Abstract
Dysbindin-1, a protein that regulates aspects of early and late brain development, has been implicated in the pathobiology of schizophrenia. As the functional roles of the three major isoforms of dysbindin-1, (A, B, and C) remain unknown, we generated a novel mutant mouse, dys-1A−/−, with selective loss of dysbindin-1A and investigated schizophrenia-related phenotypes in both males and females. Loss of dysbindin-1A resulted in heightened initial exploration and disruption in subsequent habituation to a novel environment, together with heightened anxiety-related behavior in a stressful environment. Loss of dysbindin-1A was not associated with disruption of either long-term (olfactory) memory or spontaneous alternation behavior. However, dys-1A−/− showed enhancement in delay-dependent working memory under high levels of interference relative to controls, ie, impairment in sensitivity to the disruptive effect of such interference. These findings in dys-1A−/− provide the first evidence for differential functional roles for dysbindin-1A vs dysbindin-1C isoforms among phenotypes relevant to the pathobiology of schizophrenia. Future studies should investigate putative sex differences in these phenotypic effects.
Introduction
Dystrobrevin-binding protein 1 (DTNBP1), more commonly known as dysbindin-1, is a protein with diverse physiological roles that extend particularly to the nervous system, where it regulates aspects of early and late brain development and neuronal functions that include gene transcription, axonal and dendritic spine formation, receptor trafficking, synaptic vesicle biogenesis, and exocytosis (Talbot et al, 2009; Jia et al, 2014; Mullin et al, 2015). This protein is of special clinical interest given that variation in the DTNBP1 gene has been associated with risk for schizophrenia (Allen et al, 2008; Ayalew et al, 2012) and that DTNBP1 gene and/or protein expression are down regulated in multiple brain regions (ie, the hippocampal formation (HF: Talbot et al, 2004, 2011; Weickert et al, 2008), dorsolateral prefrontal cortex (PFC: Weickert et al, 2004; Tang et al, 2009), and auditory association cortices (Talbot et al, 2011)) of schizophrenia cases. These regions are essential elements in a hippocampal–prefrontal cortical network that is implicated in the regulation not only of memory, emotion, and other self-referential processes (Aggleton, 2012) but also of putative sexually dimorphic cognitive dysfunction in schizophrenia (Mendrek and Mancini-Marie, 2015). Indeed, the role of dysregulated dysbindin-1 gene and protein expression in schizophrenia appears most closely related to the prominent cognitive impairment in that disorder as DTNBP1 genotype influences cognition both in normal subjects and in schizophrenia (Burdick et al, 2007; Zinkstok et al, 2007; Zhang et al, 2010; Wolf et al, 2011; Baek et al, 2012; Varela-Gomez et al, 2015).
Although reductions of one or more dysbindin-1 isoforms have been found in the brains of schizophrenia cases (Talbot et al, 2004, 2009, 2011; Tang et al, 2009), it remains unclear what contributions each of these isoforms may make to increased risk and clinical features of schizophrenia. Three major dysbindin-1 isoforms are expressed in the human brain: dysbindin-1A, -1B, and -1C (Talbot et al, 2009, 2011). Dysbindin-1A (~ 50 kDa) is the full-length protein (351 amino acids in humans), is localized in synapses almost entirely postsynaptically in humans (Talbot et al, 2011), and is a component of a large protein assembly known as BLOC-1 (biogenesis of lysosomal organelles complex-1; Larimore et al, 2014; Wang et al, 2014). Dysbindin-1B (~ 37 kDa) is a truncated version of dysbindin-1A (303 amino acids in humans) in which the C-terminal region lacks the PEST domain of dysbindin-1A (Talbot et al, 2009). This second isoform, which is not expressed in mice, is localized in synapses only presynaptically (Talbot et al, 2011), and may or may not be part of BLOC-1. Dysbindin-1C (~33 kDa) is also a truncated version of dysbindin-1A (270 amino acids in humans), but the truncation occurs in the N-terminal region (NTR), which is entirely missing in dysbindin-1C (Talbot et al, 2009). This third isoform is localized in synapses both pre- and postsynaptically (Talbot et al, 2011; Wang et al, 2014) and is not part of BLOC-1 (Larimore et al, 2014; Wang et al, 2014).
Mouse mutants have been used to identify functions of dysbindin-1 isoforms and their potential contributions to schizophrenia phenotypes. The mouse most intensively studied for this purpose is the sandy (sdy) mouse, which arose from a spontaneous deletion mutation of exons 6 and 7 (along with most of introns 5–7) in the DTNBP1 gene (Li et al, 2003; Talbot, 2009; Talbot et al, 2009). This leads to expression of DTNBP1 transcripts encoding a truncated coiled-coil region on which all dysbindin-1 isoforms depend for interaction with other proteins (Talbot et al, 2009). These abnormal transcripts are not successfully translated. Thus, although wild-type (WT) mice express dysbindin-1A and -1C (but not dysbindin-1B as indicated above), homozygous sdy (dys−/−) mice express neither isoform (Talbot, 2009; Talbot et al, 2009). As a result, abnormalities found in sdy mice are not readily attributable to loss of any specific dysbindin-1 isoform.
As noted above, dysbindin-1A differs from dysbindin-1C by the presence of the NTR in the former isoform. The NTR is encoded by exons 1 and 3–5, partial or complete deletion of which should produce transcripts without the NTR and thus encode only dysbindin-1C (Talbot et al, 2009). The ability to distinguish between effects of dysbindin-1A and -1C is important given their differences in synaptic localization and presence in BLOC-1, as well as their differences in protein interactions and physiological functions (Talbot et al, 2009). Dysbindin-1A is the dysbindin isoform best known to regulate gene expression in developing and adult neurons (Oyama et al, 2009; Larimore et al, 2014; Soma et al, 2014) and differs from dysbindin-1C by having a binding site for Akt (Talbot et al, 2009), which mediates promotion of neuronal viability by dysbindin-1A (Numakawa et al, 2004). In contrast, dysbindin-1C has neuronal functions separate from dysbindin-1A, including promotion of autophagy (Yuan et al, 2015), regulation of adult neurogenesis in the dentate gyrus (Wang et al, 2014), and potential suppression of vagally induced apoptosis in the hippocampus (González-López et al, 2013).
In rodent brain, levels of dysbindin-1A (~ 50 kDa) are highest at late embryonic and early postnatal periods (Ghiani et al, 2010; Ito et al, 2010), whereas levels of dysbindin-1C (~ 33 kDa) are barely detectable before early adulthood (Ito et al, 2010). We report here that selective knockout of exon 5 in the DTNBP gene of C57Bl/6 J mice results in selective loss of dysbindin-1A without reduction of dysbindin-1C levels in the brain. We studied the behavioral phenotype in both male and female dysbindin-1A mutants given that sex influences schizophrenia incidence risk (greater in males: Aleman et al, 2003; McGrath et al, 2008; Abel et al, 2010; van der Werf et al, 2014) and peak age at onset (later in females: Häfner et al, 1998; van der Werf et al, 2014) and that schizophrenia risk associated with some genetic variants in DTNBP1 differs according to sex (Zuo et al, 2009; Voisey et al, 2010; Sacchetti et al, 2013; Tan et al, 2015). Accordingly, adult male and female heterozygous and homozygous mutants and WT mice were evaluated to determine phenotypic effects on a wide range of sensorimotor, schizophrenia-related and other behaviors. By providing the first characterization of the dysbindin-1A-null phenotype, the present paper provides the first indications of the contributions dysbindin-1A reductions may make to the pathophysiology of schizophrenia.
