Abstract
Tremendous progress in genetics and genomics led to a wide range of healthcare providers, genetic tests, and more patients who can benefit from these developments. To guarantee and improve the quality of genetic testing, a unified European-based registration for individuals qualified in biomedicine was realized. Therefore a Europe-wide recognition of the profession ‘European registered Clinical Laboratory Geneticist (ErCLG)' based on a syllabus of core competences was established which allows for harmonization in professional education. The ‘European Board of Medical Genetics division − Clinical Laboratory Geneticist' provides now since 3 years the possibility to register as an ErCLG. Applicants may be from all European countries and since this year also from outside of Europe. Five subtitles reflect the exact specialty of each ErCLG, who can reregister every 5 years. A previously not possible statistics based on ~300 individuals from 19 countries as holders of an ErCLG title provides interesting insights into the professionals working in human genetics. It could be substantiated that there are around twice as many females than males and that a PhD title was achieved by 80% of registered ErCLGs. Also most ErCLGs are still trained as generalists (66%), followed by such ErCLGs with focus on molecular genetics (23%); the remaining are concentrated either on clinical (6%), tumor (4%) or biochemical genetics (1%). In conclusion, besides MDs and genetic counselors/nurses an EU-wide recognition system for Clinical Laboratory Geneticist has been established, which strengthens the status of specialists working in human genetic diagnostics in Europe and worldwide.
Introduction
Technological advances in genetics and genomics have resulted in genetic testing being used by a wide range of healthcare providers. Multiple genetic tests are now available, increasing numbers of patients can benefit from genetic testing and genetic testing is now more publicly affordable (in both public and private laboratories).1 This situation brings benefits as well as problems, as concerns about the quality of diagnostic tests offered by non-genetic specialists have been raised for at least 10 years.1, 2, 3, 4 The European Society of Human Genetics (ESHG) was one of those who discussed early how to encounter the differences among the different countries in Europe concerning provision of genetic services. ESHG and its Public and Professional Policy Committee examined the professional and scientific views on the social, ethical and legal issues that influence genetic services throughout member states of European Union.5 One of the most important key elements in improving the quality of genetic testing in Europe was the provision of appropriate genetic education for health professionals. The work carried out by EuroGentest-Network of Excellence6 underlined the need for concerted efforts to provide professional education to a range of health professionals who play a role in providing genetic testing.7 Patient information was also considered as one of the milestones to provide an appropriate genetic counseling.8 Moreover, thanks to the work carried out by the Education Committee of the ESHG, the core competences to support preparation of health professionals in Europe were published in 2010.9
Thus, in order to potentiate the best possible healthcare and to ensure appropriate levels of competence, there is a proven need for establishing a unified European-based registration for individuals qualified in biomedicine who work in a human genetics diagnostic laboratory, that is, biology and related subjects. These professionals are mainly trained as scientists but then they were appointed in a genetic diagnostic setting. In parallel also some medical doctors decided to work full time in laboratory settings and they are also involved. Therefore a Europe-wide recognition of the profession ‘European registered Clinical Laboratory Geneticist (ErCLG)' based on a syllabus of core competences (https://www.eshg.org/fileadmin/eshg/EBMG/CLG/Core-Curriculum_2016.pdf) will allow harmonization in professional education. Similar efforts were already undertaken for genetic counselors;10 MDs working in genetic counseling are already recognized by EU-laws (https://www.eshg.org/111.0.html).
Background
An increasing need for recognition of a laboratory specialty in human genetic diagnostics was recognized by the ESHG in 2010/2011. Thus, an ‘Ad hoc core committee: laboratory genetics' was then brought into being (Jacqueline Schoumans (chair), Egbert Bakker (co-chair), et al.). After establishing the European Board of Medical Genetics (EBMG) in June 2012 (legal entity since June 2014) the ‘European Board of Medical Genetics division – Clinical Laboratory Geneticist' was founded from the aforementioned subcommittee (https://www.eshg.org/668.0.html).
The major work of this committee between 2011 and 2012 (Thomas Liehr (chair), Isabel M. Carreira (co-chair), et al.) was to introduce an ErCLG curriculum. A proposal for a core curriculum was established in 2012 and has been slightly revised twice since then (https://www.eshg.org/fileadmin/eshg/EBMG/CLG/Core-Curriculum_2016.pdf). The core curriculum has been approved by the ESHG Executive Committee Board and has additionally received support from 14 country representatives, representing 204/345 (59%) of the EU weighted (qualified majority) votes (https://www.eshg.org/668.0.html).
