Abstract
New developments in the rapid diagnosis and treatment of boys with Duchenne muscular dystrophy (DMD) have led to growing enthusiasm for instituting DMD newborn screening (NBS) in the United States. Our group has been interested in developing clinical guidance to be implemented consistently in specialty care clinics charged with the care of presymptomatically identified newborns referred after DMD-NBS. We reviewed the existing literature covering patient-centered clinical follow-up after NBS, educational material from public health and advocacy sites, and federal recommendations on effective NBS follow-up. We discussed the review as a group and added our own experience to develop materials suitable for initial parent and primary care provider education. These materials and a series of templates for subspecialist encounters could be used to provide consistent care across centers and serve as the basis for ongoing quality improvement.
Keywords: clinical, Duchenne muscular dystrophy, follow-up, long-term, management, newborn screening, public health
Duchenne muscular dystrophy (DMD) is an X-linked disorder with an incidence, based on systematic review,1 ranging from 10.7 to 27.8 per 100,000. Although no cure exists for the progressive muscle weakness of DMD, corticosteroid treatment improves muscle strength and function, and in combination with supportive medical care, is associated with markedly improved survival.2–5 New and emerging therapies are now available through promising clinical trials including antisense oligomers that show improvements in the 6-min walk test compared with historical controls.6 Effective new treatments promise to modify the severity of DMD and its outcome.
There have been concerns about the suitability of DMD for newborn screening (NBS) due to the lack of randomized controlled trials to show that treatment in early infancy improves outcomes.7,8 However, without early diagnosis, there are persistent diagnostic delays9 which, in turn, delay initiation of effective treatment until after major muscle damage has occurred. Recent developments in DMD diagnosis and genetic treatments, especially new mutation-specific agents,6,10 have increased the enthusiasm for early DMD diagnosis by means of NBS.
Mendell et al.11 reported a successful pilot program of DMD-NBS in Ohio using a 2-tiered laboratory screening approach: (1) initial determination of creatine kinase (CK) on the NBS dried blood spot card and (2) DMD gene mutational testing in those with high CK. This 2-tier methodology is likely to be adopted if broader implementation of DMD-NBS is introduced in the United States. DMD-NBS is not recommended by the Secretary of Health and Human Services Advisory Committee on Heritable Disorders in Newborns and Children (“Advisory Committee”). The Advisory Committee recommends disorders for the federal Recommended Uniform Screening Panel after reviewing the best available evidence.12 To address barriers and gaps in information that have hindered DMD-NBS in the United States, the Parent Project Muscular Dystrophy has convened a broad coalition of muscular dystrophy clinicians and researchers, NBS policy experts, DMD lay advocates, and public health professionals, to form the US National Duchenne Newborn Screening Program Taskforce. The authors of this study are members of the Task-force workgroup charged with developing detailed clinical guidance for following infants identified by DMD-NBS.
We recognized that in the current recommended panel of disorders screened in newborns, there is variability in the manner in which early diagnoses are made and how families are counseled about rare disease management. This variability in early clinical practices has long-lasting effects on later chronic disease follow-up.13 Because a goal of DMD-NBS is to avoid delays in initiating beneficial treatments, our workgroup wanted to develop consistent recommendations on the clinical care that follows NBS referral. Our vision is to provide “anticipatory guidance” for infants identified as having DMD or other muscle disorders. We have reviewed successful strategies from the cystic fibrosis (CF) NBS programs whose centers follow detailed care guidelines after NBS.14 Our recommendations will include information to counsel families whose infants are normal but have a presymptomatic “diagnosis” and to provide psychological support.15 This guidance will be reflected in a series of encounter templates to be implemented in most specialty care center (SCC) electronic medical record systems, and from which clinical data can be readily extracted and analyzed to ensure ongoing quality improvement. In this way, we hope to incorporate the NBS long-term follow-up goals outlined by the US Secretary for Health and Human Service Advisory Committee on Heritable Disorders in Newborns and Children: care coordination, evidence-based treatment, continuous quality improvement, and new knowledge discovery.13,16
MATERIALS AND METHODS
The workgroup members have extensive clinical experience in caring for children with a wide range of muscular dystrophies as well as for infants who have had presymptomatic diagnosis of DMD due to family history. Two co-authors (J.R.M., H.Z.A.) have been involved in clinical follow-up of infants referred from pilot US DMD-NBS programs, and 1 (J.M.K.) directs short- and long-term clinical follow-up of Krabbe disease and Pompe disease NBS referrals at the University of Rochester Medical Center, a NYS SCC. Several authors have experience providing neonatal family counseling and presymptomatic DMD care, because it is common to diagnose a second newborn boy in the family of an affected boy. Thus, this collective experience was also considered in the development of our guidance. Workgroup members reviewed existing information about short- and long-term follow-up provided by state NBS programs (e.g., New York State Department of Health, http://www.wadsworth.org/newborn-screening-program) and NBS advocacy Web sites (e.g., “Baby’s First Test,” http://www.babysfirsttest.org; NewS-TEPs, https://www.newsteps.org). Models of successful practices for clinical follow-up in specialty centers were reviewed. Useful materials for education of parents and medical home providers were also reviewed and adapted for DMD and other muscle disorders likely to be identified by DMD-NBS.
