Abstract
Trace amine-associated receptor 1 (TAAR1) agonists have been shown to have procognitive, antipsychotic-like, anxiolytic, weight-reducing, glucose-lowering, and wake-promoting activities. We used Taar1 knockout (KO) and overexpressing (OE) mice and TAAR1 agonists to elucidate the role of TAAR1 in sleep/wake. EEG, EMG, body temperature (Tb), and locomotor activity (LMA) were recorded in Taar1 KO, OE, and WT mice. Following a 24 h recording to characterize basal sleep/wake parameters, mice were sleep deprived (SD) for 6 h. In another experiment, mice were given three doses of the TAAR1 partial agonist RO5263397, caffeine, or vehicle p.o. Baseline wakefulness was modestly increased in OE compared with WT mice. Baseline theta (4.5–9 Hz) and low gamma (30–60 Hz) activity was elevated in KO compared with OE mice in NREM and REM sleep. Following SD, both KO and OE mice exhibited a homeostatic sleep rebound. In WT mice, RO5263397 increased waking and reduced NREM and REM sleep, decreased gamma power during wake and NREM, and decreased Tb without affecting LMA; these effects were absent in KO mice and potentiated in OE mice. In contrast, caffeine increased wake time, NREM gamma power, and LMA in all strains compared with vehicle; this effect was attenuated in KO and potentiated in OE mice. TAAR1 overexpression modestly increases wakefulness, whereas TAAR1 partial agonism increases wakefulness and also reduces NREM and also REM sleep. These results indicate a modulatory role for TAAR1 in sleep/wake and cortical activity and suggest TAAR1 as a novel target for wake-promoting therapeutics.
Introduction
Trace amines (TAs) are endogenous amino acid metabolites structurally similar to the biogenic amines (Burchett and Hicks, 2006; Grandy, 2007). TAs such as β-phenylethylamine, tyramine, octopamine, and tryptamine are synthesized in neuronal terminals from the same amino acid precursors as dopamine (DA), noradrenaline (NE), and serotonin (5-hydroxytryptamine (5-HT)) by aromatic-L-amino acid decarboxylase (AADC; EC 4.1.1.28), are colocalized and coreleased with biogenic amine transmitters, and are rapidly metabolized by monoamine oxidase (MAO; EC 1.4.3.4) A and B. In 2001, two groups (Borowsky et al, 2001; Bunzow et al, 2001) described a family of mammalian G protein-coupled receptors (GPCRs) that are activated by TAs and amphetamine in vitro. Now known as trace amine-associated receptors (TAARs), all but TAAR1 are expressed in the olfactory epithelium in mammals where they are thought to constitute a substrate for detection of volatile amines (Liberles, 2009; Liberles and Buck, 2006).
TAAR1 is expressed in limbic and monoaminergic systems, including the ventral tegmental area (VTA), dorsal raphe nucleus (DRN), amygdala, preoptic area, hypothalamus, and nucleus tractus solitarius (Lindemann et al, 2008). In monoaminergic neurons, TAAR1 tonically activates inwardly rectifying K+ channels to reduce basal firing activity (Bradaia et al, 2009; Revel et al, 2011). Taar1 knockout (KO) mice appear similar to wild-type (WT) littermates in most neurological and behavioral analyses (Lindemann et al, 2008; Wolinsky et al, 2007), except that the spontaneous firing rate of VTA dopaminergic and DRN serotonergic neurons is greatly increased (Revel et al, 2011). When challenged with d-amphetamine, Taar1 KO mice show enhanced hyperlocomotion and striatal monoamine release (Lindemann et al, 2008; Wolinsky et al, 2007). In contrast, brain TAAR1 overexpression results in attenuated hyperlocomotion and striatal DA release following amphetamine (Revel et al, 2012a). TAAR1 thus negatively modulates monoaminergic neurotransmission.
Selective TAAR1 full and partial agonists have procognitive, stress-reducing, and antipsychotic-like properties in rodents and non-human primates (Revel et al, 2012b, 2013). More recent studies have implicated TAAR1 in addiction (Pei et al, 2014, 2015) and metabolic regulation (Raab et al, 2016). TAAR1 partial agonists also dose-dependently increase wakefulness in rats, suggesting a role for TAAR1 in regulating wakefulness (Revel et al, 2012b, 2013).
Endogenous TAAR1 tone appears critical for normal regulation of monoaminergic neuronal activity (Lindemann et al, 2008; Revel et al, 2011, 2012a). Thus, TAAR1 knockout or overexpression may alter spontaneous sleep/wake patterns or EEG spectra, as has been shown in other models of monoamine dysregulation (Dzirasa et al, 2006, 2009; Wisor et al, 2001). We first assessed the functions of endogenous TAAR1 in regulating sleep/wake by characterizing basal and sleep-deprived sleep/wake physiology and EEG spectra in Taar1 KO and overexpressing (OE) mice. We then asked whether wakefulness induced by TAAR1 partial agonists is mediated specifically via TAAR1 by comparing the response to RO5263397 in Taar1 KO, OE, and WT mice.
Materials and methods
For additional methodological details, please see Supplementary Information.
Animals
Adult, 4–5-month-old male Taar1 KO mice (n=13), Taar1 OE mice (n=7), and their WT littermates (n=10 and 7, respectively) were maintained on a pure C57BL/6 background. Generation and breeding of these mice has been previously described (Lindemann et al, 2008; Revel et al, 2012a). All studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at SRI International.
EEG/EMG, LMA, and Tb Recording and Analysis
Mice were instrumented for tethered EEG and EMG recording and implanted with intraperitoneal telemetry for monitoring locomotor activity (LMA) and core body temperature (Tb). EEG/EMG data were continuously recorded using iox2 (EMKA Technologies, France) on a PC. Surgical procedures, recording, and analysis have been described previously (Fisher et al, 2013, 2016).
Experimental Protocols
Experiment 1: basal sleep/wake physiology
EEG, EMG, LMA, and Tb were recorded for 24 h in Taar1 KO, Taar1 OE, and WT mice.
Experiment 2: sleep deprivation
Starting at ZT0 (Zeitgeber time 0=lights on and ZT12=lights off), mice were sleep deprived (SD) from ZT0 to ZT6, and then left undisturbed to recover. EEG/EMG were recorded continuously.
