Abstract
Objectives:
Evaluation of the effect of low-frequency noise (LFN) on the frequency of chromosomal aberrations in the bone marrow cells and on the content of low-molecular-weight DNA (lmwDNA) in the blood plasma of rats.
Materials and Methods:
A total of 96 male Wistar rats were exposed to either single (17 min session) or multiple (17 min session repeated five times a week for 13 weeks) LFN, with the maximum range below 250 Hz and the sound pressure levels (SPLs) at 120 and 150 dB, respectively. The rats in the control groups were not subjected to any impact. The frequency of chromosomal aberrations in the bone marrow cells and the levels of lmwDNA in the blood plasma were measured afterwards.
Results:
It has been detected that a single LFN exposure with either corresponding SPLs had a significant increase in the frequency of chromosomal aberrations (more than 10-fold) compared to the controls (0.9 ± 0.3%) and resulted in the appearance of dicentric chromosomes in the aberration spectrum, both of which are evident for the occurrence of deoxyribonucleic acid double strand breaks triggered by the exposure. Furthermore, the lmwDNA levels in the blood plasma measured the following day after a single LFN exposure were significantly higher (7.7- and 7.6-fold, respectively) than that in the control group (11.0 ± 5.4 ng/ml), and such levels were maintained higher (4.8- and 2.1-fold, respectively) in the week after a single LFN exposure for the SPL of 120 and 150 dB, respectively, compared to the control group (18.8 ± 1.6 ng/ml). Similar results were obtained from the group with multiple LFN exposures (36.4- and 22.4-fold, respectively) compared to the control (17.7 ± 1.7 ng/ml) and suggest the enhancement of cellular apoptosis as a result of the LFN impact.
Conclusion:
Presumably, the LFN may have possible mutagenic effects and cause massive cell death.
Keywords: Apoptosis, blood plasma, chromosomal aberrations, extracellular low-molecular-weight DNA, low-frequency noise
Introduction
The World Health Organization recognizes low-frequency noise (LFN) as an environmental problem.[1] LFNs ranging from 20 to 300 Hz are emitted by a broad variety of sources including diverse means of transportation, in particular lorries, buses, trains, trams, airplanes, and helicopters; industrial machinery; stationary sources such as heating, cooling, and ventilation systems for buildings; compressors; and wind turbines.[2,3] Moreover, LFN covers long distances and penetrates through walls and windows with only little attenuation.[2]
LFN can cause moderate-to-severe health issues. A 15–20 min exposure to LFN with a sound pressure level (SPL) of 95–135 dB at frequencies up to 100 Hz results in undue fatigability, irritability, headaches, increased sweating, pains in the region of the heart, and difficulty in breathing.[4] Prolonged exposure to LFN with an SPL of 58–61 dB is accompanied by stress overload, sleep disturbances, vibroacoustic disease,[2] and disorders of the vestibular system, manifested as vertigo, nausea, and nystagmus.[5] However, to our best knowledge, very few publications address the problem of whether LFN is capable of causing organic damages in the body. Some research studies showed that the disturbance of sister chromatid exchange in the mice splenocytes occurred as a result of prolonged LFN and vibration influence.[6] Other recent studies suggested that prolonged LFN action with an SPL of 90 dB below 500 Hz caused perivascular structural changes in the coronary artery, leading to the development of periarterial fibrosis in the heart[7] and the enlargement of the perivascular ductal connective tissue of the parotid gland.[8] Consequently, LFN may adversely affect the genome, the tissues, and the cells of the body.
One of the criteria of impact on the genome is chromosomal aberrations, which can lead to impaired fertility, cancer and hereditary diseases, and apoptosis, wherein the consequences include internucleosomal deoxyribonucleic acid (DNA) fragmentation into fragments of 180–190 bp, circulating outside the cells. Such DNA fragments may be involved in an immune response and malignant transformation of the cell and can be used as an integral measure of apoptosis.[9] The goal of our study is to evaluate the effect of LFN on the frequency of chromosomal aberrations in the bone marrow cells and on the content of low-molecular-weight DNA (lmwDNA) in the blood plasma in rats.