RESULTS
Review of NBS program materials showed that for most disorders there were links to patient information brochures, the American College of Medical Genetics action sheets (“ACT sheets,” http://www.ncbi.nlm.nih.gov/books/NBK55832/, downloaded 1/10/16) that describe the initial actions a health professional should follow for infants with positive NBS to counsel families and confirm diagnoses, and other similar algorithms available to clinicians engaged in clinical care after NBS. We found the New York state materials on X-linked adrenoleukodystrophy helpful, as this is an X-linked disorder where NBS also identifies female carriers and infants with other peroxisomal diagnoses (see http://www.wadsworth.org/newborn-screening/adrenoleukodystrophy, downloaded 11/9/15). In a similar manner, DMD-NBS would also identify female carriers and other forms of muscular dystrophy associated with highly elevated CK levels. The information helped us to develop:
Information for the primary care provider (PCP): (a) At the time of NBS referral, the state public health laboratory will send the PCP a printed letter that provides basic information about DMD and directs the PCP to contact the SCC where the patient will be seen for confirmatory diagnostic testing and for initiation of follow-up care. (b) Before the SCC visit, we recommend that the PCP and the SCC provider arrange a conference call with the infant’s family to discuss the referral and the initial visit with the SCC. This call will be made as soon as possible after the initial NBS referral. During this call and before the initial SCC visit, the family will be told that both parents and possibly 1–2 other support people can attend. The family will be informed that this visit will involve them meeting with an MD trained in neuromuscular disorders who will examine the baby, and that they might meet additional SCC providers and staff, including genetic counselor, social worker, and clinic coordinator.
A brochure for families, Understanding Newborn Screening of Duchenne Muscular Dystrophy, which describes DMD, its management, and how the NBS program will identify infants likely to have forms of non-DMD muscle disease. (A draft brochure suitable for modification by state NBS programs is available as supplementary material, available online.)
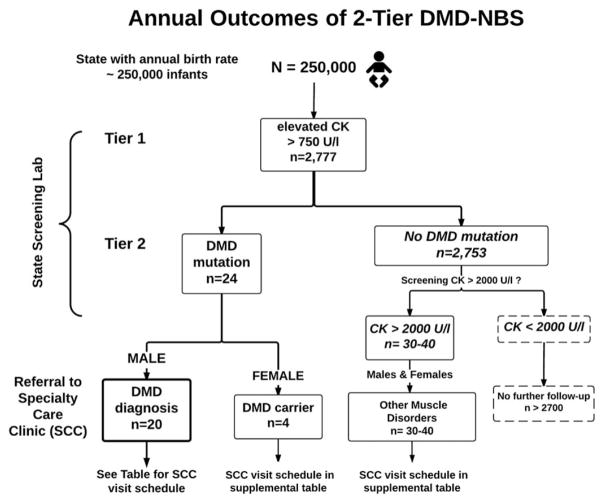
Graphical depiction of the likely results of DMD-NBS in a large state (annual birthrate of ~250,000; Fig. 1). Extrapolating from the Ohio pilot DMD-NBS program,11 such a birthrate would likely result in identification of 20 infant boys with DMD and 4 infant girls as DMD carriers. This hypothetical, large state’s DMD-NBS program would also identify approximately 30–40 infants with CK > 2000 U/l (considered “highly elevated”) but without DMD gene mutations. These infants are presumed to have other forms of muscular dystrophy.