Experiment 3
TAAR1 agonism. All mice received p.o. RO5263397 (0.1, 0.3, and 1 mg/kg), Caf (10 mg/kg), or vehicle (0.3% Tween-80) in the mid-light phase (ZT6) in balanced order with at least 3 days between treatments. Mice were acclimated to oral dosing with vehicle (Veh) for at least 3 days before data collection.
Statistical Analyses
Baseline sleep was analyzed by two-way mixed-factor ANOVA comparing genotype and time, or one-way ANOVA comparing genotype. 60-Hz noise was filtered out of spectral analyses by removing all values between 59.8 and 60.2 Hz. Baseline spectral power for KO and OE mice was normalized to the WT group average and evaluated using t-tests for each 1 Hz frequency bin. Total power for each of 6 frequency bands (delta, 0.5–4 Hz; theta, 4–9 Hz; alpha, 9–12 Hz; beta, 12–30 Hz; low gamma, 30–59.8 Hz; high gamma, 60.2–100 Hz) was compared via ANOVAs. Drug efficacy was evaluated within genotypes using two-way within-subjects ANOVAs comparing drug treatment and time, and between genotypes using two-way mixed-factor ANOVAs comparing genotype and drug treatment. Positive ANOVA results were followed by post hoc Bonferroni t-tests where appropriate. Bout architecture (Supplementary Figure S1) was analyzed within genotype using two-way within-subjects ANOVAs comparing drug treatment and bout duration; positive results were followed by planned comparisons of drug treatment vs Veh within each bout duration category.
Results
Baseline Physiological Recordings
Sleep/wake distribution and amounts
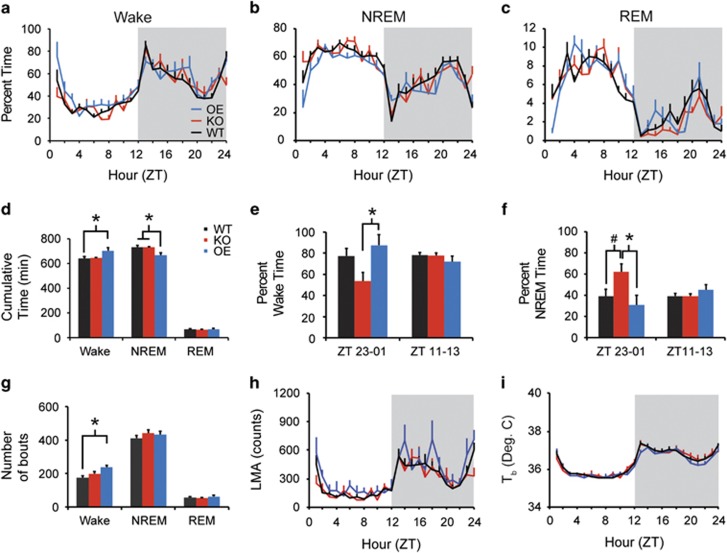
All genotypes exhibited robust daily sleep/wake rhythms (Figure 1a–c). OE mice spent more time awake across the 24 h period than WT (Figure 1d; main effect of genotype, F2, 24=4.044, p=0.031), and less time in NREM sleep than KO or WT mice (Figure 1d; main effect of genotype, F2, 24=4.913, p=0.016), decreasing the ratio of total sleep time (NREM+REM time) to wakefulness in OE mice compared with WT (F2, 24=4.459, p=0.023). There were no effects of genotype on baseline REM sleep time, or on the ratio of NREM to REM sleep time. OE mice had more frequent wake bouts than WT (Figure 1g; main effect of genotype, F2, 24=6.970, p=0.004). There were no effects on wake bout duration, NREM bout number or duration, or REM bout number or duration (Figure 1g).
Figure 1.
Taar1 OE mice have more wakefulness than WT mice. Taar1 KO and Taar1 OE mice exhibited robust diurnal rhythms in wake (a), NREM (b), and REM (c) sleep in a 24 h undisturbed baseline recording. Across the 24 h period, Taar1 OE mice had more total wakefulness and less NREM sleep than WT mice (d) as well as more bouts of wakefulness (g). During the 2 h around the lights-on transition (ZT23–ZT01), Taar1 OE mice had more wake than Taar1 KO mice (e) and Taar1 KO mice had more NREM sleep than either of the other strains at this time of day (f). The diurnal distribution of LMA (h) and Tb (i) did not differ across strains. N=8 KO, 7 OE, and 12 WT mice; *p<0.05, #p<0.06.
Despite the absence of a significant genotype–time interaction, KO mice exhibited large differences in wake and NREM time compared with WT and OE mice around the lights-on transition (ZT23–ZT01; Figure 1a and b). KO mice spent less time awake than OE mice from ZT23 to ZT01 (Figure 1e; F2, 24=4.368, p=0.024) and more time in NREM sleep than OE and WT mice over the same span (Figure 1f; F2, 24=4.727, p=0.019), without comparable effects around light offset. Thus, OE mice spent more time awake than WT over 24 h, whereas KO mice exhibited increased NREM sleep around lights-on time compared with WT and OE mice.
Baseline LMA and Tb
All three genotypes exhibited robust daily LMA and Tb rhythms (Figure 1h and i). There were no effects of genotype on basal LMA or Tb.