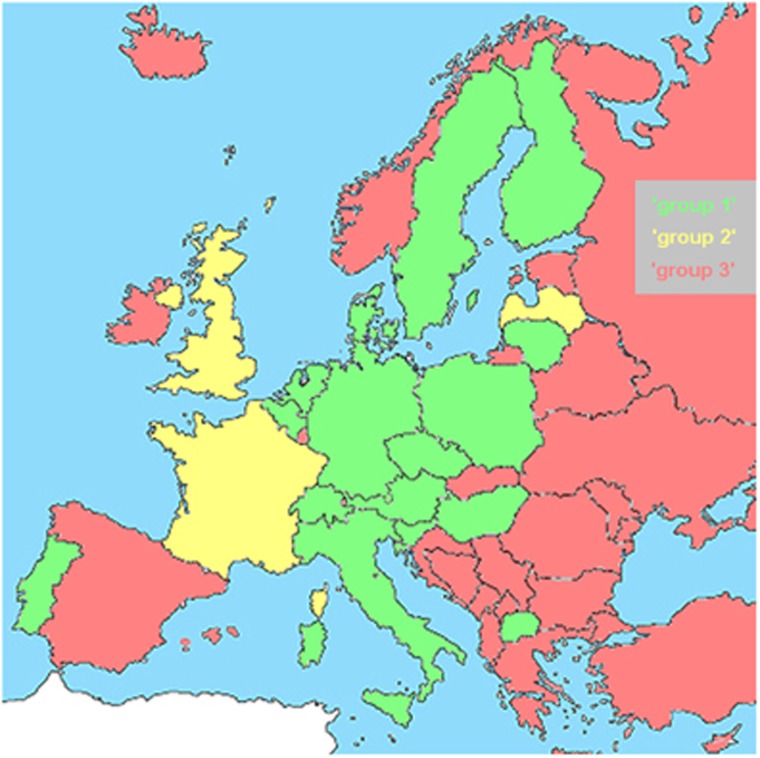
The next task in 2012 was to contact 150 individuals working as Clinical Laboratory Geneticist (CLGs) in 44 countries. They were requested to help aligning the requirements of the ErCLG core curriculum (https://www.eshg.org/fileadmin/eshg/EBMG/CLG/Core-Curriculum_2016.pdf) with those of their national guidelines for professional education. Information was returned by colleagues from 41 countries. The results led to the allocation of these countries into three different groups, those with a national CLG title (groups 1 and 2) and those without (group 3) (Figure 1). Group 1 countries have national CLG education schemes that fit to the core curriculum by 46 of 52 points as shown in Supplementary Table 1; all countries with national titles but falling below this cutoff are put in group 2, and applicants must fulfill some country-specific additional prerequisites to be eligible for the ErCLG title. Forty-four European and four non-European countries were included in this grouping (Table 1).
Figure 1.
Map of Europe indicating the distribution of countries with national CLG title (groups 1 and 2) and such without (group 3).
Table 1. European country group list in alphabetic order. In case a country is not listed yet or some changes are made in a country, please contact EBMG-ErCLG board.
| Country name | Group | National title |
|---|---|---|
| European countries | ||
| Albania | 3 | n.a. |
| Armenia | 3 | n.a. |
| Austria | 1 | Fachhumangenetiker/Fachhumangenetikerin (ÖGH) |
| Belarus | 3 | n.a. |
| Belgium | 1 | Medical Genetic Laboratory Supervisor |
| Bosnia & Herzegovina | 3 | n.a. |
| Bulgaria | 3 | n.a. |
| Croatia | 3 | n.a. |
| Cyprus | 3 | n.a. |
| Czech Republic | 1 | Clinical Bioanalytician in Clinical Genetics |
| Denmark | 1 | Klinisk laboratoriegenetiker (Clinical Laboratory Geneticist) |
| Estonia | 3 | n.a. |
| Finland | 1 | sairaalageneetikko=sjukhusgenetiker (Hospital geneticist) |
| France | 2a | Biology, option genetics |
| Georgia | 3 | n.a. |
| Germany | 1 | Fachhumangenetiker/Fachhumangenetikerin (GfH) |
| Greece | 3 | n.a. |
| Hungary | 1 | Molecular Genetic Diagnostics (MD)/Molecular Biology Diagnostics (PhD) |
| Iceland | 3 | n.a. |
| Ireland | 3 | n.a. |
| Israel | 1 | Hum. cytogeneticist, hum. molecular/biochemical geneticist |
| Italy | 1 | Genetica Medica, Genetica Applicata, Citogenetica Umana, Applicazioni Biotecnologice |
| Latvia | 2b | Geneticist – medical support person |
| Lithuania | 1 | Medical geneticist (laboratory) |
| Macedonia | 1 | Clinical Laboratory Genetics |
| Malta | 3 | n.a. |
| Moldova | 3 | n.a. |
| Montenegro | 3 | n.a. |
| Netherlands | 1 | Clinical Laboratory Geneticist |
| Norway | 3 | n.a. |
| Poland | 1 | Laboratory Medical Genetics |
| Portugal | 1 | Técnico Superior de Saúde, ramo de Genética |
| Romania | 3 | n.a. |
| Russia | 3 | n.a. |
| Serbia | 3 | n.a. |
| Slovakia | 1 | Laboratory and diagnostic methods in Clinical Genetics |
| Slovenia | 1 | Medical Genetic Laboratory Programme |
| Spain | 3 | n.a. |
| Sweden | 1 | sairaalageneetikko=sjukhusgenetiker (Hospital geneticist) |
| Switzerland | 1 | Specialist for genetic laboratory medicine FAMH |
| Turkey | 3 | n.a. |
| UK | 2a | Healthcare Scientist Training Programme |
| Ukraine | 3 | n.a. |
| Non-European countries | ||
