Abstract
Neonatal hypoxic-ischemic encephalopathy (HIE) is a devastating condition that may result in death or severe neurologic deficits in children. Neuroimaging with cranial ultrasound (US), computed tomography and magnetic resonance imaging are valuable tools in the workup of patients with HIE. The pattern of brain injury depends on the severity and duration of hypoxia and degree of brain maturation. Mild to moderate HI injury results in periventricular leukomalacia and germinal matrix bleed in preterm neonates, and parasagittal watershed infarcts in full-term neonates. Severe HI injury involves deep gray matter in both term and preterm infants. Treatment of HIE is largely supportive. The current article reviews the etiopathophysiology and clinical manifestations of HIE, role of imaging in the evaluation of the condition, patterns of brain injury in term and preterm neonates, the treatment and the prognosis.
KEYWORDS: Computed tomography, cranial ultrasound, hypoxic ischemic encephalopathy, magnetic resonance imaging
INTRODUCTION
Neonatal hypoxic-ischemic encephalopathy (HIE) is one of the most common causes of cerebral palsy (CP) and other severe neurological deficits in children. Neonatal HIE occurs in 1.5/1000 live births. It is caused by inadequate blood flow and oxygen supply to the brain resulting in focal or diffuse brain injury.[1] Asphyxia is the most significant risk factor for HIE that may occur from a variety of conditions. The pattern of brain injury depends on the severity and duration of hypoxia and degree of brain maturation. The imaging findings in full-term neonates (>36 weeks of gestation) may differ from those in preterm neonates (<36 weeks of gestation).[2] Neuroimaging modalities such as ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI) help in identification and characterization of the accurate location, extent, and severity of the brain injury. Newer imaging techniques such as diffusion-weighted imaging (DWI) and magnetic resonance spectroscopy (MRS) being more sensitive to diagnose acute brain injury have a potential role in early diagnosis and timely intervention.[3,4] The treatment is primarily supportive, aimed at correction of underlying cause. The role of neuroprotective interventions to limit the extent of brain injury caused by hypoxia-ischemia is under investigation. The prognosis of HIE depends on the severity of injury and gestational age of the infant.[5,6]
In this article, we review the etiopathophysiology and clinical manifestations of HIE, role of imaging in evaluation of the condition, patterns of brain injury in term and preterm neonates, the treatment and the prognosis.
ETIOPATHOGENESIS
Perinatal asphyxia is the most important cause of HIE. Perinatal asphyxia may occur either in utero or postnatally. Intrauterine asphyxia results due to inadequate placental perfusion and impaired gaseous exchange that may be caused by fetal factors (fetal bradycardia, fetal thrombosis, and fetal hemorrhage), maternal factors (preeclampsia, abruptio-placentae, maternal hypotension, severe anemia, asthma and chronic vascular disease), or tight nuchal cord and cord prolapse. Postnatal asphyxia results from conditions causing neonatal pulmonary failure such as severe hyaline membrane disease, meconium aspiration syndrome, pneumonia, or congenital cardiac disease.[2,3,4,5,6]
The basic physiologic processes that result in HIE, both in preterm and term neonate, is asphyxia leading to brain ischemia (reduced cerebral blood flow) and hypoxia (reduced cerebral oxygen). Hypoperfusion, in conjunction with hypoxia, leads to a cascade of events including acidosis, release of inflammatory mediators and free radical formation. These biochemical substances result in loss of normal cerebral autoregulation and diffuse brain injury (neuronal cell death). The exact nature of the injury depends on the severity and duration of hypoxia and degree of brain maturation. In term infants, myelinated fibers are more metabolically active and hence more vulnerable to HIE.[7]
CLINICAL MANIFESTATIONS
At delivery, HIE neonate may have low Apgar score (bradycardia, poor respiratory effort, hypotonia, decreased alertness, weak or absent cry, and abnormal skin color) and metabolic acidosis in cord blood. The infant usually develops seizure within first 24 h of life. Children with periventricular leukomalacia (PVL) may develop spastic CP in the form of diplegia, quadriplegia, or hemiplegia. Involvement of subcortical white matter produces severe mental retardation and impaired vision. Basal ganglia (BG) and thalamic involvement result in extrapyramidal symptoms. Multicystic encephalopathy is associated with quadriplegia, bulbar and choreoathetoid symptoms, microcephaly and mental retardation. Abnormal electroencephalographic (EEG) findings may predict adverse clinical outcome such as long-term neurologic sequelae or impending death.[6]
ROLE OF IMAGING