Power spectral analysis
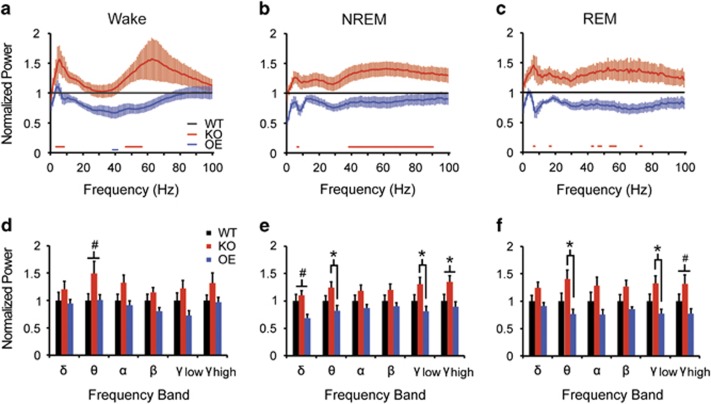
EEG spectral power during wakefulness was generally elevated in KO mice compared with WT, with significant increases between 3 and 8 Hz and from 46 to 56 Hz (Figure 2a; red bars at bottom of plot). In contrast, spectral power was generally reduced in OE mice compared with WT, with significant decreases between 38 and 41 Hz (blue bar at bottom of plot). NREM spectral power was increased in KO mice compared with WT at 6 Hz and from 38 to 90 Hz, with no significant differences between OE and WT mice (Figure 2b). EEG spectral power during REM sleep was increased in KO mice compared with WT at 6–7 Hz, 17 Hz, and intermittently between 42 and 55 Hz (Figure 2c), with no significant differences between OE and WT mice. Thus, elimination of TAAR1 increased spectral power independent of arousal state, whereas TAAR1 overexpression reduced spectral power during wakefulness.
Figure 2.
TAAR1 negatively regulates EEG theta and gamma power. (a–c) EEG spectral power is increased during wake (a), NREM (b), and REM sleep (c) in Taar1 KO mice, whereas it is attenuated in Taar1 OE mice relative to WT. Red and blue bars below traces indicate significant differences vs WT littermates (t-test, p<0.05). (d–f) Genotype effects on spectral composition were most pronounced in the theta (4–9 Hz) and gamma (low: 30–60 Hz; high: 60–100 Hz) bands in all states. N=8 KO, 7 OE, and 12 WT mice; *p<0.05, #p<0.06.
Total spectral power was analyzed for discrete power bands within each state. Consistent with the above, EEG theta power was elevated in KO compared with OE mice in NREM (Figure 2e; F2, 24=3.430, p=0.049) and REM sleep (Figure 2f; F2, 24=4.111, p=0.029); WT theta power was intermediate between these strains. Theta power during wakefulness trended toward an increase in KO compared with OE and WT mice (Figure 2d; F2, 24=3.259, p=0.056). Low gamma power was significantly greater in KO compared with OE mice during NREM (Figure 2e; F2, 24=3.854, p=0.035) and REM sleep (Figure 2f; F2, 24=3.650, p=0.041). ANOVA also revealed genotypic effects on high gamma power during NREM sleep (Figure 2e; F2, 24=3.587, p=0.043) and a trend during REM sleep (Figure 2f; F2, 24=3.287, p=0.055). Although post hoc tests were not significant for high gamma power in NREM sleep, elevated power in KO compared with OE mice was evident. There was no genotype effect on low or high gamma power in waking. There was a trend toward decreased NREM delta power in OE mice compared with WT and KO mice (Figure 2e; F2, 24=3.304, p=0.054) and a trend towards altered beta power in REM sleep (Figure 2f; F2, 24=3.103, p=0.063). Thus, theta and gamma power were elevated in Taar1 KO mice compared with OE mice, particularly in NREM and REM sleep.
Response to SD
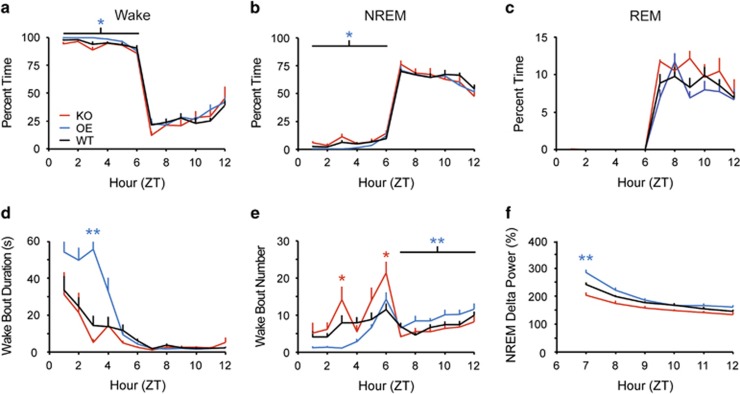
SD was effective in all genotypes, resulting in 94.9±1.2% wakefulness during the 6 h SD in WT, 92.2±1.7% wakefulness in KO, and 97.2±0.5% wakefulness in OE mice (Figure 3a). REM sleep was totally eliminated in all genotypes (Figure 3c). However, during SD, OE mice exhibited more waking (Figure 3a; F2, 19=3.810, p=0.041) and less NREM sleep (Figure 3b; F2, 19=3.601, p=0.047) than KO mice, longer wake bouts than WT and KO mice (Figure 3d; F10, 95=3.597, p<0.001), and fewer wake bouts than KO mice at ZT3 (Figure 3e; F10, 95=3.397, p<0.001). OE mice thus maintained more consolidated wakefulness during SD than either KO or WT mice.
Figure 3.
During a 6 h sleep deprivation (SD), Taar1 OE mice had more wakefulness (a) and decreased NREM sleep time (b) compared with Taar1 KO mice; REM sleep (c) did not differ across strains. The average wake bout duration during the SD was longer in Taar1 OE mice (d). Taar1 KO mice had more wake bouts during SD, whereas Taar1 OE mice had more wake bouts during recovery (e). Although recovery sleep time was unaffected by genotype (b), normalized NREM delta power during the first hour of recovery was increased in Taar1 OE mice compared with Taar1 KO and WT mice (f). N=5 KO, 7 OE, and 10 WT mice; *p<0.05 vs Taar1 KO mice, **p<0.05 vs WT and Taar1 KO mice.
Following SD, all mice exhibited a modest rebound of 110–112% of baseline NREM sleep (Figure 3b) and 113–135% of baseline REM sleep, with no effect of genotype on wake, NREM, or REM sleep time in the 6 h following SD. However, OE mice exhibited more fragmented wakefulness during recovery as reflected by an increased number of wake bouts (Figure 3e; F2, 19=7.134, p=0.005), suggesting that OE mice sustain waking ‘drive' even in the presence of a sleep debt.
NREM delta power, an index of homeostatic sleep pressure, was increased in OE compared with KO and WT mice during the first hour of recovery sleep (Figure 3f; F10, 95=7.662, p<0.001). Thus, OE mice maintained increased wakefulness and accrued a greater sleep debt during SD compared with WT and KO mice but, paradoxically, had more intrusions of wakefulness during the recovery period.
