.Kume S, Itagaki S. On the life-cycle of Dirofilaria immitis in the dog as the final host. Br Vet J. 1955;111:16–24. [Google Scholar]
- 17.Jackson RF. Proceedings of the Heartworm Symposium 1974, Auburn, AL. American Heartworm Society. 1974. The venae cavae syndrome; pp. 48–50. [Google Scholar]
- 18.Ishihara K, Kitagawa H, Ojima M, Yagata Y, Suganuma Y. Clinicopathological studies on canine dirofilarial hemoglobinuria. Jap J Vet Sci. 1978;40:525–537. doi: 10.1292/jvms1939.40.525. [DOI] [PubMed] [Google Scholar]
- 19.Atwell R, Tarish JH. The effect of oral, low-dose prednisolone on the extent of pulmonary pathology associated with dead Dirofilaria immitis in a canine lung model. In Sol MD, Knight DH (eds): Proceedings of the Heartworm Symposium. Batavia, IL: American Heartworm Society; 1995. p. 103-11.
- 20.Slocombe JOD, Villeneuve A. Heartworm in dogs in Canada in 1991. Can Vet J. 1993;34(10):630–633. [PMC free article] [PubMed] [Google Scholar]
- 21.Klotins KC, Martin SW, Bonnett BN, Peregrine AS. Canine heartworm testing in Canada: are we being effective? Can Vet J. 2000;41(12):929–937. [PMC free article] [PubMed] [Google Scholar]
- 22.Rikihisa Y. The tribe Ehrlichieae and ehrlichial diseases. Clin Microbiol Rev. 1991;4:286–308. doi: 10.1128/CMR.4.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rikihisa Y. New findings on members of the family Anaplasmataceae of veterinary importance. Ann NY Acad Sci. 2006;1078:438–445. doi: 10.1196/annals.1374.083. [DOI] [PubMed] [Google Scholar]
- 24.Gary AT, Webb JA, Hegarty BC, Breitschwerdt EB. The low seroprevalence of tick-transmitted agents of disease in dogs from southern Ontario and Quebec. Can Vet J. 2006;47:1194–1200. [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey JW, Simpson CF, Gaskin JM. Cyclic thrombocytopenia induced by a Rickettsia-like agent in dogs. J Infect Dis. 1978;137(2):182–188. doi: 10.1093/infdis/137.2.182. [DOI] [PubMed] [Google Scholar]
- 26.Harvey JW. Thromocytotropic anaplasmosis (A. platys infection) In: Greene CE, editor. Greene’s infectious diseases of the dog and cat. St. Louis, MO: Saunders Elsevier; 2006. pp. 229–231. [Google Scholar]
- 27.Harrus S, Waner T. Diagnosis of canine monocytotropic ehrlichiosis (Ehrlichia canis): an overview. Vet J. 2011;187(3):292–296. doi: 10.1016/j.tvjl.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Neer TM, Harrus S. Canine monocytotropic ehrlichiosis and neorickettsiosis (E. canis, E. chaffeensis, E. ruminantium, N. sennetsu, and N. risticii infections) In: Greene CE, editor. Infectious diseases of the dog and cat. 3. St. Louis, MO: Saunders Elsevier; 2006. pp. 203–216. [Google Scholar]
- 29.Springer YP, Eisen L, Beati L, James AM, Eisen RJ. Spatial distribution of counties in the continental United States with records of occurrence of Ambylomma americanum (Ixodida: Ixodidae) J Med Entomol. 2014;51(2):342–351. doi: 10.1603/ME13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Connor TP, Esty KJ, Hansom JL, Shields P, Philipp MT. Dogs vaccinated with common Lyme disease vaccines do not respond to IR6, the conserved immunodominant region of the VlsE surface protein of Borrelia burgdorferi. Clin Diagn Lab Immunol. 2004;11:458–462. doi: 10.1128/CDLI.11.3.458-462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chandrashekar R, Mainille CA, Beall MJ, O’Connor T, Eberts MD, et al. Performance of a commercially available in-clinic ELISA for the detection of antibodies against Anaplasma phagocytophilum, Ehrlichia canis, and Borrelia burgdorferi and Dirofilaria immitis antigens in dogs. Am J Vet Res. 2010;71:1443–1450. doi: 10.2460/ajvr.71.12.1443. [DOI] [PubMed] [Google Scholar]
