Abstract
Comprehension of the brain function can be helpful for therapy of neurodegenerative diseases. The brain consists of various types of neuron sets, which organize in three-dimensional complex networks and form neural circuits underlying different behaviors. The circuits act based on the patterns that encode the brain functions. Recognition of the neural patterns requires methods to manipulate the neurons. Electrical stimulation may be the most common method. However, it has significant drawbacks including failure to identify specific neurons in experiments. As an alternative, optical stimulation is a new method that acts in combination with genetic approaches. The novel, optogenetic technology makes it feasible to manipulate either the specific cell types or the neural circuits. This is associated with minimum tissue damages as well as side effects. In this study, a new technology has been introduced, and then its optical and genetical tools have been investigated.
Keywords: Cell-type specificity, neural circuit, neural probes, opsin proteins, optical manipulation, optogenetics, patterned stimulation
Introduction
A compactness disbalance in the chemical substances of the peripheral and/or the central neural system results in psychiatric and neurological disorders. Scientists use appropriate pharmaceuticals together with new, feedback control methods to maintain a balanced concentration.[1] Therefore, many mental disorders can be cured by this idea. Stroke, epilepsy, and Parkinson’s disease are the common neurological diseases that are treated by medication. For instance, stroke is one of the foremost reasons for death, but it yet has very limited treatment options available, so that most survivors have long-lasting defects.[2,3,4]
On one hand, the progress in technology of the brain interface tools has created a modern research field for psychic disease remedy. Interventional psychiatry is a novel approach that manipulates the neuronal interconnections electrically. Deep-brain stimulation (DBS) is an example for this method, which has successfully treated some neurological illnesses. However, it has significant disadvantages. For example, the electrodes implanted in the patients stimulate nontarget cells in their range in addition to the specific cell types. This may lead to additional sensory problems and motor control defects.[5,6]
On the other hand, electrical signaling, which happens among the different and multiple neurons in the brain, determines the brain functions. The generated electrical signals are in the order of milliseconds. These signals are spatiotemporally encoded, because the neurons have been arranged in intricate three-dimensional (3D) circuits in the brain.[7,8,9,10] Over time, the electrical signals form activity patterns of the brain. Actually, these patterns encode our thoughts, skills, feelings, and memories, and thereby control the brain function and then its resulting behavior. However, how this process is done is unknown.[11,12,13,14] Simulation of the real, neuronal activity patterns helps scientists to control the brain nervous system and, therefore, plays an important role in understanding the brain activities and treatment of its diseases.
Recently, optical stimulation methods exhibited significant capabilities in recognizing the nervous system function. Therefore, the brain stimulation patterns can be generated by optical stimulation techniques based on the light modulation methods.[15,16] For this purpose, some new molecules have been identified that target the specific cells using genetic tools and make it possible to investigate the effect of the specific subsets of the cells in complex activities of the brain.[17,18,19,20,21,22] The result is the well-known optogenetic method that its development depends on either molecular genetics or optics. Optogenetics is a neuromodulation approach that controls the neural activity using light.
For the study of neuroscience, optical techniques are more useful tools than the pharmaceutical and the electrical ones because of their higher speed and accuracy and less damage to tissue. Optogenetics is a very powerful neuroscience method. This method functions on the basis of the bioengineered light-sensitive proteins. It can optionally stimulate or silence particular cell types and neuronal circuits with millisecond temporal accuracy. This method allows more temporal resolution for analyzing a specific neural circuit operation in different diseases.[23,24,25,26,27,28,29,30]
Light-Sensitive Proteins: A Toolbox for Optogenetics
The brain’s function is determined by the neuronal accordant activity. The neurons are distributed in the form of 3D networks in the brain. These circuits consist of tens of different cellular subtypes and form functional subnetworks. An external stimulus generates the intricate temporal and spatial function patterns through these neuronal circuits. The generated patterns are responsible for the brain activity and the resulting behavior.[7,8]
Understanding of the nervous physiologic mechanisms requires tools that can control the activity of neurons. Furthermore, difficulties in manipulating the functions of the neurons because of the elaboration and size of their interconnections lead to the demand for more spatiotemporally accurate physiological research tools, because the neurons respond to an external exciter within a few milliseconds.[31]
Advancement of optics and molecular genetics has created fundamental improvements in the study levels of the central nervous system, mostly by the feasibility for detecting and manipulating the neuronal activity and, thereupon, in the understanding of the brain operation.
As aforementioned, optogenetics as a photostimulation technique allows the modulation of the neuronal activity by light. In this manner, a set of light-gated, microbial opsin proteins acts on being stimulated by light. They are expressed in the neurons, which are genetically targeted. In addition, appropriate wavelengths of light are needed to expose these neurons. Bidirectional management of the neural function and also genetical targeting of the specific cell types are possible by optogenetics. Therefore, it is a powerful method for experimental investigations of the brain circuitries related to different disorders.[24,32,33]
Microbial opsins are of different types such as bacteriorhodopsin, channelrhodopsin (ChR),[25] and halorhodopsin (HR).[24] As the primary microbial opsin, channelrhodopsin-2 (ChR2) of Chlamydomonas reinhardtii is sensitive to blue light and controls the action potentials with millisecond resolution.[34,35]
Retinal is one of the many forms of vitamin A, and all-trans-retinal is also an essential component of microbial opsins. As shown in Figure 1, in these molecules, light transforms 11-cis-retinal to all-trans-retinal, which cycles back to 11-cis-retinal with the dark condition. 11-cis-Retinal can covalently compound with the protein ChR2 and then alter to all-trans-retinal by a blue photon with a wavelength of 470 nm. This process causes the formation of a 6°A open pore [Figure 2].[36] As the light turns off, retinal returns back to its 11-cis form, and the pore is blocked as short as a few milliseconds.
Figure 1.

Isomerization of 11-cis-retinal by illumination
Figure 2.
