Abstract
Background
ACOSOG Z1031 demonstrated that neoadjuvant endocrine therapy (NET) increased breast conserving surgery (BCS) rates for postmenopausal patients with clinical tumor stage 2–4c estrogen receptor positive breast cancer. We evaluated national trends in NET use in relation to Z1031 trial conduct and the impact of NET on rates of BCS.
Methods
Using the National Cancer Data Base (NCDB), we identified all cT2-4c hormone receptor positive breast cancer patients age ≥50 from 2004–2012. Time intervals of pre-Z1031 (2004–2006), during-Z1031 (2007–2009), and post-Z1031 (2010–2012) were examined. Adjusted analyses were performed using multivariable logistic regression.
Results
Of 77,272 patients, 2,294 (3.0%) received NET. Clinical T stage distribution was 66,885 (86.6%) cT2, 7,318 (9.5%) cT3, and 3,069 (4.0%) cT4a-c. There was a small but statistically significant increase in NET use from 2.7% pre-Z1031 to 3.2% post-Z1031; the adjusted OR for NET was 1.28 (95% CI: 1.13–1.45, p<0.001) for post-Z1031 versus pre-Z1031. NET use varied by clinical T stage; in cT2 patients, it increased from 1.8% pre-Z1031 to 2.4% post-Z1031 (p<0.001); in cT3 patients from 6.3% pre-Z1031 to 7.4% post-Z1031 (p=0.02). Patients receiving NET were more likely to undergo BCS compared with patients undergoing primary surgery (46.4% vs 43.9%, p=0.02) with adjusted OR 1.60 (95% CI: 1.46–1.75, p<0.001).
Conclusions
NET use has increased slowly since Z1031, however overall use remains low. NET significantly increased rates of BCS in patients with hormone receptor positive clinical T2-4c breast cancer. Clinicians should consider NET use for patients with hormone receptor positive breast cancer interested in BCS.
Keywords: National Cancer Data Base, neoadjuvant endocrine therapy, breast cancer, breast conservation, lumpectomy
INTRODUCTION
The use of endocrine therapy in the neoadjuvant setting was initially explored in Europe and is still more commonly used in Europe compared with the US. Two large international studies led in Europe were the P024 trial and the IMPACT trial. The P024 trial compared neoadjuvant letrozole to tamoxifen in T2-4c hormone receptor positive breast cancer, and showed that letrozole was superior to tamoxifen in overall response rates and rates of breast conservation in women who were not eligible for breast conserving surgery (BCS).1 The IMPACT trial compared neoadjuvant tamoxifen, anastrozole and the combination of tamoxifen and anastrozole in postmenopausal women with hormone receptor positive breast cancer. There were similar response rates across the three arms, but higher rates of breast conservation with anastrozole compared to tamoxifen.2 These trials showed that aromatase inhibitors were associated with better response rates compared with tamoxifen as neoadjuvant therapy. These trials also showed that NET is a well-tolerated, reasonable treatment option for strongly hormone receptor positive breast cancer patients who are interested in breast conservation. Similar to neoadjuvant chemotherapy, NET can downstage tumors and importantly also provides information on the tumors’ endocrine responsiveness.
The American College of Surgeons Oncology Group (ACOSOG) Z1031was a randomized phase II neoadjuvant endocrine therapy trial comparing response rates between three aromatase inhibitors conducted in the US. A total of 377 postmenopausal women with clinical stage II or III estrogen receptor (ER) positive breast cancer were enrolled from 2007 to 2009. Eligible patients were randomized to exemestane 25 mg daily, letrozole 2.5 mg daily, or anastrozole 1 mg daily for 16 to 18 weeks prior to surgery. The overall clinical response rate was 69% (258/374) with no differences across the three AIs.3 Furthermore, 51.5% (84/163) of the patients who were deemed by the surgeon to require mastectomy prior to therapy were able to successfully undergo BCS after treatment with NET. ACOSOG Z1031 results were initially presented at the American Society of Clinical Oncology Annual Meeting in 2010 and published in the Journal of Clinical Oncology in 2011.