Materials and methods
Animals
Male and female breeding pairs and experimental mice were housed in opaque plastic cages (30 × 16 × 12 cm). Standard laboratory mouse chow and water were available ad libitum. Mice were maintained at a constant temperature of 21±1 °C on a 12 h light/dark schedule. Offspring were left with breeding pairs until they reached weaning age of 19–22 days. Experimental animals were generated from heterozygous breeding pairs selected from different litters. Once genotyped, mice were housed in groups of 3–5 per cage. Male and female WT and mutant mice were from litters of the same generational age and were studied during young adulthood in groups of indistinguishable current age. All experiments were approved by the Research Ethics Committee of the Royal College of Surgeons in Ireland under license from the Department of Health and Children, in accordance with Irish legislation and the European Communities Council Directive 86/609/EEC for the care and use of experimental animals, and from the Environmental Protection Agency in relation to the contained use of genetically modified organisms.
Construction and Validation of Exon 5-DTNBP1 Knockout Mice
We generated a novel mutant mouse with deletion in exon 5 of the DTNBP1 gene and resultant isoform-specific loss of brain expression of dysbindin-1A protein, hereafter referred to as dys-1A−/−, together with heterozygous mutants (dys-1A+/−) and WT (dys-1A+/+). Gene targeting was performed in C57Bl/6 J KSR5.4 embryonic stem cells. Male chimaeras were crossed with C57Bl/6 J females to produce N1F0 offspring. Null alleles were generated by crossing animals containing the primary targeted allele with a germline Cre deleter transgenic strain (SA-Cre: Taconic-Artemis, Germany). Heterozygous animals carrying the null allele were mated with one another to produce F1 animals. PCR genotyping was performed using WT and targeted allele-specific primers. Detailed descriptions of the construction and genotyping of this mutant line are given in Supplementary Information.
To confirm isoform-specific knockout, dysbindin-1A and -1C proteins were evaluated by western blot analysis using a fully validated and well-characterized antibody (Oxford PA3111; Benson et al, 2001; Talbot et al, 2009, 2011). Western blots were analyzed by densitometry using ImageJ software (NIH, Bethesda, MD). A detailed description of these procedures is given in Supplementary Information.
General Health and Sensorimotor Function
Mice were evaluated for any general health problems and sensorimotor deficits that might interfere with performance in behavioral tasks, using multiple assessments from previously described procedures: Comprehensive Observational Assessment (Irwin, 1968) and SHIRPA (SmithKline Beecham, Harwell, Imperial College, Royal London Hospital phenotype assessment; Rogers et al, 1997). A detailed description of these assessments is given in Supplementary Information Table S1.
Exploratory Activity
Automated analysis of exploratory activity in a novel environment was conducted as described previously (O'Tuathaigh et al, 2010). A detailed description of this behavioral assay is given in Supplementary Information.
Anxiety-Related Behavior
Elevated plus-maze
The elevated plus-maze was used to test anxiety-related behavior, as described previously (O'Tuathaigh et al, 2010). A detailed description of this behavioral assay is given in Supplementary Information.
Light/dark box
Anxiety-related behavior was also assessed in the light–dark box, as described previously (O'Tuathaigh et al, 2012). A detailed description of this behavioral assay is given in Supplementary Information.
Prepulse Inhibition
Habituation of startle response and prepulse inhibition was assessed as described previously (O'Tuathaigh et al, 2012). A detailed description of this behavioral assay is given in Supplementary Information.
Social Behavior
Sociability and social novelty preference
The three-chamber test of sociability and social novelty was conducted as described previously (O'Tuathaigh et al, 2008). A detailed description of this behavioral assay is given in Supplementary Information.
Dyadic social interaction
Using ethologically based indices of social behavior, dyadic social interaction with an unfamiliar conspecific was evaluated as described previously (O'Tuathaigh et al, 2008). A detailed description of this behavioral assay is given in Supplementary Information.
Long-Term (Olfactory) Memory
Long-term olfactory memory was assessed using the social transmission of food preference test, employing a previously described protocol (Wrenn et al, 2003). A detailed description of this behavioral assay is given in Supplementary Information.
Spontaneous Alternation Behavior
The Y-maze was used in a continuous spontaneous alternation procedure, inter alia a putative index of delay-independent working memory (Dudchenko, 2004), as described previously (O'Tuathaigh et al, 2010). A detailed description of this behavioral assay is given in Supplementary Information.
Delay/Interference-Dependent Working Memory
In the 8-arm radial maze, a delayed non-match-to-position paradigm (Dudchenko, 2004; Malleret et al, 2010) was employed; the procedure involved a low working memory load with two levels of interference: limited interference or high interference. A detailed description of this behavioral assay is given in Supplementary Information.
Statistical Analysis
This followed procedures similar to those described previously (O'Tuathaigh et al, 2010). Multivariate analysis of variance (ANOVA) was applied to assess group differences. Repeated measures ANOVA was performed for exploratory behavior, behavior in the light/dark box, prepulse inhibition, social behavior, and behavior in the radial arm maze. Where the data were not normally distributed, analyses were conducted following square-root transformation. Statistically significant effects in each ANOVA were followed by post hoc comparisons using the Least Significant Difference test. As the present studies on dys-1A−/− mice were conducted using both males and females, findings are presented for each sex separately. All results are presented as means±SEM. A p-value<0.05 was considered significant. Statistical analyses were performed using the Statistical Package for the Social Sciences program 19.0 (SPSS, Chicago, IL).
Results
Selective Loss of Dysbindin-1A Expression in Dys-1A −/−
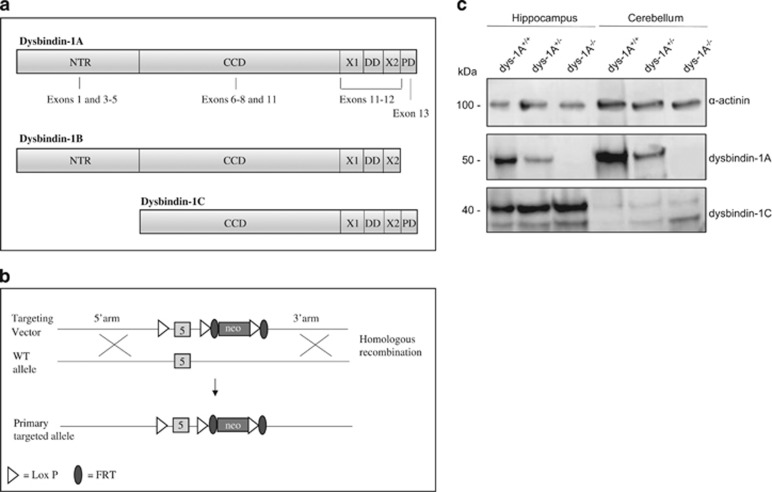
Dysbindin-1A (~ 50 kDa) was expressed in the HF and cerebellum of WT but was reduced in dys-1A+/- and absent in dys-1A−/−. In contrast, expression of dysbindin-1C (40 kDa) in the HF was evident across all genotypes (Figure 1). We confirmed findings in both humans and mice (Talbot et al, 2009, 2011; Wang et al, 2014) by showing that dysbindin-1C expression is at or below the threshold of detectability in the cerebellum, irrespective of genotype.
Figure 1.
(a) Adapted representation of dysbindin-1A, -1B, and -1C from Talbot et al (2009). Compared with the full long isoform dysbindin-1A, dysbindin-1B has a shorter CTR and dysbindin-1C lacks the NTR that is encoded by exons 1 and 3–5. (b) Selective abrogation of dysbindin-1A is obtained by the formation of ES cells with DTNBP1 exon 5-targeted disruption. Exogenous DNA is introduced into ES cells from C57BL/6 mice via homologous recombination. Recombinant cells in which one copy of the DTNBP1 gene is disrupted was obtained by using a recombinant vector that carries the DTNBP1 gene disrupted with neor, a neomycin-resistance gene. Subsequently, homologous recombination in neomycin-resistant ES cells was confirmed by Southern blot. (c) Western blots showing expression of dysbindin-1A (50 kDa) and dysbindin-1C (40 kDa) in wild-type (WT, dys-1A+/+) mice and mice with heterozygous (dys-1A+/−) or homozygous (dys-1A−/−) deletion of dysbindin-1A, using Oxford PA3111 antibody, with α-actin (100 kDa) as loading control. In the hippocampus, expression of dysbindin-1A is reduced in dys-1A+/- and absent in dys-1A−/−, whereas expression of dysbindin-1C is intact across all genotypes. Similarly, in the cerebellum expression of dysbindin-1A is reduced in dys-1A+/- and absent in dys-1A−/−, whereas expression of dysbindin-1C was essentially absent across all genotypes. A faint second band below 40 kDa has been noted previously in WT mice and may correspond to a dysbindin-1C degradation product (Talbot et al, 2009).