Materials and Methods
Experimental animals
This study was conducted in 96 male Wistar rats, 12 weeks of age, with a body weight of 170 ± 35 g. The rats were obtained from the animal breeding farm “Rappolovo” (Leningrad Region, Russian Federation). The animals were kept in a vivarium under standard-type barrier conditions in polypropylene cages (six rats per cage) under 14/10 h light/dark regimes at 21–23°C. They received a standard laboratory diet of pellets for rodents, which was PK-120 (Laboratorkorm Ltd., Moscow, Russian Federation), supplemented by water ad libitum. The animals were kept and maintained in accordance with the National Standard of “The Principles of Good Laboratory Practice,” Russian Federation, GOST Р-53434-2009. The design of the experiments was approved by the Ethics Committee of N.N. Petrov Research Institute of Oncology (Saint Petersburg, Russian Federation).
Exposure to low-frequency noise
A electrodynamics transducer “JBL 2225” (USA) was used as the acoustic generator, which was connected with the waveguide using a metal-truncated cone. A metal tube with a diameter of 40 cm and length of 3 m was used as the waveguide. A chamber was made in the middle of the waveguide, with a length of 0.6 m, and fenced off on both the sides of the coarse mesh. The chamber was designed to accommodate the experimental animals in free space without the use of anesthesia. For exposure, the animals were transferred from the home cage into the chamber in groups and were free to move. A microphone was used to record the acoustic signal with subsequent processing by a computer. Using specialized custom software, emitter generated impulse noise with an impulse duration of 0.17 s, the maximum spectral density in 2–40 Hz, and the total exposure time − 17 min. For the evaluation of the repeated influence of the LFN groups of animals, these animals were exposed to multiple acoustic treatments that comprised five subsequent sessions per week followed by a break for 2 days. The control groups of animals were placed in the chamber in the absence of an acoustic treatment.
Assessment of the chromosomal aberrations
We randomized 24 rats into the following four groups of six animals per group: single 17-min LFN exposition with an SPL of 120 dB; single 17-min LFN exposition with an SPL of 150 dB; and two control groups without any LFN impact for each of the first two LFN groups. Both the control groups were then combined for subsequent analysis.
Chromosomal aberrations were determined in the metaphase plates of the rat’s bone marrow cells by the method described in the previous study.[9] The animals were injected intraperitoneally with 0.2 ml of 0.025% colchicine (Sigma–Aldrich, St. Louis, MO, USA) 2 h prior to euthanasia by cervical dislocation method after 24 h following the LFN exposure. The bone marrow samples were obtained by washing the tibia with Media 199 (Mechnikov Biomed, Moscow, Russian Federation) at 37°C. The collected cell suspensions were centrifuged at 150×g for 6 min, then resuspended in 0.56% potassium chloride solution, and fixed in a 1:3 mixture of ice-cold acetic acid and methanol. The samples were stained according to Giemsa with standard solution of azure eosin (Unimed, Moscow, Russian Federation) for 40 min and analyzed under a light microscope. At least 100, well-spread metaphases from each rat were analyzed in a blind fashion. The percentage of the cells with chromosomal aberrations was calculated, and the total frequency of chromosomal aberrations, dicentric frequency, and single and paired fragments were counted.
Evaluation of low-molecular-weight DNA content in the blood plasma
We randomized 72 rats into nine groups (eight animals each): single 17-min LFN exposition with an SPL of 120 or 150 dB, collecting the blood after 24 h; one control group without any LFN impact for both of the first two LFN groups; single 17-min LFN exposition with an SPL of 120 or 150 dB, collecting the blood after 7 days; one control group without any LFN impact for both of the second two LFN groups; multiple 17-min sessions five times a week during 13 weeks LFN exposition with an SPL of 120 or 150 dB, collecting the blood after 24 h following the last exposition; one control group without any LFN impact for the third two LFN groups.
