Abstract
Purpose
Cancer-related insomnia is associated with diminished quality of life (QOL), suggesting that improvement in insomnia may improve QOL in cancer survivors. Cognitive behavioral therapy for insomnia (CBT-I) has been shown to improve insomnia, but less is known regarding its effect on QOL and whether improvement in insomnia corresponds to improved QOL. The present analysis examines the effects of CBT-I, with and without armodafinil, on QOL both directly and indirectly through improvements of insomnia.
Methods
This is an analysis of 95 cancer survivors for a specified secondary aim of a 4-arm randomized controlled trial assessing the combined and individual effects of CBT-I and armodafinil to improve insomnia. QOL and insomnia severity were assessed before, during the intervention, at post-intervention, and three months later by Functional Assessment of Cancer Therapy-General and Insomnia Severity Index, respectively.
Results
Mean change in QOL from pre- to post-intervention for CBT-I+placebo, CBT-I+armodafinil, armodafinil, and placebo was: 9.6 (SE=1.8; p<0.0001), 11.6 (SE=1.8; p<0.0001), −0.2 (SE=3.2; p=0.964), and 3.3 (SE=2.0; p=0.124), respectively. ANCOVA controlling for pre-intervention scores showed that participants receiving CBT-I had significantly improved QOL at post-intervention compared to those not receiving CBT-I (p<0.0001, effect size=0.57), with benefits being maintained at the three-month follow-up. Path analysis revealed that this improvement in QOL was due to improvement in insomnia severity (p=0.002), and Pearson correlations showed that changes in QOL from pre- to post-intervention were significantly associated with concurrent changes in insomnia severity (r=−0.56; p<0.0001). Armodafinil had no effect on QOL for those who did or did not receive it (p=0.976; effect size=−0.004).
Conclusion
In cancer survivors with insomnia, CBT-I resulted in clinically significant improvement in QOL via improvement in insomnia. This improvement in QOL remained stable even three months after completing CBT-I.
Implications for Cancer Survivors
Considering the high prevalence of insomnia and its detrimental impact on QOL in cancer survivors and the effectiveness of CBT-I in alleviating insomnia, it is important that evidence-based non-pharmacological sleep interventions such as CBT-I be provided as an integral part of cancer care.
Keywords: Cancer, Cancer Survivors, CBT-I, Quality of life, Insomnia
Introduction
The number of cancer survivors has been steadily increasing due to better detection methods and improved treatments [1]. Living longer, however, doesn’t necessary imply living well, as cancer survivors experience both short- and long-term side effects from cancer and its treatments. These psychological and physical side effects negatively impact quality of life (QOL) in survivors, resulting in increased disability and healthcare utilization [2]. QOL is a multi-dimensional construct encompassing physical, functional, emotional, and social aspects of an individual’s overall well-being [3], and is recognized as a vital outcome of cancer care. Maintaining a good QOL is of increasing importance to patients and health care providers, as it frequently decreases after diagnosis and treatment [4, 5]. A recent US survey showed that approximately 25% of cancer survivors had poor physical QOL and 10% had poor mental QOL compared with 10% and 6%, respectively, of adults without cancer [6]. Therefore, it is important to explore possible ways to maintain and improve overall QOL during and after the cancer care trajectory.
Reduced QOL is associated with sleep disturbances [7–10], and sleep disturbance is a significant and frequent problem in cancer survivors. Studies indicate that 30–60% of individuals with cancer experience difficulties with initiating or maintaining sleep [11, 12], while 19–30% meet diagnostic criteria for insomnia disorder [13, 14] with high rates of insomnia persisting well beyond the completion of cancer treatments [15]. Gooneratne et al. showed that lung cancer survivors with poorer sleep quality had significantly worse QOL than those with good sleep quality [16]. Others have also shown that prolonged daytime napping and shorter nighttime sleep negatively affects QOL [17] while maintenance of consistent sleep-wake patterns is associated with better QOL [18]. Thus, interventions for the management of insomnia could be very effective in improving QOL.
Growing evidence from randomized controlled trials (RCT) has shown that cognitive behavioral therapy for insomnia (CBT-I) is an effective method for reducing insomnia in cancer patients and survivors [19–22], and that acceptability of this intervention by patients is high [23]. CBT-I is a multimodal intervention consisting of sleep restriction, stimulus control, cognitive restructuring, and education to re-establish a regular sleep pattern [24]. In the general population, CBT-I is considered the gold standard treatment for insomnia disorder [25]. To date, eight controlled and four uncontrolled trials of CBT-I have been conducted in cancer survivors [18, 26]. A recent systematic review of these RCTs showed a large effect size for the self-reported insomnia severity (d=0.77) pooled across studies for survivors who received CBT-I compared to those who did not [26]. Of the 12 studies, only a few have evaluated the effect of CBT-I on QOL, which revealed mixed results with some studies finding improvements in QOL while some did not [19–21, 27–29]. Further, no study to date has evaluated whether the effects of CBT-I on QOL are mediated by insomnia reductions and whether improvement in insomnia in general corresponds to improved QOL.
We recently reported on a four-arm RCT of CBT-I with and without armodafinil compared to armodafinil alone and placebo alone in 96 cancer survivors with insomnia [30]. CBT-I resulted in significant improvement in insomnia severity and sleep quality, with no additive benefit provided by armodafinil. Armodafinil alone had no significant effects on insomnia severity and sleep quality. The objective of the present analysis is to report on a specified secondary aim of this four-arm RCT to examine the effect of the intervention on QOL. We also explored whether any effects of the intervention on QOL were mediated by changes in insomnia severity.