General Health and Sensorimotor Function
No general health problems or sensorimotor deficits were detected in dys-1A−/−, including ability to nest, deliver pups and provide milk to offspring, basic visual, auditory and olfactory function, pain sensitivity, and motor coordination. As observed in relation to the sdy mutant, the dys-1A−/− coat color is sandy owing to the fact that absence of dysbindin-1A should interfere with BLOC-1 function, one of which is synthesis of melanin (Li et al, 2003). As with the sdy mutant, dys-1A−/− also exhibited prolonged bleeding time following tail biopsy, owing to platelet abnormalities (Li et al, 2003). No other overt phenotypic abnormalities were observed (Supplementary Information Table S1).
Dysregulation of Novelty-Induced Exploration and Habituation in Dys-1A −/−
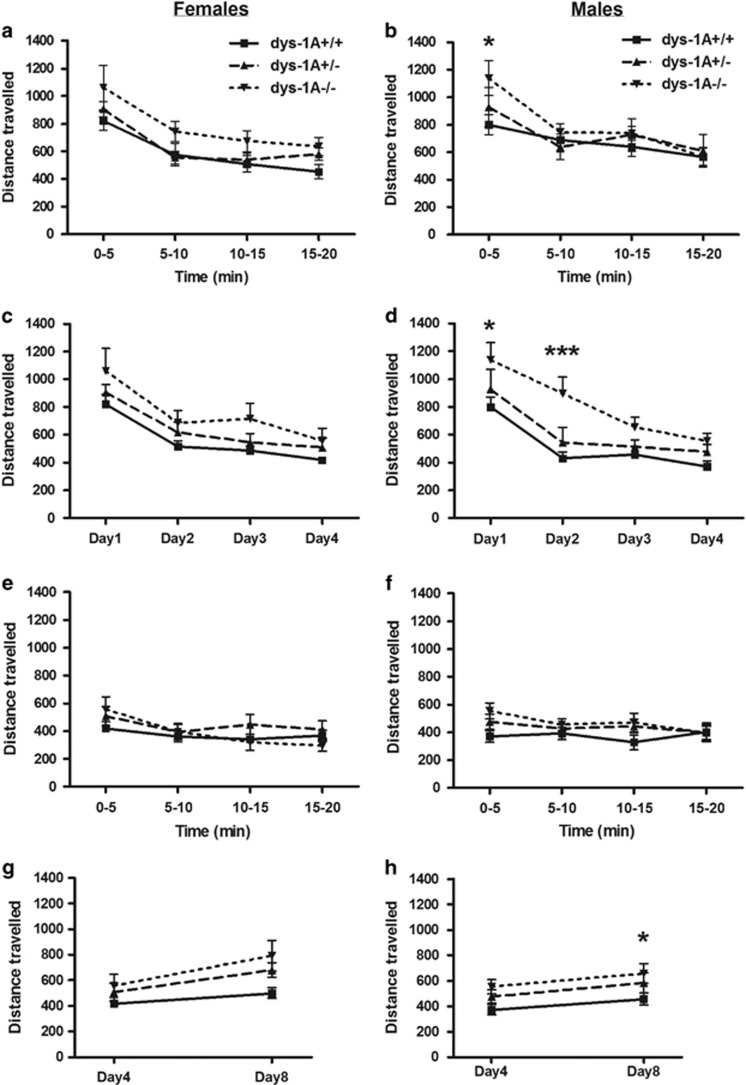
On day 1, male dys-1A−/− mice showed locomotor hyperactivity during initial exposure to the novel environment of the open-field, but habituated rapidly within this initial session (time × genotype interaction: F(6,34)=3.12, p<0.01; dys-1A−/− mice traveled a greater distance than WT mice during the first 5 min of exposure, p<0.05); no significant effects were evident among females (Figures 2a and b). On repeated exposure to the open-field, male dys-1A−/− mice showed hyperactivity during the first 5 min of exposure across days 1–4 (effect of genotype: F(2,34)=4.87, p=0.01; dys-1A−/− mice traveled a greater distance than WT mice during the first 5 min of exposure on day 1, p<0.05, and day 2, p<0.001); no significant effects were evident among females (Figures 2c and d). The level of habituated locomotor activity following completion of assessment on day 4 was indistinguishable between the genotypes for both sexes (Figures 2e and f). Following a 4-day retention interval and re-exposure to the open-field on day 8, activity during the first 5 min of exposure across days 4–8 was increased among male dys-1A−/− mice (effect of genotype: F(2,34)=3.77, p<0.05; dys-1A−/− mice traveled a greater distance than WT mice during the first 5 min of exposure on day 8, p<0.05); no significant effects were evident among females (Figures 2g and h).
Figure 2.
Novelty-induced exploration and habituation in 25 WT (dys-1A+/+; 13 male, 12 female), 24 dys-1A+/− (12 male, 12 female), and 22 dys-1A−/− (12 male, 10 female) mice. Data are presented as means±SEM. (a,b) Distance traveled on day 1 in males and females, respectively, during the four successive 5-min periods constituting a 20-min exposure to the open-field; *p<0.05 male dys-1A−/− vs WT. (c,d) Distance traveled on days 1–4 in males and females, respectively, during initial 5-min periods of exposure to the open-field on successive days; ***p<0.001, *p<0.05 male dys-1A−/− vs WT. (e,f) Distance traveled on day 4 in males and females, respectively, during the four successive 5-min periods constituting the 20-min exposure to the open-field. (g,h) Distance traveled on day 4 and, following a retention interval of 4 days, on re-introduction to the open-field on day 8 in males and females, respectively, during initial 5-min periods of exposure to the open-field on each day; *p<0.05 male dys-1A−/− vs WT.
In summary, an increase in novelty-induced exploratory activity was observed in male but not female dys-1A−/−.
Dysregulation of Anxiety-Related Behavior in Dys-1A −/−
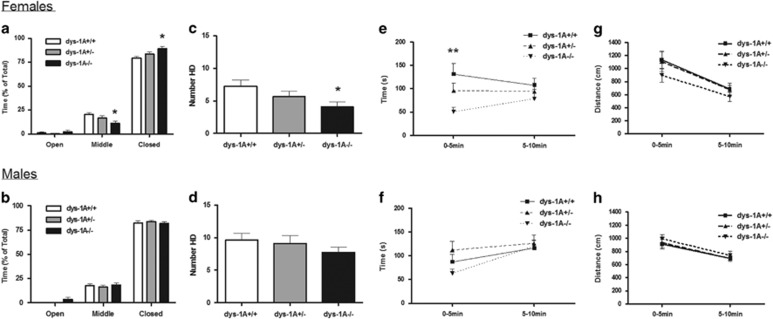
In the elevated plus-maze, the total number of entries into either arm did not differ between the genotypes for either sex (Supplementary Information Figure S1a,b). Female dys-1A−/− mice spent the majority of time in the closed arms, a minority of time in the middle area and little time in the open arms, in a manner that differed between the genotypes (compartment × genotype interaction: F(4,90)=3.74, p<0.01; dys-1A−/− mice spent more time in the closed arms, p<0.05, and less time in the center area, p<0.05); no significant effects were evident among males (Figures 3a and b). To further characterize these findings, we evaluated ethological behaviors that have been shown to not only enhance discrimination between general activity and emotional reactivity but also enhance the sensitivity of the test to anxiety-related behavior (Carobrez and Bertoglio, 2005). Female dys-1A−/− mice made fewer head-dips in the center hub (effect of genotype: F(2,30)=3.36, p<0.05; dys-1A−/− mice made fewer head-dips than WT mice, p<0.05), a decision point for approach/avoidance conflict, with no alteration in stretched-attend postures in the center hub or in the number of closed arm returns or rearing-to-wall in the closed arms (data not shown); no significant effects were evident among males (Figures 3c and d).