The lmwDNA content in the blood plasma was determined by the method described in the previous study.[9] The blood samples were collected after decapitation of the rats under ether anesthesia. For each sample, the plasma was separated after centrifuging at 900×g for 10 min at 4°C. To remove the cells and cell debris, the plasma was further centrifuged twice at 2200×g for 10 min each. The nucleic acids were then extracted from each processed plasma sample by deproteinization using phenol and chloroform and precipitation with ethanol. The dried pellets were dissolved in deionized water (1 μl per 1 ml of original plasma sample). They were then incubated with RNase (Sigma–Aldrich) and evaluated in 2/16 (%) gradient polyacrylamide electrophoresis gels (Amresco, Louisville, KY, USA) stained with ethidium bromide (Amresco). For each sample, lmwDNA (160–180 bp) were quantified against a standard prepared using BspR1/pBR322 (Sigma–Aldrich).[9]
The experimental results were statistically processed in the GraphPad Prism 6 program using analysis of variance (ANOVA) with Tukey’s post-hoc test.
Results
The effects of low-frequency noise on the frequency of chromosomal aberrations
The effects of a single LFN exposure with different SPLs on chromosomal aberrations in the bone marrow of rats are presented in Figure 1. The overall spontaneous chromosomal aberration frequency in the control group amounted to 0.9 ± 0.3% and increased by 12.6- and 11.1-fold in the LFN-exposed groups with an SPL of 120 and 150 dB, respectively. There was a different spectrum of chromosomal aberrations in the LFN-exposed groups in contrast to the control. In particular, there were zero decentrics in the control group, unlike the groups exposed to LFN of 120 and 150 dB. The frequency of single fragments in the control was 0.3 ± 0.2%, whereas in the LFN-exposed groups, it was increased by 5.3 and 4.7 times, respectively. The frequency of paired fragments in the control amounted to 0.6 ± 0.3%, whereas in the LFN-exposed groups, it was higher by 12.5- and 11.8-fold, respectively. One-way ANOVA was performed for each type of chromosomal aberration, which showed a significant effect of LFN on the frequency of paired fragments (F(2,21) = 23.34, P < 0.0001) and for the total frequency of aberrations (F(2,21) = 53.34, P < 0.0001). A Tukey’s post-hoc test revealed that the frequency of paired fragments and the total frequency of aberrations were significantly higher after exposure to both 120 and 150 dB (P < 0.0001 for every comparison) compared to the intact control. The effect of LFN on the frequency of dicentrics was close to significance (F(2,21) = 3.183, P = 0.062), and Tukey’s post-hoc test results were close to significance only for the 120 dB group (P = 0.0683). It may be interesting to note that the increase in SPL had no effect on the damage of chromosomes; the total frequency of chromosomal aberrations and their spectrum in the groups with 120 and 150 dB did not differ significantly [Figure 1].
Figure 1.