Methods
Patients
As described in full detail previously [30], cancer survivors with chronic insomnia were recruited from outpatient cancer clinics, via letters to patients, and from posters and advertising in two Northeastern U.S. cities between September 2008 and November 2012. Eligibility criteria remained essentially unchanged since December, 2008, when the original criterion of having breast cancer was broadened to include having any cancer and the criterion of receiving chemotherapy was broadened to include radiation therapy. Thus, participants were cancer survivors at least 18 years of age with any cancer type who had completed all chemotherapy and/or radiotherapy ≥ 1 month before study start, had no measurable disease, and were required to discontinue any prescribed or over-the-counter sleep medications for 1 week prior to the collection of baseline data as well as during the 11-week study period. Participants who had ever taken modafinil/armodafinil, had undergone CBT-I, or had a previous history of seizures, severe headaches, sleep apnea, or an unstable medical or psychiatric illness were not eligible. The institutional review boards of the University of Rochester and University of Pennsylvania approved the protocol, and subjects provided written informed consent. This trial is registered with ClinicalTrials.gov, number NCT01091974.
Study Design and Treatments
Participants were randomized to one of the four groups: CBT-I+placebo, CBT-I+armodafinil, armodafinil, or placebo, i.e., a factorial design of CBT (yes/no) versus armodafinil (yes/no). Randomization was done using an online computer-generated random table with block size 8 and stratified by city and gender. The random assignment was conveyed to a pharmacist, who provided the study coordinator with the appropriate study medications. All study personnel and participants were blinded regarding the medication assignment but not the CBT-I condition. The CBT-I intervention was 7 weeks long, was provided on an individual basis by therapists trained in CBT-I, and followed a published treatment manual [24]. There were 7 individual weekly CBT-I sessions, with sessions 1, 2, and 4 (30–60 min) conducted in person, and sessions 3, 5, 6, and 7 (15–30 min) conducted over the telephone. Armodafinil is a CNS stimulant and the R-enantiomer of modafinil. It was included in the study because of its wakefulness promoting properties and we had hypothesized that it would increase adherence to CBT-I by reducing the daytime sleepiness associated with the sleep restriction component of CBT-I. Participants took armodafinil (50 mg) or placebo in the morning and again in the afternoon for 47 days. For titration purposes, a placebo capsule was substituted for the afternoon doses of armodafinil on the first 3 days and the last 4 days of the 47-day medication period.
Assessments
Participants completed on-study questionnaires at consent, providing demographic and clinical information.
QOL was assessed by the Functional Assessment of Cancer Therapy-General (FACT-G), a 27-item instrument designed to assess physical, functional, social, and emotional well-being on a scale of 0–108, with higher score indicating better QOL. The FACT-G has been demonstrated to have good validity and reliability in cancer patients and the US general population [31]. The minimal clinically important difference (CID) for the FACT-G is defined as a score change ≥ 4 points [32].
Insomnia severity was assessed by the Insomnia Severity Index (ISI), a 7-item validated instrument for measuring insomnia on a scale of 0–28, with higher score indicating worse insomnia [33].
Both the FACT-G and the ISI were completed at pre-intervention (average of weeks 1 and 2), during intervention, post-intervention (average of weeks 10 and 11), and 3 months later (average of weeks 23 and 24).
Statistical Analyses
Sample size calculations were performed for the primary analysis and have been reported previously [30]. In the original study protocol, the target accrual was 226 participants and the planned primary analysis was a longitudinal analysis using estimation by generalized estimating equation and possible linear splines to represent trajectories in each arm. Due to our ability to randomize only 96 participants, and that the original planned analysis for the primary and secondary outcomes would be problematic with this smaller sample size, we changed the analyses to ANCOVA using pre-intervention score as covariate to evaluate the effects of CBT-I and armodafinil. Changes in insomnia severity and QOL from pre- to post-intervention were calculated as Post - Pre. Descriptive statistics were used for the pre-intervention, post-intervention, follow-up, and change scores. One-sample T-tests were performed on the mean changes to identify those that were significantly nonzero. For the analysis of covariance (ANCOVA), we started with a linear model including post-intervention QOL as the dependent variable, pre-intervention QOL as a covariate, CBT-I (yes/no), armodafinil (yes/no), and CBT-I by armodafinil interaction as fixed effects, and assessed the statistical significance of the interaction by type III F tests. Since the interaction was not statistically significant at the 0.05 level, it was removed from the model and the model was re-fitted. Bonferroni adjustment for multiple comparisons was done for the two main effects of CBT-I and armodafinil. One of the 96 randomized participants had missing baseline data and was omitted from all analysis. The analyses were done by intention to treat, although 29.5% of the 95 participants did not provide post-intervention data for QOL. Since there was missing data, the ANCOVA analysis was performed for both complete cases (i.e., observations with missing post-intervention data were omitted from the analysis) and multiple imputation (MI). The missing value patterns were examined through visual inspection and logistic regression modeling of dropout versus intervention group and relevant clinical and demographic characteristics. We also tested whether the rate of dropout depended on pre-intervention QOL levels but found no association. Assuming a missing at random (MAR) mechanism, we performed MI (using Fully Conditional Specification method) for post-intervention and follow-up QOL. We also examined changes in QOL from post-intervention to follow-up using one-way ANOVA among the four intervention arms.
We used Pearson correlations to examine the association between QOL and insomnia severity change scores. To determine if insomnia severity mediated the effect of the intervention on QOL, we performed a path analysis using a structural equation model (SEM) with maximum likelihood estimation. In the model, we included direct effects from CBT-I (yes/no) and armodafinil (yes/no) on both QOL and insomnia change scores. For the indirect effects, we included insomnia change score as a predictor of QOL change score. All statistical analyses were performed using SPSS version 22 and STATA IC version 14 for analyses as appropriate.