Response to TAAR1 Agonism
WT mice
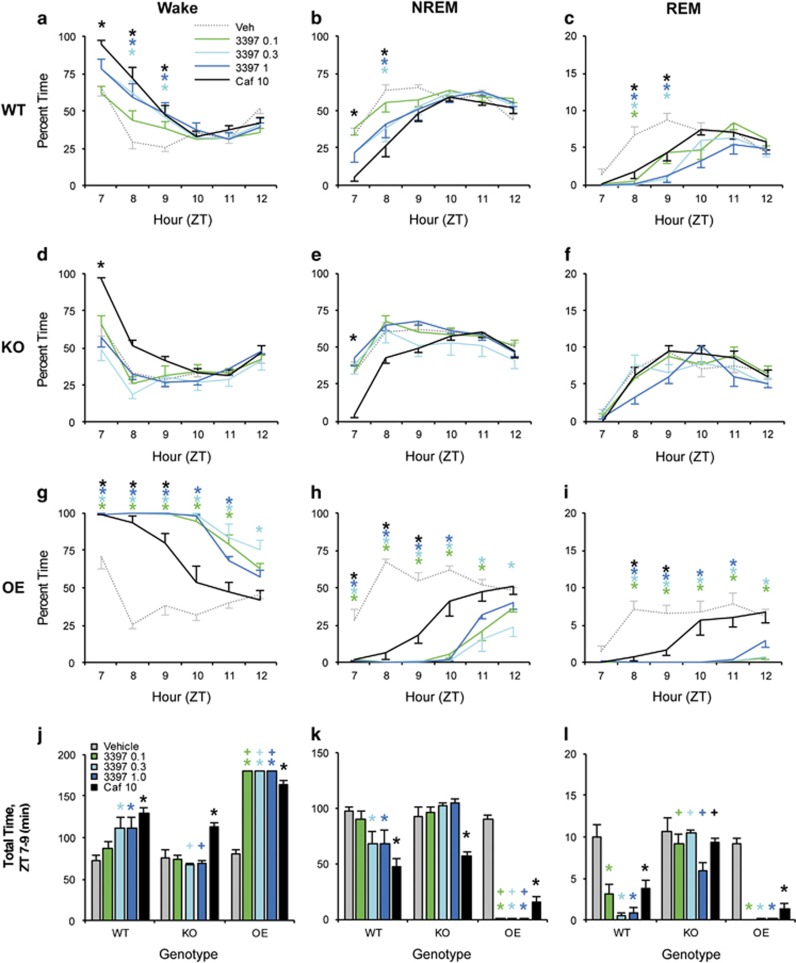
RO5263397 (0.3 and 1.0 mg/kg) increased wake time compared with Veh during ZT8–9 (Figure 4a; F20, 220=5.735, p<0.001). This increase did not differ from that produced by caffeine (Caf); however, only Caf significantly increased waking in the first hour after dosing (ZT7). RO5263397 (0.3 and 1.0 mg/kg) also decreased NREM sleep compared with Veh at ZT8 (Figure 4b; F20, 220=5.288, p<0.001). As with wake time, Caf exhibited a faster onset of action than RO5263397, but NR time from ZT8 to ZT9 did not differ between 0.3 mg/kg, 1.0 mg/kg, and Caf. All concentrations of RO5263397 suppressed REM sleep for 2–3 h after dosing compared with Veh (Figure 4c; F20, 220=3.826, p<0.001) and did not differ from Caf. There were no effects of drug treatment on wake, NREM, or REM sleep time from ZT10 to ZT12.
Figure 4.
The wake-promoting and REM-inhibiting effects of RO5263397 are TAAR1 dependent. RO5263397 increased wake time (a) and decreased NREM (b) and REM (c) sleep time vs Veh in WT mice following dosing at ZT6. RO5263397 did not affect sleep or waking in Taar1 KO mice (d–f), whereas the effects of RO5263397 on sleep/wake states were potentiated in Taar1 OE mice (g–l). Caffeine increased wakefulness (j) and reduced NREM sleep (k) in all genotypes. N=8 KO, 7 OE, and 12 WT mice; *p<0.05 vs Veh; +p<0.05 vs WT. Asterisks are color coded to indicate dose.
KO mice
RO5263397 did not alter wake, NREM, or REM sleep time in KO mice compared with Veh at any dose (Figure 4d–f), whereas Caf significantly increased wake time (F20, 140=3.282, p<0.001) and decreased NREM time compared with Veh in the first hour after dosing (F20, 140=3.683, p<0.001). REM sleep time was not altered by any drug treatment in KO mice. Thus, the duration of the Caf response was somewhat attenuated in KO compared with WT mice.
OE mice
All doses of RO5263397 powerfully increased W time in OE mice for 5–6 h compared with Veh (Figure 4g; F20, 120=8.824, p<0.001). NREM sleep was suppressed for 4–6 h (Figure 4h; F20, 120=9.185, p<0.001) and REM sleep was almost completely suppressed for 6 h after all doses of RO5263397 (Figure 4i; F20, 120=4.283, p<0.001). Caf increased waking and decreased NREM and REM sleep for up to 3 h after dosing. Interestingly, Caf significantly decreased NREM time for 3 h in OE mice compared with 2 h in WT.
Between-genotype comparisons
To directly compare the effect of genotype on drug response, we analyzed total wake, NREM, and REM time between ZT7 and ZT9, when wake promotion by RO5263397 and Caf was maximal in WT mice (Figure 4a–c). RO5263397 (0.3 and 1.0 mg/kg) increased wake time in WT mice (Figure 4j; F8, 96=14.468, p<0.001). This effect was abolished in KO mice and reached a ceiling (180 min) in OE mice (Figure 4j). Caf increased wake time compared with Veh in all genotypes; although this increase appeared attenuated in KO and potentiated in OE mice, there was no between-genotype effect.
RO5263397 (0.3 and 1.0 mg/kg) decreased NREM time in WT mice (Figure 4k; F8, 96=15.371, p<0.001). RO5263397 did not alter NREM time at any dose in KO mice, but completely eliminated NREM sleep in OE mice at all doses. Caf decreased NREM time compared with Veh in all genotypes without a between-genotype effect.
