Key Points
Ezh2 requires Hsp90 to maintain Ezh2 protein stability and function in alloreactive T cells.
Pharmacological inhibition of Hsp90 destabilizes Ezh2 protein in alloreactive T cells and reduces GVHD but preserves graft-versus-leukemia effects.
Abstract
Modulating T-cell alloreactivity has been a main strategy to reduce graft-versus-host disease (GVHD), a life-threatening complication after allogeneic hematopoietic stem-cell transplantation (HSCT). Genetic deletion of T-cell Ezh2, which catalyzes trimethylation of histone H3 at lysine 27 (H3K27me3), inhibits GVHD. Therefore, reducing Ezh2-mediated H3K27me3 is thought to be essential for inhibiting GVHD. We tested this hypothesis in mouse GVHD models. Unexpectedly, administration of the Ezh2 inhibitor GSK126, which specifically decreases H3K27me3 without affecting Ezh2 protein, failed to prevent the disease. In contrast, destabilizing T-cell Ezh2 protein by inhibiting Hsp90 using its specific inhibitor AUY922 reduced GVHD in mice undergoing allogeneic HSCT. In vivo administration of AUY922 selectively induced apoptosis of activated T cells and decreased the production of effector cells producing interferon γ and tumor necrosis factor α, similar to genetic deletion of Ezh2. Introduction of Ezh2 into alloreactive T cells restored their expansion and production of effector cytokines upon AUY922 treatment, suggesting that impaired T-cell alloreactivity by inhibiting Hsp90 is achieved mainly through depleting Ezh2. Mechanistic analysis revealed that the enzymatic SET domain of Ezh2 directly interacted with Hsp90 to prevent Ezh2 from rapid degradation in activated T cells. Importantly, pharmacological inhibition of Hsp90 preserved antileukemia activity of donor T cells, leading to improved overall survival of recipient mice after allogeneic HSCT. Our findings identify the Ezh2-Hsp90 interaction as a previously unrecognized mechanism essential for T-cell responses and an effective target for controlling GVHD.
Introduction
Allogeneic hematopoietic stem-cell transplantation (HSCT) is potentially curative therapy for malignant and nonmalignant hematological diseases. This beneficial effect is largely derived from reconstitution of normal donor hematopoiesis and immune system in the recipient and donor T-cell–mediated graft-versus-leukemia (GVL) activity. However, alloreactive T cells mediate graft-versus-host disease (GVHD), which affects 20% to 70% of patients receiving transplants and accounts for approximately 15% to 20% of deaths.1,2 GVHD involves complex interactions of immune cells, and induction of alloreactive T-cell responses requires orchestrated expression of myriad genes encoding inflammatory cytokines, cytotoxic molecules, and transcription factors. Targeting a single effector molecule has limited effect on controlling severe GVHD.1,2
Histone methylation is considered to be crucial for establishing cell-type–specific gene expression patterns.3,4 Depending on the site and degree of methylation, histone methylation can be associated with gene repression (trimethylation of histone H3 at lysine 27 [H3K27me3], H3K9me3, H3K9me2) or activation (H3K4me3, H4K9me3).5,6 For example, in naïve CD4+ T cells, high levels of repressive H3K27me3 are present at regulatory regions of IFNG and IL4 genes. Upon Th1 cell differentiation, the regulatory region of IFNG showed a reduction of H3K27me3 but an increase of H3K4me3 and H3K9me2.7-9 However, emerging evidence indicates that histone methyltransferases, which selectively catalyze methylation, may regulate effector T-cell differentiation and proliferation through a mechanism independent of catalyzing histone methylation.10,11
Ezh2 catalyzes H3K27me3 and acts primarily as a gene silencer.12-14 Our studies and others suggest that Ezh2 plays an important role in regulating effector survival and lineage differentiation of CD4+ Th1 and Th2 cells.15-17 Using mouse models of allogeneic HSCT, we discovered that loss of T-cell Ezh2 led to GVHD inhibition.16 Thus, targeting Ezh2 may represent an effective therapeutic strategy for GVHD prevention and treatment. Selective inhibitors of Ezh2 have been discovered, including GSK126, UNC1999, and EPZ6438.18-21 These potent compounds specifically reduce H3K27me3 without altering Ezh2 protein.18-21 However, whether specifically inhibiting H3K27me3 may reduce GVHD, and whether selectively depleting Ezh2 protein may be required for controlling the disease, have not been previously determined.
Herein, we report that specifically reducing H3K27me3 by Ezh2 inhibitors fails to inhibit GVHD in mice. However, destabilizing Ezh2 protein by Hsp90 inhibitor AUY922, which is in clinical trials for cancer treatment, reduces GVHD and retains antileukemia activity, leading to significantly improved overall survival of mice undergoing allogeneic HSCT.
Materials and methods
Mice
B6/SJL (H-2b, CD45.1+), C3H.SW (H-2b, CD45.2+), C57BL/6 (B6, H-2b, CD45.2+), BALB/c (H-2d), and DBA/2 (H-2d) mice were purchased from Jackson Laboratories and the Chinese Academy of Sciences (Shanghai, China). B6×DBA/2 F1 (BDF1, H-2b/d) mice were generated by breeding B6 and DBA/2 mice. Animals were housed in specific pathogen-free facilities at the Chinese Academy of Sciences and Temple University. Animal protocols were approved by the Institutional Review Board of the Institute of Health Sciences and the Temple University Committee on Use and Care of Animals.
Induction of GVHD and GVL
Mouse models of allogeneic HSCT were performed as described.22,23 Briefly, T-cell–depleted (TCD) bone marrow (BM; 5 × 106) derived from C3H.SW mice, together with or without C3H.SW CD8+ T cells (2 × 106), was transferred into lethally irradiated B6/SJL mice (10 Gy). B6/SJL spleen cells (3 × 107) were transferred into unirradiated BDF1 recipients. B6/SJL TCD-BM (5 × 106) together with or without B6/SJL spleen cells (1 × 107) was transferred into lethally irradiated BDF1 mice (11.5 Gy). GVHD was monitored over time as described.24 In GVL experiments, leukemic cells were transferred 2 hours before HSCT, which reflects the residual disease in human transplant recipients.25 Leukemic growth was monitored by using bioluminescence imaging (LB983 NC100 system; Berthold Technologies).
Statistical analysis
Survival in different groups was compared using the log-rank test. Comparison of two means was analyzed using the two-sided two-sample Student t test and Mann-Whitney U test.