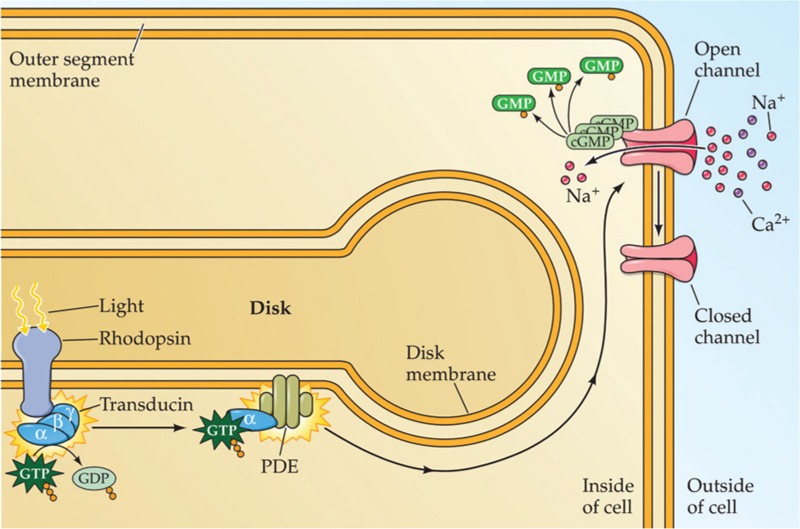
Schematic of channel opening upon illumination
Actually, blue light stimulation opens ChR2; therefore, cations stream into the neural cell through its membrane. This is cell depolarization that eventually leads to neuronal firing.
However, halorhodopsin obtained from the halophilic bacterium Natronobacterium pharaonis (NpHR) is the principal rhodopsin that inhibits the neuronal activity. NpHR acts as a chloride pump. It is sensitive to yellow light with a wavelength of 580 nm; therefore, one chloride ion enters into the cell through its membrane per one yellow photon that the nerve cell receives. This process hyperpolarizes the neuron.[37]
Figure 3 shows the activation and the inhibition of the neural activity by appropriate wavelengths.
Figure 3.
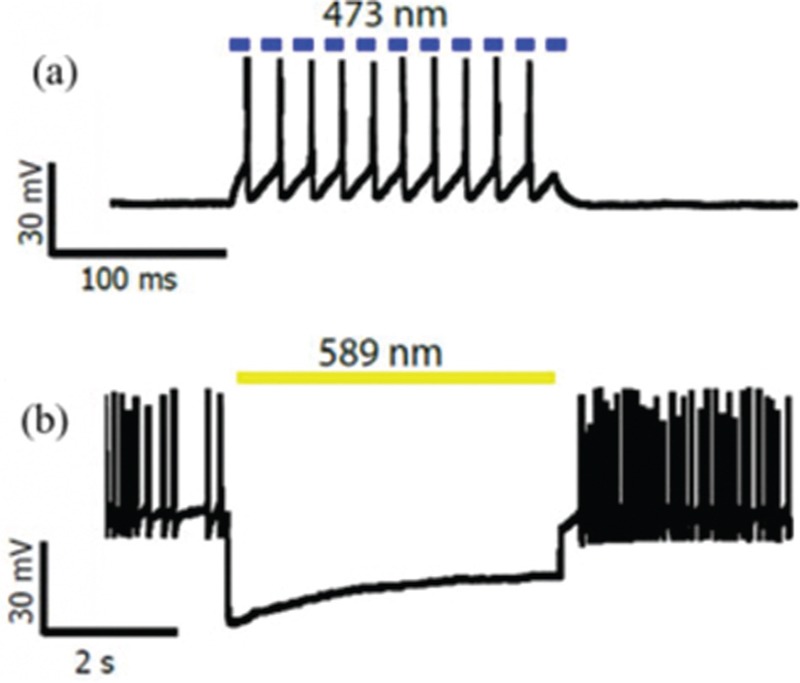
(a) Action potentials triggering by blue light pulse illumination (b) Inhibition of neural activity by yellow light illumination
Figure 4.
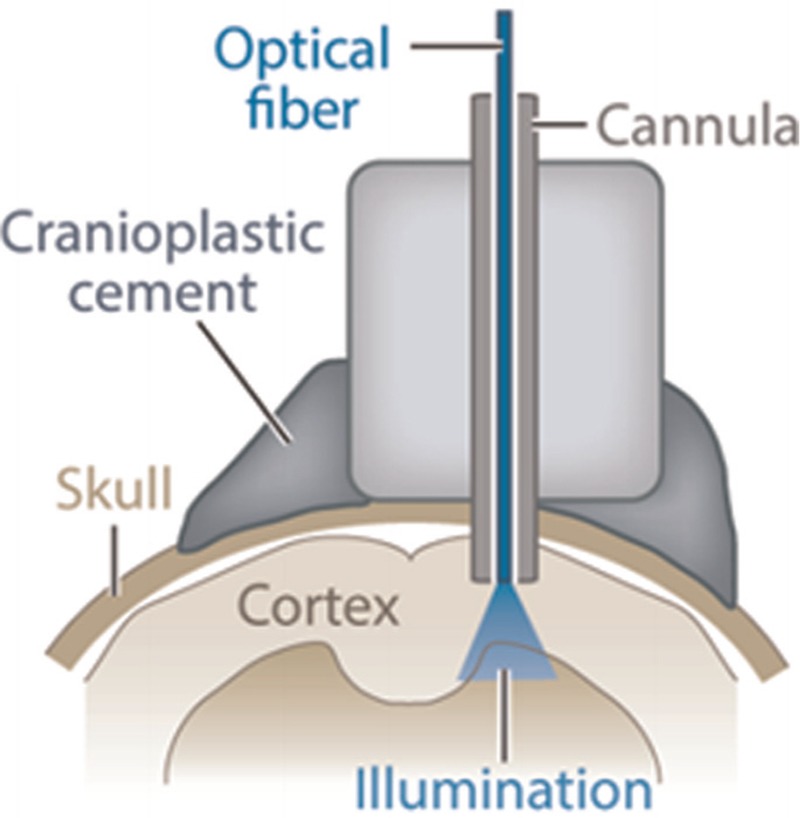
An optical fiber implanted into the brain for light delivery
Figure 5.