RO5263397 (all doses) decreased REM time compared with Veh in WT mice (Figure 4l; F8, 96=4.424, p<0.001). In KO mice, RO5263397 did not alter REM time compared with Veh at any concentration, and REM time was significantly greater than in WT mice at all concentrations. In OE mice, RO5263397 completely eliminated REM sleep at all doses. Caf decreased REM time compared with Veh in WT and OE but not KO mice, with REM time in WT and OE mice significantly decreased compared with KO mice.
Sleep architecture
For the first 3 h following dosing, RO5263397 increased the frequency of wake bouts and short NREM sleep bouts in WT mice, whereas it decreased long (4–8 min) NREM bouts and decreased REM bouts of all durations (Supplementary Figure S1a–c). RO5263397 thus produced more fragmented wakefulness than Caf that decreased the frequency of short wake bouts, long NREM bouts, and both short and long REM bouts.
In KO mice, there were no effects of drug treatment on wake, NREM, or REM bout organization (Supplementary Figure S1d–f). In OE mice, RO5263397 consolidated bout architecture into a single wake episode for the first 3 h after dosing, whereas Caf decreased the frequency of short (<1 min) wake bouts, long (>2 min) NREM bouts, and all but the longest REM bouts (Supplementary Figure S1g–i).
Power spectral analysis
In WT mice, RO5263397 dose-dependently decreased EEG spectral power in mid to high frequencies during wake (Supplementary Figure S2) and NREM sleep (Supplementary Figure S3), with the greatest changes occurring in beta and gamma band activity during wakefulness and alpha and beta band activity in NREM sleep. In contrast, Caf increased gamma band activity in NREM sleep (Supplementary Figure S3), whereas it decreased beta power in wake (Supplementary Figure S2).
LMA and Tb
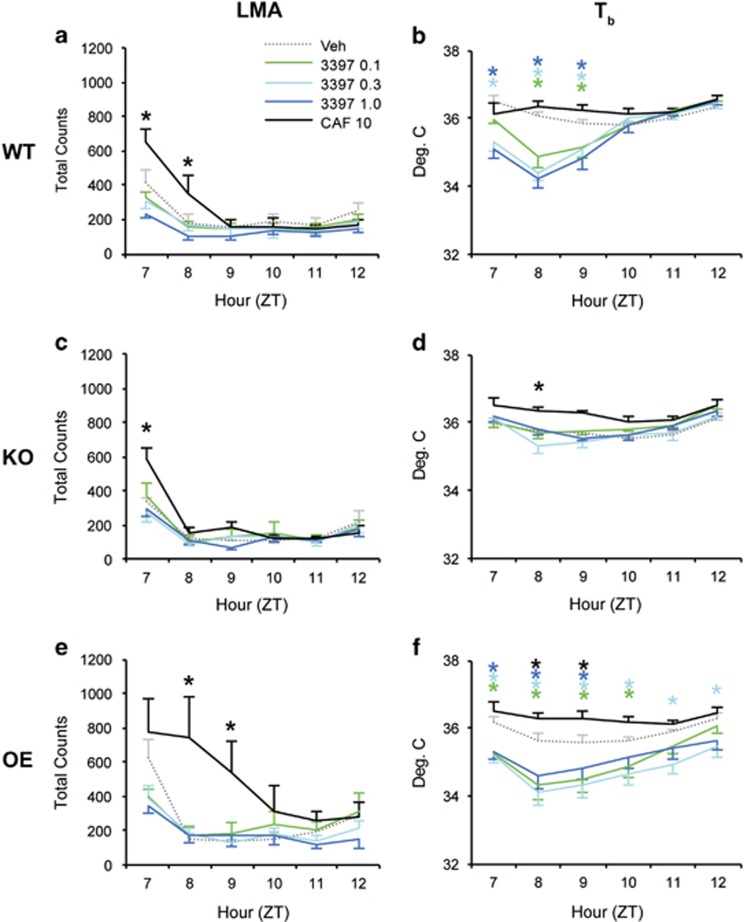
Whereas Caf increased LMA in all genotypes, RO5263397 did not affect LMA in WT (Figure 5a; F20, 220=4.182, p<0.001), KO (Figure 5c; F20, 100=3.449, p<0.001), or OE mice (Figure 5e; F20, 120=2.679, p<0.001). As observed for sleep/wake state, the duration of the Caf response was slightly attenuated in KO and potentiated in OE mice compared with WTs. In contrast, RO5263397 decreased Tb at all doses in WT (Figure 5b, F20, 220=9.4, p<0.001) but not KO mice (Figure 5d; F20, 100=1.9, p=0.020). RO5263397 significantly decreased Tb in OE mice (Figure 5f; F20, 120=5.26, p<0.001). Caf moderately increased Tb in KO and OE but not WT mice (Figure 5b, d, and f). Thus, RO5263397-induced waking was not accompanied by hyperactivity, but was characterized by mild hypothermia in WT and OE mice.
Figure 5.
Although locomotor activity is unaffected by TAAR1 partial agonism, Tb is reduced. LMA does not differ between Veh and any dose of RO5263397 in WT (a), Taar1 KO (c), and Taar1 OE mice (e), although caffeine transiently causes hyperactivity in all three strains. In contrast, RO5263397 causes a dose-dependent decrease in Tb (b) that is absent in Taar1 KO mice (d) and prolonged in Taar1 OE mice (f). In contrast, caffeine elevates Tb, at least in Taar1 KO (d) and Taar1 OE (f) mice. N=8 KO, 7 OE, and 12 WT mice; *p<0.05 vs Veh; asterisks are color coded to indicate dose.