Results
Selective inhibition of H3K27me3 fails to control GVHD
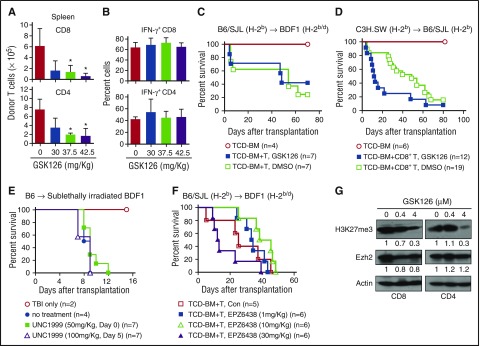
To assess if reducing H3K27me3 only might influence GVHD, we transferred B6/SJL splenocytes into unirradiated haploidentical BDF1 mice, treated with various doses of GSK126. Administration of GSK126 reduced the total number of host-reactive donor T cells in the spleen (Figure 1A) in a dose-dependent manner but did not affect their production of IFN-γ (Figure 1B). To induce GVHD, we transferred B6/SJL T cells together with TCD-BM into lethally irradiated BDF1 mice. Administration of GSK126 (37.5 mg/kg) failed to reduce GVHD (Figure 1C). Increasing the dose up to 100 mg/kg caused toxicity, manifested by rapid weight loss and hunched posture (data not shown). Using another minor histocompatibility antigen (miHA)–mismatched major histocompatibility complex (MHC)–identical GVHD model, where C3H.SW CD8+ T cells were transplanted into lethally irradiated B6/SJL mice, we found that GSK126 treatment worsened survival (Figure 1D; supplemental Figure 1, available on the Blood Web site). Further, neither UNC1999 nor EPZ6438 showed an effect on preventing GVHD (Figure 1E-F). Western blot analysis validated that GSK126 decreased total amount of H3K27me3 but not Ezh2 protein in activated T cells (Figure 1G). Thus, selectively reducing H3K27me3 is insufficient to reduce GVHD.
Figure 1.
The Ezh2-specific inhibitor GSK126 is unable to control GVHD in mice. (A-B) Spleen mononuclear cells (3 × 107) were isolated from B6/SJL mice and transplanted into unirradiated BDF1 recipient mice. On days 4, 5, and 6, various doses of GSK126 (0, 30, 37.5, and 42.5 mg/kg/day intraperitoneally) were injected into these recipient mice. Donor T cells were recovered on day 7 after transplantation. Graphs show the donor T-cell number (A) and the fraction of interferon γ (IFN-γ)–expressing donor T cells (B). (C-D) Lethally irradiated (11.5 Gy) BDF1 recipient mice received B6/SJL TCD-BM (5 × 106) or TCD-BM plus spleen-cell (1 × 107) transplants (C), and lethally irradiated (10 Gy) B6/SJL recipient mice received C3H.SW TCD-BM (5 × 106) or TCD-BM plus CD8+ T-cell (2 × 106) transplants (D). GSK126 (37.5 mg/kg) was administered to the mice for 12 doses from day 0 to day 27 after transplantation. Survival was monitored over time. (E) Sublethally irradiated (6.5 Gy) BDF1 recipient mice received B6 lymph node–cell (1 × 106) transplants, followed by UNC1999 treatment daily for 10 doses (50 mg/kg) from the day of transplantation or 5 doses (100 mg/kg) from day 5 after transplantation. Survival was monitored over time. (F) Lethally irradiated BDF1 recipient mice received B6/SJL TCD-BM (5 × 106) plus spleen cells (1 × 107) transplants. EPZ6438 (1, 10, or 30 mg/kg) was administered for 12 doses from day 0 to 27 after transplantation. Survival was monitored over time. (G) B6 mouse–derived CD4+ and CD8+ naïve T cells were stimulated with anti-CD3 antibody (3 μg/mL), anti-CD28 antibody (2 μg/mL), and recombinant murine interleukin-2 (5 ng/mL). One day later, different doses of GSK126 were used to treat the cells for another 2 days. The expressions of Ezh2 and H3K27me3 were measured. Error bars indicate mean ± standard deviation. Results are representative of 2 to 3 independent experiments. *P < .05. TBI, total-body irradiation.
Ezh2 SET domain positively regulates Ezh2 protein stability
The failure to prevent GVHD by reducing H3K27me3 stands in contrast to our published work showing that Ezh2 deficiency or depletion of Ezh2 protein by DZNep led to reduction of GVHD in mice.16,26 Because DZNep is not available for clinical trials, we wanted to identify other pharmacological inhibitors that can potentially deplete Ezh2 protein and are in clinical trials.
We examined under what circumstances Ezh2 protein was degraded. Ezh2fl/fl mice27 were crossed with CD4-Cre B6 mice to generate T-cell–specific Ezh2-knockout (Ezh2−/−) mice. In these Ezh2−/− T cells, the exons encoding the SET domain of Ezh2 are flanked with loxP sequences and deleted by Cre recombinase. The SET domain, located in Ezh2 from amino acids 612 to 727,28-31 was chosen as a target for Ezh2 inactivation because this domain is essential for Ezh2 enzymatic activity.27,32 Cells homozygous for the Ezh2 deletion contained messenger RNA (mRNA) corresponding to the Cre-modified Ezh2 gene. T-cell receptor (TCR) activation of Ezh2−/− CD8+ T cells increased Ezh2 mRNA, although to a lesser extent as compared with activated wild-type (WT) T cells (supplemental Figure 2A). Ezh2 protein lacking the SET domain (Ezh2-ΔSET) was detected in these activated T cells early after stimulation (supplemental Figure 2B, lower band). When compared with Ezh2 protein in activated WT CD8+ T cells, Ezh2-ΔSET in activated Ezh2−/− CD8+ T cells decreased 3 days after stimulation and disappeared by day 4 (supplemental Figure 2B). Addition of the proteasome inhibitor MG115 increased Ezh2-ΔSET protein in these activated Ezh2−/− T cells (supplemental Figure 2C), suggesting a critical role of the Ezh2 SET domain in regulating Ezh2 protein stability in activated T cells.
To assess if Ezh2-ΔSET might have an active role in Ezh2−/− T cells, we made MigR1 vector–encoding mutant Ezh2, in which the threonine at amino acid 689 was replaced by alanine (named Ezh2-H689A). Ezh2-H689A retains the SET domain but loses its enzymatic activity.32 Ezh2-H689A protein was stably maintained in Ezh2−/− T cells (supplemental Figure 2D-E) and associated with impaired expansion of activated Ezh2−/− T cells compared with MigR1–green fluorescent protein (GFP) control (supplemental Figure 2F). In addition, 7 days after transduction, MigR1-Ezh2 fully rescued the recovery rate of Ezh2−/− T cells to the WT T-cell level (supplemental Figure 2F). These data indicate that overexpressing Ezh2 may abrogate the negative effect of Ezh2-ΔSET on T-cell expansion. However, because Ezh2-ΔSET underwent rapid degradation within 4 days after activation (supplemental Figure 2B), its negative effect may not persist.
Ezh2 and Hsp90 form a complex to stabilize Ezh2 protein in T cells
Hsp90 is a molecular chaperone required for the stability and function of several key signaling intermediates, such as Akt, Raf1, and Erk1/2.33,34 Hsp90-specific inhibitors promote degradation of Hsp90 client proteins via the ubiquitin/proteasome pathway.33-36 It has been shown that Ezh2 is subject to ubiquitination and degradation by proteasome.37 We reasoned that if Ezh2 directly interacted with Hsp90 in T cells and if this interaction stabilized Ezh2 protein, Hsp90 inhibitors could be used to modulate GVHD.