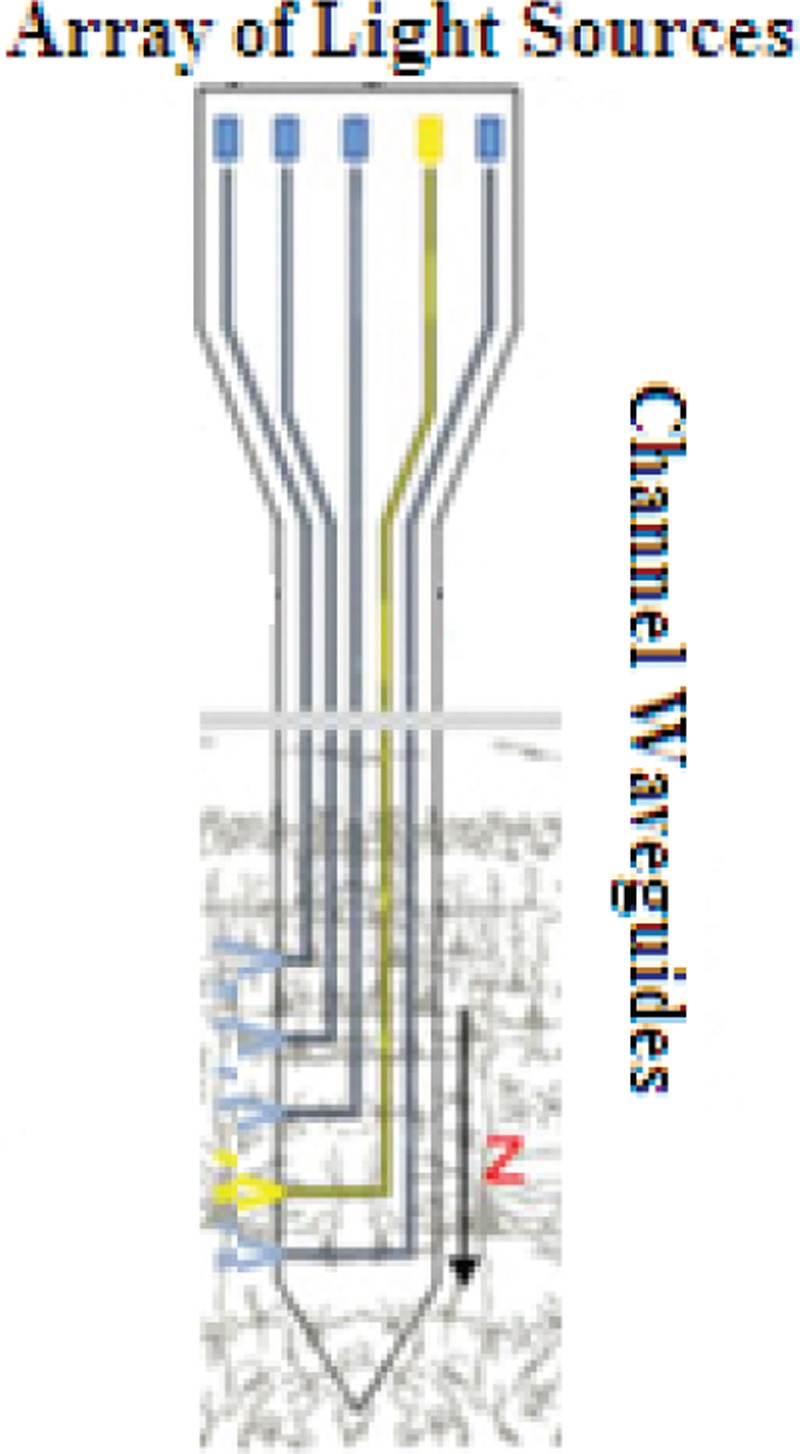
2D multichannel waveguides
Figure 6.
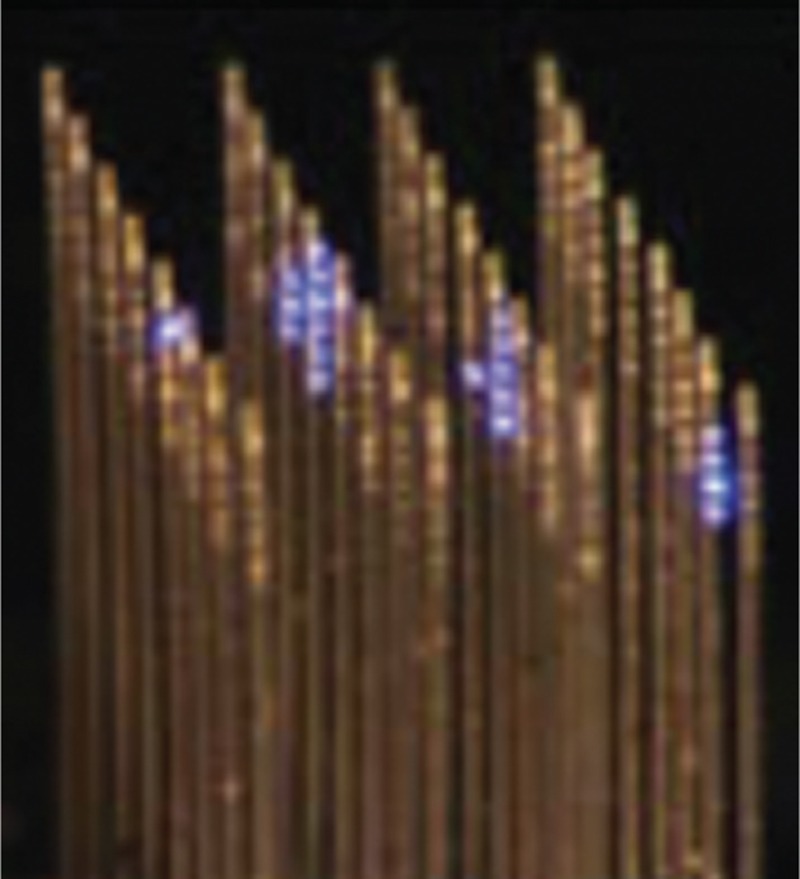
3D multichannel waveguides
Hence, each neuron can be depolarized and also hyperpolarized only with the correct choice of the light wavelength that it receives. In this manner, manipulation of the neuronal activities becomes possible for the researchers.
