Abstract
Background
Plasmodium vivax uses multiple ligand-receptor interactions for preferential invasion of human reticulocytes. Several of these ligands have been identified by in silico approaches based on the role displayed by their orthologs in other Plasmodium species during initial adhesion or invasion. However, the cell adhesion role of proteins that are exclusive to species that specifically invade reticulocytes (as P. vivax and P. cynomolgi) has not been evaluated to date. This study aimed to characterise an antigen shared between Plasmodium species that preferentially infect reticulocytes with a focus on assessing its binding activity to target cells.
Results
An in silico analysis was performed using P. vivax proteome data to identify and characterise one antigen shared between P. vivax and P. cynomolgi. This led to identification of the pvrbsa gene present in the P. vivax VCG-I strain genome. This gene is transcribed in mature schizonts and encodes a protein located on the parasite surface. rPvRBSA was antigenic and capable of binding to a population of reticulocytes with a different Duffy phenotype. Interestingly, the molecule showed a higher percentage of binding to immature human reticulocytes (CD71hi).
Conclusions
This study describes for the first time, a molecule involved in host cell binding that is exclusive in reticulocyte-infecting Plasmodium species. This suggest that PvRBSA is an antigenic adhesin that plays a role in parasite binding to target cells.
Electronic supplementary material
The online version of this article (doi:10.1186/s13071-017-2185-6) contains supplementary material, which is available to authorized users.
Keywords: Plasmodium vivax, Antigenic protein, Adhesin, Reticulocyte
Background
Basic research in P. vivax has been delayed, mainly due to difficulties associated with its in vitro propagation, resulting from the predilection of this species for invading immature erythrocyte cells (reticulocytes) [1, 2]. Consequently, bioinformatics approaches represent a good solution for identifying in silico vaccine candidates in P. vivax by comparative analysis, bearing in mind that many invasion-associated proteins from other Plasmodium species have already been described. Information derived from omics studies of P. vivax (genome [3], transcriptome [4] and proteome [5–8]) has been useful for large-scale analysis of gene composition, transcripts and parasite proteins and, importantly, facilitate in silico predictions on the function of many P. vivax proteins.
Furthermore, in silico tools have been instrumental in characterising some P. vivax molecules interacting with reticulocytes, such as the Duffy binding protein (DBP) [9], reticulocyte binding proteins (RBP) [10–12], merozoite surface protein-1 (MSP-1) [13], rhoptry neck protein-5 (RON5) [14] and, recently, the P. vivax GPI-anchored micronemal antigen (GAMA) protein (manuscript in press). However, the number of P. vivax target cell binding proteins identified to date is low compared to available information on P. falciparum, suggesting that further studies are required to supplement the current set of P. vivax adhesin data, to improve our understanding of the molecular basis of parasite invasion.
Identifying P. vivax molecules with a role in host cell invasion by their similarity with proteins in P. falciparum has been a very promising approach. However, this has limitations when identifying those molecules involved in parasite recognition and invasion of reticulocytes. This study aimed to characterise a specific molecule from species infecting reticulocytes (e.g. P. vivax and P. cynomolgi) by determining its target cell binding profile.
Methods
Bioinformatics analysis, primer design and peptide synthesis
The currently available information published in P. vivax proteome studies [5–8] was used as the source for analysing in silico proteins which might be vaccine candidates. The criteria for selecting proteins included: a prominent expression of the codifying genes > 35 h post-invasion (required) according to transcriptome study of the P. vivax intra-erythrocyte life-cycle [4]; a positive prediction by SignalP 4.1 [15] and BaCelLo [16] of a secretion signal sequence and extracellular localisation, respectively; the presence (or not) of a GPI anchor sequence using FragAnchor software [17], as well as the presence of repeats having 90% similarity in amino acid (aa) sequences using T-REKS algorithm [18]. The Phobius [19], HMMTOP [20] and TMHMM [21] servers were used to predict transmembrane regions. The selected genes were analysed to identify orthologs in other Plasmodium species according to the PlasmoDB [22] and the Kyoto Encyclopedia of Genes and Genomes ortholog clusters (KEGG OC) [23] databases. The sequence of any gene selected for being characterised was scanned in the PlasmoDB database and used for manually designing specific primers (using Generunner software, version 3.05), the same as for B-cell linear epitopes all along their encoding sequence, predicting the highest average values for hydrophilicity, solvent accessibility and Parker’s antigenicity using ANTHEPROT software [24].
Propagating VCG-I strain parasites and isolating schizonts
Vivax Colombia Guaviare-I (VCG-I) strain parasites were propagated six years ago and used as the source of biologic material, as previously described in detail [25]. The blood sample containing parasite-infected cells was collected in heparin tubes and passed through a discontinuous Percoll gradient (GE Healthcare, Uppsala, Sweden), according to an already-established protocol [26]. The schizont-stage enriched parasites were isolated from cells by incubating them for 5 min in 0.02 mM saponin buffer containing 7 mM K2HPO4, 1 mM NaH2PO4, 11 mM NaHCO3, 58 mM KCl, 56 mM NaCl, 1 mM MgCl2 and 14 mM glucose, pH 7.5 and then washed extensively with PBS, pH 7.0.
Extracting biological material
Isolated parasites were used as RNA, genomic DNA (gDNA) and total protein source. Total RNA was extracted from the sample using the Trizol method and treated with RQ1 (RNA-qualified) RNase-free DNase (Promega, Madison, USA) according to the manufacturer’s recommendations. SuperScript III enzyme (RT+) (Invitrogen, Carlsbad, USA) was used for synthesising complementary DNA (cDNA) in the following conditions: 65 °C for 5 min, 50 °C for 1 h and 70 °C for 15 min. An additional reaction without the SuperScript III enzyme (RT-) was used as negative control, following 15 min incubation at 37 °C with RNase (Promega). A Wizard Genomic purification kit (Promega) was used for obtaining the gDNA. Regarding protein extraction, the parasites were homogenised in lysis buffer containing 5% SDS, 10 mM PMSF, 10 mM iodoacetamide, 1 mM EDTA and then spun at 16,000× g for 5 min. The proteins were recovered from the supernatant and quantified using a BCA protein assay kit (Thermo Scientific, Rockford, USA). RNA, cDNA, gDNA and total protein were stored at -70 °C until later use.
