Abstract
Intensity modulated radiation therapy (IMRT) and three-dimensional conformal radiation therapy (3D CRT) are two treatment modalities in prostate cancer, which provide acceptable dose distribution in tumor region with sparing the surrounding normal tissues. IMRT is based on inverse planning optimization; in which, intensity of beams is modified by using multileaf collimators and also compensators with optimum shapes in step and shoot (SAS) and compensator-based method, respectively. In the recent study, some important parameters were compared in two IMRT and 3D CRT methods. Prescribed dose was 80 Gy for both IMRT procedures and 70 Gy for 3D CRT. Treatment plans of 15 prostate cancer candidates were compared to target the minimum dose, maximum dose, V 76 Gy (for IMRT plans) V 66.5 Gy (for 3D CRT), mean dose, conformity index (CI), and homogeneity index (HI). Dose conformity in compensators-based IMRT was better than SAS and 3D CRT. The same outcome was also achieved for homogeneity index. The target coverage was achieved 95% of prescribed dose to 95% of planning target volume (PTV) in 3D CRT and 95% of prescribed dose to 98% of PTV in IMRT methods. IMRT increases maximum dose of tumor region, improves CI and HI of target volume, and also reduces dose of organs at risks.
Keywords: 3D CRT, compensator, conformity index, homogeneity index, IMRT, step and shoot
Background
Prostate cancer is the third most common cancer in men and the second most lethal cancer in the United States, people diagnosed with this cancer is increasing worldwide.[1,2] Radiation therapy is one of the treatments that the tumor cells are destructed by high-energy radiation. The most crucial point in this treatment is to deliver 100% of the prescribed dose of 100% of the tumor volume homogeneously and to reduce the dose of the adjacent healthy tissues such as bladder, rectum, and femoral head.[3]
Nowadays, various radiation therapy methods are used, such as three-dimensional conformal radiation therapy (3D CRT), intensity modulated radiation therapy (IMRT), intensive modulation arc therapy, and volumetric modulation arc therapy. But out of the above techniques, 3D CRT and IMRT are the most accessible and applied in many radiotherapy centers around the world.[3]
IMRT is an established treatment for prostate cancer, since it has the theoretical advantage of an increased flexibility in highly conformal plans by using several numbers of radiation fields and modulated beams. Besides, IMRT provides a specific sparing of organs at risk (OAR) such as rectum, bladder, and femoral head.[4] It is based on an inverse planning optimization, which modulates the intensity of beams via multileaf collimators (MLC) in step and shoot (SAS) IMRT technique and by a compensator with optimum shapes, for compensator-based IMRT.[4,5]
IMRT with a conventional linear accelerator equipped MLC was adapted to treat the prostate cancer in 1995 (of course IMRT by compensator was being performed earlier).[6] In SAS IMRT, the MLC leaves stay immobile during irradiation, and move to reshape the beam while the radiation is turned off.[7] Advantages of this technique include precise delivery of dose, easy verification, and general availability; although a prolonged treatment time is its drawback, radiation has to be constantly switched on and off to allow MLC leaves to reshape.[8]
Compensator-based is another method of IMRT for delivering high amount of radiation dose to tumors inside patients’ body. In this method, the modulated radiation for a certain therapeutic field with a single invariant beam is obtained by compensators with the specific shapes, which have been designed by Treatment Planning System (TPS), based on patient’s computed tomography (CT) scans. Modulators are milled similar to the tumor shape and size, and made of specific alloys such as brass, which is immobile during the treatment time and has not technical problems like temporal fluctuations of smaller sub-fields.[9]
3D CRT is a sophisticated procedure that starts with the obtained, personalized, CT scans of the tumor and normal tissues. These images are utilized for treatment planning to deliver highly precise conformed dose distribution to the target region and to spare healthy tissues.[10] Conformation of dose distribution with tumor structure causes 3D CRT to raise the control rate of tumor while reducing negative side effects; thus this technique is used to treat patients with the complex tumor shapes.[10,11] Generally, the aim of the 3D CRT planning is not only to provide adequate dose coverage of planned target volume (PTV) and to deliver a homogeneous dose distribution but also to spare OARs and planning organ at risk volumes.[11]
To investigate of recent aim in treatment planning, dose volume histogram (DVH) curves are handy and useful tools that are used to define 3D dose distribution inside the treatment volume to indicate the highest, lowest, and average dose values delivered to each volume of interest.[3] Using DVH as a mighty analyzing tool, interpretation of dose distribution in target volume would be very simple, because it shows the isodose curves around specific percentage of target volume and healthy tissues.[12] The homogeneity index (HI) and conformity index (CI) are also appropriate tools to treatment plan analysis in 3D CRT and IMRT.[13,14]
In this study, two IMRT methods were compared with 3D CRT of prostate cancer by evaluating the HI and CI as well as DVH curves.