Furthermore, the cation channels and the anion pumps can express concurrently in one particular cell type, because NpHR and ChR2 have wavelengths of spectral separation.[24]
The optogenetic opsin proteins family is quickly advancing. Newly found opsins are sensitive to higher wavelengths. Yellow/red-shifted ChR types such as channelrhodopsin-1 from Volvox carteri (VChR1), A red- shifted channelrhodopsin (ReaChR), and Chrimson are some of the recent ones.[38,39,40] These wavelengths are more suitable because they have less absorption and scattering in the brain tissue; therefore, they penetrate deeper and allow even the activation of the deep neurons optically.[38]
The consecutive high-power laser pulses are the main problem in optogenetics, because the generated heat may damage the neuron. Another family of ChR overcomes this problem and is known as step-function opsin (SFO). It activates the neuron during the light pulse time, and also after the pulse is stopped. Therefore, it is proposed for studies that require longer activation time without constant laser illumination. SFO can also be deactivated by yellow light.[41]
Similar improvements have been made for the inhibition tools of optogenetics. IC1C2 is a new ChR. It is an inhibitory combination from channelrhodopsin-1 (ChR1) and channelrhodopsin-2 (ChR2), acts as a chloride conductor, and is 200 times more light sensitive. It inhibits the neurons using blue light. After stimulation, iC1C2 will open for 1 min and be disabled by red light soon. These properties lead to less light exposure and heat damage of the tissue.[42]
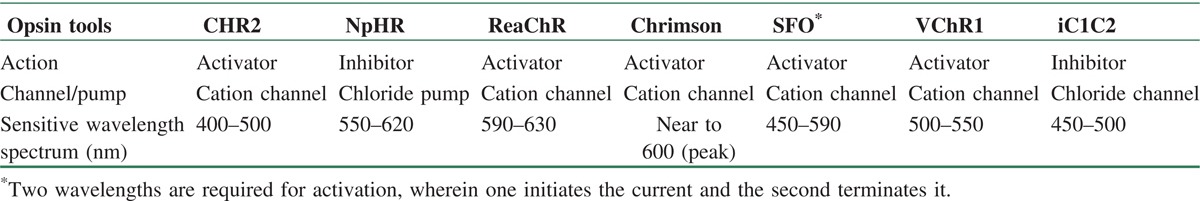
Currently, the opsin toolbox has become so diverse that there is the possibility to choose a tool for different experiments according to its unique properties such as its selectivity of the specific ions, spectrum of absorption, subcellular localization, and sensitivity to light.[43] Table 1 categorizes the mentioned optogenetic proteins with details.
Table 1.
The common optogenetic proteins for neural activity modulation
The result of an optogenetic manipulation of the neurons especially in most in vivo experiments depends on several factors. First, the number of photons that reach the opsin-expressing cells in the tissue, which is difficult to know because of light scattering and absorption in the tissue. Second, the protein expression extent into the cell, which is not necessarily the same for all cells. Third, the biophysical characteristics of the neuronal cells; for instance, the neurons with little input resistance are stimulated with much light-induced current.[44] Hence, the number of cells optically involved and modification processes of their properties are really unknown.
Light Delivery Mechanisms
In optogenetic brain studies, scientists aim to plan efficient mechanisms to give light to the brain surface and also inside its tissue. For example, the optical fibers implanted into the brain transfer laser pulses to the deep depths in the brain for stimulation.[45,46]
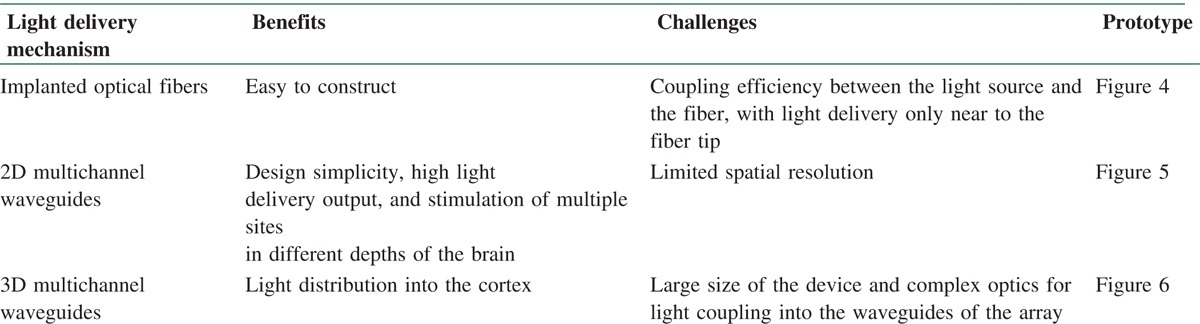
There are some drawbacks with optical fibers. First, their delivery light is limited to around the tip. Second, because the brain structure is 3D, the simple fibers cannot distribute the light into it. In addition, all the neurons containing opsin, which are placed near to the fiber point, will activate simultaneously. Therefore, the fibers cannot control the neurons spatially. Finally, more effective setups are needed, which may be more complex as well.[47,48,49,50] Table 2 contains a summary of the main methods used so far.[45,51]
Table 2.
The basic mechanisms for light delivery in the optogenetic tests
Existing methods have not the ability for showing spike timing through the various neurons and cannot repeat the spatiotemporal activation patterns related to the stimulated neuronal groups.
The use of small virus volumes or the tapered optical fibers can provide more accurate manipulation and spatial control for the optogenetic tests. Therefore, the number of stimulated cells can be fewer. In addition, the cell’s properties such as its level of electrical activity are used to express opsin precisely.[43] However, the relation of spiking activity with ChR2 expression is yet unknown.
One complete approach is patterned stimulation that controls the light spatiotemporally. Figure 7 displays the difference between the types of illumination.
Figure 7.