| Canada | 1 | Clinical Cytogenetics, Clinical Molecular Genetics and/or Clinical Biochemical Genetics title accredited by the Canadian Board of Medical Genetics |
| Hong Kong | 3 | n.a. |
| Saudi Arabia | 3 | n.a. |
| USA | 1 | Clinical Cytogenetics, Clinical Molecular Genetics and/or Clinical Biochemical Genetics title accredited by the American Board of Medical Genetics |
Special requirements for group 1, 2a, 2b and 3 countries can be found at https://www.eshg.org/587.0.html
In 2014/2015 the registration process for professionals to attain the ErCLG title was opened for the first time. Only applicants from group 1 countries were eligible to apply in that year. However, 1 year later (in the 2015/2016 call) applicants from all three groups, that is, all European countries were able to apply for the ErCLG title.
The overpowering response to the first two calls was that at present almost 300 individuals from 19 countries are holders of an ErCLG title, which is valid for five years, before renewal is required. In the 2016/2017 call >80 further individuals applied for the title. The possibility for recognition of professionals working in Human Genetics diagnostic laboratories to be recognized as ‘affiliated ErCLG' from countries outside of Europe was opened in this recent call.
Overall, all our actions aim to get a EU-recognition, based on approved EU Directive 2005/36/EC – policy developments (https://www.eshg.org/fileadmin/eshg/EBMG/CLG/Direttiva_36_EN.pdf) and Proposal for modernizing the Professional Qualifications Directive=EU Directive 2013/55/EU (https://www.eshg.org/fileadmin/eshg/EBMG/CLG/Direttiva_55_EN.pdf). The latter has been implemented by 16th January 2016. To be achieved nine EU countries need to have their national CLG-title accepted as a registered profession by their national parliaments. This is only the case for Hungary (http://net.jogtar.hu/jr/gen/hjegy_doc.cgi?docid=A1200022.EMM) and Slovenia (https://www.uradni-list.si/1/content?id=103432), at present.
The ErCLG title
The ‘Clinical Laboratory Geneticists Professional Branch Board, European Board of Medical Genetics' offers the possibility for recognition of professionals working in human genetics diagnostics laboratories to be recognized as ErCLG.
From the professional point of view, and in order to provide best possible patient care, individuals working in human genetics diagnostics laboratories should master the following core competencies:
understanding and correct interpretation of basic mechanisms in human genetics,
having knowledge of the patterns and modes of inheritance,
having a basic knowledge of genetically inherited disorders including, for example, metabolic disorders,
having a comprehensive knowledge of common international genetic nomenclature systems,
having completely independency in the use of basic as well as molecular cytogenetic techniques, to provide a best possible diagnostic results in their own work field,
being familiar with medical ethical issues in diagnostics and research in general and according to state laws, and
having basic knowledge about genetic tests including risks and limitations.
During their work all applicants are obliged to follow the ‘Code of professional practice for clinical laboratory geneticists in Europe' (https://www.eshg.org/fileadmin/eshg/EBMG/CLG/EBMG_Code_of_professional_practice_2015.pdf).