Gene cloning and sequencing
The gDNA and cDNA (RT+ and RT-) samples were used as template in 25 μl PCR reactions containing 1× KAPA HiFi HotStart ReadyMix (KAPA Biosystems, Woburn, MA, USA), 0.3 μM primers and DNAse-free water for completing the reaction volume. Specific primers were designed for amplifying the entire P. vivax reticulocyte binding surface antigen (pvrbsa) gene (Forward 5'-ATG AAA GGA ATA ATG AAT GG TT-3' and Reverse 5'-ATA ACC ATC CAA ATC GTC AAA-3') or for producing the recombinant protein excluding the signal peptide and the transmembrane region (Forward 5'-ATG ATA TTG TAC AGC GAC GAC TC-3' and Reverse 5'-GCT ATC TTT CTT CAC ATT ATA C-3').
The PCR began with a denaturing step at 98 °C for 3 min, followed by 35 cycles at 98 °C for 20 s, 56 °C for 15 s and 72 °C for 30 s. A Wizard PCR preps kit (Promega) was used for purifying gene amplicons obtained from three independent PCRs done with the RT+ and gDNA samples, once quality had been evaluated on agarose gel. Purified products were ligated to the pEXP5 CT/TOPO expression vector or in a new in house designed vector (pELMO) [27] for the gene obtained from gDNA and transformed in E. coli TOP10 chemically competent cells (Invitrogen). Several clones were grown for purifying the plasmid using an UltraClean mini plasmid prep purification kit (MO BIO Laboratories, California, USA). The insert integrity and correct orientation were then confirmed by sequencing, using an ABI-3730 XL sequencer (MACROGEN, Seoul, South Korea). ClustalW (NPS) software [28] was used for comparing manually the gene sequences from the Sal-I reference strain [3] and the primate-adapted VCG-I strain.
Recombinant protein expression and extraction
E. coli BL21-DE3 (Invitrogen) cells which had been previously transformed with the recombinant plasmids were grown in Luria-Bertani (LB) medium containing 100 μg/ml ampicillin, overnight at 37 °C using a Lab-line Incubator Shaker. The initial inoculum was seeded in 1 l LB and handled in the aforementioned conditions until reaching 0.5 OD600. After the culture was incubated on ice for 30 min, IPTG 1 mM was then used to induce expression for 16 h at room temperature (RT) at ~200 rpm. The cells were harvested by spinning at 2,400× g for 20 min and used for native extraction procedures. A new protocol for extracting proteins in a soluble form was used. Briefly, cellular pellet obtained from E. coli expressing PvRBSA was freeze/thawed for 3 cycles and then homogenised in native extraction buffer (NEB) (50 mM Tris-Cl, 300 mM NaCl, 25 mM imidazole, 0,1 mM EGTA and 0.25% Tween-20, pH 8.0). The mixture was then incubated for 1 h at 4 °C at 10 rpm using a tube rotator (Fisher Scientific, Waltham, USA) and the supernatant was collected by spinning at 16,000× g for 1 h.
Protein purification
Solid-phase affinity chromatography was used for protein purification. The Ni+2-NTA resin (Qiagen, Valencia, CA, USA) was pre-equilibrated with NEB buffer, incubated with E. coli lysate overnight at 4 °C and the protein-resin mixture was then placed on a column. The unbound proteins were eluted by washing with 20 ml NEB buffer containing 0.1% Triton X-114 followed by 50 ml of the same buffer without detergent. Bound proteins were eluted with PBS containing imidazole at increasing concentrations (50 mM to 500 mM) in 3 ml fractions. The purification was confirmed by Coomassie blue staining and the fractions pooled and dialysed extensively in PBS, pH 7.2. The protein was quantified using a micro BCA protein assay kit (Thermo Scientific) and bovine serum albumin (BSA) as reference curve.
Obtaining polyclonal antibodies
The VCG-I strain PvRBSA sequence was used for designing two 20 aa-long peptides (CG-KRNSSVSSLDSDMGSYKNKS-GC (peptide 39478) and CG-VFGKGRKKPMKVKKGGGKIS-GC (peptide 39480)) which were then synthesised, according to a previously-established methodology [29], polymerised, lyophilised and characterised by RP-HPLC and MALDI-TOF MS. New Zealand rabbits were immunised with a 500 μg dose of each synthetic peptide emulsified in Freund’s complete adjuvant (FCA) (Sigma, Missouri, USA) on day 0, whilst the same emulsified mixture in Freund’s incomplete adjuvant (FIA) was inoculated on days 21 and 42. The pre-immune sera were collected before the first immunisation and hyper-immune sera were collected 20 days after the last dose. Specific antibodies were purified by affinity chromatography using CNBr-activated Sepharose 4B (Amersham, Uppsala, Sweden). Briefly, 5 μmol of peptide were diluted in coupling buffer (0.1 M NaHCO3, 0.5 M NaCl pH 8.3) and then incubated for 16 h at 4 °C with Sepharose resin. After washing ligand excess with 5 volumes of coupling buffer, the resin-free groups were blocked with 0.1 M buffer Tris–HCl for 2 h at quiescence at RT, followed by washing the resin 3 times with alternate pH solutions (0.1 M acetate buffer with 0.5 M NaCl, pH 4.0 and 0.1 M Tris-HCl with 0.5 M NaCl, pH 8.0). Five ml of each rabbit hyper-immune serum (diluted at 1:1 ratio with buffer coupling) were passed through the resin after being homogenised with PBS. Unbound antibodies were washed with 10 ml buffer coupling while strongly bound antibodies were eluted with 1 ml elution buffer (0.1 M glycine pH 7, 6, 5, 3.9 and 2.9) at descendant pH and neutralised with 1 M Tris pH 8.0 in a 1:9 ratio (elution buffer:neutralisation buffer). The antibodies were incubated with 45% ammonium sulphate for 1 h on ice with constant stirring and then for 16 h at 4 °C without shaking. After spinning at 16,000× g for 15 min, the pellet was homogenised in 100 μl PBS and the sample was extensively dialysed and stored at -20 °C until use.