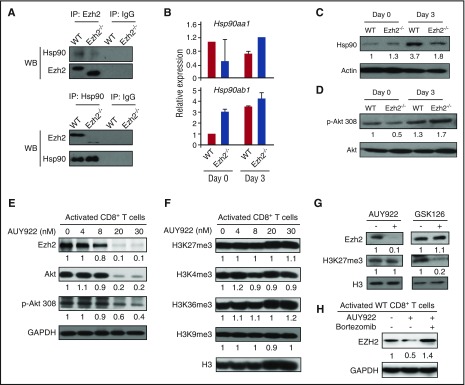
To test it, we first used reciprocal coimmunoprecipitation of TCR-activated CD8+ T-cell lysates and revealed that Ezh2 and Hsp90 directly interacted with each other (Figure 2A). We next measured the impact of Ezh2 deficiency on Hsp90 expression in CD8+ T cells. TCR activation induced higher levels of Hsp90ab1 mRNA (encoding Hsp90β protein) and Hsp90 protein in WT CD8+ T cells (Figure 2B-C). The absence of Ezh2 caused a twofold reduction of Hsp90 protein (Figure 2C) without changing the expression of Hsp90 client protein Akt and p-Akt (Figure 2D), suggesting that Ezh2 deficiency does not decrease Hsp90 function.
Figure 2.
Ezh2 and Hsp90 form a complex to stabilize Ezh2 protein in T cells. CD8+ T cells were isolated from B6 mice and stimulated with anti-CD3 antibody (3 μg/mL), anti-CD28 antibody (2 μg/mL), and recombinant murine interleukin-2 (5 ng/mL) for 3 days. (A) Cell lysates were prepared for coimmunoprecipitation (IP) assay using antibodies specific to Hsp90 and Ezh2. Western blots (WBs) show the levels of Ezh2 and Hsp90. (B) mRNA levels of Hsp90aa1 and Hsp90ab1 were detected in unstimulated (day 0) and activated (day 3) WT and Ezh2−/− T cells. (C-D) Protein levels of Hsp90, Akt, and phosphorylated Akt (p-Akt) in WT and Ezh2−/− T cells were determined by WB. (E-F) Activated T cells were treated with different doses of AUY922 for 16 hours. The expression levels of Ezh2, Akt, p-Akt, and histone methylation were examined. (G) Activated CD8+ T cells were treated with or without AUY922 (20 nM) or GSK126 (4 μM) for 48 hours. The levels of Ezh2 and H3K27me3 were determined. (H) Activated CD8+ T cells were treated with or without AUY922 (20 nM) for 48 hours. Bortezomib was added to the culture in the final 4 hours. The Ezh2 level was determined. Data are shown as mean ± standard deviation and representative of 2 to 4 independent experiments.
We then assessed whether inhibiting Hsp90 affected Ezh2 protein levels in activated T cells. The Hsp90 inhibitor AUY922 selectively targets the isoform of Hsp90α/β and has been tested as antitumor therapy in patients with cancer.33-36,38,39 We found that 16 hours after incubation with AUY922, TCR-activated CD8+ T cells decreased expression of p-Akt (Figure 2E), which is in agreement with previous reports.33-36 AUY922 treatment caused a dose-dependent reduction of Ezh2 protein in these T cells (Figure 2E). Interestingly, although addition of 30-nM AUY922 caused 2.5-fold reduction of p-Akt, it led to 10-fold decrease of Ezh2 (Figure 2E), without changing the amount of H3K27me3, H3K4me3, H3K36me3, or H3K9me3 (Figure 2F). In contrast, GSK126 did not affect Ezh2 protein, although it reduced H3K27me3 level (Figure 2G). Thus, inhibiting Hsp90 by AUY922 reduces Ezh2 protein in activated T cells. This effect differs from DZNep, which causes reduction of both Ezh2 protein and multiple histone methylation marks (eg, H3K27me3 and H3K4me3).26
We confirmed the role of proteasome in AUY922-mediated Ezh2 degradation using bortezomib (Figure 2H), which has been shown to acerbate GVHD severity when administered 5 days after transplantation.40 In addition, AUY922 decreased in a dose-dependent manner the recovery of human CD4+ and CD8+ T cells activated by allogeneic dendritic cells (DCs) in culture (supplemental Figure 3A) and depleted Ezh2 protein in these T cells (supplemental Figure 3B), suggesting the translational potential of AUY922.
Depletion of Ezh2 is important for AUY922-reduced GVH reaction
To assess the effect of Hsp90 inhibition on allogeneic T-cell responses, we transferred carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled B6/SJL T cells (H-2b) into unirradiated BDF1 mice (H-2b/d) to induce GVH reaction, administered AUY922 on days 4, 5, and 6, and collected donor T cells on day 7 to measure the impact of AUY922 on T-cell response. In this model, dividing (CFSElow) T cells represent alloantigen-activated cells, whereas nondividing (CFSEhigh) T cells contain alloantigen-unresponsive cells.41 AUY922 treatment caused a dose-dependent decrease in percentage and number of donor CFSElow CD8+ T cells but not CFSEhigh CD8+ T cells in the spleen and liver (supplemental Figure 4A-C). It also significantly decreased IFN-γ production by both donor CD8+ and CD4+ T cells (supplemental Figure 4D). These results resemble the effect of Ezh2 inhibition by DZNep or genetic inactivation, as we previously reported.16,26
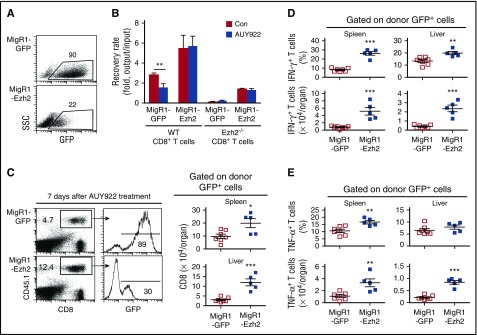
Hsp90 may affect activation of several key signaling intermediates such as Akt and Erk1/2.33,34,36 To determine if depletion of Ezh2 plays an important role in AUY922-mediated reduction of T-cell responses, we generated MigR1 virus bicistronically encoding Ezh2 and GFP (named MigR1-Ezh2) and introduced Ezh2 into WT and Ezh2−/− CD8+ T cells. Three days later, when these cells overexpressed Ezh2, AUY922 was added to the culture (Figure 3A). Introduction of Ezh2 significantly restored survival and expansion of both activated Ezh2−/− and WT CD8+ T cells treated by AUY922 (Figure 3B). This could be further improved when prolonging culture of Ezh2-transuced T cells to 7 days, shown in prior experiments (supplemental Figure 2F). In contrast, control MigR1-GFP–infected Ezh2−/− T cells diminished in cultures (Figure 3B). These data suggest that Ezh2 is modulated by AUY922 to reduce T-cell responses.
Figure 3.
