Abstract
Adaptive optics is a relatively new field, yet it is spreading rapidly and allows new questions to be asked about how the visual system is organized. The editors of this feature issue have posed a series of question to scientists involved in using adaptive optics in vision science. The questions are focused on three main areas. In the first we investigate the use of adaptive optics for psychophysical measurements of visual system function and for improving the optics of the eye. In the second, we look at the applications and impact of adaptive optics on retinal imaging and its promise for basic and applied research. In the third, we explore how adaptive optics is being used to improve our understanding of the neurophysiology of the visual system.
Keywords: Adaptive optics, retina, retinal physiology, vision science
1. Background
Adaptive optics (AO) is playing an increasing role as an enabling technology in vision science. By allowing scientists to more precisely control the visual stimulus, to image the retina at higher resolution, and to measure chemical and physiological responses of the retina directly, it has the potential to become a mainstay of the scientific armmentarium. Because there are no common commercial platforms for using AO, individual scientists have used a variety of approaches to generate their own unique systems. This article presents the reader with the responses of some of these experts to a series of structured questions. As AO systems are being applied to new areas, and to adopt differing technologies, the editors believe that gathering the thoughts and views of leaders seemed timely. The question and answer approach provides insights to prospective users of how each implementation is best suited to particular applications and allows them to envision the prospects for the technology. This format allows the reader to evaluate both common approaches as well as unique aspects of these systems. The questions and answers are divided into three sections. The first section covers approaches to investigating the evaluation of vision and the visual system (including the optics of the eye). The second section concentrates on retinal imaging. The third section concentrates on systems for neurophysiological investigations. Each section asks a series of questions on the current state of the art, future applications, and future advancements. While responses have been edited for brevity and clarity, we have tried to leave the unique character of individual responses intact. In each section, comments are presented in order of the respondent’s last name. Original responses have been edited to avoid redundancies.
2. Adaptive optics for visual evaluation
Bypassing the optics of the eye has been of interest for years to visual psychophysicists. Avoiding the optics, allows the assessment of the spatial limits of the neural system, beyond the degradation produced by the ocular optics. In earlier experiments visual stimuli projected directly on the retina unaffected by the optics were limited to gratings (Campbell & Green 1965) produced by the interference created by two points at the pupil plane. Alternatively, patients could see visual stimuli through small pupils, but although the effect of aberration is reduced, spatial resolution is limited by diffraction (Cheng et al. 2010, Ravikumar et al. 2012). The advent of AO opened the possibility of projecting aberration-free images on the retina with large pupil diameters. The first accounts of AO systems for vision science described both the use of AO systems in retinal imaging and visual psychophysics (Liang et al. 1997). Over the years, several laboratories have developed AO systems specifically addressed to measure visual function under manipulated optics, allowing revisiting classical visual psychophysics, eliminating optical degradation, or probing the spatial limits of vision and neural adaptation. For example, the impact of correcting aberrations on the contrast sensitivity function and visual acuity at different luminances or facial recognition has been measured (Liang et al. 1997, Dalimier et al. 2008, Marcos et al. 2008, Sawides et al. 2010a). Also, AO has allowed evaluation of the impact of monochromatic aberrations on chromatic aberration, or the visual benefit of correcting monochromatic aberrations on polychromatic vision (Yoon et al. 2002, Vinas et al. 2015a). The impact of aberrations on accommodative lag and accommodation response has been assessed through AO (Chen et al. 2006; Hampson et al. 2009; Gambra et al. 2009). Expanding correction beyond the isoplanatic region has also opened the possibility of testing aberration-free vision outside the fovea (Venkataraman et al. 2016; Baskaran et al. 2012). With AO it is possible to correct a subject’s native aberrations and impose those of a different subject. A number of studies have shown that the same optical degradation is perceived differently by different subjects, and visual perception and visual function is better with the subject’s native optics than with another subject’s optics. For example, patients with increased optical degradation due to corneal disease (keratoconus) perform better than normal subjects “looking through” the artificially imposed keratoconic eye’s aberration patterns (Sabesan et al. 2009; Rouger et al. 2010), and visual perception is better with the subject’s own aberration pattern than rotated versions of it (Artal et al. 2004). AO has helped to demonstrate that subjects are adapted to their own aberration pattern and has allowed estimates of the internal code for blur (Sawides et al. 2013). Interestingly, subjects can also, to some extent, adapt to other blur levels and aberration patterns (Sawides et al. 2011a). This results in practical implications as disease, aging and more specifically optical or surgical corrections (ophthalmic lenses, contact lenses, IOLs and corneal treatments) alter the ocular aberration pattern.
One of the applications of AO is to simulate the impact of certain corrections on vision, before they are given to a patient or even prior to manufacture. For example, the effect of inducing aberrations or combinations of aberrations on expanding the depth-of-focus has been explored through AO aberrometers (Piers et al. 2004; de Gracia et al. 2011a, Legras et al. 2012). Corrections such as segmented and diffractive multifocal intraocular lenses and the optical changes produced by refractive surgery have been simulated using AO. The specifications of the AO element (whether it is an electromagnetic deformable mirror or spatial light modulator) determine the type of corrections that can be simulated. As AO visual simulators enter a new area, with various systems based on different technologies entering the market, revisiting the know-how gathered in different laboratories experienced in developing AO technology for visual psychophysical applications appears timely. Presenting these views will yield insights to prospective users as to which implementation is best suited to a particular application.
2.1 Adaptive optics and visual perception and function
How is adaptive optics helping you to further understand visual perception and visual function?