Cranial ultrasound (US) is the initial investigation of choice in suspected cases of neonatal HIE as it is inexpensive, portable and imparts no radiation exposure. Cranial US is highly sensitive for detecting intracranial hemorrhage, hydrocephalus, and cystic PVL. Increased resistive index (RI) of the middle cerebral artery (MCA) on Doppler sonography helps to identify severe HIE. Normally, RI decreases with increasing gestational age. Despite the above advantages, cranial US has several limitations such as low sensitivity for detecting cortical lesions, marked interobserver variability and operator dependency. CT and MRI have greater sensitivity for the detection of cortical injury and markedly lower interobserver variability than sonography.[2,8]
CT is less sensitive and specific than MRI to diagnose neonatal HIE. However, multidetector CT may be used for screening intracranial hemorrhage in very sick neonates without need for sedation. The major disadvantage of CT in neonates is radiation exposure.[2]
MRI is the most sensitive and specific imaging modality for evaluating suspected neonatal HIE. In neonatal brain imaging as compared to the adult brain, a relatively higher repetition time for both T1 (800 ms) and T2 (6500 ms) is used to optimize the signal-to-noise ratio and gray-white matter differentiation. Conventional MRI is less sensitive than newer imaging techniques like DWI and MRS in diagnosing acute brain injury; however, they can help to exclude other causes of encephalopathy such as congenital malformation, neoplasm, cerebral infarction and hemorrhage. On conventional MRI, HI injury to gray matter (cortex and deep gray matter) demonstrates characteristic T1-hyperintensity and variable T2-hyperintensity depending on duration of the imaging and pathological condition such as hemorrhage, encephalomalacia, or gliosis. White matter injury results in T1-hypointensity and T2-hyperintensity due to ischemia-induced edema or cystic encephalomalacia. Whereas, white matter injury with abnormal T1 hyperintensity and without marked T2 hypointensity denotes astrogliosis. The fluid attenuation inversion recovery (FLAIR) sequence is particularly useful for demonstrating cystic leukomalacia and gliosis. Gradient recalled echo-T2*-weighted sequence or susceptibility weighted imaging is particularly sensitive for detecting hemorrhage and distinguishing it from astrogliosis.[2,9] DWI may demonstrate cytotoxic edema (due to hypoxic brain injury) in acute phase before the signal intensity changes are evident on conventional T1- or T2-weighted images. Cytotoxic edema can be seen as diffusion restriction on DWI evidenced by increased signal intensity on DWI and decrease signal intensity on corresponding apparent diffusion coefficient mapping. The limitation of DWI is that it may give false negative result if performed within first 24 h of HI injury. DWI changes can be typically seen for only 10–12 days after tissue death and pseudonormalization occurs thereafter. Early DWI is excellent for the detection of white matter injury. However, DWI cannot detect the severity of HI brain injury or predict adverse clinical outcome.[3,4,10] MRS performed within first 24 h after birth in a full-term neonate is very sensitive to the severity of HI brain injury and can predict adverse outcome. Elevated lactate/creatine ratio on day 1 of life is a predictor of adverse neurological outcome, whereas absence of lactate predicts a normal outcome. Decreased N-acetylaspartate (NAA), increased choline and glutamine-glutamate peaks are also seen in neonatal HIE. MRS is not recommended in preterm neonates as they usually show higher lactate and lower NAA peaks. Two major drawbacks of MRI is limited access and need for sedation in neonates.[3,4,10]
PATTERNS OF BRAIN INJURY
Although the pattern of brain injury may differ in preterm (<36 weeks of gestation) and term neonates (>36 weeks of gestation) depending on the severity and duration of hypoxia and degree of brain maturation, some overlapping features may exist.[2]
In preterm neonates with immature brain, periventricular white matter (supplied by ventriculopetal penetrating arteries) is most vulnerable to the HI injury. Mild to moderate HI injury results in PVL, that may be focal (adjacent to frontal horns and trigones) or diffuse [Figure 1]. Progressive necrosis results in loss of periventricular white matter, passive ventriculomegaly (irregular margins of the bodies and trigones of lateral ventricles) and thinning of corpus callosum [Figures 2 and 3]. Cavitation and cyst formation represents end-stage PVL and is best demonstrated on FLAIR sequence [Figure 4]. Periventricular cysts are usually ≤2–3 mm, larger cysts carry poorer prognosis. DWI is a promising technique for early detection of PVL, before any abnormality appears on conventional MRI. Germinal matrix hemorrhage (GMH) is typically seen in preterm infants with HI injury. Subsequent reperfusion to ischemic brain tissue may result in GMH from rupture of weak capillaries and increased venous pressure. Depending on severity, GMH can be graded into subependymal hemorrhage (Grade 1), intraventicular hemorrhage without (Grade 2) and with (Grade 3) ventricular dilatation or parenchymal extension of the bleed with coexisting venous infarction (Grade 4). Gradient recalled echo-T2*-weighted sequence is highly sensitive to detect GMH [Figure 5]. Coexisting periventricular white matter injury and germinal matrix bleed may be present. Thalami, brainstem, and cerebellum in the immature brain have high metabolic activity and hence are more susceptible to injury in severe HI injury [Figure 6].[11,12,13]
Figure 1.