To reflect that the field of human laboratory genetics is diverse, five different areas of specializations were defined; however, there is no difference in competence levels between the five ErCLG titles, they simply indicate in which field the holder of the title has core competences. (In case, eg, an ‘ErCLG with focus on Tumorgenetics' changed during the last 3 years the field and does now do only pre- and postnatal diagnostics he/ she may go for recertification for an ‘ErCLG focus on Clinical Genetics' title.) The five titles are the following:
General: This title confirms expertise in cytogenetics and molecular genetics (ie, prenatal, postnatal and molecular genetics, or tumorcytogenetics and molecular genetics in oncology – knowledge on Biochemical Genetics is optional). The title confirms knowledge of how to organize/establish and lead a cytogenetic and molecular genetic laboratory.
Clinical Genetics: This title confirms expertise in cytogenetics and/or molecular genetics in prenatal and or postnatal settings. The title confirms knowledge of how to organize/establish and lead a cytogenetic and/or molecular genetics/cytogenetic laboratory.
Molecular Genetics: This title confirms expertise in molecular genetics in prenatal and/or postnatal diagnostics; tumorgenetics may be included. The title confirms knowledge of how to organize/establish and lead a molecular genetic laboratory.
Biochemical Genetics: This title confirms expertise in biochemical genetics as applied eg in metabolic disorders or mitochondriopathies. The title confirms knowledge on how to organize/establish and lead a laboratory specialized in biochemical genetics.
Tumor Genetics: This title confirms expertise in cytogenetics and/or molecular genetics in oncological field. The title confirms knowledge of how to organize/establish and lead a cytogenetic and molecular genetics/cytogenetic laboratory.
The set of core competence stated above applies to all ErCLG subtypes. The registration process is based on availability of individual national titles/national certification system (via specialization or national society). As every country has different education schemes and/or different national society curriculum, the prerequisites for registration are based on the core curriculum of the ErCLG (https://www.eshg.org/fileadmin/eshg/EBMG/CLG/Core-Curriculum_2016.pdf).
Registration process
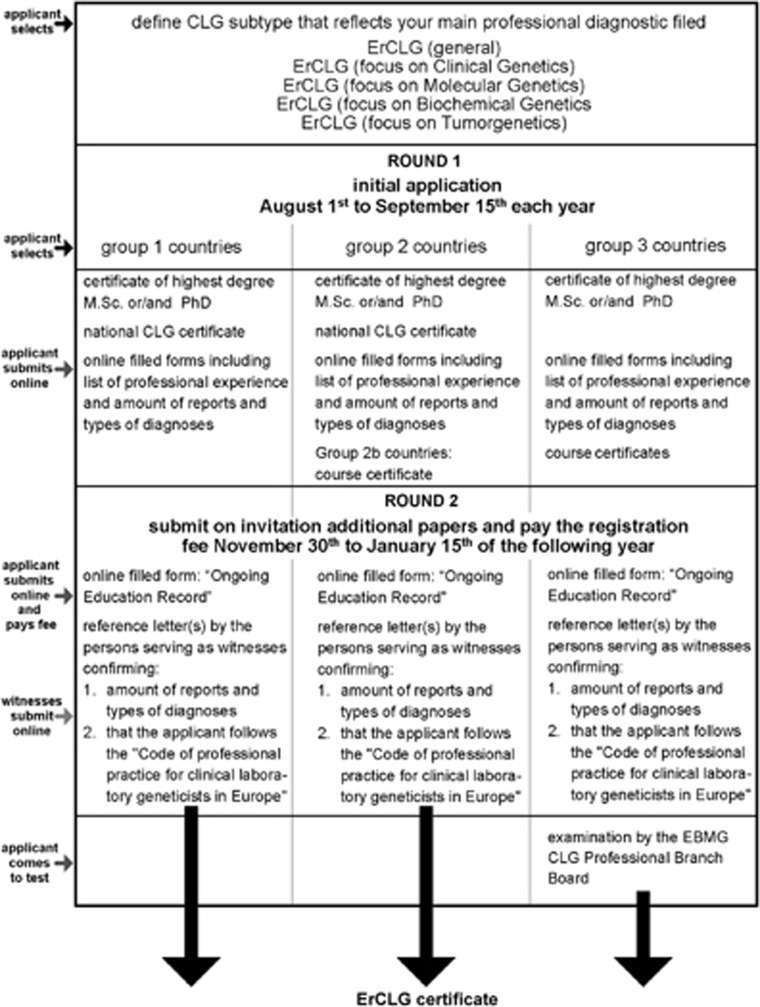
The registration process includes a two-step online submission procedure: first, the applicant has to submit information on his/her CV and upload certificates (Table 2) for the first round of evaluation. If an applicant does not fulfill the necessary criteria, he or she is informed about the reasons and there is no fee to pay. Up to now more than 95% of applicants passed round 1 and were then asked to submit additional papers and pay a registration fee. Witnesses are invited to submit supporting letters which confirm the disclosures applicant made about his or her own diagnostic activities.