Discussion
TAAR1 negatively regulates DA and 5-HT release and is expressed in monoaminergic and limbic areas associated with arousal state regulation (Lindemann et al, 2008). Although DA was initially thought to be unimportant in sleep/wake regulation because the firing rate of mesencephalic dopaminergic neurons was reported to be state invariant (Miller et al, 1983), subsequent cellular neurophysiological (Dahan et al, 2007), pharmacological (Wisor et al, 2001), functional (Dzirasa et al, 2006, 2009; Lu et al, 2006), and clinical studies (Avorn et al, 2005; Rye, 2004) prompted re-examination of its role in sleep/wake. TAAR1 partial agonists increase firing of VTA dopamine and DRN 5-HT neurons and dose-dependently increase wakefulness in rats (Revel et al, 2012b, 2013). Accordingly, we investigated the phenotype of Taar1 KO and OE mice. Although the effects on basal sleep/wake distribution in these two strains were modest, TAAR1 overexpression reduced sleep time across the 24 h period and TAAR1 deletion increased NREM sleep specifically around lights-on. Moreover, TAAR1 expression levels profoundly altered EEG spectra, with KO mice having elevated spectral power in the theta and gamma bands associated with waking, exploration, and cognition. Using the TAAR1 partial agonist RO5263397, we replicated the wake-promoting and REM sleep-inhibiting effects of partial agonism in WT mice, and showed that these effects were TAAR1 dependent and greatly exacerbated in OE mice. Finally, we found that waking induced by RO5263397 was characterized by decreased gamma band activity without the hyperlocomotion produced by Caf.
TAAR1 Expression and Basal Physiology
When assessing whether particular genes are involved in a behavioral or physiological response, gene elimination and overexpression studies must be interpreted with the caveat of potential developmental compensation. The constitutive knockout and ectopic overexpression of TAAR1 may cause complex alterations in monoaminergic systems. Accordingly, we have interpreted our results within the context of known TAAR1–monoamine interactions, and how these mutations affect monoamine signaling.
LMA and Tb rhythms were unaffected by Taar1 manipulation. Taar1 overexpression yielded a modest wakefulness increase and NREM sleep decrease across the 24 h period. Increased wakefulness in OE mice resulted from more frequent wake bouts and was particularly evident at the lights-on transition; in contrast, KO mice had more NREM sleep than either WT or OE mice at this time of day. These results suggest that TAAR1 may modulate transmission of photic information to the circadian and/or sleep/wake regulatory systems.
To assess the integrity of the sleep homeostat, we imposed 6 h SD at light onset. Consistent with the phenotype of increased basal wakefulness, OE mice exhibited more wake during SD through longer wake bouts. All strains showed sleep homeostasis as evidenced by a declining trend in NREM delta power during the 6 h recovery. However, OE mice exhibited greater NREM delta power during the first hour of recovery than the other strains, indicating a greater buildup of sleep debt during SD—yet, these mice also had more wake bouts during the recovery period. Together, these results indicate that TAAR1 expression levels subtly modulate sleep/wake amounts and sleep architecture both during baseline conditions and in response to homeostatic perturbation. In particular, TAAR1 overexpression may increase waking ‘drive'.
TAAR1 Partial Agonists Promote Wakefulness
The TAAR1 partial agonist RO5263397 increased waking and decreased NREM and REM sleep in WT mice as previously described in rats (Revel et al, 2013). These effects were completely absent in KO mice, indicating TAAR1 dependence, and were greatly exacerbated in OE mice. Increased wakefulness in WT mice occurred predominantly in short bouts, coincident with decreased numbers of long NREM bouts, suggesting that TAAR1 partial agonism increases waking drive. In contrast, the decline in REM sleep induced by RO5263397 in WT mice was accompanied by decreases in the number of REM bouts of all durations. Tb also declined in WT mice for the first 3 h at all doses tested; this effect was blocked in KO mice and exacerbated in OE mice. As a hypothermic effect was not observed in rats treated with RO5263397 (Revel et al, 2013), the wake-promoting effects of RO5263397 are likely independent of any changes in Tb in contrast to the results reported in a recent study of the serotonergic system (Murray et al, 2015).
TAAR1 may promote wakefulness by modulating DA and 5-HT signaling in the ascending reticular activating system. TAAR1 regulates monoamine transporters and interacts with DA D2 and 5-HT1A receptors (Espinoza et al, 2011; Leo et al, 2014; Revel et al, 2011; Xie and Miller, 2007). In vitro, RO5263397 increases firing rates in dopaminergic and serotonergic neurons (Revel et al, 2012b, 2013), mimicking the effects of the TAAR1 antagonist EPPTB (Bradaia et al, 2009; Revel et al, 2011). In contrast, the full agonist RO5256390, which is not wake promoting in rats (Revel et al, 2012b, 2013) or mice (Black et al, in press), decreases DA and 5-HT neuronal firing. RO5263397 could therefore promote arousal by increasing monoaminergic activity. However, basal wakefulness was paradoxically increased and NREM sleep decreased in OE mice compared with WT. Basal firing rates of DA and 5-HT neurons are elevated in TAAR1 KO and OE mice (Lindemann et al, 2008; Revel et al, 2012a). In KO mice, this increase results from the absence of TAAR1 in monoaminergic neurons (Lindemann et al, 2008; Revel et al, 2011), whereas in OE mice, ectopic TAAR1 expression in GABA interneurons yields decreased GABAergic tone, disinhibition of monoaminergic activity, and elevated basal monoamine release (Revel et al, 2012a). This ectopic disinhibition likely contributes to elevated basal wakefulness in OE mice. In contrast, TAAR1 full agonists decrease, and partial agonists increase, monoaminergic neuronal firing in OE mice as in WT mice (Revel et al, 2012a), consistent with our observation that RO5263397 increased waking in WT and OE mice. Thus, elevating monoaminergic tone directly (via TAAR1 partial agonism) or indirectly (via ectopic TAAR1 overexpression) increases wakefulness. Although we did not observe a similar increase in basal wakefulness in TAAR1 KO mice, which also have elevated monoaminergic tone (Leo et al, 2014), high-frequency EEG activity was increased in these mice, consistent with increased basal cortical activation. Both DA and 5-HT have been implicated in sleep/wake regulation; future studies should assess the relative contribution of each to TAAR1 agonist-induced wakefulness.
The effects of Caf, used here as a positive control, were surprisingly modulated by TAAR1 expression levels. Although total wake, NREM, and REM sleep time following Caf did not differ between genotypes, KO mice exhibited a faster offset of Caf-induced arousal (Figure 4d and e) compared with WT mice; similarly, increases in LMA, Tb, and gamma band EEG activity following Caf were exacerbated in OE and attenuated in KO mice. These effects suggest that TAAR1 is important for the arousing response to Caf, probably through its effects on monoaminergic signaling.