AUY922 treatment reduces alloreactive T-cell response dependent on Ezh2. B6-derived CD8+ T cells were stimulated with anti-CD3 antibody (3 μg/mL), anti-CD28 antibody (2 μg/mL), and recombinant murine interleukin-2 (5 ng/mL). One day later, T cells were infected with retrovirus MigR1-GFP or MigR1-GFP–encoding Ezh2. (A) Dot plots show the fraction of GFP-expressing cells 4 days after culture. (B) The GFP+ cells were sorted and cultured for an additional 3 days in the presence or absence of AUY922. Cell recovery rate after culture was determined. (C-E) Retrovirally infected WT B6/SJL CD8+ T cells were transferred into unirradiated BDF1 mice, followed by AUY922 treatment on days 4, 5, and 6 after transplantation. On day 7, cells were recovered from recipient mice. (C) Dot plots and histograms show the fraction of donor T cells and GFP+ donor T cells, respectively. Graphs show the number of GFP+ donor T cells. (D) The fraction and number of IFN-γ–expressing GFP+ donor T cells. (E) The fraction and number of tumor necrosis factor α (TNF-α)–expressing GFP+ donor T cells. Error bars indicate mean ± standard deviation. Data are representative of 2 independent experiments. *P < .05; **P < .01; ***P < .001.
To assess if depleting T-cell Ezh2 was an important mechanism by which AUY922 reduced GVH responses in vivo, we retrovirally infected WT B6/SJL CD8+ T cells with MigR1-Ezh2 or MigR1-GFP, transferred them into unirradiated BDF1 mice, administered AUY922 treatment on days 4, 5, and 6, and recovered donor T cells on day 7. Mice receiving MigR1-Ezh2–infected T cells had approximately two- and fourfold more donor T cells recovered from spleen and liver, respectively, compared with MigR1-GFP controls (Figure 3C). Ezh2 overexpression led to significantly increased percentage and absolute number of IFN-γ– and TNF-α–producing T cells both in spleen and liver (Figure 3D-E). These data suggest that Ezh2 depletion is important for AUY922-mediated inhibition of GVH reaction both in vitro and in vivo.
AUY922 treatment reduces GVHD against miHAs
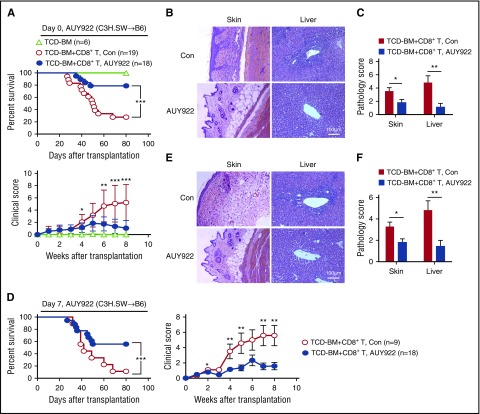
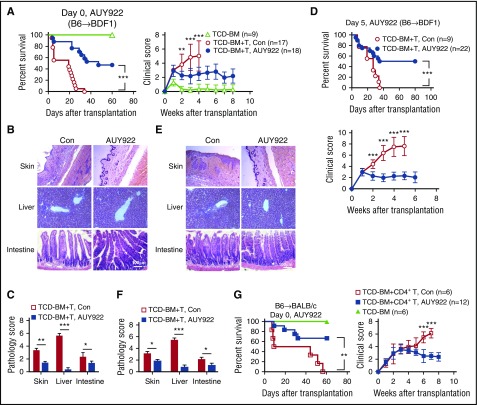
To test the effect of AUY922 treatment on GVHD against miHAs, we transplanted C3H.SW TCD-BM, with or without C3H.SW CD8+ T cells, into lethally irradiated B6/SJL mice. Fourteen doses of AUY922 (50 mg/kg) were administered to the B6/SJL recipients from day 0 to day 27 after transplantation. As expected, 80% of control B6/SJL mice receiving donor TCD-BM plus CD8+ T cells died as a result of GVHD (Figure 4A). AUY922 treatment inhibited GVHD in B6/SJL mice receiving donor CD8+ T cells, as evidenced by improved survival (80%) over a period of 80 days after transplantation and decreased clinical score of GVHD (Figure 4A). Histologic examination showed significantly reduced damage in the skin and liver (Figure 4B-C). To assess if AUY922 treatment rescued mice from ongoing GVHD, we administered AUY922 from day 7 after transplantation when clinical signs of GVHD occurred. Twelve doses of AUY922 treatment inhibited GVHD, with 60% of mice surviving without severe GVHD (Figure 4D), and reduced inflammation in the skin and liver (Figure 4E-F). Thus, AUY922 treatment has both prophylactic and therapeutic effects on reducing GVHD against miHAs.
Figure 4.
AUY922 treatment reduces GVHD against miHAs. Lethally irradiated B6/SJL recipient mice received C3H.SW TCD-BM or TCD-BM plus CD8+ T-cell transplants. Recipients were treated with 14 doses of AUY922 from day 0 to day 27 (A-C) or 12 doses of AUY922 from day 7 to day 29 (D-F) after transplantation, with phosphate-buffered saline treatment as control (Con). (A,D) Survival and clinical score were monitored over time. (B-C,E-F) Tissues were collected on day 22 and later but before day 55 after transplantation. (B,E) Images were obtained with an Olympus BX41 microscope (magnification ×200; hematoxylin-eosin stain). (C,F) Graphs show the histologic score of GVHD. Error bars indicate mean ± standard deviation. Data are representative of 2 to 3 independent experiments *P < .05; **P < .01; ***P < .001.
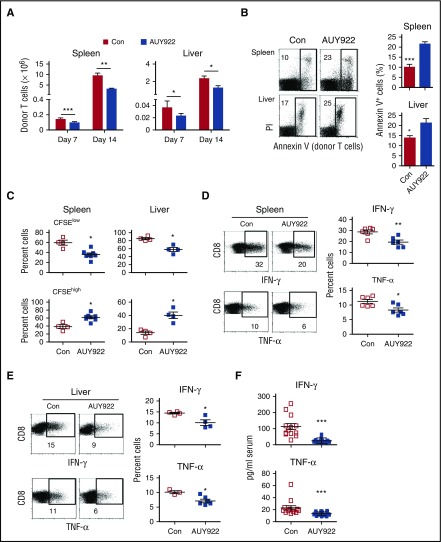
To understand the mechanism underlying the GVHD inhibition, we examined effect of AUY922 treatment on alloreactive T cells in B6/SJL mice receiving C3H.SW CD8+ T cells. As compared with phosphate-buffered saline controls, AUY922 treatment markedly decreased the number of donor CD8+ T cells by day 7 and day 14 after transplantation (Figure 5A), increased the percentage of donor CD8+ T cells undergoing apoptosis in the spleen and liver (twofold and 1.5-fold, respectively; Figure 5B), selectively reduced highly proliferative donor CD8+ T cells (CFSElow; Figure 5C), and significantly decreased both IFN-γ– and TNF-α–producing effector CD8+ T cells (Figure 5D-E). Enzyme-linked immunosorbent assay further confirmed the decrease of serum IFN-γ and TNF-α in these AUY922-treated recipients (Figure 5F).
Figure 5.
AUY922 treatment reduces alloreactive T-cell responses in vivo. Lethally irradiated B6/SJL recipient mice received C3H.SW TCD-BM transplants, together with CD8+ T cells that had been prestained with CFSE, followed by AUY922 or phosphate-buffered saline (Con) treatment. Cells were recovered from the recipient mice on day 7 (A-F) and day 14 (A) after transplantation. (A) Graphs show the absolute number of recovered donor CD8+ T cells in the spleen and liver. (B) The fraction of apoptotic (Annexin V+) donor T cells. (C) The fraction of CFSElow and CFSEhigh cells in donor T cells. (D-E) The expressions of IFN-γ and TNF-α in donor T cells were examined. (F) Levels of serum IFN-γ and TNF-α were measured by enzyme-linked immunosorbent assay. Error bars indicate mean ± standard deviation. Data are representative of 2 to 3 independent experiments. *P < .05; **P < .01; ***P < .001.