Results
Patient Characteristics
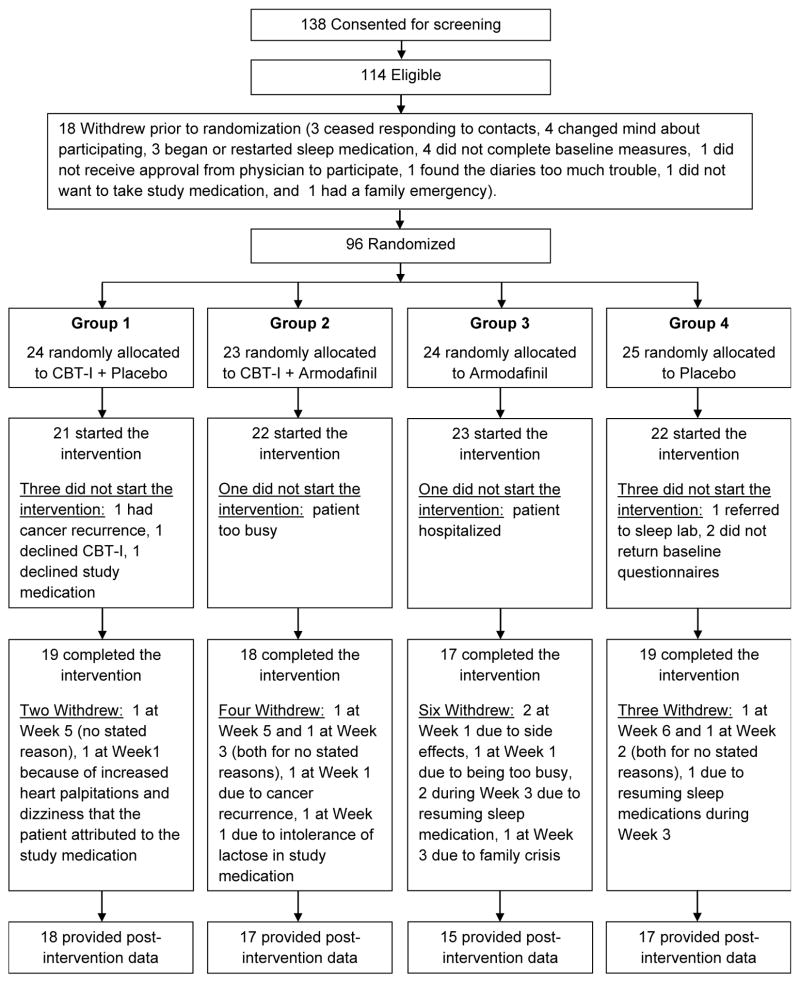
Fig. 1 shows the consort diagram, which is also provided in our published manuscript on the primary study aim of insomnia [30]. Briefly, of the 138 participants who consented to screening, 114 were eligible, 96 were randomized, 88 began the intervention, and 73 completed the intervention. No serious study-related adverse effects were reported. One grade 2 possibly related event (tingling, numbness, and weakness in legs) occurred as did two probably related grade 2 events (headaches). All three events occurred in patients assigned to CBT-I+armodafinil. As previously reported for this study, the average adherence with study medication was >90% for all intervention groups and there were no significant differences in the overall adherence between CBT-I+placebo or CBT-I+armodafinil [34]. Table 1 shows the baseline characteristics of the 95 participants who provided baseline QOL data, with 24, 23, 24, 24 having been randomized to CBT-I+placebo, CBT-I+armodafinil, armodafinil, and placebo, respectively. Mean (SD) age was 56 (10) years, 88% were female, 68% were treated for breast cancer, 90% were white, 95% were non-Hispanic or Latino, and mean time since last cancer treatment to intervention was 1292 days. Mean QOL at baseline was 77.0 (SD=16.6). As has been previously reported, these characteristics were not statistically significantly different among the four intervention groups at baseline [30].
Figure 1.
CONSORT diagram
Table 1.
Baseline characteristics of participants
| Characteristics | Total Sample (N = 95) | CBT-I + Placebo (N = 24) | CBT-I + Armodafinil (N = 23) | Armodafinil (N = 24) | Placebo (N = 24) | |
|---|---|---|---|---|---|---|
| Age | Mean (SD) | 56.2 (9.9) | 58.9 (9.9) | 56.3 (10.0) | 57.1 (7.4) | 52.8 (11.5) |
| Range | 26–75 | 30–74 | 36–73 | 43–75 | 26–69 | |
| Gender | Female | 84 (88.4%) | 21 (87.5%) | 22 (95.7%) | 23 (95.8%) | 18 (75.0%) |
| Male | 11 (11.6%) | 3 (12.5%) | 1 (4.3%) | 1 (4.2%) | 6 (25.0%) | |
| Race | White | 86 (90.5%) | 23 (95.8%) | 21 (91.3%) | 23 (95.8%) | 19 (79.2%) |
| African American | 8 (8.4%) | 1 (4.2%) | 2 (8.7%) | 1 (4.2%) | 4 (16.7%) | |
| Unknown | 1 (1.1%) | – | – | – | 1 (4.2%) | |
| Ethnicity | Non-Hispanic/Latino | 90 (94.7%) | 23 (95.8%) | 22 (95.7%) | 22 (91.7%) | 23 (95.8%) |
| Unknown | 5 (5.3%) | 1 (4.2%) | 1 (4.3%) | 2 (8.3%) | 1 (4.2%) | |
| Education | Bachelor’s or higher | 47 (49.5%) | 10 (41.7%) | 14 (60.9%) | 13 (54.2%) | 10 (41.7%) |
| Partial college training | 37 (38.9%) | 10 (41.7%) | 5 (21.7%) | 10 (41.7%) | 12 (50.0%) | |
| High school graduate or less | 10 (10.6%) | 4 (16.7%) | 3 (13.0%) | 1 (4.2%) | 2 (8.3%) | |
| Cancer Type | Breast Cancer | 65 (68.4%) | 16 (66.7%) | 17 (73.9%) | 17 (70.8%) | 15 (62.5%) |
| Blood Cancer | 9 (9.5%) | 4 (16.7%) | 1 (4.3%) | – | 4 (16.7%) | |