Protein localisation by indirect immunofluorescence (IFI)
Slides containing Aotus monkey infected reticulocytes were previously prepared, as described in previous work [30]. The samples were fixed and permeabilised by incubating them for 5 min at RT with PBS containing 4% paraformaldehyde (v/v) and then with PBS with 0.1% Triton X-100 (v/v). After blocking with 1% BSA-PBS solution (v/v) for 1 h at RT, each sample was incubated with anti-PvRBSA rabbit antibodies (1:30) or anti-PvRON2 mouse antibodies (1:20) in the same conditions. FITC-conjugated anti-rabbit IgG antibody (Sigma) at 1:30 dilution and Rhodamine-conjugated anti-mouse IgG antibody (1:200) monoclonal secondary antibodies were used for 1 h in darkness at RT. DAPI (0.5 μg/ml) was used for staining parasite nuclei for 10 min at RT and then was washed several times with PBS to remove excess reagent. The slides were examined under a florescence microscope (Olympus BX51) using 100× oil immersion objective.
Western blot analysis of recombinant and parasite proteins
Total parasite and recombinant proteins were separated on 12% SDS-PAGE and transferred to nitrocellulose membranes which were blocked with 5% skimmed milk in TBS-0.05% Tween for 1 h. The membrane was cut into strips to be incubated for 1 h at RT with rabbit anti-PvRBSA purified antibodies (1:100 dilution) and then with the phosphatase-coupled goat anti-rabbit IgG monoclonal secondary antibody (1:5,000) (Catalogue 9503 F, ICN) in the same conditions. The positive control for rPvRBSA Western blotting was a strip incubated with peroxidase-coupled mouse anti-histidine monoclonal antibody (1:4,500) (Catalogue A7058, Sigma). The blots were revealed with a BCIP/NBT colour development substrate kit (Promega) or VIP peroxidase substrate kit (Vector Laboratories, Burlingame, Canada) according to the manufacturers’ indications. Each band’s expected weight was determined by linear regression using XL-OptiProtein (Applied Biological Materials Inc, Richmond, BC, Canada) weight marker as reference.
Enzyme-linked immunosorbent assay (ELISA)
The recombinant protein was used for evaluating the presence of anti-rPvRBSA antibodies in samples taken from P. vivax-exposed individuals (who had suffered at least one episode of infection) in the municipality of Tierra Alta, Córdoba. The negative controls used here came from sera from healthy individuals who had never been affected by the disease. The ELISA was performed as described previously [30].
Cell binding assay
Cord blood samples were typified for determining the Duffy phenotype (Fya+/Fyb−; Fya−/Fyb+; Fya+/Fyb+) by standard blood banking methods using anti-Fya and Fyb sera. Five μL of cells were then incubated with 25 μg rPvRBSA for 16 h at 4 °C at 4 rpm. DBP region II and III/IV were used as positive and negative controls, respectively [9]. After washing with 1% BSA-PBS solution (v/v), the sample was incubated with mouse anti-His-PE monoclonal antibody (1:40 dilution) (MACSmolecular-Miltenyi Biotec, San Diego, CA, USA) for 30 min in darkness. Reticulocytes and white cells were stained by incubating with anti-CD71 APC-H7 Clone M-A712 (1:80 dilution) (Becton Dickinson, Franklin Lakes, NJ, USA) and anti-CD45 APC clone 2D1 (1:80 dilution) (Becton Dickinson) monoclonal antibodies for 20 min at RT. A FACSCanto II cytometer (BD, San Diego, CA, USA) was then used for quantifying erythrocyte binding and FlowjoV10 software for analysing 1 million events. PE signal intensity was evaluated as a function of CD71 signal to determine CD71 low (CD71lo) and high (CD71hi) cells.
Statistical analysis
Statistical significance was assessed by comparing means, using a 0.05 significance level. Mann-Whitney U-test analysis was used for comparing the mean of the experimental group with the control in ELISA. Differences between means were compared by Tukey’s range test when comparing multiple groups or t-test for comparing two groups for binding assays. GradhPad Software (San Diego, CA) was used for all statistical analysis. Mean values and standard deviations (SD) were calculated from the measurements of three independent experiments.
Results
Predicting P. vivax invasion-related proteins
The criteria established in the methodology led to identifying several genes encoding P. vivax molecules which play a role in cell binding (as previously reported), such as the RBPs [31], some RONs [14] and GAMA. Interestingly, one gene encoding a 48 kDa protein (PlasmoDB database ID: PVX_096055) was identified which, apart from P. vivax, was also present in Plasmodium cynomolgi (one species infecting reticulocytes). This gene was named the P. vivax reticulocyte binding surface antigen (PvRBSA) according to the results showed in this study.
Regarding the pvrbsa gene, it presence and transcription in the P. vivax VCG-I strain was confirmed by PCR using specific primers (designed using the Sal-I strain gene sequence) and schizont gDNA and cDNA as template. Fig. 1a shows a 1.4 to 1.6 kbp amplification product using gDNA (Lane 2) corresponding to the complete gene whilst a 1.2 to 1.4 kbp product was obtained using cDNA as template (Lane 4). No product was amplified in the control sample, thereby indicating that the synthesised cDNA had not become contaminated with gDNA (Fig.1a, Lane 3). Aligning the gene sequences from the Aotus monkey- adapted VCG-I strain with those from the Sal-I reference strain led to one synonymous, 9 non-synonymous mutations and one deletion being identified (Table 1). Comparing the sequences obtained from cDNA (1,269 bp) (deposited in the NCBI under GenBank access KY349105) and gDNA (1,485 bp) led to observing that the pvrbsa gene was encoded by two exons, the first covering the signal peptide according to in silico prediction (D-cutoff = 0.450) (Fig. 1b). The pvrbsa gene encoded a 423 aa long protein having a molecular weight of around ~47.09 kDa including a signal peptide and being 7 residues shorter than that for the Sal-I strain. PvRBSA had 2 transmembrane regions located between residues 332 to 377 according to prediction by TMHMM, Phobius and HMMTOP servers and one repeat region (RR) located in aa 103 to 137, consisting of residues LT(G/E)S(N/R)ES as predicted by T-REKS (Fig. 1b); these have been numbered according to the VCG-I strain PvRBSA amino acid sequence. Amplifying pvrbsa from the synthesised cDNA sample confirmed that the gene was transcribed in schizonts, coinciding with transcriptional analysis of 3 P. vivax clinical isolates where pvrbsa had a prominent expression profile during TP7-TP9 times corresponding to parasite development during the mature stage (early and late schizonts) of the intra-reticulocyte life-cycle [4].