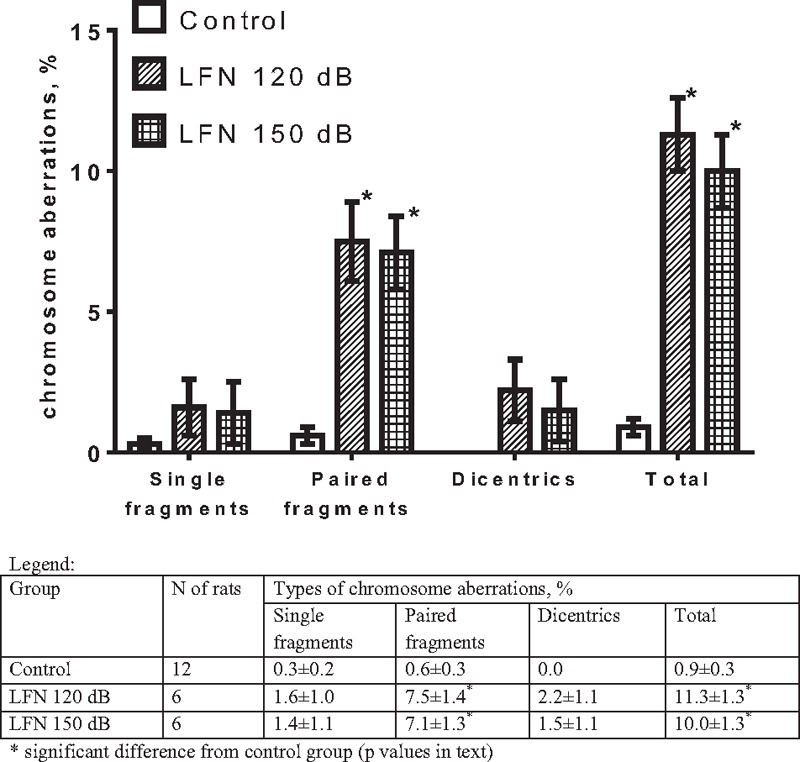
The frequency of chromosomal aberrations of the bone marrow cells after a single exposure to low-frequency noise (mean±SEM)
The effects of low-frequency noise on the content of low-molecular-weight DNA in the blood plasma
No statistical differences were found between the control groups. The effects of LFN in different modes on the content of lmwDNA in the blood plasma in rats are presented in Figure 2. Both single and multiple LFN exposure regimes increased the lmwDNA content in the blood plasma in rats. Its content in the first control group amounted to 11.0 ± 5.4 ng/ml, whereas after having a single LFN exposure with 120 and 150 dB, and blood collection the day after, the levels rose by 7.7- and 7.6-fold, respectively. Collecting the blood on the seventh day after the LFN exposure, the content of lmwDNA in the plasma remained elevated. This level in the second control group amounted to 18.8 ± 1.6 ng/ml, whereas after a single LFN exposure with 120 and 150 dB, and blood collection on the seventh day, the content of lmwDNA in the blood plasma was higher by 4.8- and 2.1-fold, respectively, wherein when exposed to 120 dB, the DNA content was 2.2 times higher when compared to the 150 dB exposure. Two-way ANOVA was performed for both the experiments with single exposure to LFN, showing a significant effect of LFN (F(2,42) = 8.383, P = 0.0009), but not time after exposure, and no interaction between these two factors. A Tukey’s post-hoc test revealed that the level of lmwDNA was significantly higher compared to the intact control after exposure to 120 dB (P = 0.006) and 150 dB (P = 0.0067) when collecting the blood after 24 h and after exposure to 120 dB (P = 0.0075), but not 150 dB, when collecting the blood after 7 days. We should also note that the difference between the 120 and 150 dB groups after 7 days was close to significance (P = 0.0565).
Figure 2.
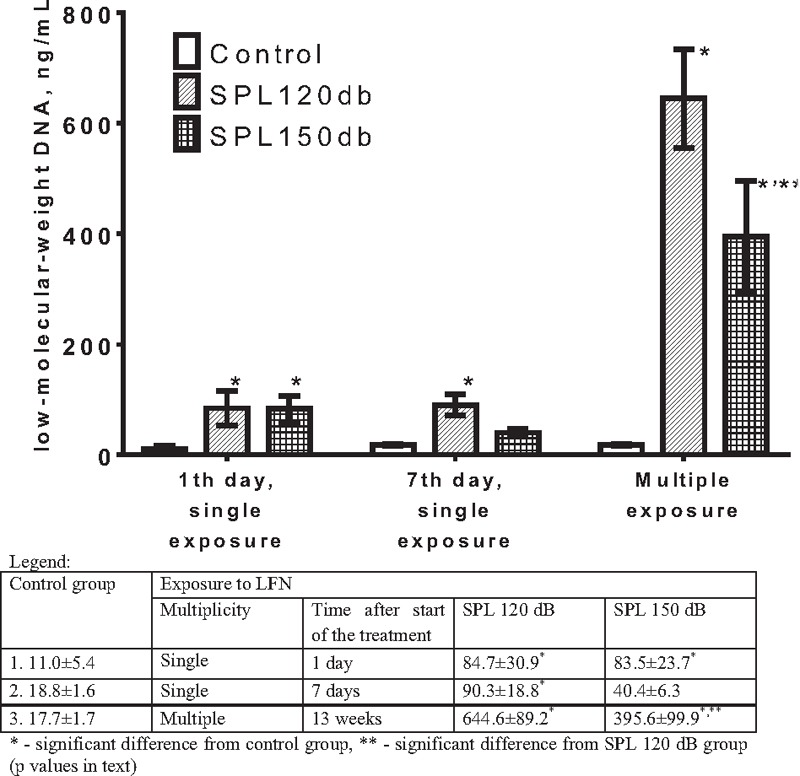
The content of low-molecular-weight DNA (ng/ml) in the blood plasma of rats exposed to low-frequency noise (mean±SEM; n=8)
Multiple LFN exposures increased the content of lmwDNA in the blood plasma even more significantly. This level in the third control group amounted to 17.7 ± 1.7 ng/ml, whereas following multiple LFN exposures with 120 and 150 dB and collecting the blood the day after, its content increased by 36.4- and 22.4-fold, wherein when exposed to 120 dB, the DNA content was 1.6 times higher when compared to the 150 dB exposure. The experiment with multiple exposures to LFN was analyzed separately, because they involved a combination of three factors (SPL, time after the beginning of exposure, and the number of exposures). One-way ANOVA has shown a significant effect of multiple exposition to LFN (F(2,21) = 33.16, P < 0.0001). A Tukey’s post-hoc test revealed that the level of lmwDNA was significantly higher after exposure to 120 dB (P < 0.0001) and 150 dB (P = 0.0003) compared to the intact control and was significantly different between the groups exposed to LFN (P = 0.0349) [Figure 2].