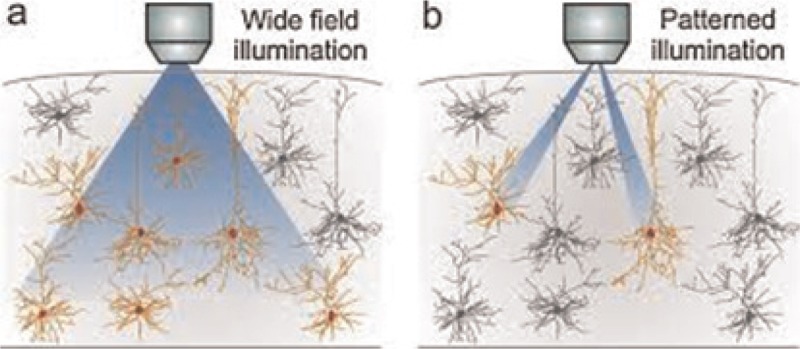
(a) usual illumination (b) patterned illumination[43]
Patterned Stimulation Methods
Deflecting a light beam randomly was the first idea for pattern generation in laser-based neurostimulation systems [Figure 8]. The beam scanned the cell body alternatively. Therefore, the neuron was stimulated more effectively. A major drawback of this system was its sequential manner.[52,53] However, the real activity patterns in the brain were spatiotemporally complicated, because tasks were done in parallel using its neural networks. Special methods that can control numerous, photostimulation locations in parallel implement these patterns.[54]
Figure 8.
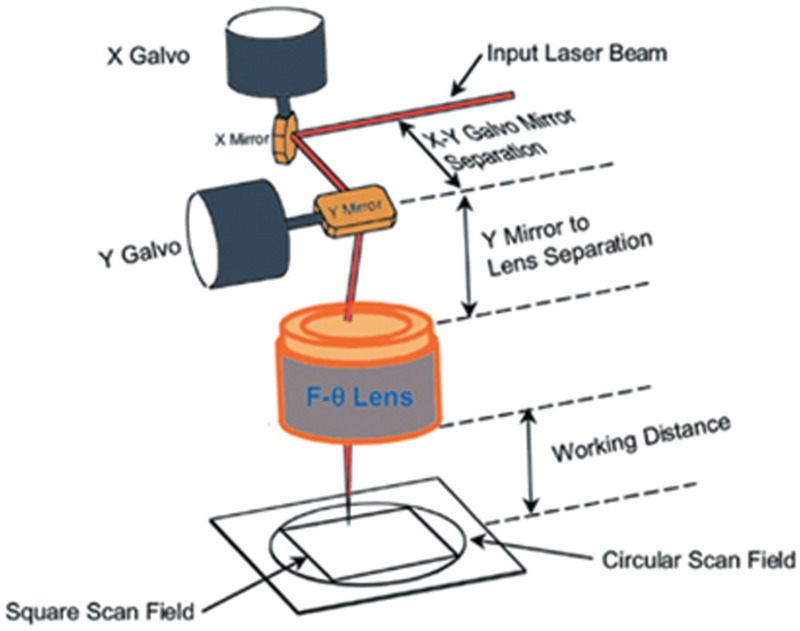
A laser scanning system prototype[8]
Photostimulation systems using spatial light modulators (SLMs) such as liquid crystal and microelectromechanical (MEMS) ones allow thousands of parallel photostimulation beams. Therefore, they are able to generate the brain activity patterns.[51]
The liquid crystal SLMs just manipulate the phase of the incoming light. Using this method, the laser beam can be concentrated on different depths simultaneously in the 3D tissue. Therefore, the desired stimulation patterns are used in the brain.[51,54,55,56]
As a MEMS SLM, the digital micromirror device (DMD) from Texas Instrument is a large array with micromirror elements. Each element is individually addressable. Then it can be tilted. It reflects the light toward the object or an absorber according to the angle of the tilt. Massively parallel patterns can be generated by timing the mirrors to be on or off. Therefore, DMD modulates the amplitude of the incident light beam.[57,58]
Two sample optogenetic setups with mentioned SLMs are shown in Figure 9.[51,59]
Figure 9.
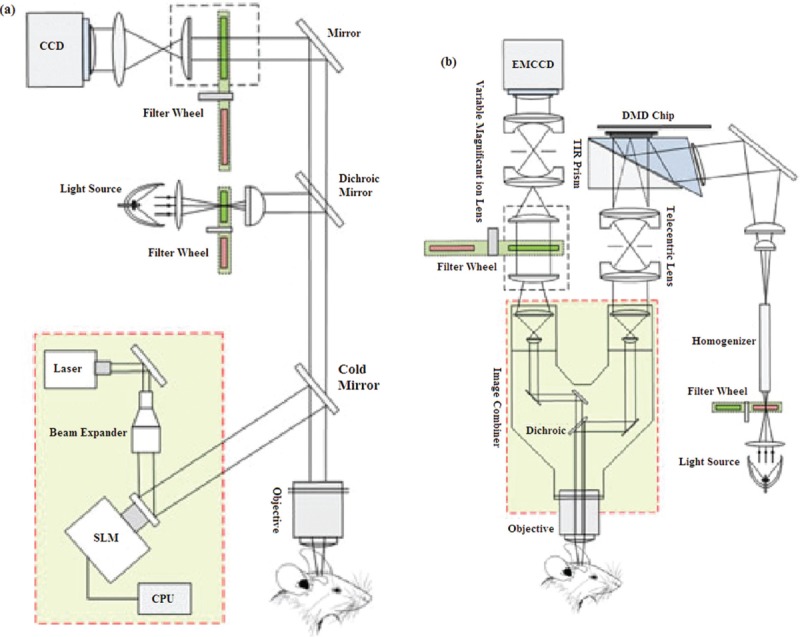
(a) A light delivery system based on liquid crystal modulator and holographic microscopy (b) A DMD based optical system design for optogenetic stimulation and imaging[51]
Table 3 shows a comparison of the light modulation techniques.[31]
Table 3.