Fig. 1.
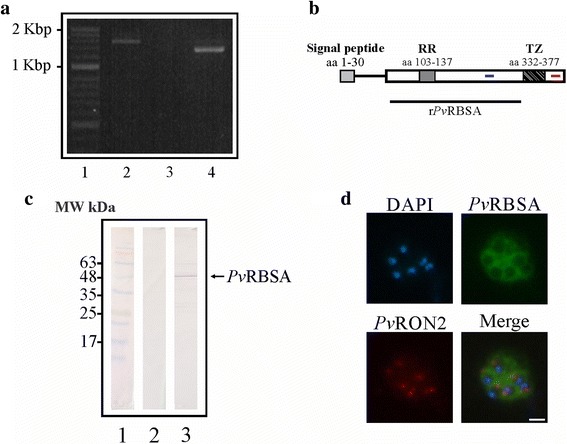
PvRBSA characterisation in the P. vivax VCG-I strain. a pvrbsa gene presence and transcription in schizonts. Lane 1 indicates the molecular weight pattern. Lanes 2 to 4 show gene amplification using gDNA, cDNA-RT- and cDNA-RT+, respectively. b In silico characterisation of PvRBSA. The diagram shows signal peptide, repeat region (RR) and transmembrane zone (TZ) location. The peptides selected for rabbit immunisation are highlighted in blue (peptide 39478) and red (peptide 39480) lines. The recombinant protein region expressed is shown by a black line. c Recognition of PvRBSA in parasite lysate. Lane 1 indicates the molecular weight (MW) marker. Lanes 2 and 3 show recognition of the protein using pre-immune and hyper-immune sera, respectively. d Sub-cellular location of PvRBSA in schizonts. The images show recognition of PvRBSA (green), PvRON2 (red) and the nuclei (blue). Scale-bar: 1 μm
Table 1.
PvRBSA mutations found by comparing the nucleotide and amino acid sequences of P. vivax VCG-I and Sal-1 strains
| Changes in PvRBSA nucleotide sequence in Sal-I and VCG-I P. vivax strainsa | Changes in PvRBSA aa sequences in Sal-I and VCG-I P. vivax strainsa | Mutation |
|---|---|---|
| c.29A > G | p.Tyr10Cys | Non-synonymous |
| c.391_411delCTAACAGGAAGTAATGAATCC | p.Leu131_Ser137delb | Deletion |
| c.533 T > G | p.Phe178Cys | Non-synonymous |
| c.796A > G | p.Lys266Gly | Non-synonymous |
| c.797A > G | p.Lys266Gly | Non-synonymous |
| c.801 T > A | p.Ser267Arg | Non-synonymous |
| c.803A > C | p.Tyr268Ser | Non-synonymous |
| c.808C > G | p.His270Asp | Non-synonymous |
| c.845C > T | p.Pro282Leu | Non-synonymous |
| c.1029 T > C | p.Lys343Lys | Synonymous |
| c.1091G > C | p.Trp364Ser | Non-synonymous |
aNucleotide and amino acid positions are numbered according to the Sal-I reference strain sequence alignment with the VCG-I strain
bRelative location for a region having 4 identical tandem repeats from amino acid position 103 to 130 in the VCG-I strain
PvRBSA characterisation by molecular biology tools
Antibodies directed against 39478 and 39480 synthetic peptides were purified and used for evaluating the protein’s presence and location in mature parasite forms (schizonts). Specific anti-PvRBSA antibodies detected one band in P. vivax VCG-I strain lysate treated in reduced conditions above the expected size by in silico analysis (43.8 kDa without the signal peptide) (Fig. 1c). Such discrepancy can be explained by anomalous migration caused by several acidic residues in the protein sequence (aspartic and glutamic acids). The antibodies also led to a surface fluorescence signal being visualised in mature schizonts like a “bunch of grapes”, this being characteristic of proteins expressed on merozoite surface (Fig. 1d). There was no signal overlap for one apical marker (PvRON2). These findings led to suggesting that the pvrbsa transcript gave a protein product in P. vivax VCG-I strain schizonts, as shown in an earlier study by mass spectrometry analysis [6].
According to the classic approach, antigenic proteins should be considered for vaccine development given that a response against them could inhibit interaction with cells. Hence rPvRBSA was expressed, purified and successfully obtained in soluble form (Additional file 1: Figure S1) to evaluate its antigenicity using sera from patients suffering P. vivax malaria and sera from people who had never suffered the disease. The screening gave 61% seropositivity in the patients group. The statistical test gave a significant difference between the means for recognition by the sera from the infected patients group (X̄ ± SD = 0.38 ± 0.24) and the control group (X̄ ± SD = 0.12 ± 0.05) (Mann-Whitney U-test: U = 52, Z = -3.66, P = 0.0001) (Fig. 2), thereby highlighting that the protein was able to induce an immune response during natural infection.
Fig. 2.