Previous studies using geldanamycin revealed that Hsp90 inhibition reduced human DC function in cultures.42 We observed that AUY922 moderately reduced surface expression of MHC II but not CD80 and CD86 in BM-derived DCs (supplemental Figure 5A). However, AUY922-treated DCs had capacity similar to that of untreated controls to drive proliferation of allogeneic CD4+ T cells in cultures (supplemental Figure 5B). Pretreatment of animals with AUY922 did not alter its potency or the time kinetics of GVHD inhibition (supplemental Figure 5C).
Collectively, these data indicate that AUY922 treatment reduces GVHD most likely by impairing expansion of alloreactive effector T cells and their production of inflammatory cytokines rather than by disrupting DC function.
AUY922 reduces GVHD in mice undergoing haploidentical allogeneic HSCT
We used a haploidentical B6/SJL anti-BDF1 mouse model of GVHD, which is increasingly used in clinic,43,44 to examine the benefit of AUY922 treatment. All control BDF1 recipients of B6 splenocytes died as a result of severe GVHD within 35 days, whereas AUY922 treatment protected BDF1 recipients of B6 splenocytes against severe GVHD, with 50% of them surviving >60 days (Figure 6A). Histologic examination confirmed the reduction of inflammation in the skin, liver, and intestine in AUY922-treated recipients (Figure 6B-C). Delayed administration of AUY922 from 5 days after transplantation, when clinical signs of GVHD occurred, protected approximately 50% of them from lethal GVHD (Figure 6D) and reduced inflammation in GVHD target organs (Figure 6E-F). We further confirmed efficacy of AUY922 in reducing GVHD using BALB/c mice receiving both MHC- and miHA-mismatched B6 CD4+ T cells (Figure 6G), ruling out a model-specific effect of AUY922.
Figure 6.
AUY922 treatment reduces GVHD in mice undergoing haploidentical allogeneic HSCT. Lethally irradiated BDF1 recipients (11.5 Gy) received B6/SJL mouse–derived TCD-BM (5 × 106) with or without spleen cells (1 × 107). Recipients were treated with 14 doses of AUY922 from day 0 to day 27 (A-C) or 12 doses of AUY922 from day 5 to day 27 (D-F) after transplantation, with phosphate-buffered saline treatment as control (Con). (A,D) Survival and clinical score were monitored over time. (B-C,E-F) Tissues were collected from day 28 to day 35 after transplantation. (B,E) Images were obtained with an Olympus BX41 microscope (magnification ×200; hematoxylin-eosin stain). (C,F) Graphs show the histologic score of GVHD. (G) B6/SJL-derived TCD-BM (5 × 106) with or without CD4+ T cells (1 × 106) were transferred into lethally irradiated (8 Gy) BALB/c mice. Fourteen doses of AUY922 (50 mg/kg) were administered to these recipients from day 0 to day 27 after transplantation. Survival rate and clinical score were monitored over time. Error bars indicate mean ± standard deviation. Data are representative of 2 to 3 independent experiments. *P < .05; **P < .01; ***P < .001.
Inhibition of Hsp90 may also affect pathways such as Akt, Jak-Stat, Erk1/2, and Lck.45 Recent studies have suggested that Ezh2 is an important downstream effector of Akt and Jak3 in promoting cancer-cell proliferation.46,47 To further prove whether Ezh2 is a major target of AUY922 for reducing GVHD, we transduced WT B6 CD8+ T cells by MigR1-Ezh2 or MigR1-GFP and transferred them into lethally irradiated BDF1 mice, followed by AUY922 treatment (supplemental Figure 6). AUY922 treatment inhibited GVHD in mice receiving MigR1-GFP T cells (supplemental Figure 6A) but not in those receiving MigR1-Ezh2 T cells (supplemental Figure 6B). AUY922 treatment was unable to reduce Ezh2 protein in these MigR1-Ezh2 T cells to the threshold required for inhibiting GVHD (data not shown). These results indicate that depletion of T-cell Ezh2 by AUY922 plays a major role in reducing GVHD.
To determine the effect of AUY922 on Ezh2 in alloreactive T cells in vivo, we measured the expression of Ezh2 and T-bet, a transcription factor regulated by Ezh2 in T cells,15,16 in donor T cells isolated from those undergoing allogeneic HSCT. As compared with untreated controls, AUY922 decreased the protein levels of Ezh2, Akt, and p-Akt and transcription of Tbx21 (encoding T-bet) in alloreactive CD8+ T cells (supplemental Figure 7A-B), without altering their production of H3K27me3, H3K4me3, H3K9me3, or H3K36me3 (supplemental Figure 7A). Ezh2-based coimmunoprecipitation assay revealed that AUY922 markedly reduced the amount of Suz12 and Eed proteins that form the polycomb repressive complex 2 with Ezh2; however, it had no direct effect on cellular level of Suz12 or Eed (supplemental Figure 7C). This suggests that AUY922 selectively depletes Ezh2 protein and consequently reduces the amount of polycomb repressive complex 2 in alloreactive T cells. Intriguingly, donor T cells from AUY922-treated mice retained 70% Ezh2 protein relative to untreated recipients (supplemental Figure 7A). In contrast, ex vivo AUY922 treatment reduced Ezh2 level to 10% of that in untreated T cells (Figure 2E-G). We therefore proposed that the in vivo AUY922 administration regimen needs optimization to achieve higher efficiency of Ezh2 depletion in alloreactive T cells.
Hsp90 inhibition preserves GVL effects
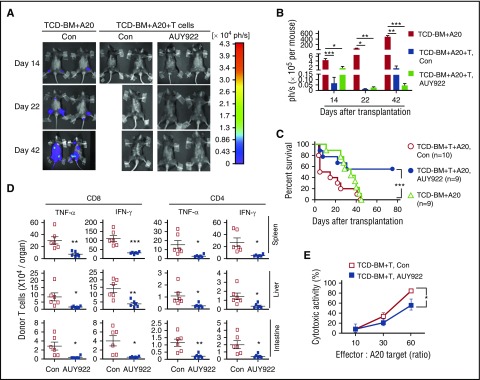
To evaluate whether AUY922 treatment retained GVL activity, we challenged BDF1 recipients with A20TGL leukemia/lymphoma cells (H-2d), mimicking residual leukemia in patients receiving treatment with allogeneic HSCT. Mice transplanted with TCD-BM and challenged with A20 cells died as a result of leukemia (Figure 7A-C), which was not affected by AUY922 treatment (data not shown). BDF1 mice receiving donor splenocytes and A20 cells died as a result of GVHD without leukemia (Figure 7A-C). B6 splenocyte recipients treated with AUY922 showed decreased GVHD and elimination of A20 cells, leading to their significantly improved survival (Figure 7A-C).
Figure 7.