Procedure
Target delineation
Initially, the CT scans with 3 mm thickness were obtained from 15 prostate cancer candidates in supine position. Digital imaging and communications in medicine images were transferred to the Module Integrated Radiotherapy System (MIRS) version 5.0.00 TCS; Then clinical target volume (CTV) was considered as prostate gland with seminal vesicles and contoured by radiation oncologist. In SAS technique and compensator-based IMRT, the PTV was determined by three-dimensional margin of 10 mm around the CTV and 8 mm towards rectum posteriorly. The PTV also defined by adding margin of 10 mm around CTV like IMRT techniques and 6 mm to the rectum in 3D CRT. All treatment plans were done by a medical physicist and approved by radiation oncologist.
Planning details and dose prescriptions
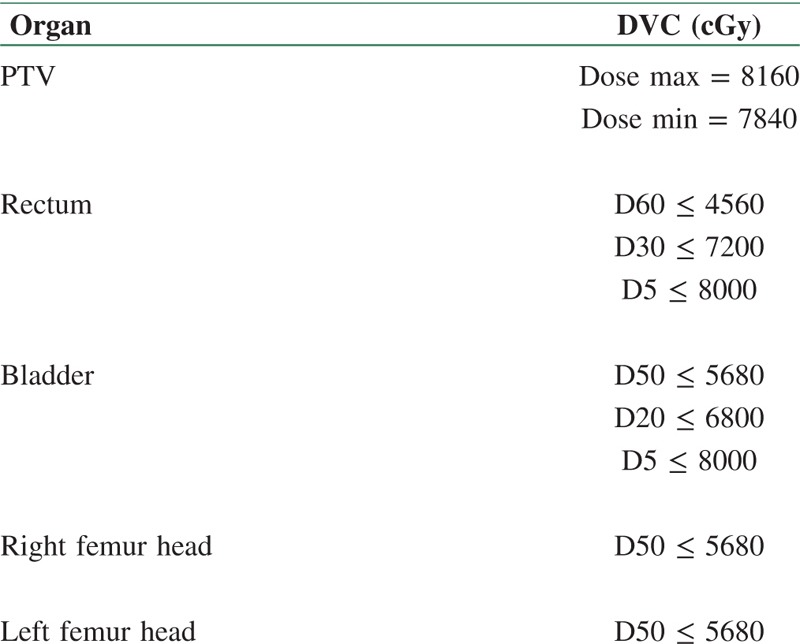
A clinical linear accelerator (Elekta, Precise Model, United Kingdom), which produced three energies (6, 10, and 18 MV) and integrated with 80 pairs of leaves (MLC) was used for SAS IMRT and 3D CRT. The therapeutic fields were the same for three methods in term of the directions and beam of views [Table 1]. The prescribed dose in both IMRT and 3D CRT was considered 80 and 70 Gy. Dose volume constraints (DVCs) selected in IMRT methods is shown in Table 2, and five fields with the same weight (40 cGy for each beam) in 3D CRT were determined for all techniques.
Table 1.
The directions and gantry angles in treatment plans

Table 2.
DVCs for SAS and compensator IMRT
Homogeneity and conformity indexes
Dose homogeneity indicates the uniformity of dose distribution within the target volume, and dose conformity is defined as the ratio between the PTV and the irradiated volume at specified prescription dose.[8,15,16] The dose conformity and uniformity were measured and estimated according to International Commission on Radiation Unit and Measurement (ICRU) 83.[9]
The CI defined as following:
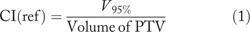
V95% is the volume of PTV covered by at least 95% of prescribed dose.
The HI defined as following:[9]
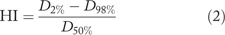
D2%, D98%, and D50% are the received dose by 2%, 98%, and 50% of target volume.