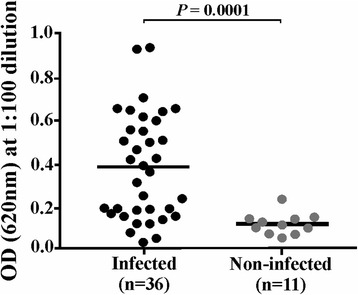
Antigenicity assay. The dot plot shows OD distribution (Y-axis) for detecting the PvRBSA in infected and non-infected patients’ sera (X-axis). A statistically significant difference between groups was observed (Mann-Whitney U-test: U = 52, Z = -3.66, P = 0.0001)
PvRBSA interaction with human reticulocytes
Flow cytometry was used for quantifying rPvRBSA ability to bind cord blood reticulocytes using a gating strategy to exclude cell debris and select the CD71 + CD45- cell population (Fig. 3). The recombinant protein had a curve shift when PE signals from rPvRBSA binding assay and control (using CD71 + CD45- cells) were compared in a histogram. rPvRBSA bound to mature erythrocytes to a much lesser extent compared with reticulocytes (t-test: t (4) = 13.74, P = 0.0001) (Fig. 4a, Table 2). The protein had similar binding activity to cells having a different Duffy phenotype (X̄ ± SD = 9.17 ± 1.4) and to positive control (X̄ ± SD = 23.8 ± 9.8) (ANOVA-Tukey: F (3,5) = 2.43, P = 0.181), whilst there was a statistically significant difference in rPvRBSA binding activity compared to negative control (X̄ ± SD = 2.0 ± 0.34) (Fig. 4b) (ANOVA-Tukey: F (3,5) = 49.53, P = 0.0001). Interestingly, rPvRBSA had higher interaction with CD71hi than CD71lo cells (Fig. 4c) (t-test: t (4) = 16.44, P = 0.0001), suggesting that this molecule binds better to the more immature reticulocyte stages.
Fig. 3.
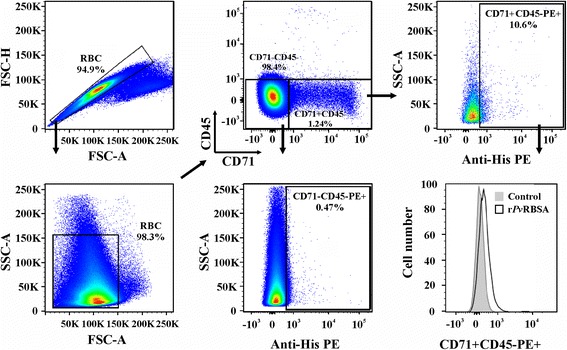
Gating strategy for selecting reticulocytes and mature erythrocyte cell population. Doublets were excluded comparing FSC-H to FSC-A cytogram. Cells were then selected by their granularity by plotting SSC-A against FSC-A. The CD45 and CD71 signals were plotted for selecting reticulocyte (CD71 + CD45-) and mature erythrocyte (CD71-CD45-) populations. The cell percentage having bound protein was calculated using the PE signal against SSC-A. A representative histogram from three independent experiments analysing the PE signal for the rPvRBSA binding assay compared with the control is shown
Fig. 4.
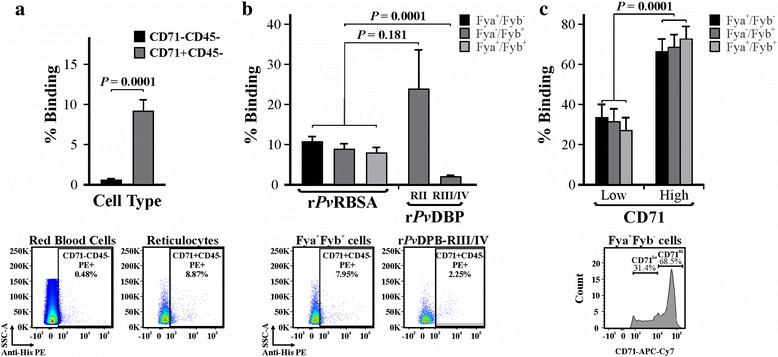
rPvRBSA binding activity to target cells. a rPvRBSA binding percentage to erythrocytes (CD71-CD45-) and reticulocytes (CD71 + CD45-). b Percentage rPvRBSA binding to reticulocytes with a different Duffy phenotype and c regarding CD71-APCH7 signal. A representative dot plot or histogram used for building the bar chart is shown in the bottom part of each figure. Binding percentage in three analyses were expressed as mean ± SD of three independent experiments
Table 2.
rPvRBSA binding percentage to mature and immature erythrocytes. The mean and standard deviation of three independent experiments is shown for each assay
| Molecule | Phenotype | % Binding to mature erythrocytes | % Binding to reticulocytes |
|---|---|---|---|
| rPvRBSA | Fya+/Fyb− | 0.47 ± 0.01 | 10.7 ± 1.29 |
| Fya−/Fyb+ | 0.48 ± 0.22 | 8.87 ± 0.65 | |
| Fya+/Fyb+ | 0.79 ± 0.23 | 7.95 ± 1.94 |
Discussion
Plasmodium vivax has several proteins with essential functions in target cell binding and invasion. An important amount of such proteins has recently been identified in P. vivax by proteomics analysis which, combined with in silico analysis, has led to partly understanding the complex protein machinery used by the parasite and predicting the functions which some parasite proteins may have [5–8]. Exploiting the information available in proteome studies of P. vivax, a large-scale analysis was made for predicting protein vaccine candidates, taking into account the parameters described in the methodology. The screening identified PvRBSA, a molecule whose unique homologue is in P. cynomolgi, a species which invades reticulocytes and which is taxonomically very close to P. vivax [32].
The in silico analysis showed that PvRBSA has the characteristics of a good vaccine candidate, as reported for other parasite proteins. Two transmembrane regions were predicted. Transmembrane helices are usually 20 amino acids long, suggesting that the two helices identified for PvRBSA require a very tight loop to both fit into the membrane. Given these findings (predicted by several programmes), it was considered that the region spanning amino acids 332 to 377 is a transmembrane zone, though future investigation is necessary to ascertain their architecture.