AUY922 treatment preserves donor T-cell–mediated GVL effects. Lethally irradiated BDF1 recipients (11.5 Gy) received B6/SJL mouse–derived TCD-BM (5 × 106) transplants with or without spleen cells (1 × 107) and challenged with A20TGL cells (1 × 106). Fourteen doses of AUY922 were administered from day 0 to day 27 after transplantation. (A-B) In vivo image analysis on days 14, 22, and 42 after transplantation. Graph shows the total-body luminescence intensity. (C) Survival rate was monitored over time. (D) Donor T cells were recovered on day 7 after transplantation. Graphs show the fractions of TNF-α– and IFN-γ–producing donor CD4+ and CD8+ T cells. (E) Donor T cells isolated from the spleen on day 14 were used for cytolytic assay against A20TGL cells. Error bars indicate mean ± standard deviation. Data are representative of 2 to 3 independent experiments. *P < .05; **P < .01; ***P < .001.
To understand the mechanism underlying the GVL preservation, we transferred B6 T cells into BDF1 mice treated with or without AUY922. We found that the preservation of GVL effects in donor T-cell–replete recipients treated by AUY922 was associated with redirecting donor T cells away from GVHD target tissues, such as the liver and intestine. Although AUY922 treatment significantly reduced the number of IFN-γ–producing CD8+ and CD4+ T cells in the spleen, liver, and intestine (Figure 7D), it retained a substantial proportion of alloreactive T cells capable of killing A20 leukemic cells in the spleen (Figure 7E). As a consequence, this reduction of T-cell alloreactivity led to decreased clinical score of GVHD in the skin and gastrointestinal track (supplemental Figure 8).
We further validated the impact of AUY922 treatment on GVL effects in B6 mice receiving C3H.SW CD8+ T cells and EL4 thymoma cells. Necrosectomy and histologic examination showed that although in vivo administration of AUY922 treatment did not affect leukemia growth in mice receiving TCD-BM only (supplemental Figure 9A), it reduced GVHD and retained antitumor activity in B6 mice receiving C3H.SW CD8+ T cells (supplemental Figure 9B-C). As a result, approximately 60% of T-cell recipients treated by AUY922 survived >80 days without sign of tumor (supplemental Figure 9B-C).
Normal hematopoiesis and thymopoiesis after AUY922 treatment
Because Ezh2 is known to be important for hematopoiesis and thymopoiesis, we examined whether AUY922 administration may affect hematopoiesis and immune reconstitution. B6 TCD-BM (CD45.2+) was transferred into lethally irradiated B6/SJL (CD45.1+) mice. Fourteen doses of AUY922 were administered to these recipients from day 0 to day 27. We harvested BM, splenocytes, and thymocytes 40 days after transplantation. There was no difference in the number of CD4+ T, CD8+ T, and B220+ cells in the spleen between AUY922-treated and phosphate-buffered saline–treated recipients (supplemental Figure 10A). AUY922 treatment impaired neither thymic reconstitution (supplemental Figure 10B-C) nor the engraftment of BM cells (supplemental Figure 10D) or lineage-negative Sca-1+C-kit+ hematopoietic stem/progenitor cells (supplemental Figure 10E). Thus, a normal reconstitution of hematopoiesis and thymopoiesis occurs in mice undergoing HSCT treated by AUY922.
Discussion
Identifying the molecular pathway that can control T-cell Ezh2 expression and function will be beneficial for reducing harmful effects of allogeneic HSCT. We demonstrate that Ezh2 requires Hsp90 to maintain its stability and function in alloreactive T cells. Inhibition of Hsp90 induced a rapid Ezh2 degradation and impaired survival and expansion of alloreactive T cells, similar to genetic inactivation of Ezh2. Introduction of Ezh2 in activated T cells markedly restored their survival and expansion upon Hsp90 inhibition. In vivo administration of AUY922 showed both preventive and therapeutic effects on reducing GVHD while preserving GVL, improving the overall survival of mice after allogeneic HSCT and leukemia challenge. Our findings identify the Ezh2-Hsp90 interaction as a previously unrecognized mechanism essential for T-cell responses and an effective target for controlling GVHD.
Histone methylation is considered to be crucially involved in the regulation of T-cell differentiation and proliferation. For example, G9a, which catalyzes H3K9me2, promotes the production of Th2 cytokines in CD4+ T cells.10 SUV39H1, which catalyzes H3K9me3,48,49 is important for maintaining the stability of differentiated Th2 cells.11 Several excellent studies have examined histone methylation changes at individual genes in effector versus memory T cells7,8,50-53; however, whether inhibiting histone methylation per se is sufficient to reduce GVHD has not been previously determined. We found that GSK126 reduced H3K27me3 in activated T cells without affecting Ezh2 expression. In vivo administration of GSK126 was unable to reduce the production of IFN-γ by alloreactive T cells and failed to prevent GVHD. These results are in contradictory to our previous reports that genetic deletion of Ezh2 inhibited the production of alloreactive T cells.15,16,26 Thus, Ezh2 may have additional effects on regulating GVH reaction via a noncanonical mechanism.
Our findings suggest that targeting noncanonical effect of Ezh2 on alloreactive T cells may lead to GVHD inhibition. Emerging evidence indicates that H3K27me3-independent Ezh2 function plays important roles in the regulation of cancer-cell proliferation and survival. Ezh2 served as an adaptor protein linking estrogen receptor to β-catenin to enhance their transcriptional activity in prostate cancer.54 Ezh2 enhanced NFκB activity through direct interaction with RelA and RelB in basal-like breast cancer cells.55 Ezh2 interacted with and methylated STAT3 in cancer cells to enhance STAT3 activity.56 Furthermore, a role for Ezh2 in NOTCH activation was also demonstrated in breast cancer cells.57 We recently found that Ezh2 protected T-bet from proteasome-mediated degradation in Th1 cells.14 All these data argue the notion that depletion of Ezh2 by AUY922 may lead to a complex effect on alloreactive T cells.
The present study has significant implications in the translation of pharmacologic depletion of Ezh2 using AUY922 to treatment of patients. Earlier studies suggested that Hsp90 inhibition did not impair survival or proliferation of cytomegalovirus-specific CD8+ T cells,45,58 allowing preservation of antiviral immunity. Recent studies have suggested that Hsp90 inhibition using 17-AAG–protected intestine paneth cells against alloreactive T-cell–mediated injury, leading to improved epithelium regeneration and reduction of damage to the gastrointestinal track.59 However, whether this protective effect of 17-AAG may result in long-term protection against GVHD remains unclear. We demonstrate that Hsp90 inhibition by AUY922 has both preventive and therapeutic effects on GVHD and significantly improves the overall survival of mice undergoing allogeneic HSCT and leukemia challenge. Thus, Hsp90 inhibition by AUY922 seems to be beneficial to allogeneic HSCT in several aspects.
Because we have shown that the capacity of donor T cells to mediate GVHD relies on their expression of Ezh2,16 it is likely that the partial protection of AUY922 treatment against GVHD in MHC-mismatched mouse models may result from insufficient depletion of Ezh2 in donor T cells. Toward this end, additional studies will investigate novel strategies that may enhance the efficacy of AUY922 in depleting T-cell Ezh2 to improve its overall protection against GVHD in all those receiving treatment with allogeneic HSCT.