TAAR1 Modulation of EEG Spectra
Perhaps the most dramatic result during basal conditions were the effects on EEG spectra in which TAAR1 elimination increased spectral power in a portion of the theta and low gamma bands across all arousal states, whereas overexpression tended to reduce power in these frequencies. Similarly, RO5263397 acutely decreased spectral power across a range of frequencies from alpha through gamma band activity in waking and NREM sleep. These results indicate that TAAR1 activation profoundly affects cortical activity, perhaps by modulation of DA release (Lindemann et al, 2008). However, TAAR1 also modulates cortical NMDA receptor-mediated glutamate transmission (Espinoza et al, 2015). As NMDA receptor antagonists increase gamma (Ehrlichman et al, 2009; Hakami et al, 2009; Lazarewicz et al, 2010; Pinault, 2008) and theta (Lazarewicz et al, 2010) power, TAAR1 modulation of glutamatergic neurotransmission may be a critical component underlying EEG activity.
Conclusions
Abnormal levels of TAs are associated with schizophrenia, depression, and Parkinson's disease (Berry, 2007; Burchett and Hicks, 2006; Davis, 1989; Grandy, 2007; Sotnikova et al, 2009). Since its identification in 2001, TAAR1-mediated regulation of dopaminergic, serotonergic, and glutamatergic systems has been implicated in arousal, cognition, addiction, and weight and glucose regulation (Espinoza et al, 2015; Lindemann et al, 2008; Lynch et al, 2013; Pei et al, 2014; Raab et al, 2016; Revel et al, 2011, 2012a; Thorn et al, 2014). Here, we demonstrate that endogenous TAAR1 signaling contributes to sleep/wake regulation and, moreover, that global brain function as reflected in the EEG spectra is profoundly altered by manipulating TAAR1 activity. Sleep and EEG disturbances are a common feature of psychiatric and neurological diseases; sleep disturbances may exacerbate symptoms and affect treatment efficacy. Consequently, TAAR1-based therapeutics may offer a new way to address sleep and arousal-based disturbance in mental illness.
Funding and disclosure
The authors declare no conflict of interest. Dr Hoener is an employee of F Hoffmann-La Roche. Over the past 2 years, Dr Morairty has received research support from CHDI Foundation, F Hoffmann-LaRoche, Sunovion Pharmaceuticals, and Merck Pharmaceuticals. Over the past 2 years, Dr Kilduff has served as a consultant for NIH, the Japanese Society for the Promotion of Science, Merck Pharmaceuticals, and Pfizer; made paid educational presentations for the benefit of APSS, and the Physician's Postgraduate Press; and received research support from F Hoffmann-LaRoche, Sunovion Pharmaceuticals, and Inscopix.
Acknowledgments
We thank Kelsie Bogyo, Tsui-Ming Chen, Webster Lincoln, Spenser Smith, Deepti Warrier, Dr Lars Dittrich, and Dr Alexia Thomas for technical assistance and Drs A Harmeier, J-L Moreau, and J Wettstein for valuable scientific input. This research was supported by NIH R01NS082876, R21NS083639, and R21NS085757 to TSK.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Avorn J, Schneeweiss S, Sudarsky LR, Benner J, Kiyota Y, Levin R et al (2005). Sudden uncontrollable somnolence and medication use in Parkinson disease. Arch Neurol 62: 1242–1248. [DOI] [PubMed] [Google Scholar]
- Berry MD (2007). The potential of trace amines and their receptors for treating neurological and psychiatric diseases. Rev Recent Clin Trials 2: 3–19. [DOI] [PubMed] [Google Scholar]
- Black SW, Schwartz MD, Chen T-M, Yamanaka A, Hoener MC, Kilduff TS TAAR1 agonists as narcolepsy therapeutics. Biol Psychiatry (in press). [DOI] [PMC free article] [PubMed]
- Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL et al (2001). Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci USA 98: 8966–8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradaia A, Trube G, Stalder H, Norcross RD, Ozmen L, Wettstein JG et al (2009). The selective antagonist EPPTB reveals TAAR1-mediated regulatory mechanisms in dopaminergic neurons of the mesolimbic system. Proc Natl Acad Sci USA 106: 20081–20086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI et al (2001). Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharmacol 60: 1181–1188. [DOI] [PubMed] [Google Scholar]
- Burchett SA, Hicks TP (2006). The mysterious trace amines: protean neuromodulators of synaptic transmission in mammalian brain. Prog Neurobiol 79: 223–246. [DOI] [PubMed] [Google Scholar]
- Dahan L, Astier B, Vautrelle N, Urbain N, Kocsis B, Chouvet G (2007). Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology 32: 1232–1241. [DOI] [PubMed] [Google Scholar]
- Davis BA (1989). Biogenic amines and their metabolites in body fluids of normal, psychiatric and neurological subjects. J Chromatogr 466: 89–218. [DOI] [PubMed] [Google Scholar]
- Dzirasa K, Ribeiro S, Costa R, Santos LM, Lin SC, Grosmark A et al (2006). Dopaminergic control of sleep-wake states. J Neurosci 26: 10577–10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzirasa K, Santos LM, Ribeiro S, Stapleton J, Gainetdinov RR, Caron MG et al (2009). Persistent hyperdopaminergia decreases the peak frequency of hippocampal theta oscillations during quiet waking and REM sleep. PLoS One 4: e5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlichman RS, Gandal MJ, Maxwell CR, Lazarewicz MT, Finkel LH, Contreras D et al (2009). N-methyl-d-aspartic acid receptor antagonist-induced frequency oscillations in mice recreate pattern of electrophysiological deficits in schizophrenia. Neuroscience 158: 705–712. [DOI] [PubMed] [Google Scholar]