In spite of the difficulty involved in basic research regarding P. vivax, given the intrinsic characteristics of its biology [1], the PvRBSA was characterised due to adapting the P. vivax VCG-I strain in primates [25], which led to sufficient biological material being obtained for developing the experimental assays. The methods used here showed that the pvrbsa gene was transcribed and translated for a surface protein in P. vivax VCG-I strain mature schizonts (Fig. 1a, d), thereby coinciding with the finding of PvRBSA peptides being detected in the first proteomic study in Colombia of a primate model-adapted P. vivax strain [6]. It has been found that parasite transcripts are strictly controlled during the development of the intra-erythrocyte life-cycle [4, 33] and that their codifying products correlate with having a specialised function. For example, more than 50 different P. falciparum transcripts having maximum expression during mature stages (>35 h post-invasion) encode proteins that play an important role during cell invasion [34]. The previous statement, added to the results concerning pvrbsa presence and expression in P. vivax schizonts, suggested that the molecule could have a function during reticulocyte adhesion.
Another important characteristic regarding proteins to be included in a vaccine is that they should be antigenic since it has been seen that an immune response induced during infection is related to naturally-acquired immunity [35, 36]. It was found that PvRBSA could trigger an immune response during natural P. vivax malaria infection (Fig. 2), as described for other surface antigens in the P. vivax VCG-I strain, such as PvMSP-10 [37], Pv12 [38] and PvARP [30]. Once PvRBSA localisation pattern and ability to trigger an immune response had been determined, it was ascertained whether the protein could bind to the most immature human reticulocytes using anti-CD71 monoclonal antibody (a specific marker for the cells [39]). rPvRBSA was able to interact with the youngest reticulocyte population (CD71hi) having different Duffy phenotypes in similar percentages (Fig. 4b). This binding pattern to cells with different Duffy phenotypes has also been reported for DBP [40].
On the other hand, although rPvRBSA was able to bind to mature erythrocytes, its interaction was much greater with reticulocytes (Fig. 4a, Table 2). Such preferential binding to this type of cells has also been observed in other P. vivax proteins such as DBP [9], MSP-1 [13], the erythrocyte binding protein (EBP) [12] and some RBPs [11, 41]. In the case of MSP-1, it was initially thought that target cell selection occurred at a later stage when RBPs were secreted. However, further receptor-ligand studies using PvMSP-1-derived 20-mer long peptides have shown that several peptides bind more strongly to reticulocytes than to erythrocytes, suggesting that this protein participates in the pre-selection of P. vivax target cells [13]. Furthermore, it has been shown that Aotus monkeys vaccinated with MSP-1 recombinant fragments containing reticulocyte-binding peptides have developed protective immunity against P. vivax challenge [42].
A recent study assessing five RBPs’ target cell preference has shown the preferential binding to reticulocytes of just one of them (RBP2b). Interestingly, antibodies against RBP2b, acquired during natural P. vivax infection, have shown a strong protective effect [41]. These studies highlight the significant role for this type of molecule in interaction with P. vivax target cells. According to the results shown here, PvRBSA was localised on parasite surface and displayed a preferential binding profile for the more immature reticulocyte stages. It can thus be suggested that RBPs are not only participating in P. vivax preferential binding to reticulocytes (as was initially thought) but that other ligands are also pre-selecting this cell population, such as PvMSP-1, EBP, DBP and now, rPvRBSA.
Conclusions
This study has described for the first time, an exclusive reticulocyte-infecting Plasmodium species molecule’s characterisation and role in binding. The findings highlight that PvRBSA is present in the P. vivax VCG-I strain genome, produces a transcript and encodes a protein having a surface location pattern. PvRBSA is antigenic and is an adhesin protein able to bind preferentially to human reticulocytes. Future studies should be undertaken aimed at assessing the protective efficacy induced when immunising with PvRBSA in the Aotus monkey experimental model.
Acknowledgements
We would like to thank Ana María Perdomo and Bernardo Camacho from the Hemocentro Distrital (Bogotá) for supplying the umbilical cord blood, Diana Diaz for technical support in cytometry and Jason Garry for translating this manuscript.
Funding
This research was financed by the Colombian Science, Technology and Innovation Department (COLCIENCIAS), contract RC#0309-2013.
Availability of data and materials
All data generated or analysed during this study are included within this article and its additional file.
Authors’ contributions
DAMP devised and designed the study; DAMP, LAB and DMCA performed the experiments; DAMP, LAB and MAP analysed the results; DAMP and MAP wrote the manuscript. All authors read and approved the final manuscript.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
New Zealand rabbits were handled in strict accordance with Colombian Law 84/1989 and resolution 504/1996 and EU Directive 2010/63/EU for animal experiments, following established guidelines for the care and use of laboratory animals (National Institute of Health, USA). All efforts were made to minimise animal suffering. Sera were collected from 36 patients who had suffered episodes of P. vivax infection as well as from 11 healthy individuals who had never been affected by the disease. The newborn umbilical cord blood samples used in this research were collected by the Hemocentro Distrital (Bogotá). All individuals (progenitors regarding umbilical cord samples) signed an informed consent form after having received detailed information regarding the study’s goals. All procedures were approved by FIDIC’s ethics committee.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ANOVA
Analysis of variance.
- CD71hi
CD71 high
- CD71lo
CD71 low
- cDNA
Complementary DNA
- DBP
Duffy binding protein
- EBP
Erythrocyte binding protein
- ELISA
Enzyme-linked immunosorbent assay
- GAMA
GPI-anchored micronemal antigen
- gDNA
Genomic DNA
- LB
Luria-Bertani
- MSP-1
Merozoite surface protein-1
- NEB
Native extraction buffer
- OD
Optical density
- PvRBSA
P. vivax reticulocyte binding surface antigen
- RBP
Reticulocyte binding protein
- RON5
Rhoptry neck protein-5
- RT
Room temperature
- SD
Standard deviation
- VCG-I
Vivax Colombia Guaviare I
Additional file
Recombinant PvRBSA purification. Lane 1: the proteins’ molecular marker; Lanes 2–6: eluted protein using buffer with increasing concentration of imidazole (50 mM, 100 mM, 200 mM, 300 mM and 500 mM) stained with Coomassie blue; Lane 7: recognition of rPvRBSA by Western blot using anti-polyhistidine antibodies. (TIF 1539 kb)
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s13071-017-2185-6) contains supplementary material, which is available to authorized users.