Magnetic resonance imaging of brain, fluid attenuation inversion recovery (axial) images in a 5-month old preterm infant showing feature of mild-moderate hypoxic-ischemic injury in form of periventricular leukomalacia seen as periventricular white matter hyperintensity
Figure 2.

Noncontrast computed tomography head (axial) in a 4-month old preterm infant showing features of mild-moderate hypoxic-ischemic injury in the form of significant loss of periventricular white matter and passive ventriculomegaly with irregular margins
Figure 3.

Noncontrast computed tomography head (sagittal), in a 6-month old preterm infant with neonatal hypoxia showing features of periventricular leukomalacia (arrow), passive ventriculomegaly (asterisk) and thinning of corpus callosum (arrow head)
Figure 4.
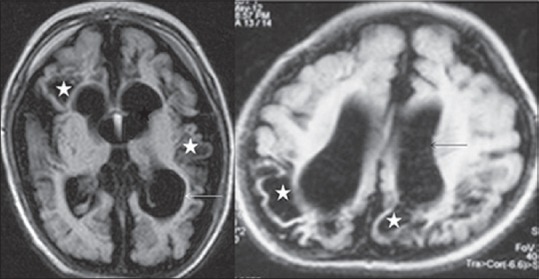
Magnetic resonance imaging brain, fluid attenuation inversion recovery (axial) images, in two different preterm infants showing features of end-stage periventricular leukomalacia in form of multicystic encephalomalacia (asterisk), best appreciated on fluid attenuation inversion recovery image. Passive ventriculomegaly (arrow) and prominent subarachnoid space is also present
Figure 5.
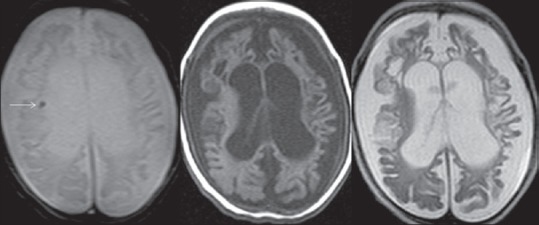
Magnetic resonance imaging brain, Gradient recalled echo and T1/T2 weighted (axial) images, in a 5-month old preterm infant showing subependymal germinal matrix bleed (Grade 1) on gradient recalled echo image (arrow). Note bleed is not evident on T1/T2 weighted images
Figure 6.

Noncontrast computed tomography head (axial) in 3-month old preterm infant showing features of both severe and mild-moderate hypoxia-ischemic injury in form of bilateral basal ganglia and thalamic injury (hyperdense basal ganglia and thalami) typical of severe hypoxia (thin arrow); and multicystic encephalomalacia (asterisk) and passive ventriculomegaly (thick arrow) typical of mild-moderate hypoxia. Dystrophic cortical calcification (arrow head) is also present
In term neonates, mild to moderate HI injury produces parasagittal watershed zone infarcts between anterior/MCA and middle/posterior cerebral artery. Both the cortex and underlying subcortical white matter are involved [Figure 7]. Severe HI injury results in injury to metabolically active tissues such as ventrolateral thalami, posterior putamina, hippocampi, brainstem, corticospinal tracts, and sensorimotor cortex [Figures 8–11]. BG injury is more common than parasagittal pattern and carries the worst prognosis. DWI and proton MRS demonstrate BG injury earlier than any other imaging modality. Severe global hypoxia may also lead to diffuse cerebral edema [Figure 12]. MR scan of term infants with chronic HIE may reveal cortical atrophy and thinning (ulegyria) and multicystic encephalomalacia [Figure 13].[2,12,14]
Figure 7.

Noncontrast computed tomography head (axial) in a 4-month old term infant showing features of mild-moderate hypoxic-ischemic injury in form of acute watershed zone infarcts (wedge shaped low density areas) involving bilateral frontal and parieto-occipital region (arrow)
Figure 8.