CRT treatment planning
A treatment plan was administrated by using five fields, which were set up to minimize the received dose to rectum, bladder, right and left femur heads, and to maximize the target dose coverage for 3D CRT. The radiant beams were applied to acquire adequate dose coverage for whole PTV while critical organs were shielded by MLC without compromising PTV covering. Weight of each beams was adjusted the same to optimize coverage and improve homogeneity of dose distribution in PTV, which received at least 95% (66.50 Gy) of the prescribed dose.
IMRT treatment planning
Treatment plans were prepared to achieve the minimum criteria (98% of PTV receives 95% of the prescribed dose in both IMRT techniques). In therapeutic fields of SAS IMRT, 11 subfields were defined whiles in compensator-based IMRT, each patient had the unique compensator for therapeutic fields. The dose constraints of the target and critical organs for both IMRT methods are mentioned in Table 2. DVHs were utilized to calculate the volumes received mean (D50%), maximum (D2%), and minimum (D98%) dose, and the covered volume of PTV with reference dose.
Results
Treatment planning was performed in all methods as mentioned before, while directions and beam of views were the same with three energies (6, 10, and 18 MV) and five fields [Table 1]. DVCs were determined by radiation oncologist based on acceptable radiation doses for PTV and OARs. Maximum and minimum received dose and also Dx were ascertained for PTV and OARs, respectively [Table 2]. Target coverage in IMRT methods and 3D CRT as well as Dmax, Dmin and Dmean of PTV were extracted from DVH curves, and finally HI and CI were calculated in three energies [Table 3].
Table 3.
Data from DVHs of treatment plans in IMRT methods and 3D CRT for 6, 10 and 18 MV
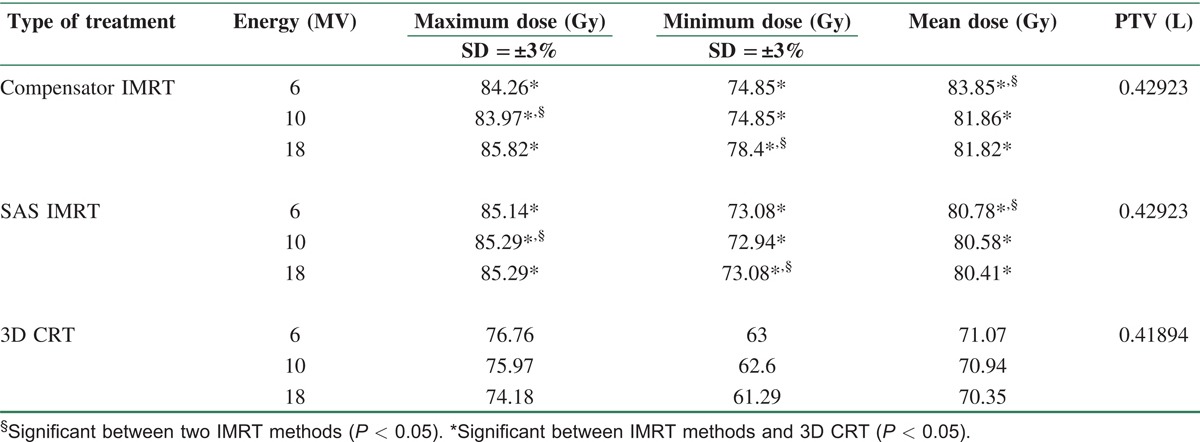
On the basis of Table 3, Dmax (hot spot) in PTV in 3D CRT is significantly higher than that in two other IMRT methods in all energies. Between IMRTs, Dmax in SAS was higher than that in compensator IMRT in 6 and 10 MV. 3D CRT and compensator IMRT showed the lowest and highest Dmin in PTV, respectively, in all energies while there was a significant difference in Dmin between two IMRT methods in 18 MV. Substantially, Dmean in PTV in IMRT methods was higher than that in 3D CRT.
The dose distribution in sagittal sections is shown for 10 MV in three methods [Figure 1]. In IMRT methods, the OARs (rectum and bladder) spared more and received D95% less than that in 3D CRT, and the coverage of prostate with D95% was more acceptable in both IMRT approaches.
Figure 1.