Contributor Information
Darwin A. Moreno-Pérez, Email: darandmorper@gmail.com
Luis A. Baquero, Email: labs1990@gmail.com
Diana M. Chitiva-Ardila, Email: diana_marcela8@hotmail.com
Manuel A. Patarroyo, Email: mapatarr.fidic@gmail.com
References
- 1.Moreno-Perez DA, Ruiz JA, Patarroyo MA. Reticulocytes: Plasmodium vivax target cells. Biol Cell. 2013;105:251–260. doi: 10.1111/boc.201200093. [DOI] [PubMed] [Google Scholar]
- 2.Patarroyo MA, Calderón D, Moreno-Pérez DA. Vaccines against Plasmodium vivax: a research challenge. Expert Rev Vaccin. 2012;11:1249–60. [DOI] [PubMed]
- 3.Carlton JM, Adams JH, Silva JC, Bidwell SL, Lorenzi H, Caler E, et al. Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature. 2008;455:757–763. doi: 10.1038/nature07327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bozdech Z, Mok S, Hu G, Imwong M, Jaidee A, Russell B, et al. The transcriptome of Plasmodium vivax reveals divergence and diversity of transcriptional regulation in malaria parasites. Proc Natl Acad Sci USA. 2008;105:16290–5. [DOI] [PMC free article] [PubMed]
- 5.Roobsoong W, Roytrakul S, Sattabongkot J, Li J, Udomsangpetch R, Cui L. Determination of the Plasmodium vivax schizont stage proteome. J Proteomics. 2011;74:1701–1710. doi: 10.1016/j.jprot.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreno-Perez DA, Degano R, Ibarrola N, Muro A, Patarroyo MA. Determining the Plasmodium vivax VCG-1 strain blood stage proteome. J Proteom. 2014;113C:268–280. doi: 10.1016/j.jprot.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Anderson DC, Lapp SA, Akinyi S, Meyer EV, Barnwell JW, Korir-Morrison C, et al. Plasmodium vivax trophozoite-stage proteomes. J Proteom. 2015;115:157–176. doi: 10.1016/j.jprot.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acharya P, Pallavi R, Chandran S, Dandavate V, Sayeed SK, Rochani A, et al. Clinical proteomics of the neglected human malarial parasite Plasmodium vivax. PLoS One. 2011;6:e26623. doi: 10.1371/journal.pone.0026623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ocampo M, Vera R, Eduardo Rodriguez L, Curtidor H, Urquiza M, Suarez J, et al. Plasmodium vivax Duffy binding protein peptides specifically bind to reticulocytes. Peptides. 2002;23:13–22. doi: 10.1016/S0196-9781(01)00574-5. [DOI] [PubMed] [Google Scholar]
- 10.Cantor EM, Lombo TB, Cepeda A, Espinosa AM, Barrero CA, Guzman F, et al. Plasmodium vivax: functional analysis of a highly conserved PvRBP-1 protein region. Mol Biochem Parasitol. 2001;117:229–234. doi: 10.1016/S0166-6851(01)00355-3. [DOI] [PubMed] [Google Scholar]
- 11.Han JH, Lee SK, Wang B, Muh F, Nyunt MH, Na S, et al. Identification of a reticulocyte-specific binding domain of Plasmodium vivax reticulocyte-binding protein 1 that is homologous to the PfRh4 erythrocyte-binding domain. Sci Rep. 2016;6:26993. doi: 10.1038/srep26993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ntumngia FB, Thomson-Luque R, Torres Lde M, Gunalan K, Carvalho LH, Adams JH. A novel erythrocyte binding protein of Plasmodium vivax suggests an alternate invasion pathway into Duffy-positive reticulocytes. mBio. 2016;7:e01261–16. [DOI] [PMC free article] [PubMed]
- 13.Rodriguez LE, Urquiza M, Ocampo M, Curtidor H, Suarez J, Garcia J, et al. Plasmodium vivax MSP-1 peptides have high specific binding activity to human reticulocytes. Vaccine. 2002;20:1331–1339. doi: 10.1016/S0264-410X(01)00472-8. [DOI] [PubMed] [Google Scholar]
- 14.Arevalo-Pinzon G, Bermudez M, Curtidor H, Patarroyo MA. The Plasmodium vivax rhoptry neck protein 5 is expressed in the apical pole of Plasmodium vivax VCG-1 strain schizonts and binds to human reticulocytes. Malaria J. 2015;14:106. doi: 10.1186/s12936-015-0619-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 16.Pierleoni A, Martelli PL, Fariselli P, Casadio R. BaCelLo: a balanced subcellular localization predictor. Bioinformatics. 2006;22:e408–416. doi: 10.1093/bioinformatics/btl222. [DOI] [PubMed] [Google Scholar]
- 17.Poisson G, Chauve C, Chen X, Bergeron A. FragAnchor: a large-scale predictor of glycosylphosphatidylinositol anchors in eukaryote protein sequences by qualitative scoring. GPB Beijing Genom Inst. 2007;5:121–130. doi: 10.1016/S1672-0229(07)60022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jorda J, Kajava AV. T-REKS: identification of Tandem REpeats in sequences with a K-meanS based algorithm. Bioinformatics. 2009;25:2632–2638. doi: 10.1093/bioinformatics/btp482. [DOI] [PubMed] [Google Scholar]
- 19.Kall L, Krogh A, Sonnhammer EL. A combined transmembrane topology and signal peptide prediction method. J Mol Biol. 2004;338:1027–1036. doi: 10.1016/j.jmb.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Tusnady GE, Simon I. The HMMTOP transmembrane topology prediction server. Bioinformatics. 2001;17:849–850. doi: 10.1093/bioinformatics/17.9.849. [DOI] [PubMed] [Google Scholar]