| Brain & Peripheral Nervous System Tumor | 2 (2.1%) | 1 (4.2%) | – | 1 (4.2%) | – | |
| Head & Neck Cancer | 6 (6.3%) | – | 4 (17.4%) | 2 (8.3%) | – | |
| Alimentary Track Cancer | 6 (6.3%) | – | 1 (4.3%) | 3 (12.5%) | 2 (8.3%) | |
| Genitourinary Tract Cancer | 3 (3.2%) | 1 (4.2%) | – | – | 2 (8.3%) | |
| Gynecologic Cancer | 3 (3.2%) | 1 (4.2%) | – | 1 (4.2%) | 1 (4.2%) | |
| Skin Cancer | 1 (1.1%) | 1 (4.2%) | – | – | – | |
| Previous Tx | Surgery | 11 (11.6%) | 3 (12.5%) | 2 (8.7%) | 5 (20.8%) | 1 (4.2%) |
| Chemotherapy | 76 (80.0%) | 17 (70.8%) | 17 (73.9%) | 22 (91.7%) | 20 (83.3%) | |
| Radiation Therapy | 71 (74.7%) | 19 (79.2%) | 18 (78.3%) | 17 (70.8%) | 17 (70.8%) | |
| Time | Last cancer Tx to intervention (days) | 1292 | 1587 | 1588 | 1358 | 647 |
| Range (days) | 48–10034 | 136–7071 | 48–10034 | 104–6115 | 112–1957 | |
| Insomniaa | Mean (SD) | 14.1 (4.8) | 14.4 (4.6) | 13.3 (5.8) | 13.6 (3.9) | 15.2 (5.0) |
| QOLb | Mean (SD) | 77.0 (16.6) | 78.9 (15.3) | 79.8 (17.8) | 77.4 (12.9) | 71.9 (19.7) |
NOTE: Data might not add to 100% because of rounding.
Abbreviations: SD, standard deviation; Tx, treatment; QOL, quality of life.
By Insomnia Severity Index.
By Functional Assessment of Cancer Therapy-General.
Effect of the intervention on QOL
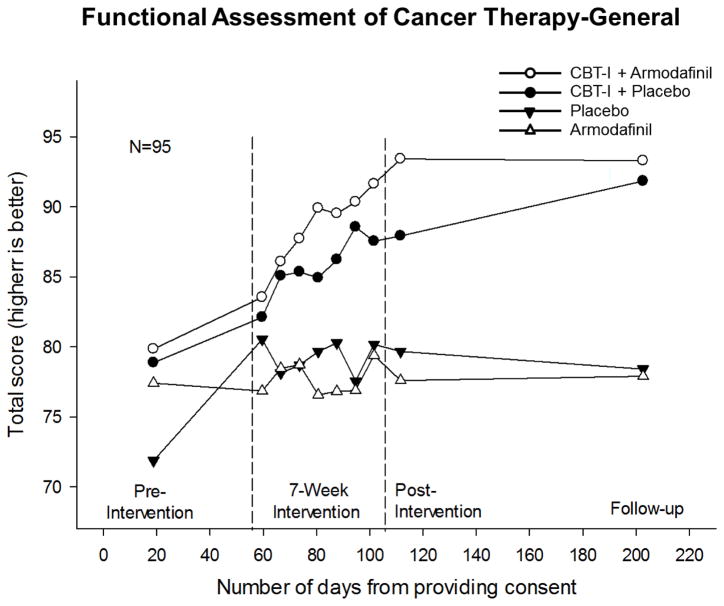
The unadjusted means for QOL from pre-intervention to follow-up for the four intervention groups are shown in Fig. 2. Post-intervention QOL means for CBT-I+placebo, CBT-I+armodafinil, armodafinil, and placebo (complete cases) were: 87.9 (SD=13.9), 93.4 (SD=13.5), 77.6 (SD=15.3), and 79.7 (SD=15.7), respectively. T-tests showed that there were no statistically significant differences between CBT-I+placebo and CBT-I+armodafinil (difference=5.5; SE=4.6; 95% CI=−3.9, 14.9; p=0.243) and between armodafinil and placebo (difference=2.1; SE=5.5; 95% CI=−9.1, 13.3; p=0.706). Change in QOL from pre- to post-intervention for the CBT-I+placebo, CBT-I+armodafinil, armodafinil, and placebo were: 9.6 (SE=1.8; 95% CI=5.7, 13.4; p<0.0001), 11.6 (SE=1.8; 95% CI=7.7, 15.5; p<0.0001), −0.2 (SE=3.2; 95% CI=−6.9, 6.6; p=0.964), and 3.3 (SE=2.0; 95% CI=−1.0, 7.6; p=0.124), respectively. The CBT-I by armodafinil interaction was not statistically significant for post-intervention QOL for both complete cases (p=0.160) and when using MI (p=0.100). ANCOVA for complete cases, while controlling for pre-intervention QOL, showed that CBT-I significantly improved QOL at post-intervention (estimate=9.5; 95% CI=5.3, 13.7; p<0.0001). This indicates that CBT-I led to a mean increase of 9.5 units i.e., improvement compared to no CBT-I. Armodafinil, on the other hand, had no significant effect on QOL at post-intervention (estimate=−0.1; 95% CI=−4.3, 4.1; p=0.976). Details on ANCOVA for complete cases and MI are provided in Table 2.