- Espinoza S, Lignani G, Caffino L, Maggi S, Sukhanov I, Leo D et al (2015). TAAR1 modulates cortical glutamate NMDA receptor function. Neuropsychopharmacology 40: 2217–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza S, Salahpour A, Masri B, Sotnikova TD, Messa M, Barak LS et al (2011). Functional interaction between trace amine-associated receptor 1 and dopamine D2 receptor. Mol Pharmacol 80: 416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SP, Schwartz MD, Black SW, Thomas AM, Chen T-M, Miller MA et al (2016). Quantitative EEG analysis provides an early-stage indicator of disease onset and progression in the zQ175 knock-in mouse model of Huntington's disease. Sleep 39: 379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SP, Black SW, Schwartz MD, Wilk AJ, Liu H, Chen T-M et al (2013). Longitudinal analysis of the sleep phenotype and EEG signatures in the R6/2 model of Huntington's disease. Brain 136: 2159–2172. [DOI] [PubMed] [Google Scholar]
- Grandy DK (2007). Trace amine-associated receptor 1-Family archetype or iconoclast? Pharmacol Ther 116: 355–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakami T, Jones NC, Tolmacheva EA, Gaudias J, Chaumont J, Salzberg M et al (2009). NMDA receptor hypofunction leads to generalized and persistent aberrant gamma oscillations independent of hyperlocomotion and the state of consciousness. PLoS One 4: e6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarewicz MT, Ehrlichman RS, Maxwell CR, Gandal MJ, Finkel LH, Siegel SJ (2010). Ketamine modulates theta and gamma oscillations. J Cogn Neurosci 22: 1452–1464. [DOI] [PubMed] [Google Scholar]
- Leo D, Mus L, Espinoza S, Hoener MC, Sotnikova TD, Gainetdinov RR (2014). Taar1-mediated modulation of presynaptic dopaminergic neurotransmission: role of D2 dopamine autoreceptors. Neuropharmacology 81: 283–291. [DOI] [PubMed] [Google Scholar]
- Liberles SD (2009). Trace amine-associated receptors are olfactory receptors in vertebrates. Ann NY Acad Sci 1170: 168–172. [DOI] [PubMed] [Google Scholar]
- Liberles SD, Buck LB (2006). A second class of chemosensory receptors in the olfactory epithelium. Nature 442: 645–650. [DOI] [PubMed] [Google Scholar]
- Lindemann L, Meyer CA, Jeanneau K, Bradaia A, Ozmen L, Bluethmann H et al (2008). Trace amine-associated receptor 1 modulates dopaminergic activity. J Pharmacol Exp Ther 324: 948–956. [DOI] [PubMed] [Google Scholar]
- Lu J, Jhou TC, Saper CB (2006). Identification of wake-active dopaminergic neurons in the ventral periaqueductal gray matter. J Neurosci 26: 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch LJ, Sullivan KA, Vallender EJ, Rowlett JK, Platt DM, Miller GM (2013). Trace amine associated receptor 1 modulates behavioral effects of ethanol. Subst Abuse 7: 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JD, Farber J, Gatz P, Roffwarg H, German DC (1983). Activity of mesencephalic dopamine and non-dopamine neurons across stages of sleep and walking in the rat. Brain Res 273: 133–141. [DOI] [PubMed] [Google Scholar]
- Murray NM, Buchanan GF, Richerson GB (2015). Insomnia caused by serotonin depletion is due to hypothermia. Sleep 38: 1985–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y, Lee J, Leo D, Gainetdinov RR, Hoener MC, Canales JJ (2014). Activation of the trace amine-associated receptor 1 prevents relapse to cocaine seeking. Neuropsychopharmacology 39: 2299–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y, Mortas P, Hoener MC, Canales JJ (2015). Selective activation of the trace amine-associated receptor 1 decreases cocaine's reinforcing efficacy and prevents cocaine-induced changes in brain reward thresholds. Prog Neuropsychopharmacol Biol Psychiatry 63: 70–75. [DOI] [PubMed] [Google Scholar]
- Pinault D (2008). N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry 63: 730–735. [DOI] [PubMed] [Google Scholar]
- Raab S, Wang H, Uhles S, Cole N, Alvarez-Sanchez R, Kunnecke B et al (2016). Incretin-like effects of small molecule trace amine-associated receptor 1 agonists. Mol Metab 5: 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel FG, Meyer CA, Bradaia A, Jeanneau K, Calcagno E, Andre CB et al (2012. a). Brain-specific overexpression of trace amine-associated receptor 1 alters monoaminergic neurotransmission and decreases sensitivity to amphetamine. Neuropsychopharmacology 37: 2580–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel FG, Moreau JL, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R et al (2011). TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity. Proc Natl Acad Sci USA 108: 8485–8490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel FG, Moreau JL, Gainetdinov RR, Ferragud A, Velazquez-Sanchez C, Sotnikova TD et al (2012. b). Trace amine-associated receptor 1 partial agonism reveals novel paradigm for neuropsychiatric therapeutics. Biol Psychiatry 72: 934–942. [DOI] [PubMed] [Google Scholar]
- Revel FG, Moreau JL, Pouzet B, Mory R, Bradaia A, Buchy D et al (2013). A new perspective for schizophrenia: TAAR1 agonists reveal antipsychotic- and antidepressant-like activity, improve cognition and control body weight. Mol Psychiatry 18: 543–556. [DOI] [PubMed] [Google Scholar]
- Rye DB (2004). The two faces of Eve: dopamine's modulation of wakefulness and sleep. Neurology 63: S2–S7. [DOI] [PubMed] [Google Scholar]
- Sotnikova TD, Caron MG, Gainetdinov RR (2009). Trace amine-associated receptors as emerging therapeutic targets. Mol Pharmacol 76: 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn DA, Jing L, Qiu Y, Gancarz-Kausch AM, Galuska CM, Dietz DM et al (2014). Effects of the trace amine-associated receptor 1 agonist RO5263397 on abuse-related effects of cocaine in rats. Neuropsychopharmacology 39: 2309–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM (2001). Dopaminergic role in stimulant-induced wakefulness. J Neurosci 21: 1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky TD, Swanson CJ, Smith KE, Zhong H, Borowsky B, Seeman P et al (2007). The Trace Amine 1 receptor knockout mouse: an animal model with relevance to schizophrenia. Genes Brain Behav 6: 628–639. [DOI] [PubMed] [Google Scholar]
- Xie Z, Miller GM (2007). Trace amine-associated receptor 1 is a modulator of the dopamine transporter. J Pharmacol Exp Ther 321: 128–136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.