The goal of our study was to assess whether the results of the Z1031 trial have impacted clinical practice in the US. We evaluated national trends in the use of NET in the years before and after the release of trial results as well as the impact of NET on rates of BCS in the US using data from the National Cancer Data Base (NCDB).
MATERIALS AND METHODS
Study design
The NCDB is a clinical oncology database jointly sponsored by the American College of Surgeons and the American Cancer Society. The NCDB is sourced from a hospital based registry collected in more than 1,500 Commission on Cancer accredited facilities and represents approximately 70 percent of newly diagnosed cancer cases in the US.
The NCDB was queried from 2004 to 2012 for breast cancer patients ≥50 years of age with tumor stage cT2-4c, cN0-3, hormone receptor positive breast cancer. Hormone receptor positive disease was defined as tumors that were both estrogen receptor (ER) positive and progesterone receptor (PR) positive. Patients who received neoadjuvant chemotherapy and/or neoadjuvant radiation therapy were excluded. We defined patients as receiving NET if endocrine therapy was initiated more than 30 days prior to their surgical intervention. The following variables were examined: patient age at diagnosis, sex, race, Charlson-Deyo comorbidity score, facility location, facility type, and county type (metro/urban/rural).
Statistical Analysis
The use of NET was evaluated in three time periods defined as pre-Z1031 (2004–2006), during-Z1031 (2007–2009), and post-Z1031 (2010–2012). Trends across the three time periods were evaluated using Cochran-Armitage trend tests. The associations of other demographic and clinical factors with the use of NET were evaluated using chi-square tests for nominal variables, trend tests for ordinal variables, and two-sample t-tests for continuous variables. Impact of NET on BCS was analyzed by comparing the proportion undergoing BCS between NET and primary surgery patients overall and within clinical T stage subgroups using chi-square tests. Adjusted analyses were performed using multivariable logistic regression. P-values < 0.05 were considered statistically significant. Analysis was performed using SAS (SAS Institute Inc., Cary, NC, Version 9.4).
RESULTS
We identified 85,884 patients ≥50 years of age with clinical tumor stage T2-4c hormone receptor (ER and PR) positive breast cancer. Of these, 8,612 (10.03%) received neoadjuvant chemotherapy and were excluded from this analysis, leaving a cohort of 77,272 patients included in this study. Among these 2,294 (3.0%) received NET. The use of NET showed a small but statistically significant increase over time, from 2.7% pre-Z1031 and during-Z1031 to 3.2% post-Z1031 (p<0.001). NET use varied by clinical T stage. For patients with cT2 tumors, NET use increased from 1.8% pre-Z1031 and 2.0% during-Z1031 to 2.4% post-Z1031 (p<0.001). In cT3 patients, NET use was 6.3% pre-Z1031, 5.1% during-Z1031 and increased to 7.4% in the post-Z1031 period (p=0.02). There was no significant trend among cT4a-c patients (p=0.68) (Figure 1).
Figure 1.
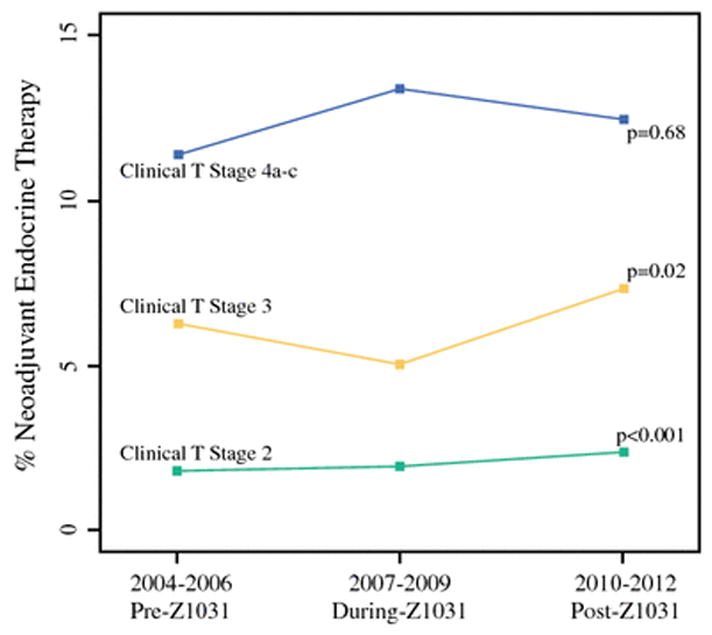
Neoadjuvant endocrine therapy use by clinical T stage over time.