Written recommendations and guidance for follow-up of all infants referred to the SCC with expertise in counselling, diagnosing, and treating neuromuscular disorders. Because 3 general categories of infant will be referred to the SCC, materials were developed to provide follow-up care specific to each category (i.e., boys with DMD mutations, girls with DMD mutations [carriers], and children without DMD mutations but with highly elevated CK). The table shows an outline of important elements covered in the first few SCC visits of boys with DMD after being identified by the 2-tier DMD-NBS. Similar guidance has been developed for girls identified as carriers and for infants with very high CK who may have other forms of muscular dystrophy (see Supplementary Tables, which are available online).
FIGURE 1.
Projected outcome of 2-tier DMD-NBS in a large state with a high birthrate. The numbers for males with a given DMD status and other muscle diseases were derived from data of the Ohio pilot DMD-NBS program.11 The results of this pilot provide the following estimates of population frequencies: (a) 1/90 infants with CK >750 U/L; (b) 1/3,800 to 1/4,500 infants with CK > 2,000 U/L; and (c) 1/6291 male infants with DMD gene mutations. Females with DMD gene mutations were not found in the Ohio population-based pilot, thus the frequency of females with DMD mutations (carriers) was estimated as 20% of that in males.
Table 1.
Visits with the specialty care center (SCC) for a newborn boy with a verified Duchenne muscular dystrophy (DMD) gene mutation.
| Visit attribute | Initial visit* | Follow-up visits at 6–9 months; 15–18 months; 24–30† months |
|---|---|---|
| Visit goals |
|
|
| History, including | Health history since birth: include feeding, sleeping, elimination behaviors | Interim history |
| Physical exam, including |
|
|
| End of visit |
|
|
While the initial visit can occur anytime during the first 2 months of life, the family’s needs and level of anxiety should be considered in the timing of this visit.
Starting at 30–36 months of age, visits will be every 6 months and will be constructed to adhere to recommendations of the DMD Care Considerations Working Group.33
Meeting with the genetic counsellor may occur at any of the scheduled visits, and the key genetic counselling services recommended may also be spread across multiple visits.
The 2-tier method is designed to reduce the number of false positive results that might occur with CK screening alone. The number of false negatives is unknown, but it is likely to be small if we consider DMD the phenotype of interest. The initial CK cut-off of 750 U/L will make it less likely to miss infants who present in early childhood with the DMD phenotype. Some boys with a Becker muscular dystrophy phenotype and later onset of weakness may be missed, because their CK at birth is not highly elevated yet.11,17
DISCUSSION
DMD-NBS has been controversial because of the lack of early treatments and the perception that early diagnosis did not improve outcome.7,8 We now know that significant muscle injury is present in very young DMD boys, and the need to initiate treatment early is supported by promising results from clinical trials of new treatments that improve outcomes, especially if they are given before significant muscle injury has occurred.10,18 Fine and gross motor skills in DMD infants, as assessed by the Bayley III scale, are significantly delayed relative to controls, which confirms the presence of measurable deficits at an early age.19 This clinical finding is corroborated by the finding of highly elevated CK levels, a biomarker of membrane fragility and active muscle degeneration, in DMD boys at birth and during the first year of life.11,20 Histological evidence of dystrophic pathology has been described in DMD fetuses21 and is evident on muscle biopsies as early as the perinatal period.22 Furthermore, early pathology of the pelvic girdle musculature can be identified by magnetic resonance imaging in DMD boys in the first 2 years of life.23 The therapeutic benefit of cortico-steroid treatment in prolonging ambulation may be more pronounced in DMD patients treated at an early age.24 This could be the result of having a higher percentage of viable skeletal muscle tissue responsive to treatment, which is seen in emerging therapies that were most beneficial in subjects with a lesser degree of fibrosis as measured by MRI.18,25 For many boys with DMD, these observations mean that newer treatments could be considered shortly after birth.