Discussion
Our results show for the first time the effect of LFN on the genome of the cell. More specifically, both 120 and 150 dB LFN resulted in a 10-fold increase in the overall chromosomal aberrations range compared to the spontaneous chromosomal mutation rates in the bone marrow cells in rats, leading to the occurrence of dicentrics that were not detected in the control. This significant increase in chromosomal aberration rates is evidence for the LFN’s ability to cause DNA double strand breaks in the cells. Dicentrics are the most substantial type of chromosomal mutations that result from rearrangements occurring simultaneously in two chromosomes and which are capable of killing the cell.[10] We have previously shown that a single, full-body ionizing irradiation of rats at a dose of 2 Gy increases the total frequency of chromosomal aberrations in the bone marrow cells by more than 10 times as compared to the control and results in the occurrence of dicentrics with a 2.1% frequency.[10] Consequently, the damaging effect of LFN on the chromosomes of the bone marrow is comparable to that of the ionizing radiation. It is known that the long-term effects of ionizing radiation include an increased risk of leukemia and other malignancies.[11] We can assume that LFN exposures may also have a carcinogenic effect. The evaluation of the possible carcinogenic effects of LFN in animal experiments and epidemiological studies in terms of its long-term effects could clarify the LFN’s carcinogenic risks, as we did not find any previous data on the carcinogenic effect of LFN.
The detection of a nucleosome DNA that circulates in the blood plasma and other biological fluids is currently used as a cell death marker both in clinical practice and fundamental scientific studies.[12] The DNA released by the apoptotic cells is subjected to internucleosomal degradation into 160–180 bp fragments, which demonstrate a characteristic laddering pattern in electrophoretic gels.[13] They are defined as extracellular lmwDNA and can correlate with the severity of apoptosis in the body.[9,14]
In this study, we present novel findings, which show that LFN elevates the lmwDNA content in the blood plasma that may be associated with cell death. Specifically, a single acoustic LFN exposure significantly increases the lmwDNA content in the blood plasma, whereas multiple exposures continue to elevate lmwDNA levels. It also has been observed that LFN leads to a stable (at least 7 days) increase of lmwDNA in the blood plasma, even though it is known that lmwDNA is rapidly excreted from the body.[15] It can be assumed that long-term persistence of increased DNA content in the blood plasma indicates the ongoing cell death in rats after LFN exposure and the weaknesses of the organism’s ability to restore cellular homeostasis after exposure to LFN.