Comparison of patient characteristics, including patient demographics, Charlson-Deyo comorbidity score, facility location, facility type, and county type between those receiving NET and those undergoing primary surgery (PS) is shown in Table 1. Patients undergoing NET were older (mean age 71 years) compared to those undergoing primary surgery (PS) (mean age 67 years, p<0.001). Use of NET was more common in females, with 3.0% of women being treated with NET compared to 1.5% of men (p=0.001).
Table 1.
Patient Characteristics
| Neoadjuvant Endocrine Therapy (N=2294) | Primary Surgery (N=74978) | Total (N=77272) | p value | |
|---|---|---|---|---|
| Age at Diagnosis | <0.00011 | |||
| N | 2294 | 74978 | 77272 | |
| Mean (SD) | 71.1 (10.4) | 66.7 (11.1) | 66.8 (11.1) | |
| Median | 72.0 | 65.0 | 65.0 | |
| Q1, Q3 | 63.0, 79.0 | 57.0, 75.0 | 58.0, 75.0 | |
| Range | (50.0–90.0) | (50.0–90.0) | (50.0–90.0) | |
| Sex | 0.00142 | |||
| Male | 22 (1.0%) | 1402 (1.9%) | 1424 (1.8%) | |
| Female | 2272 (99.0%) | 73576 (98.1%) | 75848 (98.2%) | |
| Race | 0.06842 | |||
| White | 1940 (84.6%) | 64626 (86.2%) | 66566 (86.1%) | |
| Black | 255 (11.1%) | 7093 (9.5%) | 7348 (9.5%) | |
| Other | 77 (3.4%) | 2547 (3.4%) | 2624 (3.4%) | |
| Unknown | 22 (1.0%) | 712 (0.9%) | 734 (0.9%) | |
| Charlson-Deyo Score | 0.01172 | |||
| 0 | 1796 (78.3%) | 59554 (79.4%) | 61350 (79.4%) | |
| 1 | 372 (16.2%) | 12262 (16.4%) | 12634 (16.4%) | |
| 2+ | 126 (5.5%) | 3162 (4.2%) | 3288 (4.3%) | |
| Facility Location | 0.00032 | |||
| New England | 168 (7.3%) | 4075 (5.4%) | 4243 (5.5%) | |
| Middle Atlantic | 343 (15.0%) | 10395 (13.9%) | 10738 (13.9%) | |
| South Atlantic | 477 (20.8%) | 16085 (21.5%) | 16562 (21.4%) | |
| East North Central | 441 (19.2%) | 14469 (19.3%) | 14910 (19.3%) | |
| East South Central | 113 (4.9%) | 4877 (6.5%) | 4990 (6.5%) | |
| West North Central | 204 (8.9%) | 6074 (8.1%) | 6278 (8.1%) | |
| West South Central | 165 (7.2%) | 5771 (7.7%) | 5936 (7.7%) | |
| Mountain | 118 (5.1%) | 4014 (5.4%) | 4132 (5.3%) | |
| Pacific | 265 (11.6%) | 9218 (12.3%) | 9483 (12.3%) | |
| Facility Type | <0.00012 | |||
| Community Cancer Program | 230 (10.0%) | 9796 (13.1%) | 10026 (13.0%) | |
| Comprehensive Community Cancer Program | 1158 (50.5%) | 44761 (59.7%) | 45919 (59.4%) | |
| Academic/Research Program | 892 (38.9%) | 20322 (27.1%) | 21214 (27.5%) | |
| Other specified types of cancer programs | 14 (0.6%) | 99 (0.1%) | 113 (0.1%) | |
| County Type | 0.04032 | |||
| Metro Counties | 1921 (83.7%) | 61323 (81.8%) | 63244 (81.8%) | |
| Urban Counties | 269 (11.7%) | 10011 (13.4%) | 10280 (13.3%) | |
| Rural Counties | 29 (1.3%) | 1290 (1.7%) | 1319 (1.7%) | |
| Unknown | 75 (3.3%) | 2354 (3.1%) | 2429 (3.1%) |
Wilcoxon
Chi-Square
NET varied significantly by facility type (p<0.001) and was more frequently utilized in academic centers where 4.2% of patients received NET compared to cancer community programs (2.3%) and comprehensive community cancer programs (2.5%). Patients with more co-morbidities had higher use of NET, with 3.8% of patients with Charlson-Deyo score of 2 or more receiving NET compared to 2.9% of patients with Charlson-Deyo score of 0 or 1 (p=0.01).