The light modulation methods

Probes in Optogenetics
Detailed understanding of neural circuits structures underlying behaviors and also their dynamics need to direct electronic recordings of neural activity.[29] It is possible by using neural probes. The simplest probe available is a single metallic microwire (∼50–100 μm in diameter). Gold, tungsten, platinum, and steel are the fundamental materials used for the electrodes. An array of multiple microwires can also be designed to record more neuronal sites.[60,61]
These invasive electrodes are probes that penetrate within the brain tissue. Any stimulation leads to the movement of ions through the membrane of a neuron and changes their concentrations around the electrode consequently. In this manner, single spikes are generated and then recorded by these individual electrodes.[62]
The optical fibers are most common in the optogenetic tests; therefore, it is possible to integrate them with electrophysiological probes. The new integrated device is called an optrode, and comprises of a metal electrode that externally sticks to an optical fiber.[32,63,64,65]
Optogenetics: Advantages and Applications in Neuroscience
As aforementioned, optogenetics is a potent experimental method for analysis of the neural circuitries, which are involved in the psychiatric and neurological disorders. In this method, expression of the light-sensitive proteins in the interested cell type in the brain tissue can be controlled by a gene delivery mechanism. Therefore, specific cell type targeting becomes feasible.
Another advantage of optogenetics is its bidirectional control of the neural activities simultaneously. This makes it possible to manipulate activities of the neurons even in large networks such as the cortex.
There are many different applications of optogenetic neuromodulation in literature.[26,27,28,46] However, we can classify them into three major classes.
Control of the neural activity in optogenetics includes both its activation and inhibition. Temporal resolution is in the order of milliseconds. In addition, any firing pattern can be produced by a sequence of light pulses, which represents a neural code. Thereafter, the effect of this code can be studied on the postsynaptic neurons. This process is known as cracking neural codes. We need to know the neural code, because we need to measure the activity in the neural circuits and understand how that activity draws the behind behavior.
Neural circuits interrogation is the next important application. Analysis of mental disease circuitries and perception of their effect in the disease are all provided by the activity modulation of the different specific cell types of the neurons.
Bidirectional control of the neural circuits is also used for pattern generation of neural diseases reversibly. Therefore, new models of neurological disorders are produced, which can be studied to find new treatments. Generally, because the neuronal cells can be controlled selectively, efficient mental treatments with minimum side effects can be achieved.
A complex network of cortical and subcortical connections governs both the motor and sensory functions of the brain. Herein, there are some case studies of optogenetics applications in neuroscience.
Injury to the brain such as by stroke affects not only the damaged area but also parts linked to it remotely. If remapping of the brain connections arises immediately after stroke, no new structural connections will be formed at first. Structural changes will occur over time after stroke. Therefore, understanding the progression of the neural circuit dynamics is important to improve the poststroke recovery strategies. Recently, Lim research group used the optogenetic method combined with voltage-sensitive dye imaging to study remapping of the functional cortical connections.[66]
Cortical excitability is one of the main problems after stroke that has been studied by the optogenetic approaches. Anenberg group did a complete research in this field using a stroke model that they generated by blocking arterioles of the motor cortex.[66]
Optogenetic microelectrocorticography (micro-ECoG) is a new plan that combines optical stimulation using optogenetics with electrical recording of produced action potentials of the stimulated neurons. ECoG array is implanted on the cortex surface. Previously, micro-ECoG arrays were used only for electrophysiological recordings. In addition, desired illumination pattern sequences can be generated using optogenetic micro-ECoG setups equipped to SLMs. In this method, the light patterns are projected onto the cortical surface, the neurons are stimulated, and their potentials mapped simultaneously by the micro-ECoG array [Figure 10].[51,65]
Figure 10.
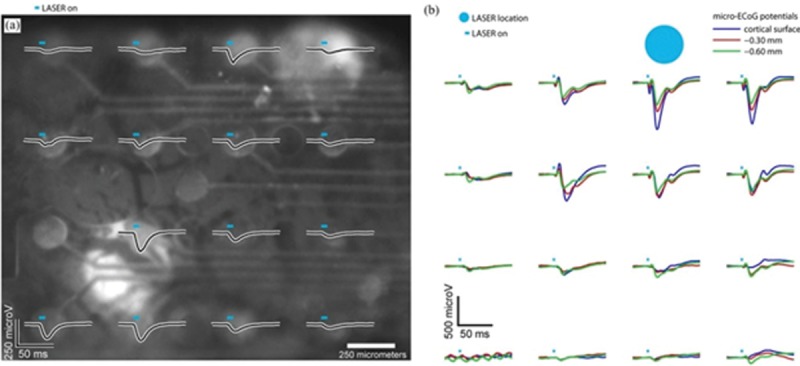
(a) Optogenetically evoked cortical potentials driven with a SLM and recorded by a micro-ECoG array (b) Potentials recorded on the cortical surface with micro-ECoG in response to photostimulation at three depths into the cortex with a fiber coupled to the laser[51]
Conclusion
The existing brain stimulation techniques such as the DBS, the transcranial direct current stimulation, and the transcranial magnetic stimulation lack the ability of specific cell type and neural circuit targeting. Optogenetics has overcome this problem and made manipulations with temporal precision to study the mechanisms of neurological disorders. However, for its application in humans, other involved sciences including gene therapy, opsin engineering, and optoelectronics must develop as well.
Recent developments in the brain interface systems have shown satisfactory results for human researches.
The main challenge for optogenetic implementation in humans is the expression of adequate amount of opsins, which need to be activated for neuronal stimulation and specific behavior extraction, without heat damage. A high photocurrent opsin or an infrared one may be suitable for the purpose. In addition, a low-heat light source needs to be developed.