Table 2. Entering criteria and requirements for application based on country group.
| Country group | National CLG certificate | Academic qualification | Minimum working experience | number of signed report/year | Ongoing education/Additional requirements |
|---|---|---|---|---|---|
| 1 | Yes | MSc and/or PhD | 5a years | 2000 | 35 h per year (15 h internal and 20 h external)b |
| 2a | |||||
| 2b | 35 h per year (15 h internal and 20 h external)b At least one specialized course in human genetic diagnostics with a certificate proofing successful passing of a final test or daily tests and duration of the course of at least 3 daysc | ||||
| 3 | Not applicable | 8d years | At least one specialized course in human genetic diagnostics with a certificate proofing successful passing of a final test or daily tests and a duration of the course of at least 3 daysc At least one specialized course in human genetic diagnostics with a certificate proofing participation and a duration of the course of at least 3 daysc Examination by the EBMG Professional Branch Board for Clinical Laboratory Geneticists that includes written exam to test theoretical knowledge and individual oral discussion checking experience in diagnosticsc |
If working for more than 7 years reports should be signed during at least three of the last 5 years.
Recorded for the last 2 years before application.
If working for more than 10 years reports should be signed during at least three of the last 5 years.
A second evaluation round is then undertaken by the CLG branch board members. A detailed description of the registration process is presented in Figure 2 and can be found elsewhere.7 Finally an ErCLG certificate that is valid for 5 years is issued.
Figure 2.
A detailed schema of ErCLG registration process. Before applying determine which group your country belongs to and check entering criteria and requirements for specific country. Not requested or evaluated are scientific records, like publication lists.
Since the 2016/2017 call also non-European residents can apply for an ‘affiliated ErCLG' title. If the country of origin of a potential applicant is not listed (Table 1) the interested person is invited to contact the chair of the Clinical Laboratory Geneticists Professional Branch Board, European Board of Medical Genetics for a discussion about an application.
It may be important to add that each ErCLG has to pass at least once a kind of examination in front of a board of colleagues. In group 1 and 2 countries such an exam is obligatory to receive the corresponding national CLG title. Thus for candidates from group 3 countries such an exam is part of the ErCLG scheme. Besides, no other tests or centralized exams for ErCLG applicants are foreseen yet be the EBMG.
Reaccreditation
The recognition as an ErCLG is valid for a 5-year period. If afterwards the recognition is for the same or another ErCLG subtype, applicants present documents confirming that they are still working in the same field or accordingly have changed it. They must also demonstrate that they are involved in signing reports, they have ongoing education (~35 h per year) and they can present the previous ErCLG registration.
Some statistics
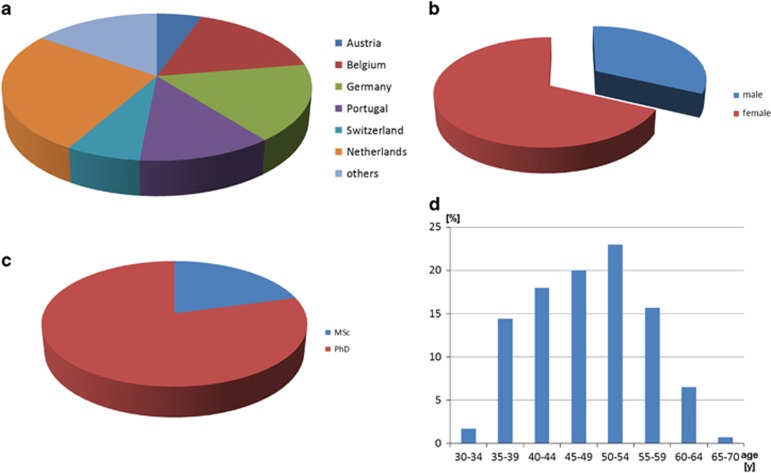
The ErCLG certification has enabled us to collect data on the professionals working in human genetics diagnostics laboratories throughout Europe. The corresponding data, based on 294 holders of ErCLG title, are summarized in Figure 3.
Figure 3.