Magnetic resonance imaging brain in 1-year-old term male showing feature of severe hypoxic-ischemic injury involving ventrolateral thalami (arrow head), posterior putamina (thin arrow), and prirolandic cortex (thick arrow), as fluid attenuation inversion recovery hyperintensity
Figure 11.

Noncontrast computed tomography head (axial) in a 3-year-old term male showing features of severe hypoxic-ischemic injury in the form of hyperdense basal ganglia (thin white arrow) and thalami (arrow head), loss of periventricular white matter, passive ventriculomegaly with irregular margin (thick black arrow) and atrophy of sensory motor cortex (thick white arrow)
Figure 12.

Noncontrast computed tomography head (axial) in a 4-month old term infant showing features of severe global hypoxia in the form of diffuse cerebral edema (asterisk). Thalami and basal ganglia are spared
Figure 13.
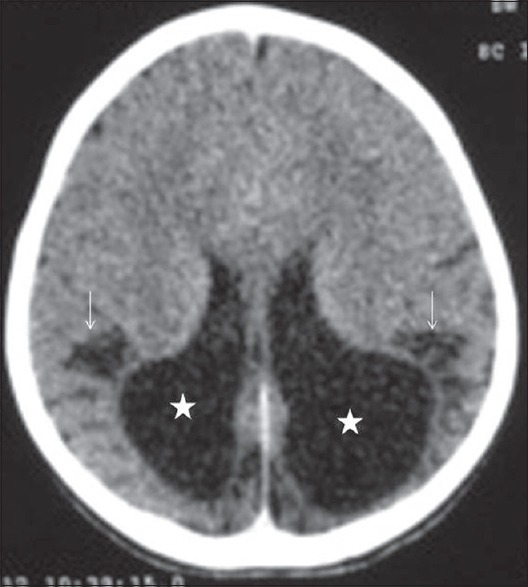
Noncontrast computed tomography head (axial) in 6-month old term infant showing features of chronic mild-moderate hypoxic-ischemic injury in form of chronic watershed zone infarct involving bilateral parieto-occipital region with areas of cystic encephalomalacia/gliosis (arrow), focal loss of white matter and colpocephaly (dilated occipital horns) (asterisk)
Figure 9.
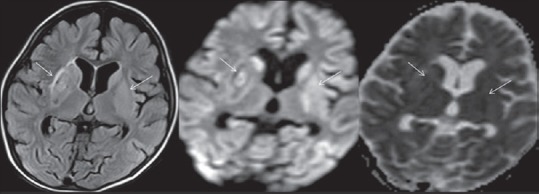
Magnetic resonance imaging brain in 2.5-year-old term male showing feature of severe hypoxic-ischemic injury in the form of acute basal ganglia infarcts appearing hyperintense on fluid attenuation inversion recovery sequence and showing diffusion restriction on diffusion-weighted imaging and corresponding apparent diffusion coefficient mapping (arrow)
Figure 10.
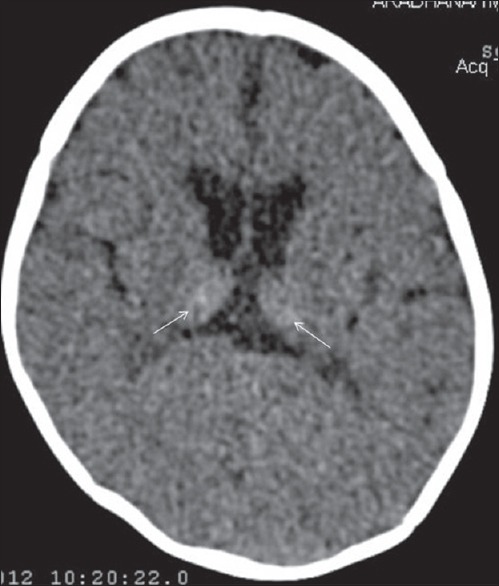
Noncontrast computed tomography head (axial) in 1-month old term infant showing feature of severe hypoxia in form of Primarily thalamic injury (hyperdense thalami) (arrow)
TREATMENT AND PROGNOSIS
Early diagnosis and timely intervention is the main objective in the management of a neonate with suspected HIE. The estimated therapeutic window is very short, of about 2–6 h during which intervention may be efficacious in reducing the severity of brain injury. Recent trials have shown that therapeutic hypothermia in the form of selective brain cooling is neuroprotective and helps to improve neurological outcome among HIE infants with moderate EEG abnormalities. Research on the potential benefit of other neuroprotective agents (like calcium channel blockers) is going on. The treatment is primarily supportive with correction of the underlying cause. The supportive care includes maintenance of adequate ventilation, metabolic status and vitals, and control of brain edema and seizure.[5,6,15]
The prognosis of HIE depends on the severity of injury and gestational age of the infant. Cortex and BG involvement on conventional MRI, diffusion restriction on DWI, increased lactate on MRS within 24 h of life and severe EEG abnormalities predict poor outcome. Term infants with mild encephalopathy generally have good prognosis and show complete recovery; however, 20% of infants may die in the neonatal period and another 25% may develop significant neurological deficit. Preterm infants, compared with term infants have poor prognosis.[5,6]
CONCLUSION
HIE is an important cause of morbidity and mortality in the neonatal period. Cranial US, CT and MRI show characteristic pattern of brain injury and help to exclude other causes of encephalopathy. Imaging plays an important role in early diagnosis and timely intervention, thereby reducing the severity of neonatal brain injury.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors express their sincere gratitude to the respected Late Dr. Sachchida Nand Yadav (Sr. Radiologist, PGIMER, Dr. RML Hospital) for always being helpful unconditionally. He was a gentleman in true sense. May his noble soul rest in peace!