Figure 2.
Unadjusted mean QOL over time by intervention groups. Numbers per intervention group at beginning of intervention, post-intervention, and follow-up were as follows: cognitive behavioral therapy for insomnia (CBT-I)+placebo, 24, 18, and 16; CBT-I+armodafinil, 23, 17, and 16; armodafinil, 24, 15, and 14; and placebo, 24, 17, and 19, respectively.
Table 2.
Comparison of QOL at post-intervention
| Coefficient | Estimate | SE | P-value | 95% CI for Estimate | ES | 95% CI for ES |
|---|---|---|---|---|---|---|
| CBT-I (Yes-No) | ||||||
| Complete Case | 9.50 | 2.10 | <0.0001 | 5.30 to 13.70 | 0.57 | 0.08 to 1.06 |
| MI | 7.31 | 1.80 | <0.0001 | 3.73 to 10.90 | 0.44 | 0.03 to 0.85 |
| Armodafinil (Yes-No) | ||||||
| Complete Case | −0.06 | 2.10 | 0.976 | −4.26 to 4.13 | −0.004 | −0.48 to 0.48 |
| MI | −0.90 | 1.80 | 0.619 | −4.46 to 2.67 | −0.05 | −0.46 to 0.35 |
| CBT-I by Armodafinil interaction | ||||||
| Complete Case | 0.160 | |||||
| MI | 0.100 | |||||
NOTE: Analyses are presented as both complete case and using multiple imputation (MI) because of missing data. Estimates and associated statistics refer to adjusted differences between groups in mean change from pre-intervention. Effect size (ES) is standardized mean difference. 95% CI is the lower and upper 95 % confidence limits. P values denote improvements using ANCOVA while controlling for pre-intervention values.
Abbreviations: SE, standard error; QOL, quality of life.
Mean follow-up QOL for CBT-I+placebo, CBT-I+armodafinil, armodafinil, and placebo (complete cases) were: 91.4 (SD=15.0), 93.8 (SD=11.8), 78.7 (SD=15.3), and 78.6 (SD=20.6), respectively. A one-way ANOVA showed that there was no statistically significant difference between post-intervention and follow-up QOL (p=0.327).
Direct and Indirect Effects of CBT-I on QOL
The correlational analysis showed that changes in insomnia severity from pre- to post-intervention were significantly correlated with concurrent changes in QOL (r=−0.56, p<0.0001), indicating that an improvement in insomnia is associated with an improvement in QOL.
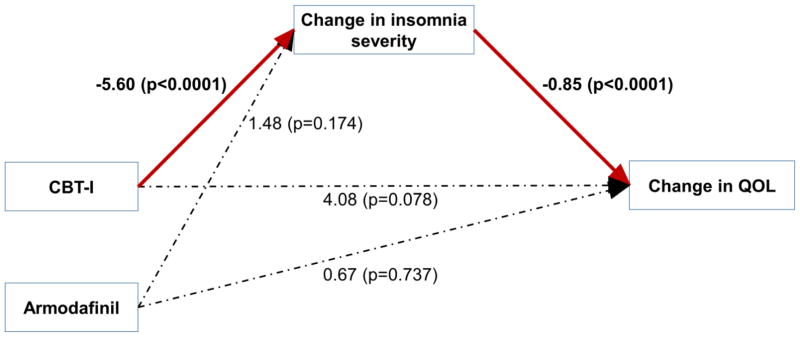
The path analysis (Fig. 3) showed that the only statistically significant direct effects (path coefficient, coef) were from CBT-I on the insomnia severity change score (coef=−5.60; p<0.0001) and from the insomnia severity change score on the QOL change score (coef=−0.85; p<0.0001). CBT-I did not have a significant direct effect on the QOL change score (coef=4.08; p=0.078). Armodafinil also did not have any significant direct effects on either the insomnia severity change score (coef=1.48; p=0.174) or QOL change score (coef=0.67; p=0.737). CBT-I had significant indirect effect on the QOL change score via the insomnia severity change score (coef=4.78; p=0.002). This indicates that CBT-I has only indirect beneficial effects on QOL, through its positive effect on insomnia severity. The path model fit the data very well with root mean square error of approximation (RMSEA) <0.0001. We note that a RMSEA <0.1 is considered ideal.
Figure 3.
Path diagram for the structural equation model. Changes in insomnia severity and QOL are from pre- to post-intervention. Statistically significant paths are in red with relevant path coefficients marked (p≤0.05). Dotted lines indicate non-significant paths.
Discussion
We have previously reported that our 7-week CBT-I treatment program was effective in improving insomnia severity and sleep quality [30], sleep continuity [34], and fatigue [35] in cancer survivors suffering from chronic insomnia. The goal of this secondary aim of the study was to assess the ability of CBT-I to improve QOL in cancer survivors and determine whether positive effects of CBT-I on QOL were mediated by decreased insomnia severity. This understanding is particularly important considering that QOL is often diminished in cancer survivors having chronic insomnia and for assessing whether symptom specific interventions have the potential to improve QOL.
The results from our study indicate that survivors treated with CBT-I showed a clinically and statistically significant improvement in QOL at post-intervention as assessed by the self-reported questionnaire, FACT-G, compared with those who were not treated with CBT-I. Armodafinil did not enhance the potency of CBT-I nor did it have any effects on QOL. CBT-I improved QOL by 9.5 points (95% CI=5.3, 13.7) in these participants, which is more than double the recommended CID for the FACT-G [32]. Thus, it is promising that an insomnia intervention, namely CBT-I, resulted in clinical improvement in QOL.