Despite the aforementioned obstacles, optogenetics provides greater advantages and fewer side effects compared to the other brain stimulation techniques.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Levinson DF. The genetics of depression: A review. Biol Psychiatry. 2006;60:84–92. doi: 10.1016/j.biopsych.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 2.Wiśniewski A, Ksiazkiewicz B. Seizures − Symptom or cause of stroke. Przegl Lek. 2014;71:193–8. [PubMed] [Google Scholar]
- 3.Caplan LR, van Gijn J. Stroke Syndromes. New York: Cambridge University Press; 2012. [Google Scholar]
- 4.Chase A. Stroke: Improved lesion-symptom mapping in poststroke aphasia. Nat Rev Neurol. 2014;10:674. doi: 10.1038/nrneurol.2014.217. [DOI] [PubMed] [Google Scholar]
- 5.Talan J. Deep Brain Stimulation in a New Treatment Shows Promise in the Most Difficult Cases. New York, NY, USA: Dana Press; 2009. [Google Scholar]
- 6.Chou KL, Grube S, Patil P. Deep Brain Stimulation in a New Life for People with Parkinson’s, Dystonia and Essential Tremor. New York, NY, USA: Demos Health; 2012. [Google Scholar]
- 7.Kandel ER, Schwartz JH, Jessel TM. Principles of Neural Science. New York: McGraw-Hill; 2012. [Google Scholar]
- 8.Bear MF, Connors BW, Paradiso MA. Neuroscience: Exploring the Brain. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 9.Purves D, Augustine GJ, Fitzpatrick D, Hall WC, LaMantia S, White LE. Neuroscience. Sunderland, MA: Sinauer Associates; 2011. [Google Scholar]
- 10.Mainen ZA, Sejnowski TJ. Reliability of spike timing in neocortical neurons. Science. 1995;268:1503–6. doi: 10.1126/science.7770778. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell JF, Sundberg KA, Reynolds JH. Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron. 2009;63:879–88. doi: 10.1016/j.neuron.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Froudarakis E, Berens P, Ecker AS, Cotton RJ, Sinz FH, Yatsenko D, et al. Population code in mouse V1 facilitates readout of natural scenes through increased sparseness. Nat Neurosci. 2014;17:851–7. doi: 10.1038/nn.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Averbeck BB, Latham PE, Pouget A. Neural correlations, population coding and computation. Nat Rev Neurosci. 2006;7:358–66. doi: 10.1038/nrn1888. [DOI] [PubMed] [Google Scholar]
- 14.O’Connor DH, Huber D, Svoboda K. Reverse engineering the mouse brain. Nature. 2009;461:923–9. doi: 10.1038/nature08539. [DOI] [PubMed] [Google Scholar]
- 15.Katona G, Szalay G, Maák P, Kaszás A, Veress M, Hillier D, et al. Fast two-photon in vivo imaging with three-dimensional random-access scanning in large tissue volumes. Nat Methods. 2012;9:201–8. doi: 10.1038/nmeth.1851. [DOI] [PubMed] [Google Scholar]
- 16.Ahrens MB, Orger MB, Robson DN, Li JM, Keller PJ. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat Methods. 2013;10:413–20. doi: 10.1038/nmeth.2434. [DOI] [PubMed] [Google Scholar]
- 17.Miyawaki A, Griesbeck O, Heim R, Tsien RY. Dynamic and quantitative Ca2+ measurements using improved cameleons. Proc Natl Acad Sci U S A. 1999;96:2135–40. doi: 10.1073/pnas.96.5.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, et al. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–7. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- 19.Heim N, Garaschuk O, Friedrich MW, Mank M, Milos RI, Kovalchuk Y, et al. Improved calcium imaging in transgenic mice expressing a troponin C-based biosensor. Nat Methods. 2007;4:127–9. doi: 10.1038/nmeth1009. [DOI] [PubMed] [Google Scholar]
- 20.Akemann W, Mutoh H, Perron A, Park YK, Iwamoto Y, Knöpfel T. Imaging neural circuit dynamics with a voltage-sensitive fluorescent protein. J Neurophysiol. 2012;108:2323–37. doi: 10.1152/jn.00452.2012. [DOI] [PubMed] [Google Scholar]
- 21.Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levitz J, Pantoja C, Gaub B, Janovjak H, Reiner A, Hoagland A, et al. Optical control of metabotropic glutamate receptors. Nat Neurosci. 2013;16:507–16. doi: 10.1038/nn.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: Optical technologies for probing neural signals and systems. Nat Rev Neurosci. 2007;8:577–81. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- 24.Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–9. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 25.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–8. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 26.Deisseroth K. Optogenetics. Nat Methods. 2011;8:26–9. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, et al. An optical neural interface: In vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng. 2007;4:S143–56. doi: 10.1088/1741-2560/4/3/S02. [DOI] [PubMed] [Google Scholar]
- 30.Beltramo R, D’Urso G, Dal Maschio M, Farisello P, Bovetti S, Clovis Y, et al. Layer-specific excitatory circuits differentially control recurrent network dynamics in the neocortex. Nat Neurosci. 2013;16:227–34. doi: 10.1038/nn.3306. [DOI] [PubMed] [Google Scholar]
- 31.Jerome J, Heck DH. The age of enlightenment: Evolving opportunities in brain research through optical manipulation of neuronal activity. Front Syst Neurosci. 2011;5:95. doi: 10.3389/fnsys.2011.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gradinaru V, Thompson KR, Zhang F, Mogri M, Kay K, Schneider MB, et al. Targeting and readout strategies for fast optical neural control in vitro and in vivo. Neuroscience. 2007;27:14231–8. doi: 10.1523/JNEUROSCI.3578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattis J, Tye KM, Ferenczi EA, Ramakrishnan C, O’Shea DJ, Prakash R, et al. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat Methods. 2011;9:159–72. doi: 10.1038/nmeth.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams JC, Denison T. From optogenetic technologies to neuromodulation therapies. Sci Transl Med. 2013;5:177–81. doi: 10.1126/scitranslmed.3003100. [DOI] [PubMed] [Google Scholar]
- 35.Nagel G, Ollig D, Fuhrmann M, Kateriya S, Musti AM, Bamberg E, et al. Channelrhodopsin-1: A light-gated proton channel in green algae. Science. 2002;296:2395–8. doi: 10.1126/science.1072068. [DOI] [PubMed] [Google Scholar]
- 36.Lanyi JK, Oesterhelt D. Identification of the retinal-binding protein in halorhodopsin. J Biol Chem. 1982;257:2674–7. [PubMed] [Google Scholar]