- 21.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 22.Aurrecoechea C, Brestelli J, Brunk BP, Dommer J, Fischer S, Gajria B, et al. PlasmoDB: a functional genomic database for malaria parasites. Nucleic Acids Res. 2009;37:D539–543. doi: 10.1093/nar/gkn814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakaya A, Katayama T, Itoh M, Hiranuka K, Kawashima S, Moriya Y, et al. KEGG OC: a large-scale automatic construction of taxonomy-based ortholog clusters. Nucleic Acids Res. 2013;41:D353–357. doi: 10.1093/nar/gks1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deleage G, Combet C, Blanchet C, Geourjon C. ANTHEPROT: an integrated protein sequence analysis software with client/server capabilities. Comp Biol Med. 2001;31:259–267. doi: 10.1016/S0010-4825(01)00008-7. [DOI] [PubMed] [Google Scholar]
- 25.Pico de Coana Y, Rodriguez J, Guerrero E, Barrero C, Rodriguez R, Mendoza M, et al. A highly infective Plasmodium vivax strain adapted to Aotus monkeys: quantitative haematological and molecular determinations useful for P. vivax malaria vaccine development. Vaccine. 2003;21:3930–3937. doi: 10.1016/S0264-410X(03)00278-0. [DOI] [PubMed] [Google Scholar]
- 26.Andrysiak PM, Collins WE, Campbell GH. Concentration of Plasmodium ovale- and Plasmodium vivax-infected erythrocytes from nonhuman primate blood using Percoll gradients. Am J Trop Med Hyg. 1986;35:251–254. doi: 10.4269/ajtmh.1986.35.251. [DOI] [PubMed] [Google Scholar]
- 27.Ramos AE, Munoz M, Moreno-Perez DA, Patarroyo MA. pELMO, an optimised in-house cloning vector. AMB Express. 2017;7:26. doi: 10.1186/s13568-017-0324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houghten RA. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci USA. 1985;82:5131–5. [DOI] [PMC free article] [PubMed]
- 30.Moreno-Perez DA, Saldarriaga A, Patarroyo MA. Characterizing PvARP, a novel Plasmodium vivax antigen. Malar J. 2013;12:165. doi: 10.1186/1475-2875-12-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galinski MR, Medina CC, Ingravallo P, Barnwell JW. A reticulocyte-binding protein complex of Plasmodium vivax merozoites. Cell. 1992;69:1213–1226. doi: 10.1016/0092-8674(92)90642-P. [DOI] [PubMed] [Google Scholar]
- 32.Tachibana S, Sullivan SA, Kawai S, Nakamura S, Kim HR, Goto N, et al. Plasmodium cynomolgi genome sequences provide insight into Plasmodium vivax and the monkey malaria clade. Nat Genet. 2012;44:1051–1055. doi: 10.1038/ng.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bozdech Z, Llinas M, Pulliam BL, Wong ED, Zhu J, DeRisi JL. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. 2003;1:E5. doi: 10.1371/journal.pbio.0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez LE, Curtidor H, Urquiza M, Cifuentes G, Reyes C, Patarroyo ME. Intimate molecular interactions of P. falciparum merozoite proteins involved in invasion of red blood cells and their implications for vaccine design. Chem Rev. 2008;108:3656–3705. doi: 10.1021/cr068407v. [DOI] [PubMed] [Google Scholar]
- 35.Dent AE, Nakajima R, Liang L, Baum E, Moormann AM, Sumba PO, et al. Plasmodium falciparum protein microarray antibody profiles correlate with protection from symptomatic malaria in Kenya. J Infect Dis. 2015;212:1429–38. [DOI] [PMC free article] [PubMed]
- 36.Longley RJ, Sattabongkot J, Mueller I. Insights into the naturally acquired immune response to Plasmodium vivax malaria. Parasitology. 2016;143:154–170. doi: 10.1017/S0031182015000670. [DOI] [PubMed] [Google Scholar]
- 37.Perez-Leal O, Sierra AY, Barrero CA, Moncada C, Martinez P, Cortes J, et al. Identifying and characterising the Plasmodium falciparum merozoite surface protein 10 Plasmodium vivax homologue. Biochem Biophys Res Commun. 2005;331:1178–1184. doi: 10.1016/j.bbrc.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 38.Moreno-Perez DA, Areiza-Rojas R, Florez-Buitrago X, Silva Y, Patarroyo ME, Patarroyo MA. The GPI-anchored 6-Cys protein Pv12 is present in detergent-resistant microdomains of Plasmodium vivax blood stage schizonts. Protist. 2013;164:37–48. doi: 10.1016/j.protis.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Malleret B, Li A, Zhang R, Tan KS, Suwanarusk R, Claser C, et al. Plasmodium vivax: restricted tropism and rapid remodeling of CD71-positive reticulocytes. Blood. 2015;125:1314–1324. doi: 10.1182/blood-2014-08-596015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King CL, Adams JH, Xianli J, Grimberg BT, McHenry AM, Greenberg LJ, et al. Fy(a)/Fy(b) antigen polymorphism in human erythrocyte Duffy antigen affects susceptibility to Plasmodium vivax malaria. Proc Natl Acad Sci USA. 2011;108:20113–8. [DOI] [PMC free article] [PubMed]
- 41.Franca CT, He WQ, Gruszczyk J, Lim NT, Lin E, Kiniboro B, et al. Plasmodium vivax reticulocyte binding proteins are key targets of naturally acquired immunity in young Papua New Guinean children. PLoS Negl Trop Dis. 2016;10:e0005014. doi: 10.1371/journal.pntd.0005014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barrero CA, Delgado G, Sierra AY, Silva Y, Parra-Lopez C, Patarroyo MA. Gamma interferon levels and antibody production induced by two PvMSP-1 recombinant polypeptides are associated with protective immunity against P.vivax in Aotus monkeys. Vaccine. 2005;23:4048–4053. doi: 10.1016/j.vaccine.2005.02.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included within this article and its additional file.