The clinical diagnosis of DMD remains persistently delayed,9 which not only exacerbates underlying health disparities26 but also keeps DMD clinical care centers from delivering high quality clinical care that could meaningfully improve outcomes. Currently, among the multidisciplinary clinics that provide high quality neuromuscular care (such as the Muscular Dystrophy Association clinics and Parent Project Muscular Dystrophy Certified Duchenne Care Centers), there is growing awareness of the need for more consistent care driven by registries.27,28 However, reducing undesired variability in clinical care and outcomes requires timely DMD diagnosis, which is difficult to accomplish without NBS due to challenges of counseling and disparities in access to health care.26,29
The US National Duchenne Newborn Screening Program Taskforce was formed to systematically address the key issues raised by NBS policy experts who have reviewed the possibility of early diagnosis of DMD by means of NBS.8,30 Our work-group was asked to address the clinical needs of infants and their families identified by DMD-NBS programs. We assumed that a 2-tier approach, such as modelled in the Ohio pilot program,11 would be used. Based on their screening outcomes,11 implementation of DMD-NBS in a large state with ~250,000 births annually, will likely result in diagnosis of 20 infant boys with DMD and another 30–40 infants with highly elevated CK levels who may develop some form of muscle disease.11,17 Extrapolating to the entire US, where the annual birth rate is 4 million infants, there will be an estimated ~300–350 new DMD diagnoses each year. While costs associated with this screening effort are a relevant concern, addressing them will require cost-benefit analyses that extend beyond the scope of our intended discussion. Our goal has been to articulate the components of an effective clinical follow-up program for infants identified as being at risk after DMD-NBS.
We reviewed the evidence for providing high-quality short-term and initiating long-term follow-up care to families of infants identified by state NBS programs.
We have taken advantage of our group’s own shared history of counseling and managing pre-symptomatic boys born into families with a history of Duchenne muscular dystrophy. In addition, we reviewed the long-term follow-up strategies used in states, such as California, with a strong history of multidimensional stakeholder involvement in long-term follow-up efforts31 as well as the successful quality improvement initiatives in cystic fibrosis NBS32 in developing a prototype of the schedule for following these infants.
Our proposed schedule of follow-up visits as well as the stated goals for each visit lays the foundation for effective lifetime chronic disease care. The earliest visits are made in close consultation with the primary care provider, setting the stage for effective long-term care coordination. While the ideal is for patients to have a medical home that can coordinate their care, the reality is that the SCC must be involved as a medical home partner. For example, neuromuscular SCCs have access to DMD clinical registries that are the basis for continuous quality improvement and evidence-based treatments. It will take partnerships between primary and specialist care providers to move DMD care forward and to realize the goals of NBS long-term follow-up set forth by the federal Advisory Committee and NBS advocates.13
Acknowledgments
The authors thank Rodolfo Valdez, PhD, and other reviewers from the Centers for Disease Control and Prevention, for their careful reading of this manuscript.
Abbreviations
- CK
creatine kinase
- DMD
Duchenne muscular dystrophy
- NBS
newborn screening
- PCP
primary care provider
- SCC
specialty care clinic
Footnotes
Additional supporting information may be found in the online version of this article.
References
- 1.Mah JK, Korngut L, Dykeman J, Day L, Pringsheim T, Jette N. A systematic review and meta-analysis on the epidemiology of Duchenne and Becker muscular dystrophy. Neuromuscul Disord. 2014;24:482–491. doi: 10.1016/j.nmd.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Mendell JR, Moxley RT, Griggs RC, Brooke MH, Fenichel GM, Miller JP, et al. Randomized, double-blind six-month trial of prednisone in Duchenne’s muscular dystrophy. N Engl J Med. 1989;320:1592–1597. doi: 10.1056/NEJM198906153202405. [DOI] [PubMed] [Google Scholar]