Figure 3.
Anxiety-related behavior in 24 WT (dys-1A+/+; 13 male, 11 female), 24 dys-1A+/− (12 male, 12 female) and 23 dys-1A−/− (13 male, 10 female) mice. Data are presented as means±SEM. (a,b) %Time spent in the open and closed arms and center hub (middle) area of the elevated plus-maze during the 5-min test period; *p<0.05 female dys-1A−/− vs WT. (c,d) Number of head-dips (HD) in the center hub during the 5-min test period; *p<0.05 female dys-1A−/− vs WT. (e,f) %Time spent in the light compartment of the light/dark box during each of two successive 5-min periods constituting a 10-min test; **p<0.01 female dys-1A−/− vs WT. (g,h) Distance traveled in the light/dark box during each of two successive 5-min periods constituting a 10-min test.
In the light/dark box, on initial exposure to the apparatus, female dys-1A−/− mice spent less time in the light compartment (effect of genotype: F(2,29)=3.37, p<0.05; during the first 5-min period, dys-1A−/− mice spent less time in the light compartment than WT mice, p<0.01), with no genotypic difference in distance traveled as an index of locomotor activity (Figures 3e and g); no significant effects were evident among males (Figures 3f and h).
In summary, across two measures of anxiety-related behavior, an increase in anxiety was observed in female but not male dys-1A−/−.
Prepulse Inhibition
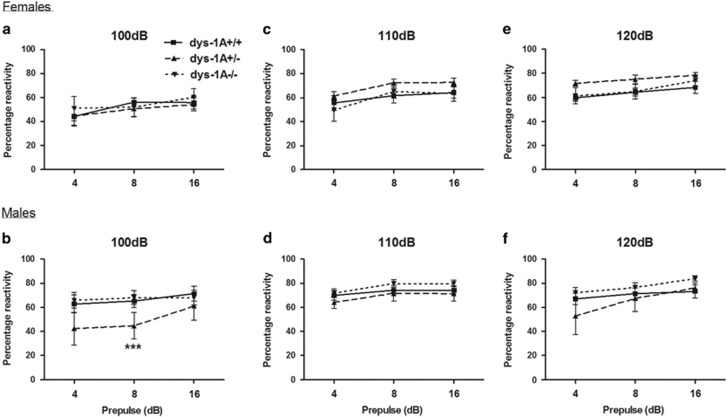
Reactivity to stimuli increased with both prepulse and pulse intensities, and habituated between block 1 and block 2 of the procedure, in a manner that did not differ between the genotypes (Supplementary Information Figure S2a–h). The only genotypic finding was that at pulse intensity 100 dBA male dys-1A+/- mice, but not dys-1A−/− mice, showed reduced % PPI (prepulse intensity × genotype interaction: F(4,52)=4.44, p<0.001; no significant effects were evident among females (Figures 4a and f). Although there are precedents for phenotypic effects in mice heterozygous but not homozygous for other mutations (Gogos et al, 1998; Babovic et al, 2008), as neither %PPI nor the internal control indices of responsivity to prepulse or pulse intensities alone and habituation to pulses were altered in either male or female dys-1A−/− mice, there appeared to be no fundamental disruption to the sensorimotor gating processes that underlie PPI in mice with the null mutation.
Figure 4.
Prepulse inhibition (PPI) in 23 WT (dys-1A+/+; 11 male, 12 female), 20 dys-1A+/- (8 male, 12 female) and 19 dys-1A−/− (10 male, 9 female) mice. Data are presented as means±SEM. (a,b) %PPI in mice subjected to prepulses of 4, 8, and 16 dBA at pulse intensity 100 dBA; ***p<0.001 male dys-1A+/- vs WT. (c) and (d) %PPI in mice subjected to prepulses of 4, 8, and 16 dBA at pulse intensity 110 dBA. (e,f) %PPI in mice subjected to prepulses of 4, 8, and 16 dBA at pulse intensity 120 dBA.
In summary, under one specific pulse intensity condition, a deficit in PPI was observed in male but not female dys-1A+/−, and not in dys-1A−/−, relative to all other groups.
Social Behavior
On assessment of sociability, both male and female spent the majority of time in the chamber containing Stranger 1, a minority of time in the opposite, empty chamber and little time in the center area, in a manner that did not differ between the genotypes (Supplementary Information Figure S3a,b). On subsequent assessment of social novelty preference, both males and females switched preference to spend the majority of time in the chamber containing the newly introduced Stranger 2, a minority of time in the opposite chamber containing the now familiar Stranger 1 and little time in the center area, in a manner that did not differ between the genotypes (Supplementary Information Figure S3c,d). On assessment of dyadic social interaction, for both males and females the number of olfactory investigations, time spent in olfactory investigations, number of walkovers, and time spent in walkovers did not differ between the genotypes (data not shown).
In summary, there appeared to be no genotype-related disruption to social behavior that involves either indirect or direct interaction with a conspecific.
Long-Term (Olfactory) Memory
On assessment of social transmission of food preference, male and female mice ate more food cued by the demonstrator than non-cued food at 24 h and at 7 days following interaction with the demonstrator, in a manner that did not differ between the genotypes (Supplementary Information Figure S4a–d).
Spontaneous Alternation Behavior
On assessment in the Y-maze, for both males and females, each of spontaneous alternation performance, percentage alternate-arm returns, and percentage same-arm returns did not differ between the genotypes (Supplementary Information Figure S5a–f).
Dysregulation of Delay/Interference-Dependent Working Memory in Dys-1A −/−
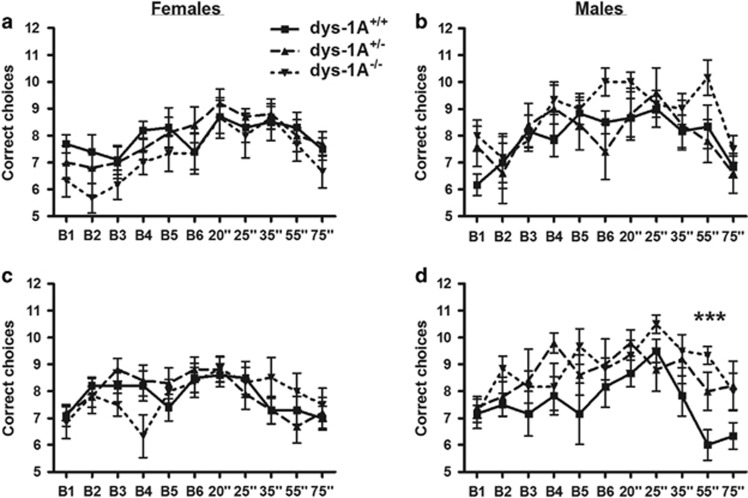
In the low-interference working memory task using the radial maze, the number of correct choices increased with training trials during acquisition for both sexes (males, effect of trial: F(5,70)=4.48, p<0.001; females, effect of trial: F(5,115)=2.71, p<0.05), followed by a delay-dependent decrease in number of correct choices (males, effect of trial: F(4,56)=6.23, p<0.001; females, effect of trial: F(492)=3.66, p<0.01), in a manner that did not differ between the genotypes (Figures 5a and b).
Figure 5.
Delay-dependent working memory in 16 WT (dys-1A+/+; 6 male, 10 female), 15 dys-1A+/- (5 male, 10 female), and 12 dys-1A−/− (6 male, 6 female) mice. Data are presented as means±SEM per 2-day block of trials. (a,b) Number of correct choices in the radial arm maze during acquisition trials (B1-6) and trials with increasing delay (20–75” (s)) under conditions of low interference. (c,d) Number of correct choices in the radial arm maze during acquisition trials (B1-6) and trials with increasing delay (20–75” (s)) under conditions of high interference; ***p<0.001 male dys-1A−/− vs WT.