Comparisons of tumor characteristics, including tumor stage, grade, and type of breast surgery performed are shown in Table 2. Clinical T stage distribution was clinical T2 in 66,885 patients (86.6%), clinical T3 in 7,318 patients (9.5%) and clinical T4a-c in 3,069 patents (4.0%). NET use increased with increasing T stage from 2.2% in cT2, 6.4% in cT3 and 12.5% in cT4 disease. NET was more commonly used in node positive disease, with 2.7% of clinically node negative patients receiving NET compared to 4.0% of patients with node positive disease. NET use was highest in well differentiated tumors (4.0%), compared to moderately differentiated (3.0%) and poorly differentiated tumors having the lowest rate (2.0%).
Table 2.
Tumor Characteristics
| Neoadjuvant Endocrine Therapy (N=2294) | Primary Surgery (N=74978) | Total (N=77272) | p value | |
|---|---|---|---|---|
| Clinical N stage | <0.00011 | |||
| Missing | 3 | 109 | 112 | |
| N0 | 1455 (63.5%) | 52835 (70.6%) | 54290 (70.4%) | |
| N1 | 560 (24.4%) | 13373 (17.9%) | 13933 (18.1%) | |
| N2 | 151 (6.6%) | 3237 (4.3%) | 3388 (4.4%) | |
| N3 | 43 (1.9%) | 1275 (1.7%) | 1318 (1.7%) | |
| NX | 82 (3.6%) | 4149 (5.5%) | 4231 (5.5%) | |
| Clinical T stage | <0.00011 | |||
| T2 | 1439 (62.7%) | 65446 (87.3%) | 66885 (86.6%) | |
| T3 | 470 (20.5%) | 6848 (9.1%) | 7318 (9.5%) | |
| T4 | 385 (16.8%) | 2684 (3.6%) | 3069 (4.0%) | |
| Grade | <0.00011 | |||
| Well differentiated | 539 (23.5%) | 12553 (16.7%) | 13092 (16.9%) | |
| Moderately differentiated | 1161 (50.6%) | 37660 (50.2%) | 38821 (50.2%) | |
| Poorly differentiated/Undifferentiated | 418 (18.2%) | 21033 (28.1%) | 21451 (27.8%) | |
| Cell type not determined | 176 (7.7%) | 3732 (5.0%) | 3908 (5.1%) | |
| Breast Surgery Type | 0.01641 | |||
| Missing | 1 | 61 | 62 | |
| Lumpectomy/BCS | 1064 (46.4%) | 32871 (43.9%) | 33935 (44.0%) | |
| Mastectomy | 1229 (53.6%) | 42046 (56.1%) | 43275 (56.0%) |
Chi-Square
In multivariable analysis, post-Z1031 years demonstrated significantly higher use of NET as compared to pre-Z1031 (adjusted OR 1.28, 95% CI: 1.13–1.45, p<0.001) (Table 3). The odds of NET also generally increased with age; patients 60–69, 70–79, and 80–89 demonstrated adjusted odds ratios of 1.94 (95% CI: 1.70–2.22), 3.18 (95% CI: 2.79–3.63), and 3.14 (95% CI: 2.72–3.62), respectively, vs age 50–59 (all p<0.001). Clinical T3 and T4a-c tumors were significantly more likely than cT2 tumors to undergo NET with adjusted odds ratios of 3.08 (95% CI: 2.75–3.44) and 6.11 (95% CI: 5.38–6.94) as were clinically node positive patients (adjusted OR 1.28, 95% CI: 1.17–1.41, p<0.001). Higher grade tumors were significantly less likely to be treated with NET. After adjusted for patient and clinical factors, there remained a significant effect for academic/research programs versus community cancer programs (adjusted OR 2.14, 95% CI: 1.84–2.49, p<0.001) (Table 3).