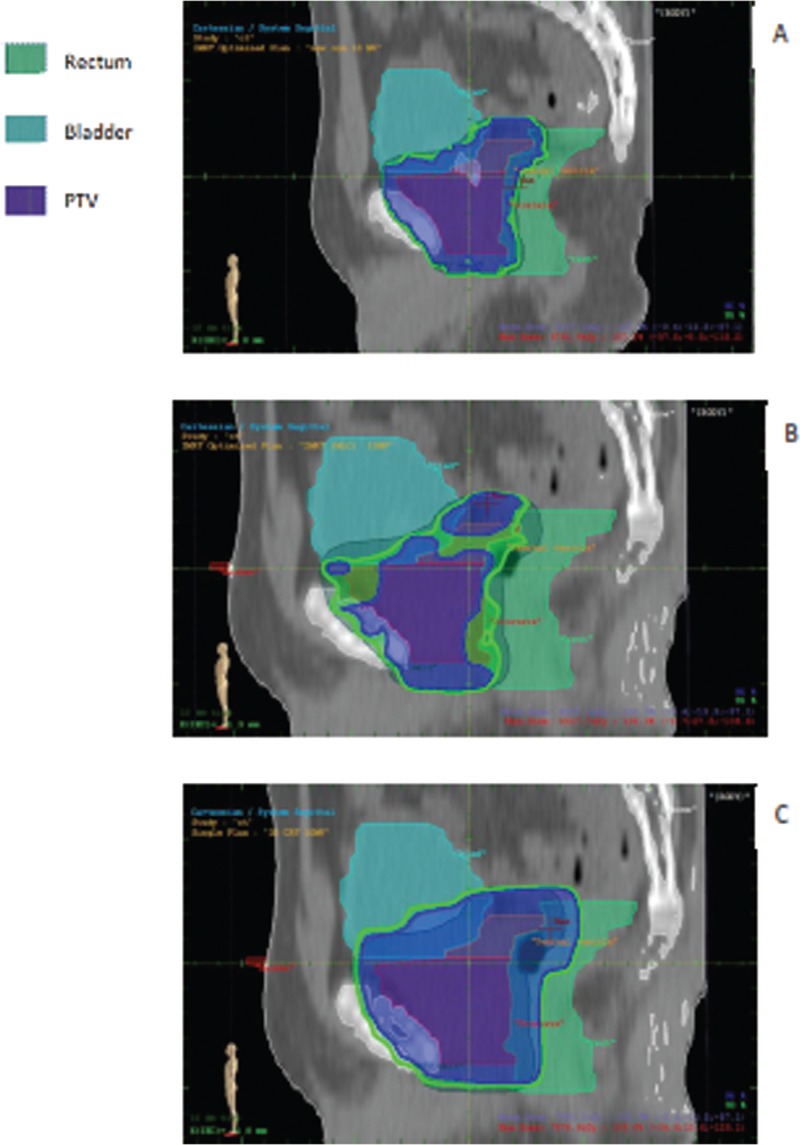
Sagittal section of dose distribution in (A) compensator IMRT, (B) SAS IMRT, and (C) 3D CRT
DVH curves of a treatment plan of prostate were calculated in all three methods in Figure 2. In DVH curves, some organs such as bone, seminal vesicles, right and left femur, prostate, bladder, and rectum were determined.
Figure 2.