In the high-interference working memory task, number of correct choices again increased with training trials during acquisition for both sexes (males, effect of trial: F(5,70)=2.47, p<0.05; females, effect of trial: F(5,115)=3.71, p<0.01), in a manner that did not differ between the genotypes. However, in male dys-1A−/− mice the delay-dependent decrease in number of correct choices differed between the genotypes (effect of trial: F(4,56)=6.58, p<0.001; effect of genotype: F(2,56)=10.46, p<0.01; dys-1A−/− made more correct choices than WT at the 55- s delay, p<0.01); in female dys-1A−/− the delay-dependent decrease in number of correct choices (effect of trial: F(4,92)=4.56, p<0.01) did not differ between the genotypes (Figures 5c and d).
In summary, male but not female dys-1A−/− showed enhancement in delay-dependent working memory under high levels of interference relative to controls, ie, impairment in sensitivity to the disruptive effect of such interference.
Discussion
We have demonstrated that targeted knockout of DTNBP1 exon 5 in mice on a C57BL/6 J background results in a novel dys-1A−/− mutant mouse with selective loss of dysbindin-1A protein expression. This is in contrast to the DTNBP1 mutation that arose spontaneously in the DBA2/J mouse and was later back-crossed into C57Bl/6 J mice, which results in loss of both dysbindin-1A and -1C (Talbot, 2009). Thus, the present studies in dys-1A−/− mutants inform, for the first time, on the functional roles of dysbindin-1A in schizophrenia-related and other phenotypes.
Heightened Initial Exploratory Behavior in Dys-1A −/− Mice
This finding related primarily to the very early phase of the interaction of dys-1A−/− mutants with the novel environment and subsequent habituation. The hyper-exploratory response to novelty, a predictive model of positive psychotic symptoms in schizophrenia (van den Buuse, 2010), appears to involve transient enhancement of perception and arousal, with early triggering of the mesocorticolimbic system to promote release of dopamine in the HF (Schomaker and Meeter, 2015). That the present findings were evident primarily in males would be consistent with evidence, from lower organisms through rodents to humans, that exploratory drive is stronger in males, in a manner that involves hippocampal more than accumbal processes (Clinton et al, 2011; Cross et al, 2013; Videlier et al, 2015). Recent studies in patients with schizophrenia, the majority of whom were male, have likewise reported over-activity in a novel, human open-field paradigm, with disruption to habituation of initial exploratory activity (Perry et al, 2009, 2010). Several DTNBP1 genetic variants (usually SNPs) in introns 1, 3, 4, and/or 7 are more closely associated with schizophrenia risk in males than in females, either increasing (Voisey et al, 2010; Tan et al, 2015) or decreasing (Zuo et al, 2009; Sacchetti et al, 2013) risk depending on the specific variants. If the genetic variants associated with increased risk for schizophrenia in males affect expression of exon 5 and thus dysbindin-1A, this could relate to the novelty-induced exploratory activity observed in male dys-1A−/−. If so, this may reflect an inhibitory role of dysbindin-1 on exploratory drive, and potentially other sexually dimorphic features of schizophrenia, and may help explain why males show a higher incidence, younger age at onset, and more severe psychopathology in schizophrenia (Aleman et al, 2003; McGrath et al, 2008; Abel et al, 2010; van der Werf et al, 2014).
Heightened Anxiety-Related Behavior in Dys-1A −/− Mice
This finding was robust across two anxiogenic paradigms. The anxiety response to stress appears to involve functional neuronal networks in which local circuits transfer information across different brain areas in an integrative manner, including the amygdala, PFC, ventral HF, and nucleus accumbens (Luthi and Luscher, 2014). That the anxiety response was significant only in females is consistent with evidence from rodents and humans that anxiety-related behaviors are more evident in females due in part to baseline and stress-induced hypothalamic-pituitary-adrenal axis responses that involve the brain areas mentioned above (Simpson and Kelly, 2012; Altemus et al, 2014; Luthi and Luscher, 2014). Thus, dysbindin-1A may have an inhibitory role in these processes in females. Anxiety is a prominent comorbid psychopathology in schizophrenia, more commonly than in the general population, where it is more evident in females than in males (Braga et al, 2013; Pallanti et al, 2013; Altemus et al, 2014). It is unknown if related effects in dys-1A−/− reflect effects of DTNBP1 genetic variants in introns 1 and 4 that affect risk for schizophrenia more in females than males, either positively (Zuo et al, 2009) or negatively (Sacchetti et al, 2013) depending on the specific genetic variant.
Dysregulation of Specialized Delay/Interference-Dependent Working Memory in Dys-1A −/− Mice
Although long-term (olfactory) memory, spontaneous alternation behavior, and delay-dependent working memory under low levels of interference were unaltered, dys-1A−/− showed enhancement in delay-dependent working memory under high levels of interference relative to controls, ie, impairment in sensitivity to the disruptive effect of such interference, revealing a highly specialized role for dysbindin-1A in cognition. The PFC has long been implicated in working memory processes (Fuster, 2000), including delay-period activity (Liu et al, 2014), and in the cognitive dysfunction of schizophrenia (Keefe and Harvey, 2012). Recent evidence indicates a role for parallel processing of information between PFC and HF in such functions for shorter delay times, with the balance shifting toward involvement of HF with increasing delay (Malleret et al, 2010). Therefore, the present findings suggest an important role for dysbindin-1A in regulating the sensitivity of delay-dependent working memory to higher levels of interference. This process has been shown to involve plasticity in the DG (Malleret et al, 2010), with a particular role for neurogenesis (Saxe et al, 2007), including circuit-dependent integration of immature neurons in effective DG function (Rangel et al, 2013). Schizophrenia cases show diminished vulnerability to proactive interference owing to their greater loss of previous working memory traces and disruption of coupling between PFC and subcortical (limbic/thalamic) regions, shown with functional imaging (Anticevic et al, 2012; Kaller et al, 2014), that would confer greater autonomy to temporal lobe regions.
The fact that these cognitive abnormalities were significant only in males may be consistent with reports noted earlier that some genetic variants in DTNBP1 modify risk for schizophrenia in males more than in females (Zuo et al, 2009; Voisey et al, 2010; Sacchetti et al, 2013; Tan et al, 2015). As also noted earlier, these variants are in non-coding regions of the DTBP1 gene, specifically introns 1, 3–4, and/or 7 close to exons encoding the NTR of dysbindin-1A (ie exons 1 and 3–5; Talbot et al, 2009). In contrast to other locations in the gene, variability in these NTR-associated DTNBP1 introns is associated with early-onset psychosis (Gornick et al, 2005; Fatjo-Vilas et al, 2011) and cognitive impairment in schizophrenia (Zinkstok et al, 2007; Wessman et al, 2009; Zhang et al, 2010).
Comparing Behavioral Phenotypes in Dys-1A −/− and sdy −/− Mice
The present findings include genotypic effects that are present in one sex but not in the other, and it is now recommended that sex differences in biological effects should be assumed until proven otherwise (McCarthy, 2015). Though these can be attested by significant genotype × sex interactions, that they here fall short of a conventional level of statistical significance likely reflects that detection of such interactions requires sample sizes ~4 × those needed to detect main effects. Thus, confirmation of sex differences in phenotypic effects awaits studies with larger sample sizes.