- 37.Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100:13940–5. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY. ReaChR: A red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat Neurosci. 2013;16:1499–508. doi: 10.1038/nn.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang F, Prigge M, Beyrière F, Tsunoda SP, Mattis J, Yizhar O, et al. Red-shifted optogenetic excitation: A tool for fast neural control derived from Volvox carteri. Nat Neurosci. 2008;11:631–3. doi: 10.1038/nn.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, et al. Independent optical excitation of distinct neural populations. Nat Methods. 2014;11:338–46. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berndt A, Yizhar O, Gunaydin LA, Hegemann P, Deisseroth K. Bi-stable neural state switches. Nat Neurosci. 2009;12:229–34. doi: 10.1038/nn.2247. [DOI] [PubMed] [Google Scholar]
- 42.Berndt A, Lee SY, Ramakrishnan C, Deisseroth K. Structure-guided transformation of channelrhodopsin into a light-activated chloride channel. Science. 2014;344:420–4. doi: 10.1126/science.1252367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bovetti S, Fellin T. Optical dissection of brain circuits with patterned illumination through the phase modulation of light. J Neurosci Methods. 2015;241:66–77. doi: 10.1016/j.jneumeth.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Packer MA, Roska B, Häusser M. Targeting neurons and photons for optogenetics. Nat Neurosci. 2013;16:805–15. doi: 10.1038/nn.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warden MR, Cardin JA, Deisseroth K. Optical neural interfaces. Annu Rev Biomed Eng. 2014;16:103–29. doi: 10.1146/annurev-bioeng-071813-104733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang F, Gradinaru V, Adamantidis AR, Durand R, Airan RD, de Lecea L, et al. Optogenetic interrogation of neural circuits: Technology for probing mammalian brain structures. Nat Protoc. 2010;5:439–56. doi: 10.1038/nprot.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pashaie R, Falk R. Single optical fiber probe for fluorescence detection and optogenetic stimulation. IEEE Trans Biomed Eng. 2013;60:268–80. doi: 10.1109/TBME.2012.2221713. [DOI] [PubMed] [Google Scholar]
- 48.Zorzos AN, Boyden ES, Fonstad CG. Multiwaveguide implantable probe for light delivery to sets of distributed brain targets. Opt Lett. 2010;35:4133–5. doi: 10.1364/OL.35.004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zorzos AN, Scholvin J, Boyden ES, Fonstad CG. 3-Dimensional multiwaveguide probe array for light delivery to distributed brain circuits. Opt Lett. 2012;37:4841–3. doi: 10.1364/OL.37.004841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clements IP, Gnade A, Rush A, Patten C, Twomey M, Kravitz A. Miniaturized LED sources for in vivo optogenetic experimentation. Proc SPIE. 2013;8586:85860. [Google Scholar]
- 51.Pashaie R, Anikeeva P, Lee JH, Prakash R, Yizhar O, Prigge M, et al. Optogenetic brain interfaces. IEEE Rev Biomed Eng. 2014;7:3–30. doi: 10.1109/RBME.2013.2294796. [DOI] [PubMed] [Google Scholar]
- 52.Smedemark-Margulies N, Trapani JG. Tools, methods, and applications for optophysiology in neuroscience. Front Mol Neurosci. 2013;6:18. doi: 10.3389/fnmol.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang K, Liu Y, Li Y, Guo Y, Song P, Zhang X, et al. Precise spatiotemporal control of optogenetic activation using an acousto-optic device. PLoS One. 2011;6:e 28468. doi: 10.1371/journal.pone.0028468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Avants BW, Murphy DB, Dapello JA, Robinson JT. Neuro PG: open source software for optical pattern generation and data acquisition. Front Neuroeng. 2015;8:1. doi: 10.3389/fneng.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lutz C, Otis TS, DeSars V, Charpak S, DiGregorio DA, Emiliani V. Holographic photolysis of caged neurotransmitters. Nat Methods. 2008;5:821–7. doi: 10.1038/nmeth.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nikolenko V, Watson BO, Araya R, Woodruff A, Peterka DS, Yuste R. SLM microscopy: Scanless two-photon imaging and photostimulation with spatial light modulators. Front Neural Circuits. 2008;2:5. doi: 10.3389/neuro.04.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Introduction to Digital Micromirror Device (DMD) Technology, DMD 101: Application Report. Texas: Texas Instruments Inc. 2008 [Google Scholar]
- 58.Dudley D, Duncan W, Slaughter J. Emerging digital micromirror device (DMD) applications. Proc SPIE. 2003;4985:14–25. [Google Scholar]
- 59.Leifer AM, Fang-Yen C, Gershow M, Alkema MJ, Samuel AD. Optogenetic manipulation of neural activity in freely moving Caenorhabditis elegans. Nat Methods. 2011;8:147–52. doi: 10.1038/nmeth.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McNaughton BL, O’Keefe J, Barnes CA. The stereotrode: A new technique for simultaneous isolation of several single units in the central nervous system from multiple unit records. J Neurosci Methods. 1983;8:391–7. doi: 10.1016/0165-0270(83)90097-3. [DOI] [PubMed] [Google Scholar]
- 61.Gray CM, Maldonado PE, Wilson M, McNaughton B. Tetrodes markedly improve the reliability and yield of multiple single-unit isolation from multi-unit recordings in cat striate cortex. J Neurosci Methods. 1995;63:43–54. doi: 10.1016/0165-0270(95)00085-2. [DOI] [PubMed] [Google Scholar]
- 62.Kipke DR, Vetter RJ, Williams JC, Hetke JF. Silicon-substrate intracortical microelectrode arrays for long-term recording of neuronal spike activity in cerebral cortex. IEEE Trans Neural Syst Rehabil Eng. 2003;11:151–5. doi: 10.1109/TNSRE.2003.814443. [DOI] [PubMed] [Google Scholar]
- 63.Schalk G, Leuthardt EC. Brain-computer interfaces using electrocorticographic signals. IEEE Rev Biomed Eng. 2011;4:140–54. doi: 10.1109/RBME.2011.2172408. [DOI] [PubMed] [Google Scholar]
- 64.Viventi J, Kim DH, Vigeland L, Frechette ES, Blanco JA, Kim YS, et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat Neurosci. 2011;14:1599–605. doi: 10.1038/nn.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Richner TJ, Thongpang S, Brodnick SK, Schendel AA, Falk RW, Krugner-Higby LA, et al. Optogenetic micro-electrocorticography for modulating and localizing cerebral cortex activity. J Neural Eng. 2014;11:016010. doi: 10.1088/1741-2560/11/1/016010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng MY, Aswendt M, Steinberg GK. Optogenetic approaches to target specific neural circuits in post-stroke recovery. Neurotherapeutics. 2016;13:325–40. doi: 10.1007/s13311-015-0411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