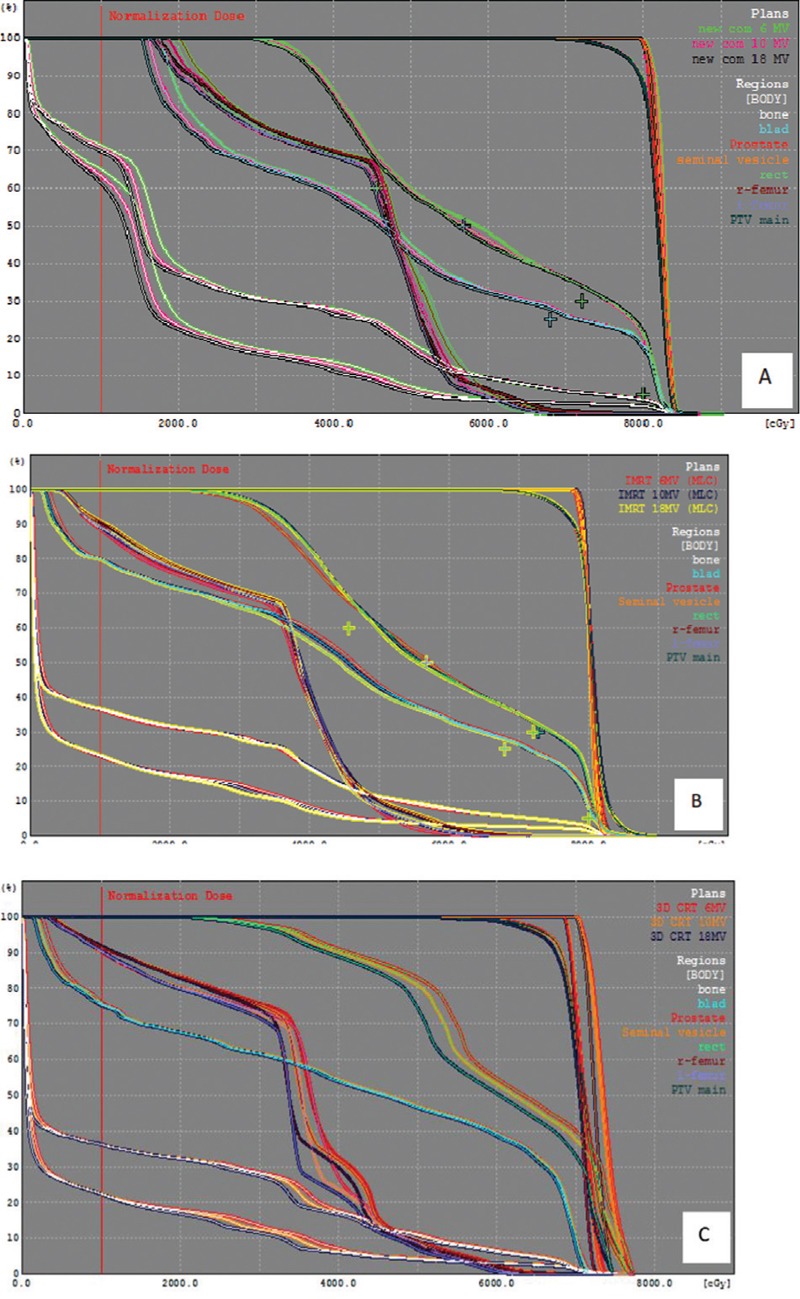
Ccumulative DVHs of PTV and OARs in 6, 10 and 18 MV in A) compensator IMRT, B) S.A.S IMRT and C) 3D CRT
The volume of PTV covered by 95% of prescribed dose (V95%) increased significantly in compensator IMRT in all energies (97.06, 97, and 96.97), but it had a steady state in all energies in SAS method. V95% in the latest approach was more than that in 3D CRT in all energies except 6 MV [Figure 3].
Figure 3.
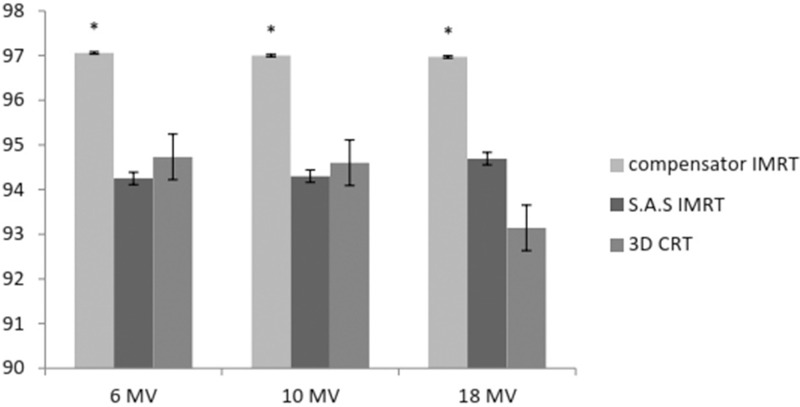
The percentage of volume of PTV covered by D95% in three energies (*P < 0.05)
HI and CI were calculated using Eqs. (1) and (2), correspondingly. The HI values had no significant difference in 6 and 10 MV between IMRT methods and 3D CRT, but in 18 MV, there was a meaningful decrease in compensator IMRT method rather than that in two other approaches [Figures 4 and 5]. Since HI = 0 is optimum, recent results revealed that compensator IMRT improves homogeneity rather than SAS IMRT and 3D CRT especially in high energies (18 MV); however HI values were almost constant in all energies in 3D CRT and SAS method, but it decreased with energy increasing in compensator IMRT [Figure 4].