In summary, we identified a previously uncharacterized molecular mechanism by which functional Hsp90 was critical for maintaining Ezh2 protein stability and function. Inhibition of Hsp90 destabilized Ezh2 protein in alloreactive T cells and reduced GVHD but preserved GVL effects in mice. These results challenge current paradigms about modulating T-cell alloimmunity by specifically reducing histone methylation and indicate that targeting the Ezh2-Hsp90 complex using AUY922 may represent a novel and clinically relevant approach to modulating the GVH response after allogeneic HSCT.
Acknowledgments
The authors thank Marcel van den Brink (Memorial Sloan Kettering Cancer Center) for providing A20TGL cells.
This work was supported by grants from the Ministry of Science and Technology of China (2015CB943300 and 2014CB943300) (Yanyun Zhang), National Natural Science Foundation of China (81373164, 81130057, and 81670540 [Yanyun Zhang]; 81670172 [S. He]), Strategic Priority Research Program of the Chinese Academy of Sciences (XDA01040000) (Yanyun Zhang), Program of Jiangsu Engineering Research Center for Tumor Immunotherapy of China (BM2014404) (Yanyun Zhang), Program of Science and Technology Commission of Shanghai Municipality of China (14ZR1446300) (M.J.), American Cancer Society (Yi Zhang), Department of Defense (Yi Zhang), and National Institutes of Health (National Cancer Institute 1R01CA172106-01 and National Heart, Lung, and Blood Institute 1R01HL127351-01A1) (Yi Zhang).
Footnotes
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Q.H., S. He, Yi Zhang, and Yanyun Zhang conceived and designed the project; Q.H., S. He, Y.T., Y.G., P.C., C.L., J.H., Y.L., H.Y., M.J., S. Hu, Q.T., and A.M. performed experiments and analyzed and interpreted the data; J.J., E.H., H.F., R.R., Yi Zhang, and Yanyun Zhang analyzed and interpreted the data; Q.H., S. He, Yi Zhang, and Yanyun Zhang wrote the manuscript; and Q.H., S. He, R.R., Yi Zhang, and Yanyun Zhang edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing conflicts of interest.
Correspondence: Yi Zhang, Fels Institute for Cancer Research and Molecular Biology, Department of Microbiology and Immunology, Temple University School of Medicine, Philadelphia, PA 19140; e-mail: yi.zhang@temple.edu; and Yanyun Zhang, Institute of Health Sciences, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences and Shanghai Jiao Tong University School of Medicine, Shanghai 200031, China; e-mail: yyzhang@sibs.ac.cn.
References
- 1.Choi SW, Reddy P. Current and emerging strategies for the prevention of graft-versus-host disease. Nat Rev Clin Oncol. 2014;11(9):536-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12(6):443-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu JI, Lessard J, Crabtree GR. Understanding the words of chromatin regulation. Cell. 2009;136(2):200-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693-705. [DOI] [PubMed] [Google Scholar]
- 5.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293(5532):1074-1080. [DOI] [PubMed] [Google Scholar]
- 6.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403(6765):41-45. [DOI] [PubMed] [Google Scholar]
- 7.Chang S, Aune TM. Dynamic changes in histone-methylation ‘marks’ across the locus encoding interferon-gamma during the differentiation of T helper type 2 cells. Nat Immunol. 2007;8(7):723-731. [DOI] [PubMed] [Google Scholar]
- 8.Wei G, Wei L, Zhu J, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30(1):155-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He S, Tong Q, Bishop DK, Zhang Y. Histone methyltransferase and histone methylation in inflammatory T-cell responses. Immunotherapy. 2013;5(9):989-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehnertz B, Northrop JP, Antignano F, et al. Activating and inhibitory functions for the histone lysine methyltransferase G9a in T helper cell differentiation and function. J Exp Med. 2010;207(5):915-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allan RS, Zueva E, Cammas F, et al. An epigenetic silencing pathway controlling T helper 2 cell lineage commitment. Nature. 2012;487(7406):249-253. [DOI] [PubMed] [Google Scholar]
- 12.Boyer LA, Plath K, Zeitlinger J, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441(7091):349-353. [DOI] [PubMed] [Google Scholar]
- 13.Bracken AP, Kleine-Kohlbrecher D, Dietrich N, et al. The polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 2007;21(5):525-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margueron R, Reinberg D. The polycomb complex PRC2 and its mark in life. Nature. 2011;469(7330):343-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tong Q, He S, Xie F, et al. Ezh2 regulates transcriptional and posttranslational expression of T-bet and promotes Th1 cell responses mediating aplastic anemia in mice. J Immunol. 2014;192(11):5012-5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He S, Xie F, Liu Y, et al. The histone methyltransferase Ezh2 is a crucial epigenetic regulator of allogeneic T-cell responses mediating graft-versus-host disease. Blood. 2013;122(25):4119-4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tumes DJ, Onodera A, Suzuki A, et al. The polycomb protein Ezh2 regulates differentiation and plasticity of CD4(+) T helper type 1 and type 2 cells. Immunity. 2013;39(5):819-832. [DOI] [PubMed] [Google Scholar]
- 18.Konze KD, Ma A, Li F, et al. An orally bioavailable chemical probe of the lysine methyltransferases EZH2 and EZH1. ACS Chem Biol. 2013;8(6):1324-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qi W, Chan H, Teng L, et al. Selective inhibition of Ezh2 by a small molecule inhibitor blocks tumor cells proliferation. Proc Natl Acad Sci USA. 2012;109(52):21360-21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCabe MT, Ott HM, Ganji G, et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012;492(7427):108-112. [DOI] [PubMed] [Google Scholar]
- 21.Knutson SK, Wigle TJ, Warholic NM, et al. A selective inhibitor of EZH2 blocks H3K27 methylation and kills mutant lymphoma cells. Nat Chem Biol. 2012;8(11):890-896. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Louboutin JP, Zhu J, Rivera AJ, Emerson SG. Preterminal host dendritic cells in irradiated mice prime CD8+ T cell-mediated acute graft-versus-host disease. J Clin Invest. 2002;109(10):1335-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao F, Zhang Y, Wang H, et al. Blockade of osteopontin reduces alloreactive CD8+ T cell-mediated graft-versus-host disease. Blood. 2011;117(5):1723-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooke KR, Kobzik L, Martin TR, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. the roles of minor H antigens and endotoxin. Blood. 1996;88(8):3230-3239. [PubMed] [Google Scholar]
- 25.Reddy P, Maeda Y, Liu C, Krijanovski OI, Korngold R, Ferrara JL. A crucial role for antigen-presenting cells and alloantigen expression in graft-versus-leukemia responses. Nat Med. 2005;11(11):1244-1249. [DOI] [PubMed] [Google Scholar]