Differential functions of dysbindin-1A vs dysbindin-1C are suggested by comparing the present findings with those on sdy−/− mice, which lack both dysbindin-1A and -1C (Talbot, 2009; see Supplementary Information Table S2 for comparative summary of behavioral phenotypes studied in mostly male sdy mutants and the present dys-1A mutants of both sexes). The many indices of sensorimotor function tested here proved to be normal in dys-1A−/− mice, as they are in sdy−/− mice on either a DBA/2 J (sdy/DBA; Takao et al, 2008) or a C57Bl/6 J (sdy/Bl6; Cox et al, 2009; Papaleo et al, 2012) background, as well as in mice selectively overexpressing human dysbindin-1A (Shintani et al, 2014). Unlike female dys-1A−/− mice, there is no clear evidence for increased anxiety in female sdy−/−/Bl6 mice (Cox et al, 2009). Unlike male dys-1A−/− mice, there is evidence to suggest increased anxiety in male sdy−/−/DBA mice under extended testing conditions (Hattori et al, 2008). Also, unlike male dys-1A−/− mice, male sdy−/−/BL6 mice show impaired prepulse inhibition (Carlson et al, 2011; Papaleo et al, 2012), and sdy−/−/DBA mice show reduced social interactions (Hattori et al, 2008; Feng et al, 2008). However, these apparent differences require further investigation, because only one study has been published on the behavior of female sdy−/−/Bl6 mice (Cox et al, 2009) and because the specific tests of working memory studied here have not been examined in sdy−/− mice. Also, sdy−/− mice have not been evaluated for long-term (olfactory) memory, which we found to be normal in dys-1A−/− mice.
Comparison of dys-1A−/− and sdy−/− mice suggests that the behavioral roles of dysbindin-1A are more subtle than those of dysbindin-1C. This may reflect the finding in mice that levels of dysbindin-1A are lower than those of dysbindin-1C in many forebrain areas, including the cerebral cortex and HF (Ito et al, 2010; Wang et al, 2014). Of the three brain areas of schizophrenia cases investigated for dysbindin-1 isoforms, reductions in dysbindin-1A have been found in auditory association cortices of the superior temporal gyrus (Talbot et al, 2011), whereas reductions in dysbindin-1C have been found in the dorsolateral PFC (Tang et al, 2009) and HF (Talbot et al, 2011).
The more subtle behavioral abnormalities of dys-1A−/− compared with sdy−/− mice provides a more selective model of certain aspects of schizophrenia. Among these is BLOC-1 reduction in this disorder, as dysbindin-1A (but not dysbindin-1C) is essential for the integrity of BLOC-1 (Li et al, 2003; Larimore et al, 2014; Wang et al, 2014), other components of which are markedly reduced in brains of schizophrenia cases (Talbot et al, in preparation). BLOC-1 reductions may help explain aspects of synaptic dysfunction in schizophrenia, as they impair synaptic protein trafficking and vesicle release (Mullin et al, 2011, 2015).
The dys-1A−/− mutant may also be a more selective model of auditory dysfunction associated with dysbindin-1 reductions in schizophrenia than is the sdy−/− model, as dysbindin-1A, but not dysbindin-1C, is reduced in auditory association cortices of schizophrenia cases (Talbot et al, 2011). Several DTNBP1 SNPs are associated with auditory, visual, and olfactory hallucinations in schizophrenia, two of which are in linkage disequilibrium with a DTNBP1 SNP reported to reduce expression of the dysbindin-1A gene (Cheah et al, 2015). In sdy−/− mice, which lack both dysbindin-1A and -1C, auditory cortices in general are atrophic (Lutkenhoff et al, 2012) and dysfunctional (Carlson et al, 2011). Future studies should thus examine the auditory cortices of dys-1A−/− mice to determine the consequences of selective dysbindin-1A loss in these brain areas. In summary, the present findings in dys-1A−/− mice on a C57Bl/6 J background provide the first evidence for selective functional roles for dysbindin-1A vis-a-vis dysbindin-1C among phenotypes relevant to the pathobiology of schizophrenia. Future studies should investigate putative sex differences in these phenotypic effects.
Funding and disclosure
This work was supported by a grant from Science Foundation Ireland (07/IN.1/B960) to JLW. JJ, AS, SAS, ADM, SW, EMS, and JNK are or were employees of GlaxoSmithKline. The authors declare no conflict of interest.
Acknowledgments
We thank A Kinsella for statistical advice and S Kerrigan, K Fantom, A West, G Malleret, and R Rutter for technical advice. This article is dedicated to the memory of James N Kew, following his tragic death.
Author contributions
JLW, EIP, BK, CMPO'T, and JNK conceived the project, designed the experiments, interpreted the results and wrote the paper. EIP, ZM, RC, CMPO'T, NC, OT, KT, DB, and DCH contributed to phenotypic studies and analyzed the data. JJ, AS, SAS, ADM, SW, and EMS constructed the mutants.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Abel KM, Drake R, Goldstein JM (2010). Sex differences in schizophrenia. Int Rev Psychiatry 22: 417–428. [DOI] [PubMed] [Google Scholar]
- Aggleton JP (2012). Multiple anatomical systems embedded within the primate medial temporal lobe: implications for hippocampal function. Neurosci Biobehav Rev 36: 1579–1596. [DOI] [PubMed] [Google Scholar]
- Aleman A, Kahn RS, Selten JP (2003). Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry 60: 565–571. [DOI] [PubMed] [Google Scholar]
- Allen NC, Bagade S, McQueen MB, Ioannidis JP, Kavvoura FK, Khoury MJ et al (2008). Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet 40: 827–834. [DOI] [PubMed] [Google Scholar]
- Altemus M, Sarvaiya N, Neill Epperson C (2014). Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol 35: 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Krystal JH, Barch DM (2012). A broken filter: prefrontal functional connectivity abnormalities in schizophrenia during working memory interference. Schizophr Res 141: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayalew M, Le-Niculescu H, Levey DF, Jain N, Changala B, Patel SD et al (2012). Convergent functional genomics of schizophrenia: from comprehensive understanding to genetic risk prediction. Mol Psychiatry 17: 887–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babovic D, O'Tuathaigh CM, O'Connor AM, O'Sullivan GJ, Tighe O, Croke DT et al (2008). Phenotypic characterization of cognition and social behavior in mice with heterozygous versus homozygous deletion of catechol-O-methyltransferase. Neuroscience 155: 1021–1029. [DOI] [PubMed] [Google Scholar]
- Baek JH, Kim JS, Ryu S, Oh S, Noh J, Lee WK et al (2012). Association of genetic variations in DTNBP1 with cognitive function in schizophrenia patients and healthy subjects. Am J Med Genet B Neuropsychiatr Genet 7: 841–849. [DOI] [PubMed] [Google Scholar]
- Benson MA, Newey SE, Martin-Rendon E, Hawkes R, Blake DJ (2001). Dysbindin, a novel coiled-coil containing protein that interacts with the dystrobrevins in muscle and brain. J Biol Chem 276: 24232–24241. [DOI] [PubMed] [Google Scholar]
- Braga RJ, Reynolds GP, Siris SG (2013). Anxiety comorbidity in schizophrenia. Psychiatry Res 210: 1–7. [DOI] [PubMed] [Google Scholar]
- Burdick KE, Goldberg TE, Funke B, Bates JA, Lencz T, Kucherlapati R et al (2007). DTNBP1 genotype influences cognitive decline in schizophrenia. Schizophr Res 89: 169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson GC, Talbot K, Halene TB, Gandal MJ, Kazi HA, Schlosser L et al (2011). Dysbindin-1 mutant mice implicate reduced fast-phasic inhibition as a final common disease mechanism in schizophrenia. Proc Natl Acad Sci USA 108: E962–E970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ (2005). Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev 29: 1193–1205. [DOI] [PubMed] [Google Scholar]