Figure 4.
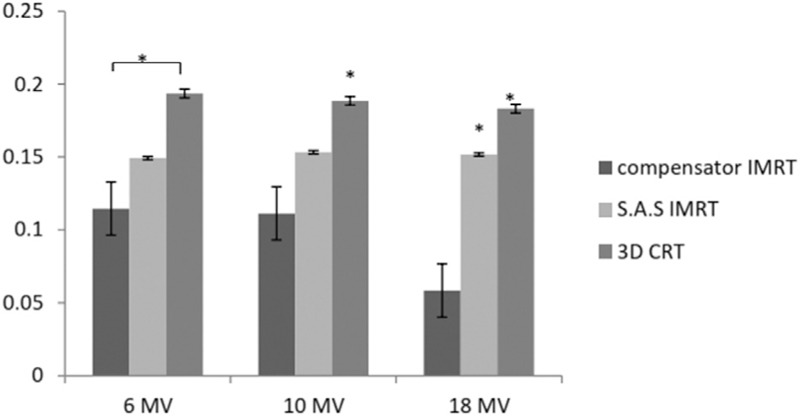
HI in three methods and energies (*P < 0.05). HI=0 is the most acceptable value
Figure 5.
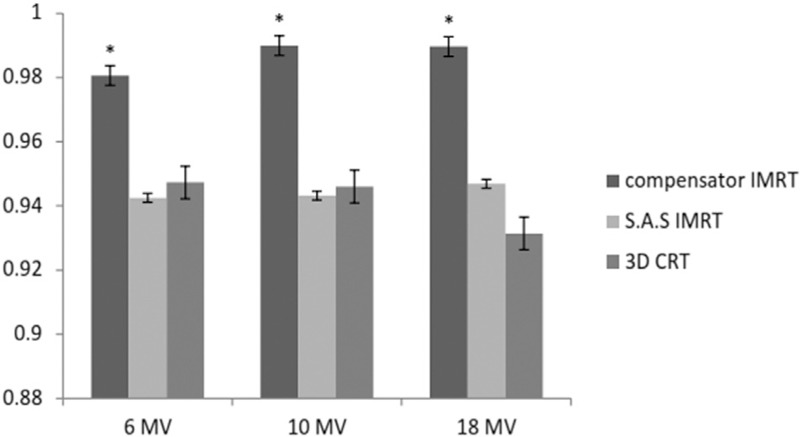
CI in three methods and energies (*P < 0.05). CI=1.0 is the most acceptable value
CI values were higher in compensator IMRT in all energies, and there was no significant difference between CI values in SAS IMRT and 3D CRT. As CI equals to one is optimum, compensator IMRT also has shown the best outcome [Figure 5].
Conclusion
In this study, calculated HI and CI by DVH curves were assessed in compensator and SAS IMRT of prostate cancer and compared with 3D CRT as a conformal method. Treatment plans were accomplished for all patients by a medical physicist and approved by a radiation oncologist. In all treatment plans, five fields were applied with the same gantry angles [Table 1] and DVCs in IMRT methods. The sagittal sections of three methods clearly showed the concave PTV coverage and exclusion of rectum and bladder during optimization by SAS and compensator IMRT while further volume of OARs like rectum and bladder received more radiation dose in 3D CRT treatment plans. Our results showed that preliminary goals of study (receiving the 95% of prescribed dose to 98% and 95% of the PTV, respectively) were achieved in compensator IMRT and 3D CRT, while these criteria were not obtained in SAS IMRT.
Generally, 3D CRT treatment plans showed several hotspots near the rectum and bladder wall, although these hot spots were in the acceptable limitation; dose distribution in IMRT methods showed better PTV coverage and sparing of OARs.
CI and HI values were acceptable in IMRT methods; thus we could mention that the arrangement of beams witch used in IMRT improved homogeneity and conformity and reduced the volume of OARs. Briefly, parameters of CI and HI were the foremost in the compensator-based IMRT.