Our findings support previous studies that have shown a secondary benefit of CBT-I in improving QOL in cancer survivors. The two largest of these were one study in 57 breast cancer survivors with chronic insomnia showing CBT-I compared to waitlist controls improved QOL in addition to ameliorating subjective sleep complaints, with these effects being maintained up to 12 months [19], and another study in cancer survivors also showing CBT-I participants had increased physical and functional QOL relative to treatment as usual at post-treatment and 6-month follow-up [20].
By contrast, a study of breast cancer survivors assigned to both the CBT-I and sleep education and hygiene control group had improved QOL for both groups, suggesting that sleep education may also have a short-term impact on QOL [28]. Another study of 242 breast cancer survivors also found that QOL significantly improved equally over time in all three groups of professionally administered CBT-I, video-based CBT-I, and no treatment group, with no significant differences between the groups [29]. In this study, however, only results on the global EORTC-QOL scale was reported, an index that may not have been sensitive enough to detect between group differences. Ritterband et. al. [36] also did not find a significant improvement in QOL with online CBT-I program compared to waitlist controls. Similarly, Matthews et al. [21] did not find a significant improvement in QOL with CBT-I compared to behavioral placebo treatment, although the authors suggested that this failure might have been due to the participants’ relatively good QOL at baseline, which provided little room for improvement. Mean QOL for our sample at baseline (i.e., 77 on FACT-G) was lower than that found in the US general population at 80.1 [37], indicating that for our study, survivors with insomnia problems had reduced QOL.
Another important finding of our study was that improvement in QOL observed in this study was due to the reduction in insomnia severity because of CBT-I and not due to the direct effects on CBT-I on QOL, and that improvement in QOL from pre to post-intervention was significantly correlated with concurrent reduction in insomnia. This finding is consistent with a study of 72 breast cancer survivors receiving CBT-I which also found through mediational analysis that the intervention did not have a direct effect on psychosocial outcomes, such as QOL, although they did not assess the indirect effect of the intervention on QOL [28].
We also found that the improvement in QOL remained higher when assessed at 3 months after the completion of CBT-I, which is consistent with previous studies showing maintenance of CBT-I treatment gains on QOL up to a year after the intervention [19, 20]. The long term benefits of CBT-I in this study may be due to the fact that because of this therapy, the individual has learned coping skills to manage acute insomnia as well as to prevent or reduce future insomnia episodes [11].
Armodafinil, a medication indicated for promoting wakefulness in order to decrease daytime sleepiness associated with sleep disorders, including narcolepsy, sleep apnea syndrome, and shiftwork disorder [38, 39], had no significant benefits on QOL. In our published manuscript on the primary study aim for insomnia [30], we also saw no effect of armodafinil on insomnia or sleep quality. This lack of significant effect is consistent with previous trials that have also demonstrated no effect of armodafinil on QOL in patients with cancer, even for doses as high as 400 mg/day [40–43]. This might be due to the fact that it may be difficult to improve insomnia beyond the effects produced by CBT-I, as several well-conducted RCTs have indicated that CBT-I produces robust clinical improvements in cancer-related sleep problems [18].
Our study includes several strengths, namely a randomized clinical trial, multi-center study, inclusion of patients with clinically diagnosed insomnia disorder at baseline, use of reliable and validated patient-reported measures, longitudinal data with 3-month follow-up, and the use of intention-to treat analyses. Conversely, the following limitations should also be considered when interpreting the study results. Participants were predominantly white women of non-Hispanic ethnicity which may limit the generalization of findings to men and to those with different racial and ethnic backgrounds. Further, this study had a small sample size, and the reduction of insomnia severity and the improvement of QOL were measured at the same time; thus, limiting firm conclusions about the directionality of the relationship. This study nonetheless has several important clinical implications. It provided additional evidence of the link between insomnia and reduced QOL, suggesting the importance of screening for clinical insomnia in cancer survivors, as survivors who have clinical insomnia may also experience poor QOL. More importantly, this study suggests that CBT-I is not only effective in treating insomnia, but that reducing insomnia has significant beneficial effects on QOL in cancer survivors; thus, highlighting the importance of offering insomnia interventions on a more routine basis to cancer survivors.
Conclusion
Given the high prevalence of insomnia in cancer survivors and its profound impact on QOL, interventions targeting insomnia could be a promising treatment for improving QOL. Our study provides evidence that CBT-I treatment is both clinically efficacious and durable for improving QOL in cancer survivors with chronic insomnia and strongly supports its use. This beneficial effect on QOL appears to be due to the reduction of insomnia severity induced by CBT-I. Armodafinil, on the other hand, did not have a detectable effect on QOL nor on the efficacy of CBT-I. Future research should focus on screening for clinical insomnia and increasing awareness and access to evidence-based non-pharmacologic insomnia interventions in order to improve cancer care.
Acknowledgments
Funding
This study was supported by the NCI grants 5 R01 CA126968, 2 R25 CA102618-01A1 and UG1 CA189961. Study medication was provided by Teva Pharmaceuticals, USA.