- Cheah S-Y, Lawford BR, Young RM, Morris CP, Voisey J (2015). Dysbindin (DTNBP1 variants are associated with hallucinations in schizophrenia. Eur Psychiatry 30: 486–491. [DOI] [PubMed] [Google Scholar]
- Clinton SM, Stead JD, Miller S, Watson SJ, Akil H (2011). Developmental underpinnings of differences in rodent novelty-seeking and emotional reactivity. Eur J Neurosci 34: 994–1005 s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MM, Tucker AM, Tang J, Talbot K, Richer DC, Yeh L et al (2009). Neurobehavioral abnormalities in the dysbindin-1 mutant, sandy, on a C57Bl/6 J genetic background. Genes Brain Behav 8: 390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross CP, Cyrenne DL, Brown GR (2013). Sex differences in sensation-seeking: a meta-analysis. Sci Rep 3: 2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudchenko PA (2004). An overview of the tasks used to test working memory in rodents. Neurosci Biobehav Rev 28: 699–709. [DOI] [PubMed] [Google Scholar]
- Fatjo-Vilas M, Papiol S, Estrada G, Bombin I, Peralta V, Rosa A et al (2011). Dysbindin-1 gene contributes differentially to early- and adult-onset forms of functional psychosis. Am J Med Genet B Neuropsychiatr Genet 3: 322–333. [DOI] [PubMed] [Google Scholar]
- Feng Y-Q, Zhou Z-Y, He X, Wang H, Guo X-L, Hao C-J et al (2008). Dysbindin deficiency in sandy mice causes reduction of snapin and displays behaviors related to schizophrenia. Schizophr Res 106: 218–228. [DOI] [PubMed] [Google Scholar]
- Fuster JM (2000). Executive frontal functions. Exp Brain Res 133: 66–70. [DOI] [PubMed] [Google Scholar]
- Ghiani CA, Starcevic M, Rodriguez-Fernandez IA, Nazarian R, Cheli VT, Chan LN et al (2010). The dysbindin-containing complex (BLOC-1) in brain: developmental regulation, interaction with SNARE proteins and role in neurite outgrowth. Mol Psychiatry 15: 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D et al (1998). Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA 95: 9991–9996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-López A, López-Alonso I, Aquirre A, Amado-Rodriguez L, Batalla-Solis E, Astudillo A et al (2013). Mechanical ventilation triggers hippocampal apoptosis by vagal and dopaminergic pathways. Am J Respir Crit Care Med 188: 693–702. [DOI] [PubMed] [Google Scholar]
- Gornick MC, Addington AM, Sporn A, Gogtay N, Greenstein D, Lenane M et al (2005). Dysbindin (DTNBP1, 6p22.3) is associated with childhood-onset psychosis and endophenotypes measured by the Premorbid Adjustment Scale (PAS). J Autism Dev Disord 35: 831–838. [DOI] [PubMed] [Google Scholar]
- Häfner H, Hambrecht M, Löffler W, Munk-Jørgensen P, Riecher-Rössler A (1998). Is schizophrenia a disorder of all ages? A comparison of first episodes and early course across the life-cycle. Psychol Med 28: 351–365. [DOI] [PubMed] [Google Scholar]
- Hattori S, Murotani T, Matsuzaki S, Ishizuka T, Kumamoto N, Takeda M et al (2008). Behavioral abnormalities and dopamine reductions in sdy mutant mice with a deletion in DTNBP1, a susceptibility gene for schizophrenia. Biochem Biophys Res Commun 373: 298–302. [DOI] [PubMed] [Google Scholar]
- Irwin S (1968). Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia 13: 222–257. [DOI] [PubMed] [Google Scholar]
- Ito H, Morishita R, Shinoda T, Iwamoto I, Sudo K, Okamoto K et al (2010). Dysbindin-1, WAVE2 and Abi-1 form a complex that regulates dendritic spine formation. Mol Psychiatry 15: 976–986. [DOI] [PubMed] [Google Scholar]
- Jia J-M, Hu Z, Nordman J, Li Z (2014). The schizophrenoa susceptibility gene dysbindin regulates dendritic spines. J Neurosci 34: 13725–13736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaller CP, Loosli SV, Rahm B, Gossel A, Schieting S, Hornig T et al (2014). Working memory in schizophrenia: behavioral and neural evidence for reduced susceptibility to item-specific proactive interference. Biol Psychiatry 76: 486–494. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Harvey PD (2012). Cognitive impairment in schizophrenia. Handbook Exp Pharmacol 213: 11–37. [DOI] [PubMed] [Google Scholar]
- Larimore J, Zlatic SA, Gokhale A, Tornieri K, Kaela S, Singleton S et al (2014). Mutations in the BLOC-1 subunits dysbindin and muted generate divergent and dosage-dependent phenotypes. J Biol Chem 289: 14291–14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhang Q, Oiso N, Novak EK, Gautam R, O'Brien EP et al (2003). Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1). Nat Genet 35: 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Gu X, Zhu J, Zhang X, Han Z, Yan W et al (2014). Medial prefrontal activity during delay period contributes to learning of a working memory task. Science 346: 458–463. [DOI] [PubMed] [Google Scholar]
- Luthi A, Luscher C (2014). Pathological circuit function underlying addiction and anxiety disorders. Nat Neurosci 17: 1635–1643. [DOI] [PubMed] [Google Scholar]
- Lutkenhoff E, Karlsgodt KH, Gutman B, Stein JS, Thompson PM, Cannon TD et al (2012). Structural and functional neuroimaging phenotypes in dysbindin mutant mice. NeuroImage 62: 120–129. [DOI] [PubMed] [Google Scholar]
- Malleret G, Alarcon JM, Martel G, Takizawa S, Vronskaya S, Yin D et al (2010). Bidirectional regulation of hippocampal long-term synaptic plasticity and its influence on opposing forms of memory. J Neurosci 30: 3813–3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM (2015). Incorporating sex as a variable in preclinical neuropsychiatric research. Schizophr Bull 41: 1016–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Sah AP, Gokhalea S, Chant D, Welham J (2008). Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev 30: 67–76. [DOI] [PubMed] [Google Scholar]
- Mullin AP, Gokhale A, Larimore J, Faundez V (2011). Cell biology of the BLOC-1 complex subunit dysbindin, a schizophrenia susceptibility gene. Mol Neurobiol 44: 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrek A, Mancini-Marie A (2015). Sex/gender differences in the brain and cognition in schizophrenia. Neurosci Biobehav Rev 30: 30111–30111. [DOI] [PubMed] [Google Scholar]
- Mullin AP, Sadanandappa MK, Ma W, Dickman DK, VijayRaghavan K, Ramaswami M et al (2015). Gene dosage in the dysbindin schizophrenia susceptibility network differentially affect synaptic function and plasticity. J Neurosci 35: 325–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numakawa T, Yagasaki Y, Ishimoto T, Okada T, Suzuki T, Iwata N et al (2004). Evidence of novel neuronal functions of dysbindin, a susceptibility gene for schizophrenia. Hum Mol Genet 13: 2699–2708. [DOI] [PubMed] [Google Scholar]
- O'Tuathaigh CM, Clarke G, Walsh J, Desbonnet L, Petit E, O'Leary C et al (2012). Genetic vs. pharmacological inactivation of COMT influences cannabinoid-induced expression of schizophrenia-related phenotypes. Int J Neuropsychopharmacol 15: 1331–1342. [DOI] [PubMed] [Google Scholar]
- O'Tuathaigh CM, Hryniewiecka M, Behan A, Tighe O, Coughlan C, Desbonnet L et al (2010). Chronic adolescent exposure to Delta-9-tetrahydrocannabinol in COMT mutant mice: impact on psychosis-related and other phenotypes. Neuropsychopharmacology 35: 2262–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Tuathaigh CM, O'Connor AM, O'Sullivan GJ, Lai D, Harvey R, Croke DT et al (2008). Disruption to social dyadic interactions but not emotional/anxiety-related behaviour in mice with heterozygous 'knockout' of the schizophrenia risk gene neuregulin-1. Prog Neuropsychopharmacol Biol Psychiatry 32: 462–466. [DOI] [PubMed] [Google Scholar]