Homogeneity index improves in IMRT in complicated treatment plans as reported by Fisher et al.[17] IMRT technique enhances normal tissue sparing and drops late effects, so patient’s quality of life improves.[18] The potential advantages of IMRT technique over 3D CRT and conventional techniques are (1) reaching to the optimal dose distribution inside the tumor volume and (2) decreasing the received dose by healthy tissues; these abilities are expected to translate into improved outcomes and reduced toxicity. Because of beam modulation during optimization compared to 3D CRT, hotspots reduces while skin dose does not increase noticeably.[19] Between two IMRT techniques, compensators (physical attenuators) are not sophisticated and without some problems such as lead positioning accuracy, interleaf leakage and transmission, rounded leaf, and finally tongue-and-groove effect that are inherent in MLC systems.[20,21,22,23] Ung et al. performed a planning study to assess dosimetric effect of systematic MLC positioning errors in SAS IMRT of prostate cancer. Their results showed dosimetric changes in end point dose of PTV and OARs (rectum and bladder) from 1 to 2.5% and reduction of CI because of synchronized MLC perturbation of 1 mm.[24]
There are some obstacles in dose calculation in compensator IMRT such as beam hardening and scatter from the filter, as Bartrum et al.[25] showed that compensator factor would increase with raising beam quality index (TPR 20, 10) in compensator IMRT.
All in all, IMRT approaches represented better homogeneity and conformity over 3D CRT and in comparison of two IMRT methods, all acceptable results can be achieved in compensator IMRT. Considering exploiting more sophisticated and costly facilities in SAS method, compensator-based can be accomplished as a suitable technique in IMRT of prostate cancer. In conclusion, results of this study showed that both IMRT methods provide better target coverage in comparison of 3D CRT. In SAS technique, maximum dose reduced compared with compensator-based IMRT while, in the later method, CI and HI improved; it must be mentioned that 3D CRT also had the acceptable HI and CI results.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Grönberg H. Prostate cancer epidemiology. Lancet. 2003;361:859–64. doi: 10.1016/S0140-6736(03)12713-4. [DOI] [PubMed] [Google Scholar]
- 2.Berger MF, Lawrence MS, Demichelis F, Drier Y, Cibulskis K, Sivachenko AY, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470:214–20. doi: 10.1038/nature09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feuvret L, Noël G, Mazeron JJ, Bey P. Conformity index: A review. Int J Radiat Oncol Biol Phys. 2006;64:333–42. doi: 10.1016/j.ijrobp.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 4.Zelefsky MJ, Levin EJ, Hunt M, Yamada Y, Shippy AM, Jackson A, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:1124–9. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 5.Senthi S, Gill SS, Haworth A, Kron T, Cramb J, Rolfo A, et al. Benchmarking dosimetric quality assessment of prostate intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:998–1005. doi: 10.1016/j.ijrobp.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Ling CC, Burman C, Chui CS, Kutcher GJ, Leibel SA, LoSasso T, et al. Conformal radiation treatment of prostate cancer using inversely-planned intensity-modulated photon beams produced with dynamic multileaf collimation. Int J Radiat Oncol Biol Phys. 1996;35:721–30. doi: 10.1016/0360-3016(96)00174-5. [DOI] [PubMed] [Google Scholar]
- 7.Webb S. The Physics of Three Dimensional Radiation Therapy: Conformal Radiotherapy, Radiosurgery and Treatment Planning. CRC Press; 1993. [Google Scholar]
- 8.Luan S, Wang C, Chen DZ, Hu XS, Naqvi SA, Yu CX, et al. A new MLC segmentation algorithm/software for step-and-shoot IMRT delivery. Med Phys. 2004;31:695–707. doi: 10.1118/1.1646471. [DOI] [PubMed] [Google Scholar]
- 9.International Commission on Radiation Units and Measurements. Prescribing, Recording, and Reporting Photon-Beam Intensity-Modulated Radiation Therapy (IMRT): ICRU Report 83. 2010 [Google Scholar]