Table 3.
Multivariable logistic regression model predicting use of neoadjuvant endocrine therapy versus primary surgery
| Variable | Level | Odds Ratio | Lower 95% CI | Upper 95% CI | p-value |
|---|---|---|---|---|---|
| Age | 60–69 vs 50–59 | 1.94 | 1.70 | 2.22 | <.001 |
| 70–79 vs 50–59 | 3.18 | 2.79 | 3.63 | <.001 | |
| 80–89 vs 50–59 | 3.14 | 2.72 | 3.62 | <.001 | |
| 90+ vs 50–59 | 1.75 | 1.32 | 2.33 | <.001 | |
| Time period | During-Z1031 vs Pre-Z1031 | 1.06 | 0.93 | 1.21 | 0.391 |
| Post-Z1031 vs Pre-Z1031 | 1.28 | 1.13 | 1.45 | <.001 | |
| Sex | Male vs Female | 0.43 | 0.28 | 0.65 | <.001 |
| Race | Black vs White | 1.06 | 0.92 | 1.21 | 0.430 |
| Other vs White | 1.08 | 0.86 | 1.37 | 0.507 | |
| Unknown vs White | 0.91 | 0.59 | 1.41 | 0.679 | |
| Charlson-Deyo Score | 1 vs 0 | 0.90 | 0.80 | 1.01 | 0.072 |
| 2+ vs 0 | 1.09 | 0.90 | 1.31 | 0.394 | |
| Clinical T stage | T3 vs T2 | 3.08 | 2.75 | 3.44 | <.001 |
| T4 vs T2 | 6.11 | 5.38 | 6.94 | <.001 | |
| Clinical N stage | N1-3 vs N0 | 1.28 | 1.17 | 1.41 | <.001 |
| NX vs N0 | 0.65 | 0.52 | 0.82 | <.001 | |
| Grade | Moderately differentiated vs Well differentiated | 0.67 | 0.60 | 0.75 | <.001 |
| Poorly differentiated/Undifferentiated vs Well differentiated | 0.41 | 0.36 | 0.47 | <.001 | |
| Cell type not determined vs Well differentiated | 0.90 | 0.75 | 1.08 | 0.258 | |
| Facility Type | Comprehensive Community Cancer Program vs Community Cancer Program | 1.16 | 1.00 | 1.34 | 0.045 |
| Academic/Research Program vs Community Cancer Program | 2.14 | 1.84 | 2.49 | <.001 | |
| Other specified types of cancer programs vs Community Cancer Program | 6.32 | 3.46 | 11.54 | <.001 |
Patients undergoing NET were significantly more likely to undergo BCS than patients treated with PS (46.4% vs 43.9%, p=0.02). This finding remained true after adjustment for patient, clinical, and facility factors in multivariable analysis with adjusted odds ratio 1.60 (95% CI: 1.46–1.75), p <0.001 when comparing odds of BCS between NET and PS patients. Within each clinical T stage rates of BCS were higher in patients treated with NET compared with those undergoing PS (Figure 2). The greatest increase was seen in cT2 disease (58.8% vs 47.9%, p<0.001). Strikingly, a quarter of patients with cT3 and cT4 disease treated with NET had BCS (26.2% vs 15.0% in cT3, 24.7% vs 20.0% in cT4a-c).
Figure 2.

Rates of Breast Conserving Surgery by clinical T stage comparing patients treated with neoadjuvant endocrine therapy and those undergoing primary surgery.
DISCUSSION
The use of neoadjuvant endocrine therapy has increased since the presentation and publication of the ACOSOG Z1031 trial results, from 2.7 percent in the pre-Z1031 era (2004–2006) to 3.2 percent post-Z1031 (2010–2012) among patients in the NCDB with adjusted odds ratio for post-Z1031 vs pre-Z1031 of 1.28 (95% CI: 1.13–1.45). The results from ACOSOG Z1031 demonstrated an overall BCS rate of 83% in women who were considered marginal candidates for BCS at presentation, and 51% in the women who were categorized as requiring mastectomy at initial presentation.3 The data from the NCDB is consistent with this, showing an increased number of patients receiving NET were able to undergo BCS compared to patients undergoing PS across all T stages from cT2 to cT4. However, despite dramatic results reported from the Z1031 trial, the overall use of NET remained low through 2012. Some possible explanations for the slow adoption of NET include that it takes time to disseminate clinical trial results and impact clinical practice and because the responses seen with NET are slower than what is seen with neoadjuvant chemotherapy and NET is less likely to see a complete response.