- 32.Duncan AW, Correa MT, Levine JF, Breitschwerdt EB. The dog as sentinel for human infection: prevalence of Borrelia burgdorferi C6 antibodies in dogs from southeastern and mid-Atlantic states. Vector Borne Zoonotic Dis. 2005;5(2):101–109. doi: 10.1089/vbz.2005.5.101. [DOI] [PubMed] [Google Scholar]
- 33.Atkins CE. Comparison of results of three commercial heartworm antigen test kits in dogs with low heartworm burdens. J Am Vet Med Assoc. 2003;222:1221–1223. doi: 10.2460/javma.2003.222.1221. [DOI] [PubMed] [Google Scholar]
- 34.Chandrashekar R, Mainville C, Daniluk D, Cambell J, Cyr K, O’Connor TP. (2007) Performance of an in-clinic test, SNAP®4Dx®, for the detection of antibodies to canine granulocytic infection, Anaplasma phagocytophilum. In: Research Abstracts of the 25th Annual ACVIM forum, Seattle, WA, 2007 June.
- 35.Stillman BA, Monn M, Liu J, Thatcher B, Foster P, et al. Performance of a commercially available in-clinic ELISA for detection of antibodies against Anaplasma phagocytophilum, Anaplasma platys, Borrelia burgdorferi, Ehrlichia canis, and Ehrlichia ewingii and Dirofilaria immitis antigen in dogs. J Am Vet Med Assoc. 2014;245(1):80–86. doi: 10.2460/javma.245.1.80. [DOI] [PubMed] [Google Scholar]
- 36.O’Connor TP, Esty KJ, Machenry P, Hansom JL, Bartol BA, Lawton T. American heartworm society triannual symposium. 2002. Performance evaluation of Ehrlichia canis and Borrelia burgdorferi peptides in a new Dirofilaria immitis combination assay; pp. 77–84. [Google Scholar]
- 37.O‘Connor TP, Hanscom JL, Hegarty BC, Groat RG, Breitschwerdt EB. Comparison of an indirect immunofluorescence assay, western blot analysis, and a commercially available ELISA for detection of Ehrlichia canis antibodies in canine sera. Am J Vet Res. 2006;67:206–210. doi: 10.2460/ajvr.67.2.206. [DOI] [PubMed] [Google Scholar]
- 38.Hegarty BC, Maggi RG, Koskinen P, Beall MJ, Eberts M, Chandrashekar R, Breitschwerdt EB. Ehrlichia muris infection in a dog from Minnesota. J Vet Intern Med. 2012;26(5):1217–20. [DOI] [PubMed]
- 39.Werden L, Lindsay LR, Barker IK, Bowman J, Gonzales EK, Jardine CM. Prevalence of Anaplasma phagocytophilum and Babesia microti in Ixodes scapularis from a newly established Lyme disease endemic area, the Thousand Islands Region of Ontario, Canada. Vector Borne Zoonotic Dis. 2015;15(10):627–629. doi: 10.1089/vbz.2015.1792. [DOI] [PubMed] [Google Scholar]
- 40.Ogden NH, Bouchard C, Kurtenbach K, Margos G, Lindsay LR, Trudel L, et al. Active and passive surveillance and phylogenetic analysis of Borrelia burgdorferi elucidate the process of Lyme disease risk emergence in Canada. Environ Health Perspect. 2010;118(7):909–914. doi: 10.1289/ehp.0901766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gabriele-Rivet V, Arsenault J, Badcock J, Cheng A, Edsall J, et al. Different ecological niches for ticks of public health significance in Canada. PLoS One. 2015;10(7):e0131282. doi: 10.1371/journal.pone.0131282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paras KL, O’Brien VA, Reiskind MH. Comparison of the vector potential of different mosquito species for the transmission of heartworm, Dirofilaria immitis, in rural and urban areas in and surrounding Stillwater, Oklahoma U.S.A. Med Vet Entomol. 2014;28:60–67. doi: 10.1111/mve.12069. [DOI] [PubMed] [Google Scholar]