The respiratory tract is considered to be one of the primary targets of LFN’s harmful impact.[16] We have previously revealed that acute LFN effects are accompanied by structural and cellular disorders of the internal organs in rats, particularly the lungs, where it appears to cause subpleural hemorrhage, occurrence of atelectasis areas, microvascular congestion, increased degranulation of the mast cells and the platelets, and destruction of the leukocytes.[17] It is quite probable that the death of the cells in the lungs and in the blood can be regarded as the major cause leading to the elevated lmwDNA concentrations in the blood plasma after an LFN exposure.
In our study, we used LFN in two modes − 120 and 150 dB, anticipating that the higher SPL would cause a more pronounced damaging effect on the cells and the chromosomes. However, no significant differences were found in the frequency of chromosomal aberrations and the blood plasma lmwDNA level 1 day after a single exposure between the two LFN groups. This suggests that the 120 dB SPL is the threshold dose for these indicators. Moreover, the level of lmwDNA in the blood plasma 7 days after a single exposure and after multiple LFN exposures was lower in the group with an SPL of 150 dB. It has been shown that lmwDNA content increases for certain types of external influences (ionizing radiation)[9,14] and pathological conditions (malignant tumors, pneumonia, etc.).[12,15] It has been suggested that the increase of this index is associated with increased cell death under these pathologies.[9,13] Uniformity of the DNA reaction to the impact of various factors, the effect of which is accompanied by the destruction of the cells, allows us to assert the nonspecific nature of the reaction as a response to massive cell death. It is practically impossible at this stage to establish the specificity and localization of pathological focus in biological objects by exploring the lmwDNA. Clearly, further research will be needed to resolve the nature of this phenomenon.Summing up the results, it can be assumed that LFN not only causes functional disorders of the body mentioned previously, including stress overload, sleep disturbances, undue fatigability, irritability, headaches, vertigo, increased sweating, nausea, and nystagmus,[2,4,5] but may also have a mutagenic effect by increasing the frequency of chromosomal aberrations, as well as damaging the cells and causing their death, which manifests itself as elevated lmwDNA content in the blood plasma. The study was performed on male rats because men are primarily exposed to industrial noise. We assume that females could be affected by noise in the same manner. The levels of 120 and 150 dB are far higher than typical human exposures in the environment, but we assume that the effects obtained at a relatively high SPL of 120–150 dB at a short 17-min exposure will likely occur with prolonged exposure to LFN with lower SPL specific to the common environment of humans and animals. Therefore, LFN is a much more harmful factor than it seemed so far.
Conclusion
A single LFN exposure in rats resulted in increased rates of chromosomal aberrations in the bone marrow cells and elevated content of lmwDNA in the blood plasma. The level of lmwDNA in the blood plasma remained high for at least 7 days after a single LFN exposure. Multiple LFN exposures resulted in even higher content of lmwDNA in the blood plasma compared to the single exposures. These data suggest that LFN may have a possible mutagenic effect and may enhance apoptosis.