(a) Distribution of holders of ErCLG title by country. (b) Distribution of gender among holders of ErCLG title. (c) Distribution of MSc and PhD among holders of ErCLG title. (d) Age distribution of holders of ErCLG title.
Most holders of ErCLG title come from the Netherlands, followed by Germany, Portugal, Belgium, Switzerland and Austria (Figure 3a). This may reflect in parts the fact that members of the Clinical Laboratory Geneticists Professional Branch Board come from these countries; thus, better advertising in other countries could help to promote the title.
Not surprisingly there are around twice as many females than males registered as an ErCLG; it is a well-known fact to all people working in genetic diagnostics that women are much more represented in the laboratories than men (Figure 3b). Furthermore, a PhD title seems to be expected from most practitioners working in Human Genetics diagnostic laboratories; 80% of all ErCLG holders have a PhD (Figure 3c). Interestingly, as yet the ErCLG-title has attracted mainly specialists at the age of >45 years of age. Only ~30% of the certificated specialists were below 44 years (Figure 3d).
Concerning the distribution of approved ErCLG subtitles: the general title was given to 66% of applicants, the molecular genetic one to 23% and the clinical genetic title to 6%. The titles ErCLG (focus on tumor genetics) and ErCLG (focus on biochemical genetics) were only requested in 4% and 1% of the applications, respectively.
The future
The ErCLG registration process is a unique chance to recognize and strengthen the status of (non-medical) specialists working in human genetic diagnostics in Europe and worldwide. ErCLGs may in future be important as well in laboratory developed tests and innovation via cowork with commercial companies. Through registration we get a feeling for and a representation of the absolute numbers we represent in this important medical field. Thus, it is important to encourage as many as possible non-registered colleagues to join us. For people coming from countries with national certificate it is easier than for those without national title. For the latter courses are offered (https://www.eshg.org/669.0.html): these are not meant to replace a national education system, but to augment the national training and can be used to enable applicants to acquire and/or demonstrate the competences that future ErCLG registrants will have.
Attaining EU-recognition as a profession for European CLGs is a major goal for the future. Each Human Genetic society in EU member states is herewith called to think about steps to bring their national parliaments towards formally accepting their national CLG-titles.
Footnotes
Supplementary Information accompanies this paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
The authors declare no conflict of interest.
Supplementary Material
References
- Lippi G, Favaloro EJ, Plebani M: Direct-to-consumer testing: more risks than opportunities. Int J Clin Pract 2011; 65: 1221–1229. [DOI] [PubMed] [Google Scholar]
- Wade CH, Wilfond BS: Ethical and clinical practice considerations for genetic counselors related to direct-to-consumer marketing of genetic tests. Am J Med Genet C Semin Med Genet 2006; 142C: 284–292. [DOI] [PubMed] [Google Scholar]
- Plebani M: Clinical laboratories: production industry or medical services? Clin Chem Lab Med 2015; 53: 995–1004. [DOI] [PubMed] [Google Scholar]
- Vrecar I, Peterlin B, Teran N, Lovrecic L: Direct-to-consumer genetic testing in Slovenia: availability, ethical dilemmas and legislation. Biochem Med (Zagreb) 2015; 25: 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godard B, Kaariainen H, Kristoffersson U, Tranebjaerg L, Coviello D, Aymé S: Provision of genetic services in Europe: current practices and issues. Eur J Hum Genet 2003; 11: S13–S48. [DOI] [PubMed] [Google Scholar]
- Cassiman JJ: EuroGentest-a European Network of Excellence aimed at harmonizing genetic testing services. Eur J Hum Genet 2005; 113: 1103–1105. [DOI] [PubMed] [Google Scholar]
- Coviello DA, Skirton H, Ceratto N, Lewis C, Kent A: Genetic testing and counselling in Europe: health professionals current educational provision, needs assessment and potential strategies for the future. Eur J Hum Genet 2007; 15: 1203–1204. [DOI] [PubMed] [Google Scholar]
- Lewis C, Kent A, Skirton H, Coviello D: EuroGentest patient information leaflets: a free resource available in over 20 languages. Eur J Hum Genet 2009; 17: 732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirton H, Lewis C, Kent A, Coviello DA: Genetic education and the challenge of genomic medicine: development of core competences to support preparation of health professionals in Europe. Eur J Hum Genet 2010; 18: 972–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paneque M, Moldovan R, Cordier C et al: Development of a registration system for genetic counsellors and nurses in health-care services in Europe. Eur J Hum Genet 2016; 24: 312–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.