Footnotes
Compliance of ethical standards
Conflict of interest
Dr. Perlis receives royalties for a variety of educational materials related to CBT-I; has received grant support for CBT-I related studies from Cephalon, Teva, and Sanofi-Aventis; has done consulting activities related to CBT-I from Lumosity, Sleep Easily, InsomniSolv, New York University, University of Buffalo and UCSD; and has received honoraria for multiple speaking engagements related to CBT-I. The remaining authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2016–2017. Atlanta: American Cancer Society; 2016. [Google Scholar]
- 2.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health, and disability. The journals of gerontology Series A, Biological sciences and medical sciences. 2003;58(1):82–91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 3.Cella DF, Tulsky DS. Quality of life in cancer: definition, purpose, and method of measurement. Cancer Invest. 1993;11(3):327–36. doi: 10.3109/07357909309024860. [DOI] [PubMed] [Google Scholar]
- 4.Trentham-Dietz A, Sprague BL, Klein R, Klein BE, Cruickshanks KJ, Fryback DG, et al. Health-related quality of life before and after a breast cancer diagnosis. Breast cancer research and treatment. 2008;109(2):379–87. doi: 10.1007/s10549-007-9653-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pockaj BA, Degnim AC, Boughey JC, Gray RJ, McLaughlin SA, Dueck AC, et al. Quality of life after breast cancer surgery: What have we learned and where should we go next? J Surg Oncol. 2009;99(7):447–55. doi: 10.1002/jso.21151. [DOI] [PubMed] [Google Scholar]
- 6.Weaver KE, Forsythe LP, Reeve BB, Alfano CM, Rodriguez JL, Sabatino SA, et al. Mental and physical health-related quality of life among U.S. cancer survivors: population estimates from the 2010 National Health Interview Survey. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21(11):2108–17. doi: 10.1158/1055-9965.EPI-12-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L, Fiorentino L, Rissling M, Natarajan L, Parker BA, Dimsdale JE, et al. Decreased health-related quality of life in women with breast cancer is associated with poor sleep. Behav Sleep Med. 2013;11(3):189–206. doi: 10.1080/15402002.2012.660589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lis CG, Gupta D, Grutsch JF. The relationship between insomnia and patient satisfaction with quality of life in cancer. Support Care Cancer. 2008;16(3):261–6. doi: 10.1007/s00520-007-0314-z. [DOI] [PubMed] [Google Scholar]
- 9.Fortner BV, Stepanski EJ, Wang SC, Kasprowicz S, Durrence HH. Sleep and quality of life in breast cancer patients. J Pain Symptom Manage. 2002;24(5):471–80. doi: 10.1016/s0885-3924(02)00500-6. [DOI] [PubMed] [Google Scholar]
- 10.Clevenger L, Schrepf A, Degeest K, Bender D, Goodheart M, Ahmed A, et al. Sleep disturbance, distress, and quality of life in ovarian cancer patients during the first year after diagnosis. Cancer. 2013;119(17):3234–41. doi: 10.1002/cncr.28188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiorentino L, Ancoli-Israel S. Insomnia and its treatment in women with breast cancer. Sleep Med Rev. 2006;10(6):419–29. doi: 10.1016/j.smrv.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savard J, Villa J, Ivers H, Simard S, Morin CM. Prevalence, natural course, and risk factors of insomnia comorbid with cancer over a 2-month period. J Clin Oncol. 2009;27(31):5233–9. doi: 10.1200/JCO.2008.21.6333. [DOI] [PubMed] [Google Scholar]
- 13.Savard J, Simard S, Blanchet J, Ivers H, Morin CM. Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep. 2001;24(5):583–90. doi: 10.1093/sleep/24.5.583. [DOI] [PubMed] [Google Scholar]
- 14.Davidson JR, MacLean AW, Brundage MD, Schulze K. Sleep disturbance in cancer patients. Social science & medicine. 2002;54(9):1309–21. doi: 10.1016/s0277-9536(01)00043-0. [DOI] [PubMed] [Google Scholar]
- 15.Savard J, Ivers H, Villa J, Caplette-Gingras A, Morin CM. Natural course of insomnia comorbid with cancer: an 18-month longitudinal study. J Clin Oncol. 2011;29(26):3580–6. doi: 10.1200/JCO.2010.33.2247. [DOI] [PubMed] [Google Scholar]
- 16.Gooneratne NS, Dean GE, Rogers AE, Nkwuo JE, Coyne JC, Kaiser LR. Sleep and quality of life in long-term lung cancer survivors. Lung Cancer. 2007;58(3):403–10. doi: 10.1016/j.lungcan.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickerson SS, Connors LM, Fayad A, Dean GE. Sleep-wake disturbances in cancer patients: narrative review of literature focusing on improving quality of life outcomes. Nat Sci Sleep. 2014;6:85–100. doi: 10.2147/NSS.S34846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garland SN, Johnson JA, Savard J, Gehrman P, Perlis M, Carlson L, et al. Sleeping well with cancer: a systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatr Dis Treat. 2014;10:1113–24. doi: 10.2147/NDT.S47790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: Sleep and psychological effects. J Clin Oncol. 2005;23(25):6083–96. doi: 10.1200/JCO.2005.09.548. [DOI] [PubMed] [Google Scholar]
- 20.Espie CA, Fleming L, Cassidy J, Samuel L, Taylor LM, White CA, et al. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol. 2008;26(28):4651–8. doi: 10.1200/JCO.2007.13.9006. [DOI] [PubMed] [Google Scholar]
- 21.Matthews EE, Berger AM, Schmiege SJ, Cook PF, McCarthy MS, Moore CM, et al. Cognitive behavioral therapy for insomnia outcomes in women after primary breast cancer treatment: a randomized, controlled trial. Oncol Nurs Forum. 2014;41(3):241–53. doi: 10.1188/14.ONF.41-03AP. [DOI] [PubMed] [Google Scholar]
- 22.Garland SN, Carlson LE, Stephens AJ, Antle MC, Samuels C, Campbell TS. Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: a randomized, partially blinded, noninferiority trial. J Clin Oncol. 2014;32(5):449–57. doi: 10.1200/JCO.2012.47.7265. [DOI] [PubMed] [Google Scholar]