- 3.Moxley RT, Ashwal S, Pandya S, Connolly A, Florence J, Mathews K, et al. Practice parameter: corticosteroid treatment of Duchenne dystrophy: report of the quality standards subcommittee of the American Academy of Neurology and the practice committee of the Child Neurology Society. Neurology. 2005;64:13–20. doi: 10.1212/01.WNL.0000148485.00049.B7. [DOI] [PubMed] [Google Scholar]
- 4.Manzur AY, Kuntzer T, Pike M, Swan AV. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2008:CD003725. doi: 10.1002/14651858.CD003725.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Schram G, Fournier A, Leduc H, Dahdah N, Therien J, Vanasse M, et al. All-cause mortality and cardiovascular outcomes with prophylactic steroid therapy in Duchenne muscular dystrophy. J Am Coll Cardiol. 2013;61:948–954. doi: 10.1016/j.jacc.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Mendell JR, Goemans N, Lowes LL, Alfano LN, Berry K, Shao J, et al. Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Ann Neurol. 2016;79:257–271. doi: 10.1002/ana.24555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross LF. Screening for conditions that do not meet the Wilson and Jungner criteria: the case of Duchenne muscular dystrophy. Am J Med Genet A. 2006;140:914–922. doi: 10.1002/ajmg.a.31165. [DOI] [PubMed] [Google Scholar]
- 8.Kemper AR, Wake MA. Duchenne muscular dystrophy: issues in expanding newborn screening. Curr Opin Pediatr. 2007;19:700–704. doi: 10.1097/MOP.0b013e3282f19f65. [DOI] [PubMed] [Google Scholar]
- 9.Ciafaloni E, Fox DJ, Pandya S, Westfield CP, Puzhankara S, Romitti PA, et al. Delayed diagnosis in Duchenne muscular dystrophy: data from the muscular dystrophy surveillance, tracking, and research network (MD-STARnet) J Pediatr. 2009;155:380–385. doi: 10.1016/j.jpeds.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodino-Klapac LR, Mendell JR, Sahenk Z. Update on the treatment of Duchenne muscular dystrophy. Curr Neurol Neurosci Rep. 2013;13:332. doi: 10.1007/s11910-012-0332-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendell JR, Shilling C, Leslie ND, Flanigan KM, al-Dahhak R, Gastier-Foster J, et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol. 2012;71:304–313. doi: 10.1002/ana.23528. [DOI] [PubMed] [Google Scholar]
- 12.Kemper AR, Green NS, Calonge N, Lam WKK, Comeau AM, Goldenberg AJ, et al. Decision-making process for conditions nominated to the Recommended Uniform Screening Panel: statement of the US Department of Health and Human Services Secretary’s Advisory Committee on Heritable Disorders in Newborns and Children. Genet Med. 2014;16:183–187. doi: 10.1038/gim.2013.98. [DOI] [PubMed] [Google Scholar]
- 13.Hinton CF, Feuchtbaum L, Kus CA, Kemper AR, Berry SA, Levy-Fisch J, et al. What questions should newborn screening long-term follow-up be able to answer? A statement of the US secretary for Health and Human Services’ Advisory Committee on Heritable Disorders in Newborns and Children. Genet Med. 2011;13:861–865. doi: 10.1097/GIM.0b013e3182209f09. [DOI] [PubMed] [Google Scholar]
- 14.Foundation CF, Borowitz D, Robinson KA, Rosenfeld M, Davis SD, Sabadosa KA, et al. Cystic fibrosis foundation evidence-based guidelines for management of infants with cystic fibrosis. J Pediatr. 2009;155:S73–S93. doi: 10.1016/j.jpeds.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey DB, Jr, Skinner D, Davis AM, Whitmarsh I, Powell C. Ethical, legal, and social concerns about expanded newborn screening: fragile x syndrome as a prototype for emerging issues. Pediatrics. 2008;121:e693–e704. doi: 10.1542/peds.2007-0820. [DOI] [PubMed] [Google Scholar]
- 16.Kemper AR, Boyle CA, Aceves J, Dougherty D, Figge J, Fisch JL, et al. Long-term follow-up after diagnosis resulting from newborn screening: statement of the US Secretary of Health and Human Services’ Advisory committee on Heritable Disorders and Genetic Diseases in Newborns and Children. Genet Med. 2008;10:259–261. doi: 10.1097/GIM.0b013e31816b64f9. [DOI] [PubMed] [Google Scholar]
- 17.Gatheridge MA, Kwon JM, Mendell JR, Scheuerbrandt G, Moat SJ, Eyskens F, et al. Identifying non–Duchenne muscular dystrophy–positive and false negative results in prior Duchenne muscular dystrophy newborn screening programs: a review. JAMA Neurol. 2016;73:111–116. doi: 10.1001/jamaneurol.2015.3537. [DOI] [PubMed] [Google Scholar]