The P024 trial was a randomized, double-blind controlled trial which compared 4 months of neoadjuvant letrozole to tamoxifen in 337 postmenopausal women with T2-4c hormone receptor positive breast cancer who were not eligible for BCS.1 The overall response rate was 55% for letrozole and 33% for tamoxifen (p<0.001). The BCS rate was significantly higher with letrozole compared to tamoxifen (45% versus 35%, respectively; p=0.022). The IMPACT trial was a phase III randomized, multicenter trial comparing neoadjuvant tamoxifen, anastrozole and the combination of tamoxifen and anastrozole in postmenopausal women with hormone receptor positive invasive breast cancer. Three hundred and thirty patients were randomized to receive neoadjuvant anastrozole, tamoxifen, or the combination of anastrozole and tamoxifen for 12 weeks. Overall response rate was 37% for anastrozole, 36% for tamoxifen, and 39% for the combination. No significant difference was seen across the three arms. Of the 124 patients considered to require mastectomy at baseline, conversion rate to BCS was 44% for anastrozole, 31% for tamoxifen, and 24% for the combination. The improvement with anastrozole compared with tamoxifen was statistically significant.2 The BCS rates from the NCDB appear similar, with 59% of cT2 tumors and 26.2% of cT3 tumors treated with NET completing BCS; however, the extent of disease and surgical recommendation prior to NET is not known in this cohort.
It is important to identify those patients who would benefit the most from receiving NET. ER and PR status are obtained on all tumors, however not all ER positive tumors are equally endocrine responsive. PAM50 intrinsic subtype appears useful in identifying non-luminal tumors which are relatively endocrine-resistant despite strong ER positivity on immunohistochemistry. In Z1031, luminal A and luminal B tumors were highly endocrine therapy sensitive.3 It has been suggested that tumors with both ER and PR positivity greater than 50% may be considered highly endocrine sensitive, whereas receptor positivity less than 50% may indicate tumors are less endocrine responsive4 Z1031 limited enrollment to patients with tumor Allred scores of 6 or higher based on the result of the letrozole P024 trial5 and this criteria may be of clinical use when selecting appropriate patients for NET. We were not able to assess degree of ER positivity or Allred score from the NCDB data and therefore limited this NCDB study to patients with both ER and PR positive disease. Degree of quantitative ER expression may be a valuable selection factor clinically in predicting endocrine therapy responsiveness.6–9
Neoadjuvant endocrine therapy has been historically used to treat patients with locally advanced breast cancer who were deemed unfit for chemotherapy because of advanced age and/or comorbidities.10,11 In our study, the mean age for patients receiving NET was slightly older at 71.1 years old compared to 66.7 years old in patients undergoing primary surgery and patients aged 70–89 years demonstrated a 3-fold increase in odds of NET as compared to patients age 50–59 years. This may reflect reluctance to utilized chemotherapy in older patients or that they may not be good surgical candidates.
The use of NET was highest in academic/research institutions, which may reflect the increased availability of clinical trials in these settings and dissemination of clinical trial results to the community cancer programs may be slower.
When deciding to treat patients with neoadjuvant systemic therapy the question of neoadjuvant chemotherapy versus NET should be considered. Low grade tumors that are strongly ER and PR positive have lower rates of response to neoadjuvant chemotherapy than triple negative or Her2 positive tumors and therefore for low grade strongly hormone receptor positive disease NET is the preferred approach. In this study approximately 10% of patients were excluded due to neoadjuvant chemotherapy. The proportion of patients treated with neoadjuvant chemotherapy is lower than seen in general for breast cancer, reflecting the hormone responsiveness of these tumors and less benefit of this tumor type from neoadjuvant chemotherapy.