REFERENCES
- 1.Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86:329–38. doi: 10.1016/j.earlhumdev.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Barkovich AJ, editor. Pediatric Neuroimaging. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. Brain and spine injuries in infancy and childhood; pp. 190–290. [Google Scholar]
- 3.Barkovich AJ, Westmark KD, Bedi HS, Partridge JC, Ferriero DM, Vigneron DB. Proton spectroscopy and diffusion imaging on the first day of life after perinatal asphyxia: Preliminary report. AJNR Am J Neuroradiol. 2001;22:1786–94. [PMC free article] [PubMed] [Google Scholar]
- 4.Zarifi MK, Astrakas LG, Poussaint TY, Plessis Ad A, Zurakowski D, Tzika AA. Prediction of adverse outcome with cerebral lactate level and apparent diffusion coefficient in infants with perinatal asphyxia. Radiology. 2002;225:859–70. doi: 10.1148/radiol.2253011797. [DOI] [PubMed] [Google Scholar]
- 5.Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–95. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- 6.Shalak L, Perlman JM. Hypoxic-ischemic brain injury in the term infant-current concepts. Early Hum Dev. 2004;80:125–41. doi: 10.1016/j.earlhumdev.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Johnston MV, Trescher WH, Ishida A, Nakajima W. Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatr Res. 2001;49:735–41. doi: 10.1203/00006450-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Benson JE, Bishop MR, Cohen HL. Intracranial neonatal neurosonography: An update. Ultrasound Q. 2002;18:89–114. doi: 10.1097/00013644-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Hüppi PS. Advances in postnatal neuroimaging: Relevance to pathogenesis and treatment of brain injury. Clin Perinatol. 2002;29:827–56. doi: 10.1016/s0095-5108(02)00049-0. [DOI] [PubMed] [Google Scholar]
- 10.Barkovich AJ, Miller SP, Bartha A, Newton N, Hamrick SE, Mukherjee P, et al. MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. AJNR Am J Neuroradiol. 2006;27:533–47. [PMC free article] [PubMed] [Google Scholar]
- 11.Barkovich AJ, Sargent SK. Profound asphyxia in the premature infant: Imaging findings. AJNR Am J Neuroradiol. 1995;16:1837–46. [PMC free article] [PubMed] [Google Scholar]
- 12.Barkovich AJ, editor. Pediatric Neuroimaging. 3rd ed. New York: Lippincott Williams & Wilkins; 2000. pp. 162–208. [Google Scholar]
- 13.Sie LT, van der Knaap MS, van Wezel-Meijler G, Taets van Amerongen AH, Lafeber HN, Valk J. Early MR features of hypoxic-ischemic brain injury in neonates with periventricular densities on sonograms. AJNR Am J Neuroradiol. 2000;21:852–61. [PMC free article] [PubMed] [Google Scholar]
- 14.Heinz ER, Provenzale JM. Imaging findings in neonatal hypoxia: A practical review. AJR Am J Roentgenol. 2009;192:41–7. doi: 10.2214/ajr.08.1321. [DOI] [PubMed] [Google Scholar]
- 15.Gulczynska E, Gadzinowski J. Therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy. Ginekol Pol. 2012;83:214–8. [PubMed] [Google Scholar]