- 18.Mendell JR, Sahenk Z, Malik V, Gomez AM, Flanigan KM, Lowes LP, et al. A phase 1/2a follistatin gene therapy trial for Becker muscular dystrophy. Mol Ther. 2015;23:192–201. doi: 10.1038/mt.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connolly AM, Florence JM, Cradock MM, Malkus EC, Schierbecker JR, Siener CA, et al. Motor and cognitive assessment of infants and young boys with Duchenne muscular dystrophy: results from the Muscular Dystrophy Association DMD clinical research network. Neuromuscular Disord. 2013;23:529–539. doi: 10.1016/j.nmd.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zatz M, Rapaport D, Vainzof M, Passos-Bueno MR, Bortolini ER, de Pavanello RC, et al. Serum creatine-kinase (CK) and pyruvate-kinase (PK) activities in Duchenne (dmd) as compared with Becker (bmd) muscular dystrophy. J Neurol Sci. 1991;102:190–196. doi: 10.1016/0022-510x(91)90068-i. [DOI] [PubMed] [Google Scholar]
- 21.Emery AE. Muscle histology and creatine kinase levels in the foetus in Duchenne muscular dystrophy. Nature. 1977;266:472–473. doi: 10.1038/266472a0. [DOI] [PubMed] [Google Scholar]
- 22.Merlini L. A 19-year-old ambulant Duchenne patient with stunted growth on long-term corticosteroids. Neuromuscular Disord. 2014;24:417–418. doi: 10.1016/j.nmd.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Li W, Zheng Y, Zhang W, Wang Z, Xiao J, Yuan Y. Progression and variation of fatty infiltration of the thigh muscles in Duchenne muscular dystrophy, a muscle magnetic resonance imaging study. Neuromuscular Disord. 2015;25:375–380. doi: 10.1016/j.nmd.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Merlini L, Gennari M, Malaspina E, Cecconi I, Armaroli A, Gnudi S, et al. Early corticosteroid treatment in 4 Duchenne muscular dystrophy patients: 14-year follow-up. Muscle Nerve. 2012;45:796–802. doi: 10.1002/mus.23272. [DOI] [PubMed] [Google Scholar]
- 25.Mendell JR, Rodino-Klapac LR, Sahenk Z, Roush K, Bird L, Lowes LP, et al. Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann Neurol. 2013;74:637–647. doi: 10.1002/ana.23982. [DOI] [PubMed] [Google Scholar]
- 26.Holtzer C, Meaney FJ, Andrews J, Ciafaloni E, Fox DJ, James KA, et al. Disparities in the diagnostic process of Duchenne and Becker muscular dystrophy. Genet Med. 2011;13:942–947. doi: 10.1097/GIM.0b013e31822623f1. [DOI] [PubMed] [Google Scholar]
- 27.Miller RG, Brooks BR, Swain-Eng RJ, Basner RC, Carter GT, Casey P, et al. Quality improvement in neurology: amyotrophic lateral sclerosis quality measures: report of the quality measurement and reporting subcommittee of the American Academy of Neurology. Neurology. 2013;81:2136–2140. doi: 10.1212/01.wnl.0000437305.37850.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scully MA, Cwik VA, Marshall BC, Ciafaloni E, Wolff JMS, Getchius TS, et al. Can outcomes in Duchenne muscular dystrophy be improved by public reporting of data? Neurology. 2013;80:583–589. doi: 10.1212/WNL.0b013e318282334e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cyrus A, Street N, Quary S, Kable J, Kenneson A, Fernhoff P. Clinic-based infant screening for Duchenne muscular dystrophy: a feasibility study. PLoS Curr. 2012:e4f99c5654147a. doi: 10.1371/4f99c5654147a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendell JR, Lloyd-Puryear M. Report of MDA muscle disease symposium on newborn screening for Duchenne muscular dystrophy. Muscle Nerve. 2013;48:21–26. doi: 10.1002/mus.23810. [DOI] [PubMed] [Google Scholar]
- 31.Feuchtbaum L, Dowray S, Lorey F. The context and approach for the California newborn screening short- and long-term follow-up data system: preliminary findings. Genet Med. 2010;12:S242–S250. doi: 10.1097/GIM.0b013e3181fe5d66. [DOI] [PubMed] [Google Scholar]
- 32.Scully MA, Farrell PM, Ciafaloni E, Griggs RC, Kwon JM. Cystic fibrosis newborn screening: a model for neuromuscular disease screening? Ann Neurol. 2015;77:189–197. doi: 10.1002/ana.24316. [DOI] [PubMed] [Google Scholar]
- 33.Bushby K, Finkel R, Bimkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: implementation of multidisciplinary care. Lancet Neurol. 2010;9:177–189. doi: 10.1016/S1474-4422(09)70272-8. [DOI] [PubMed] [Google Scholar]