Financial support and sponsorship
This work was partially financially supported by the Government of Russian Federation, Grant 074-U01.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Leventhall HG. Low frequency noise and annoyance. Noise Health. 2004;6:59–72. [PubMed] [Google Scholar]
- 2.Persson Waye K. Effects of low frequency noise on sleep. Noise Health. 2004;6:87–91. [PubMed] [Google Scholar]
- 3.Schmidt JH, Klokker M. Health effects related to wind turbine noise exposure: A systematic review. PLoS One. 2014;9:e114183. doi: 10.1371/journal.pone.0114183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schust M. Effects of low frequency noise up to 100 Hz. Noise Health. 2004;6:73–85. [PubMed] [Google Scholar]
- 5.Harrison RV. On the biological plausibility of wind turbine syndrome. Int J Environ Health Res. 2015;25:463–8. doi: 10.1080/09603123.2014.963034. [DOI] [PubMed] [Google Scholar]
- 6.Silva MJ, Dias A, Barreta A, Nogueira PJ, Castelo-Branco NA, Boavida MG. Low frequency noise and whole-body vibration cause increased levels of sister chromatid exchange in splenocytes of exposed mice. Teratog Carcinog Mutagen. 2002;22:195–203. doi: 10.1002/tcm.10012. [DOI] [PubMed] [Google Scholar]
- 7.Antunes E, Oliveira P, Oliveira MJ, Brito J, Aguas A, Martins dos SJ. Histomorphometric evaluation of the coronary artery vessels in rats submitted to industrial noise. Acta Cardiol. 2013;68:285–9. doi: 10.1080/ac.68.3.2983423. [DOI] [PubMed] [Google Scholar]
- 8.Oliveira P, Brito J, Mendes J, da Fonseca J, Águas A, Martins dos Santos J. Effects of large pressure amplitude low frequency noise in the parotid gland perivasculo-ductal connective tissue. Acta Med Port. 2013;26:237–42. [PubMed] [Google Scholar]
- 9.Vasilyeva I, Bespalov V, Baranova A. Radioprotective combination of α-tocopherol and ascorbic acid promotes apoptosis that is evident by release of low-molecular weight DNA fragments into circulation. Int J Radiat Biol. 2015;91:872–7. doi: 10.3109/09553002.2015.1087066. [DOI] [PubMed] [Google Scholar]
- 10.Friedman DA, Tait L, Vaughan AT. Influence of nuclear structure on the formation of radiation-induced lethal lesions. Int J Radiat Biol. 2016;92:229–40. doi: 10.3109/09553002.2016.1144941. [DOI] [PubMed] [Google Scholar]
- 11.Bespalov VG, Semenov AL, Aleksandrov VA, Kovan’ko EG, Ivanov SD. Anticarcinogenic activity of alpha-difluoromethylornithine, ginseng, eleutherococcus, and leuzea on radiation-induced carcinogenesis in female rats. Int J Radiat Biol. 2014;90:1191–200. doi: 10.3109/09553002.2014.932937. [DOI] [PubMed] [Google Scholar]
- 12.Holdenrieder S, Stieber P. Clinical use of circulating nucleosomes. Crit Rev Clin Lab Sci. 2009;46:1–24. doi: 10.1080/10408360802485875. [DOI] [PubMed] [Google Scholar]
- 13.Choi JJ, Reich CF, Pisetsky DS. Release of DNA from dead and dying lymphocyte and monocyte cell lines in vitro . Scand J Immunol. 2004;60:159–66. doi: 10.1111/j.0300-9475.2004.01470.x. [DOI] [PubMed] [Google Scholar]
- 14.Vasilyeva IN. Low-molecular-weight DNA in blood plasma as an index of the influence of ionizing radiation. Ann N Y Acad Sci. 2001;945:221–8. doi: 10.1111/j.1749-6632.2001.tb03889.x. [DOI] [PubMed] [Google Scholar]
- 15.Fleischhacker M, Schmidt B. Circulating nucleic acids (CNAs) and cancer − A survey. Biochim Biophys Acta. 2007;1775:181–232. doi: 10.1016/j.bbcan.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Alves-Pereira M. Noise-induced extra-aural pathology: A review and commentary. Aviat Space Environ Med. 1999;70:A7–21. [PubMed] [Google Scholar]
- 17.Vasilyeva IN, Zinkin VN. The impact of low-frequency noise on the content of low-molecular-weight DNA of blood plasma. J Nucl Acid Investig. 2011;2(Suppl 1):P1–17. [Google Scholar]