Despite several strengths of this study, including its capture of data from multiple centers, and large sample size, several limitations should be noted. Given the retrospective nature of the study, we cannot assess how the patients were selected to undergo NET. We also do not know compliance or duration of the therapy. Whether the surgical recommendations were changed after completing treatment with NET is not captured in the NCDB. In addition, NCDB lacks HER2 status information prior to 2010, and thus was not available during the earlier time periods of this study. Detailed histological information such as the degree of quantitative ER or PR expression is not available, as the NCDB defines ER or PR status as positive for expression when >1%. Thus the cohort studied may include those patients who were unsuitable for NET, making the reported proportion of patients undergoing NET lower than if the proportion was calculated from patients deemed more suitable for NET.
Evaluation of the NCDB data showed a small but statistically significant increase in the use of NET in the US since the presentation and publication of ACOSOG Z1031 from 2.7% in 2004 to 2006 to 3.2% in 2010 to 2012. While the overall use of NET in the U.S. remains low, NET significantly increased rates of BCS in patients with hormone receptor positive clinical T2-4c breast cancer. Clinicians should consider the use of NET for patients with strongly hormone receptor positive, HER2 negative breast cancer who are interested in breast conservation.
Supplementary Material
SYNOPSIS.
From 2004 to 2012, there was an increase in neoadjuvant endocrine therapy (NET) use from 2.7% to 3.2% in the US. Use of NET was associated with increase in breast conserving surgery in hormone receptor positive cT2-4c disease.
Footnotes
Disclosures: No Disclosures
References
- 1.Eiermann W, Paepke S, Appfelstaedt J, et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: A randomized double-blind multicenter study. Ann Oncol. 2001;12(11):1527–1532. doi: 10.3109/14653249.2011.613927. [DOI] [PubMed] [Google Scholar]
- 2.Smith IE, Dowsett M, Ebbs SR, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: The Immediate Preoperative Anastrozole, Tamoxifen, or Combined With Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol. 2005;23(22):5108–5116. doi: 10.1200/JCO.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Ellis MJ, Suman VJ, Hoog J, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: Clinical and biomarker outcomes and predictive value of the baseline PAM50-based int. J Clin Oncol. 2011;29(17):2342–2349. doi: 10.1200/JCO.2010.31.6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colleoni M, Bagnardi V, Rotmensz N, et al. Increasing steroid hormone receptors expression defines breast cancer subtypes non responsive to preoperative chemotherapy. Breast Cancer Res Treat. 2009;116(2):359–369. doi: 10.1007/s10549-008-0223-y. [DOI] [PubMed] [Google Scholar]
- 5.Ellis MJ, Coop A, Singh B, et al. Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: Evidence from a phase III randomized trial. J Clin Oncol. 2001;19(18):3808–3816. doi: 10.1067/mnc.2001.117181. [DOI] [PubMed] [Google Scholar]
- 6.Van De Water W, Fontein DBY, Van Nes JGH, et al. Influence of semi-quantitative oestrogen receptor expression on adjuvant endocrine therapy efficacy in ductal and lobular breast cancer-A TEAM study analysis. Eur J Cancer. 2013;49(2):297–304. doi: 10.1016/j.ejca.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 7.International Breast Cancer Study Group. Endocrine responsiveness and tailoring adjuvant therapy for postmenopausal lymph node-negative breast cancer: a randomized trial. J Natl Cancer Inst. 2002;94(14):1054–1065. doi: 10.1093/jnci/94.14.1054. http://www.ncbi.nlm.nih.gov/pubmed/12122096. [DOI] [PubMed] [Google Scholar]
- 8.Goldhirsch A, Ingle JN, Gelber RD, et al. Thresholds for therapies: Highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer. Annals of Oncol. 2009;20:1319–1329. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17(5):1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 10.Abrial C, Mouret-Reynier M, Curé H, et al. Neoadjuvant endocrine therapy in breast cancer. Breast. 2006;15(1):9–19. doi: 10.1016/j.breast.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Kaufmann M, von Minckwitz G, Mamounas EP, et al. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012;19(5):1508–1516. doi: 10.1245/s10434-011-2108-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