- 26.He S, Wang J, Kato K, et al. Inhibition of histone methylation arrests ongoing graft-versus-host disease in mice by selectively inducing apoptosis of alloreactive effector T cells. Blood. 2012;119(5):1274-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su IH, Basavaraj A, Krutchinsky AN, et al. Ezh2 controls B cell development through histone H3 methylation and Igh rearrangement. Nat Immunol. 2003;4(2):124-131. [DOI] [PubMed] [Google Scholar]
- 28.Bracken AP, Pasini D, Capra M, Prosperini E, Colli E, Helin K. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 2003;22(20):5323-5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li G, Margueron R, Ku M, Chambon P, Bernstein BE, Reinberg D. Jarid2 and PRC2, partners in regulating gene expression. Genes Dev. 2010;24(4):368-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuzmichev A, Jenuwein T, Tempst P, Reinberg D. Different EZH2-containing complexes target methylation of histone H1 or nucleosomal histone H3. Mol Cell. 2004;14(2):183-193. [DOI] [PubMed] [Google Scholar]
- 31.Cao R, Wang L, Wang H, et al. Role of histone H3 lysine 27 methylation in polycomb-group silencing. Science. 2002;298(5595):1039-1043. [DOI] [PubMed] [Google Scholar]
- 32.Su IH, Dobenecker MW, Dickinson E, et al. Polycomb group protein ezh2 controls actin polymerization and cell signaling. Cell. 2005;121(3):425-436. [DOI] [PubMed] [Google Scholar]
- 33.Neckers L. Heat shock protein 90: the cancer chaperone. J Biosci. 2007;32(3):517-530. [DOI] [PubMed] [Google Scholar]
- 34.Powers MV, Workman P. Targeting of multiple signalling pathways by heat shock protein 90 molecular chaperone inhibitors. Endocr Relat Cancer. 2006;13(suppl 1):S125-S135. [DOI] [PubMed] [Google Scholar]
- 35.Brough PA, Aherne W, Barril X, et al. 4,5-diarylisoxazole Hsp90 chaperone inhibitors: potential therapeutic agents for the treatment of cancer. J Med Chem. 2008;51(2):196-218. [DOI] [PubMed] [Google Scholar]
- 36.Voruganti S, Lacroix JC, Rogers CN, Rogers J, Matts RL, Hartson SD. The anticancer drug AUY922 generates a proteomics fingerprint that is highly conserved among structurally diverse Hsp90 inhibitors. J Proteome Res. 2013;12(8):3697-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zoabi M, Sadeh R, de Bie P, Marquez VE, Ciechanover A. PRAJA1 is a ubiquitin ligase for the polycomb repressive complex 2 proteins [published correction appears in Biochem Biophys Res Commun. 2011;410(3):705]. Biochem Biophys Res Commun. 2011;408(3):393-398. [DOI] [PubMed] [Google Scholar]
- 38.Gaykema SB, Schröder CP, Vitfell-Rasmussen J, et al. 89Zr-trastuzumab and 89Zr-bevacizumab PET to evaluate the effect of the HSP90 inhibitor NVP-AUY922 in metastatic breast cancer patients. Clin Cancer Res. 2014;20(15):3945-3954. [DOI] [PubMed] [Google Scholar]
- 39.Chiosis G, Dickey CA, Johnson JL. A global view of Hsp90 functions. Nat Struct Mol Biol. 2013;20(1):1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun K, Wilkins DE, Anver MR, et al. Differential effects of proteasome inhibition by bortezomib on murine acute graft-versus-host disease (GVHD): delayed administration of bortezomib results in increased GVHD-dependent gastrointestinal toxicity. Blood. 2005;106(9):3293-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166(2):973-981. [DOI] [PubMed] [Google Scholar]
- 42.Bae J, Mitsiades C, Tai YT, et al. Phenotypic and functional effects of heat shock protein 90 inhibition on dendritic cell. J Immunol. 2007;178(12):7730-7737. [DOI] [PubMed] [Google Scholar]
- 43.Reisner Y, Hagin D, Martelli MF. Haploidentical hematopoietic transplantation: current status and future perspectives. Blood. 2011;118(23):6006-6017. [DOI] [PubMed] [Google Scholar]
- 44.Kanakry CG, Fuchs EJ, Luznik L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat Rev Clin Oncol. 2016;13(2):132. [DOI] [PubMed] [Google Scholar]
- 45.Stuehler C, Mielke S, Chatterjee M, et al. Selective depletion of alloreactive T cells by targeted therapy of heat shock protein 90: a novel strategy for control of graft-versus-host disease. Blood. 2009;114(13):2829-2836. [DOI] [PubMed] [Google Scholar]
- 46.Xu K, Wu ZJ, Groner AC, et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is polycomb-independent. Science. 2012;338(6113):1465-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan J, Li B, Lin B, et al. EZH2 phosphorylation by JAK3 mediates a switch to noncanonical function in natural killer/T-cell lymphoma. Blood. 2016;128(7):948-958. [DOI] [PubMed] [Google Scholar]
- 48.Lachner M, O’Carroll D, Rea S, Mechtler K, Jenuwein T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410(6824):116-120. [DOI] [PubMed] [Google Scholar]
- 49.Bannister AJ, Zegerman P, Partridge JF, et al. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410(6824):120-124. [DOI] [PubMed] [Google Scholar]
- 50.Araki Y, Wang Z, Zang C, et al. Genome-wide analysis of histone methylation reveals chromatin state-based regulation of gene transcription and function of memory CD8+ T cells. Immunity. 2009;30(6):912-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Denton AE, Russ BE, Doherty PC, Rao S, Turner SJ. Differentiation-dependent functional and epigenetic landscapes for cytokine genes in virus-specific CD8+ T cells. Proc Natl Acad Sci USA. 2011;108(37):15306-15311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu KT, Kanno Y, Cannons JL, et al. Functional and epigenetic studies reveal multistep differentiation and plasticity of in vitro-generated and in vivo-derived follicular T helper cells. Immunity. 2011;35(4):622-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mukasa R, Balasubramani A, Lee YK, et al. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity. 2010;32(5):616-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi B, Liang J, Yang X, et al. Integration of estrogen and Wnt signaling circuits by the polycomb group protein EZH2 in breast cancer cells. Mol Cell Biol. 2007;27(14):5105-5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee ST, Li Z, Wu Z, et al. Context-specific regulation of NF-κB target gene expression by EZH2 in breast cancers. Mol Cell. 2011;43(5):798-810. [DOI] [PubMed] [Google Scholar]
- 56.Kim E, Kim M, Woo DH, et al. Phosphorylation of EZH2 activates STAT3 signaling via STAT3 methylation and promotes tumorigenicity of glioblastoma stem-like cells. Cancer Cell. 2013;23(6):839-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonzalez ME, Moore HM, Li X, et al. EZH2 expands breast stem cells through activation of NOTCH1 signaling. Proc Natl Acad Sci USA. 2014;111(8):3098-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berges C, Bedke T, Stuehler C, et al. Combined PI3K/Akt and Hsp90 targeting synergistically suppresses essential functions of alloreactive T cells and increases Tregs. J Leukoc Biol. 2015;98(6):1091-1105. [DOI] [PubMed] [Google Scholar]
- 59.Joly AL, Deepti A, Seignez A, et al. The HSP90 inhibitor, 17AAG, protects the intestinal stem cell niche and inhibits graft versus host disease development. Oncogene. 2016;35(22):2948. [DOI] [PubMed] [Google Scholar]