- 10.Morgan-Fletcher S. Prescribing, recording and reporting photon beam therapy (supplement to ICRU Report 50), ICRU Report 62. ICRU, pp. ix+52, 1999 (ICRU Bethesda, MD) Br J Radiol. 2001;74:294. [Google Scholar]
- 11.Tomita N, Kodaira T, Tachibana H, Nakamura T, Nakahara R, Inokuchi H, et al. A comparison of radiation treatment plans using IMRT with helical tomotherapy and 3D conformal radiotherapy for nasal natural killer/T-cell lymphoma. Br J Radiol. 2009;82:756–63. doi: 10.1259/bjr/83758373. [DOI] [PubMed] [Google Scholar]
- 12.Jabbari K, Azarmahd N, Babazade S, Amouheidari A. Optimizing of the tangential technique and supraclavicular fields in 3 dimensional conformal radiation therapy for breast cancer. J Med Signals Sens. 2013;3:107. [PMC free article] [PubMed] [Google Scholar]
- 13.Kataria T, Sharma K, Subramani V, Karrthick KP, Bisht SS. Homogeneity index: An objective tool for assessment of conformal radiation treatments. J Med Phys. 2012;37:207. doi: 10.4103/0971-6203.103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenwald J, Gaboriaud G, Pontvert D. Conformal radiotherapy: Principles and classification. Cancer Radiother. 1998;3:367–77. doi: 10.1016/S1278-3218(00)87975-5. [DOI] [PubMed] [Google Scholar]
- 15.Jabbari K. Review of fast Monte Carlo codes for dose calculation in treatment planning. J Med Signals Sens. 2011;1:73–86. [PMC free article] [PubMed] [Google Scholar]
- 16.Knöös T, Kristensen I, Nilsson P. Volumetric and dosimetric evaluation of radiation treatment plans: Radiation conformity index. Int J Radiat Oncol Biol Phys. 1998;42:1169–76. doi: 10.1016/s0360-3016(98)00239-9. [DOI] [PubMed] [Google Scholar]
- 17.Fisher J, Scott C, Stevens R, Marconi B, Champion L, Freedman GM, et al. Randomized phase III study comparing Best Supportive Care to Biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: Radiation Therapy Oncology Group (RTOG) 97-13. Int J Radiat Oncol Biol Phys. 2000;48:1307–10. doi: 10.1016/s0360-3016(00)00782-3. [DOI] [PubMed] [Google Scholar]
- 18.Luxton G, Hancock SL, Boyer AL. Dosimetry and radiobiologic model comparison of IMRT and 3D conformal radiotherapy in treatment of carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2004;59:267–84. doi: 10.1016/j.ijrobp.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 19.Moorthy S, Murthy N, Majumdar SK, El Hateer H, Mohan R, Ramanathan V. Dosimetric characteristics of IMRT versus 3DCRT for intact breast irradiation with simultaneous integrated boost. Austral-Asian J Cancer. 2012;11:221–30. ISSN 0972-2556. [Google Scholar]
- 20.Price S, Williams M, Butson M, Metcalfe P. Comparison of skin dose between conventional radiotherapy and IMRT. Australas Phys Eng Sci Med. 2006;29:272–7. doi: 10.1007/BF03178577. [DOI] [PubMed] [Google Scholar]
- 21.Jabbari K, Amouheidari A, Babazadeh S. The quality control of intensity modulated radiation therapy (IMRT) for ONCOR Siemens linear accelerators using film dosimetry. Iran J Med Phys. 2012;9:111–25. [Google Scholar]
- 22.Hashemian A, Toossi MT, Nasseri S. Design and fabrication of the control part of a prototype multileaf collimator system. J Med Signals Sens. 2014;4:300–4. [PMC free article] [PubMed] [Google Scholar]
- 23.Ezzell GA, Galvin JM, Low D, Palta JR, Rosen I, Sharpe MB, et al. Guidance document on delivery, treatment planning, and clinical implementation of IMRT: Report of the IMRT Subcommittee of the AAPM Radiation Therapy Committee. Med Phys. 2003;30:2089–115. doi: 10.1118/1.1591194. [DOI] [PubMed] [Google Scholar]
- 24.Ung N, Harper C, Wee L. Dosimetric impact of systematic MLC positional errors on step and shoot IMRT for prostate cancer: A planning study. Australas Phys Eng Sci Med. 2011;34:291–8. doi: 10.1007/s13246-011-0062-8. [DOI] [PubMed] [Google Scholar]
- 25.Bartrum T, Bailey M, Nelson V, Grace M. Linear attenuation coefficients for compensator based IMRT. Australas Phys Eng Sci Med. 2007;30:281–7. doi: 10.1007/BF03178438. [DOI] [PubMed] [Google Scholar]