- 23.Davidson JR, Feldman-Stewart D, Brennenstuhl S, Ram S. How to provide insomnia interventions to people with cancer: insights from patients. Psychooncology. 2007;16(11):1028–38. doi: 10.1002/pon.1183. [DOI] [PubMed] [Google Scholar]
- 24.Perlis ML, Jungquist C, Smith MT, Posner D. Cognitive behavioral tretament of insomnia: a session-by-session guide. Springer; New York: 2005. [Google Scholar]
- 25.National Institutes of H. National Institutes of Health State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults, June 13–15, 2005. Sleep. 2005;28(9):1049–57. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- 26.Johnson JA, Rash JA, Campbell TS, Savard J, Gehrman PR, Perlis M, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev. 2016;27:20–8. doi: 10.1016/j.smrv.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Quesnel C, Savard J, Simard S, Ivers H, Morin CM. Efficacy of cognitive-behavioral therapy for insomnia in women treated for nonmetastatic breast cancer. Journal of consulting and clinical psychology. 2003;71(1):189–200. [PubMed] [Google Scholar]
- 28.Dirksen SR, Epstein DR. Efficacy of an insomnia intervention on fatigue, mood and quality of life in breast cancer survivors. J Adv Nurs. 2008;61(6):664–75. doi: 10.1111/j.1365-2648.2007.04560.x. [DOI] [PubMed] [Google Scholar]
- 29.Savard J, Ivers H, Savard MH, Morin CM. Is a video-based cognitive behavioral therapy for insomnia as efficacious as a professionally administered treatment in breast cancer? Results of a randomized controlled trial. Sleep. 2014;37(8):1305–14. doi: 10.5665/sleep.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roscoe JA, Garland SN, Heckler CE, Perlis ML, Peoples AR, Shayne M, et al. Randomized placebo-controlled trial of cognitive behavioral therapy and armodafinil for insomnia after cancer treatment. J Clin Oncol. 2015;33(2):165–71. doi: 10.1200/JCO.2014.57.6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brucker PS, Yost K, Cashy J, Webster K, Cella D. General population and cancer patient norms for the Functional Assessment of Cancer Therapy-General (FACT-G) Eval Health Prof. 2005;28(2):192–211. doi: 10.1177/0163278705275341. [DOI] [PubMed] [Google Scholar]
- 32.Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24(6):547–61. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 33.Savard MH, Savard J, Simard S, Ivers H. Empirical validation of the Insomnia Severity Index in cancer patients. Psychooncology. 2005;14(6):429–41. doi: 10.1002/pon.860. [DOI] [PubMed] [Google Scholar]
- 34.Garland SN, Roscoe JA, Heckler CE, Barilla H, Gehrman P, Findley JC, et al. Effects of armodafinil and cognitive behavior therapy for insomnia on sleep continuity and daytime sleepiness in cancer survivors. Sleep Med. 2016;20:18–24. doi: 10.1016/j.sleep.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heckler CE, Garland SN, Peoples AR, Perlis ML, Shayne M, Morrow GR, et al. Cognitive behavioral therapy for insomnia, but not armodafinil, improves fatigue in cancer survivors with insomnia: a randomized placebo-controlled trial. Support Care Cancer. 2016;24(5):2059–66. doi: 10.1007/s00520-015-2996-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ritterband LM, Bailey ET, Thorndike FP, Lord HR, Farrell-Carnahan L, Baum LD. Initial evaluation of an Internet intervention to improve the sleep of cancer survivors with insomnia. Psychooncology. 2012;21(7):695–705. doi: 10.1002/pon.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pearman T, Yanez B, Peipert J, Wortman K, Beaumont J, Cella D. Ambulatory cancer and US general population reference values and cutoff scores for the functional assessment of cancer therapy. Cancer. 2014;120(18):2902–9. doi: 10.1002/cncr.28758. [DOI] [PubMed] [Google Scholar]
- 38.Randomized trial of modafinil as a treatment for the excessive daytime somnolence of narcolepsy: US Modafinil in Narcolepsy Multicenter Study Group. Neurology. 2000;54(5):1166–75. doi: 10.1212/wnl.54.5.1166. [DOI] [PubMed] [Google Scholar]
- 39.Perlis ML, Smith MT, Orff H, Enright T, Nowakowski S, Jungquist C, et al. The effects of modafinil and cognitive behavior therapy on sleep continuity in patients with primary insomnia. Sleep. 2004;27(4):715–25. doi: 10.1093/sleep/27.4.715. [DOI] [PubMed] [Google Scholar]
- 40.Lee EQ, Muzikansky A, Drappatz J, Kesari S, Wong ET, Fadul CE, et al. A randomized, placebo-controlled pilot trial of armodafinil for fatigue in patients with gliomas undergoing radiotherapy. Neuro Oncol. 2016;18(6):849–54. doi: 10.1093/neuonc/now007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spathis A, Fife K, Blackhall F, Dutton S, Bahadori R, Wharton R, et al. Modafinil for the treatment of fatigue in lung cancer: results of a placebo-controlled, double-blind, randomized trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32(18):1882–8. doi: 10.1200/JCO.2013.54.4346. [DOI] [PubMed] [Google Scholar]
- 42.Berenson JR, Yellin O, Shamasunder HK, Chen CS, Charu V, Woliver TB, et al. A phase 3 trial of armodafinil for the treatment of cancer-related fatigue for patients with multiple myeloma. Support Care Cancer. 2015;23(6):1503–12. doi: 10.1007/s00520-014-2486-7. [DOI] [PubMed] [Google Scholar]
- 43.Boele FW, Douw L, de Groot M, van Thuijl HF, Cleijne W, Heimans JJ, et al. The effect of modafinil on fatigue, cognitive functioning, and mood in primary brain tumor patients: a multicenter randomized controlled trial. Neuro Oncol. 2013;15(10):1420–8. doi: 10.1093/neuonc/not102. [DOI] [PMC free article] [PubMed] [Google Scholar]