- 43.Bourguinat C, Keller K, Bhan A, Peregrine A, Geary T, Prichard R. Macrocyclic lactone resistance in Dirofilaria immitis. Vet Parasitol. 2011;181(2–4):388–392. doi: 10.1016/j.vetpar.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 44.Ogden NH, Radojevic M, Caminade C, Gachon P. Recent and projected future climatic suitability of North America for the Asian tiger mosquito Aedes albopictus. Parasit Vectors. 2014;7:532–545. doi: 10.1186/s13071-014-0532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dahlgren FS, Heitman KN, Behravesh CB. Undetermined human ehrlichiosis and anaplasmosis in the United States, 2008–2012: A catch-all for passive surveillance. Am J Trop Med Hyg. 2016;94(2):229–301. doi: 10.4269/ajtmh.15-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelder MP, Russel C, Lindsay LR, Dhar B, Patel SN, et al. Population-based passive tick surveillance and detection of expanding foci of blacklegged ticks Ixodes scapularis and the Lyme disease agent Borrelia burgdorferi in Ontario, Canada. PLoS One. 2014;9(8):e105358. [DOI] [PMC free article] [PubMed]
- 47.Al Izzi S, Martin DS, Chan RY, Leutenegger CM. Babesia canis vogeli, Ehrlichia canis, and Anaplasma platys infection in a dog. Vet Clinic Pathol. 2013;42(4):471–475. doi: 10.1111/vcp.12090. [DOI] [PubMed] [Google Scholar]
- 48.Johnson DK, Schiffman EK, Davis JP, Neitzel DF, Sloan LM, et al. Human infection with Ehrlichia muris-like pathogen, United States, 2007-2013(1) Emerg Infect Dis. 2015;21(10):1794–1799. doi: 10.3201/eid2110.150143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saito TB, Walker DH. A tick vector transmission model of monocytotropic ehrlichiosis. J Infect Dis. 2015;212(6):968–977. doi: 10.1093/infdis/jiv134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castillo CG, Eremeeva ME, Paskewitz SM, Sloan LM, Lee X, et al. Detection of human pathogenic Ehrlichia muris-like agent in Peromyscus leucopus. Ticks Tick Borne Dis. 2015;6(2):155–157. doi: 10.1016/j.ttbdis.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Morshed MG, Lee MK, Man S, Renando K, Wong Q, et al. Surveillance for Borrelia burgdorferi in Ixodes ticks and small rodents in British Columbia. Vector Borne Zoonotic Dis. 2015;15(11):701–705. doi: 10.1089/vbz.2015.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peregrine AS. Rational use of diagnostic tests chapter 133. In: Ettinger SJ, Feldman EC, editors. Textbook of veterinary internal medicine: diseases of the dog and cat. sixth. St. Louis, MO: Elsevier Saunders; 2005. pp. 489–492. [Google Scholar]
- 53.Peregrine AS, Barker IK, Abrams-Ogg ACG, Woods JP. Screening dogs in Ontario for Borrelia burgdorferi and Ehrlichia canis should be selective rather than routine (Letter to the Editor) Can Vet J. 2007;48:673. [PMC free article] [PubMed] [Google Scholar]
- 54.Schurer JM, Ndao M, Quewezance H, Elmore SA, Jenkins EJ. People, pets, and parasites: one health surveillance in southeastern Saskatchewan. Am J Trop Med Hyg. 2014;90(6):1184–1190. doi: 10.4269/ajtmh.13-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaito A, Gjivoje V, Lutz S, Baxter B. Comparative analysis of the infectivity rate of both Borrelia burgdorferi and Anaplasma phagocytophilum in humans and dogs in a New Jersey community. Infect Drug Resist. 2014;7:199–201. doi: 10.2147/IDR.S68742. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
IDEXX Laboratories, Inc. (Westbrook, ME) maintains the proprietary rights to the dataset. The corresponding author can be directly contacted for examination or potential use of the dataset.