- Oyama S, Yamakawa H, Sasagawa N, Hosoi Y, Futai E, Ishiura S (2009). Dysbindin-1, a schizophrenia-related protein, functionally interacts with the DNA- dependent protein kinase complex in an isoform-dependent manner. PLoS One 4: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Yang F, Garcia S, Chen J, Lu B, Crawley JN et al (2012). Dysbindin-1 modulates prefrontal cortical activity and schizophrenia-like behaviors via dopamine/D2 pathways. Mol Psychiatry 17: 85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallanti S, Cantisani A, Grassi G (2013). Anxiety as a core aspect of schizophrenia. Curr Psychiatry Rep 15: 354. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Henry B, Kincaid M, Young JW, Geyer MA (2010). Quantifying over-activity in bipolar and schizophrenia patients in a human open field paradigm. Psychiatry Res 178: 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ et al (2009). A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Arch Gen Psychiatry 66: 1072–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel LM, Quinn LK, Chiba AA, Gage FH, Aimone JB (2013). A hypothesis for temporal coding of young and mature granule cells. Front Neurosci 7: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DC, Fisher EM, Brown SD, Peters J, Hunter AJ, Martin JE (1997). Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome 8: 711–713. [DOI] [PubMed] [Google Scholar]
- Sacchetti E, Scassellati C, Minelli A, Valsecchi P, Bonvicini C, Pasqualetti P et al (2013). Schizophrenia susceptibility and NMDA-receptor mediated signalling: an association study involving 32 tagSNPs of DAO, DAOA, PPP3CC, and DTNBP1 genes. BMC Med Genet 14: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, Sofroniew MV et al (2007). Paradoxical influence of hippocampal neurogenesis on working memory. Proc Natl Acad Sci USA 104: 4642–4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomaker J, Meeter M (2015). Short- and long-lasting consequences of novelty, deviance and surprise on brain and cognition. Neurosci Biobehav Rev 55: 268–279. [DOI] [PubMed] [Google Scholar]
- Shintani N, Onaka Y, Hashimoto R, Takamura H, Nagata T, Umeda-Yano S et al (2014). Behavioral characterization of mice overexpressing dysbindin-1. Mol Brain 7: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J, Kelly JP (2012). An investigation of whether there are sex differences in certain behavioural and neurochemical parameters in the rat. Behav Brain Res 229: 289–300. [DOI] [PubMed] [Google Scholar]
- Soma M, Wang M, Suo S, Ishiura S (2014). Dysbindin-1, a schizophrenia-related protein, interacts with HDAC3. Neurosci Lett 582: 120–124. [DOI] [PubMed] [Google Scholar]
- Takao K, Toyama K, Nakanishi K, Hattori S, Takamura H, Takeda M et al (2008). Impaired long-term memory retention and working memory in sdy mutant mice with a deletion in Dtnbp1, a susceptibility gene for schizophrenia. Mol Brain 1: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot K (2009). The sandy (sdy) mouse: a dysbindin-1 mutant relevant to schizophrenia research. Prog Brain Res 179: 87–94. [DOI] [PubMed] [Google Scholar]
- Talbot K, Eidem WL, Tinsley CL, Benson MA, Thompson EW, Smith RJ et al (2004). Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. J Clin Invest 113: 1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot K, Louneva N, Cohen JW, Kazi H, Blake DJ, Arnold SE (2011). Synaptic dysbindin-1 reductions in schizophrenia occur in an isoform-specific manner indicating their subsynaptic location. PLoS One 6: e16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot K, Ong W-Y, Blake DJ, Tang J, Louneva N, Carlson GC et al (2009) Dysbindin-1 and Its Protein Family. In: Lajtha A, Javitt D, Kantrowitz J (eds). Handbook of Neurochemistry and Molecular Neurobiology: Schizophrenia. Springer: Boston, pp 107–241. [Google Scholar]
- Tan GKN, Tee SF, Tang PY (2015). Genetic association of single nucleotide polymorphisms in dystrobrevin binding protein 1 gene with schizophrenia in a Malayasian population. Genet Mol Biol 38: 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, LeGros RP, Louneva N, Yeh L, Cohen JW, Hahn CG et al (2009). Dysbindin-1 in dorsolateral prefrontal cortex of schizophrenia cases is reduced in an isoform-specific manner unrelated to dysbindin-1 mRNA expression. Hum Mol Genet 18: 3851–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Buuse M (2010). Modeling the positive symptoms of schizophrenia in genetically modified mice: pharmacology and methodology aspects. Schizophr Bull 36: 246–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werf M, Hanssen M, Kohler S, Verkaaik M, Verhey FR et alRISE Investigators (2014). Systematic review and collaborative recalculation of 133,693 incident cases of schizophrenia. Psychol Med 44: 9–16. [DOI] [PubMed] [Google Scholar]
- Varela-Gomez N, Mata I, Perez-Iglesias R, Rodriguez-Sanchez JM, Ayesa R, Fatjo-Vilas M et al (2015). Dysbindin gene variability is associated with cognitive abnormalities in first-episode non-affective psychosis. Cogn Neuropsychiatry 20: 144–156. [DOI] [PubMed] [Google Scholar]
- Videlier M, Cornette R, Bonneaud C, Herrel A (2015). Sexual differences in exploration behavior in Xenopus tropicalis? J Exp Biol 218: 1733–1739. [DOI] [PubMed] [Google Scholar]
- Voisey J, Swagell CD, Hughes IP, Lawford BR, Young RM, Morris CP (2010). Analysis of HapMap tag-SNPs in dysbindin (DTNBP1) reveals evidence of consistent association with schizophrenia. Eur Psychiatry 25: 314–319. [DOI] [PubMed] [Google Scholar]
- Wang H, Yuan Y, Zhang Z, Yan H, Feng Y, Li W (2014). Dysbindin-1C is required for the survival of hilar mossy cells and the maturation of adult newborn neurons in dentate gyrus. J Biol Chem 289: 29060–29072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert CS, Rothmond DA, Hyde TM, Kleinman JE, Straub RE (2008). Reduced DTNBP1 (dysbindin-1) mRNA in the hippocampal formation of schizophrenia patients. Schizophr Res 98: 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert CS, Straub RE, McClintock BW, Matsumoto M, Hashimoto R, Hyde TM et al (2004). Human dysbindin (DTNBP1) gene expression in normal brain and in schizophrenic prefrontal cortex and midbrain. Arch Gen Psychiatry 61: 544–555. [DOI] [PubMed] [Google Scholar]
- Wessman J, Paunio T, Tuulio-Henriksson A, Koivisto M, Partonen T, Suvisaari J et al (2009). Mixture model clustering of phenotype features reveals evidence for association of DTNBP1 to a specific subtype of schizophrenia. Biol Psychiatry 66: 990–996. [DOI] [PubMed] [Google Scholar]
- Wolf C, Jackson MC, Kissling C, Thome J, Linden DE (2011). Dysbindin-1 genotype effects on emotional working memory. Mol Psychiatry 16: 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrenn CC, Harris AP, Saavedra MC, Crawley JN (2003). Social transmission of food preference in mice: methodology and application to galanin-overexpressing transgenic mice. Behav Neurosci 117: 21–31. [PubMed] [Google Scholar]
- Yuan Y, Wang H, Wei Z, Li W (2015). Impaired autophagy in hilar mossy cells of the dentate gyrus and its implication for schizophrenia. J Genet Genomics 42: 1–8. [DOI] [PubMed] [Google Scholar]
- Zhang J-P, Burdick KE, Lencz T, Malhotra AK (2010). Meta-analysis of genetic variation in DTNBP1 and general cognitive ability. Biol Psychiatry 68: 1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkstok JR, de Wilde O, van Amelsvoort TAMJ, Tanck MW, Baas F, Linszen DH (2007). Association between the DTNBP1 gene and intelligenece: a case-control study in young patients with schizophrenia and related disorders and unaffected siblings. Behav Brain Funct 3: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L, Luo X, Kranzler HR, Lu L, Rosenheck RA, Cramer J et al (2009). Association study of DTNBP1 with schizophrenia in a US sample. Psychiatr Genet 19: 292–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.