Pablo Artal (PA): In my laboratory we have been performing visual testing using AO Visual Simulators (Fernández et al. 2002; Fernández et al, 2009a), In fact, the term simulation may be misleading, since with AO, we are doing more than simulating, we are recreating real optics. Several of the studies we have performed are directed to better understand the phenomenon of night myopia (Artal et al. 2012; Chirre et al. 2016), to study the nature of adaptation to aberrations (Artal et al. 2004) and to better understand the impact of aberrations on spatial vision in normal eyes, and eyes with IOLs or refractive surgery (Lundström et al. 2007, Piers et al. 2007, Fernandez et al. 2010, Schwarz et al. 2014a, Schwarz et al. 2014b, Leray et al. 2015).
David Atchison (DA): AO enables the correction and manipulation of aberrations to help explore the optical and neural limitations of visual performance. I have been exploring subjective blur limits (just noticeable, just troublesome and just objectionable), (Atchison et al. 2009a; Atchison et al. 2009b; Atchison & Guo 2010; Guo & Atchison 2010), effects of aberrations on colour phenomena (Gupta et al., 2010), influences of blur adaptation on visual acuity, and effects of peripheral aberrations on contrast sensitivity (Guo et al. 2008).
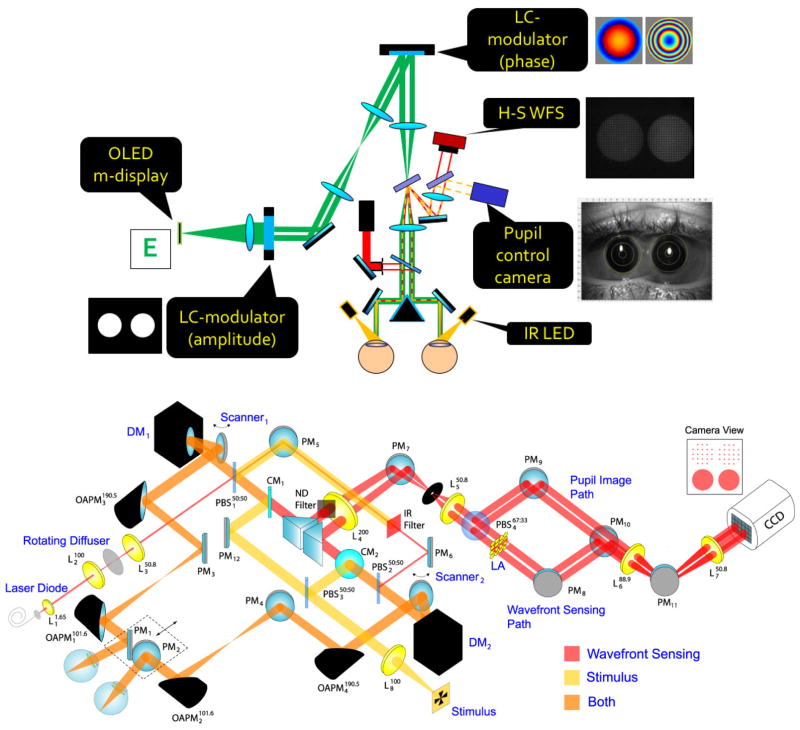
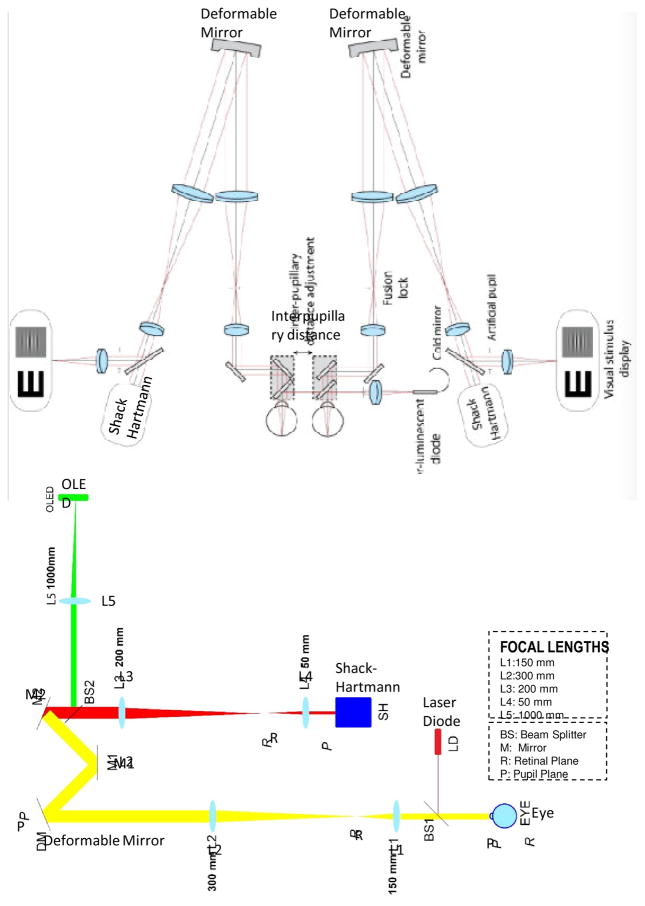
Karen Hampson (KH): We are using AO to understand the effect of higher-order monochromatic aberrations on accommodation control. We have explored the impact of aberrations on the dynamics of the accommodation step response (Chin et al. 2009a; Hampson et al. 2010), the response to predictable stimuli (Chin et al. 2009b) and microfluctuations in accommodation (Hampson et al. 2012, Hampson et al. 2013). As a number of changes in the accommodation system occur in myopic subjects, and manipulation of higher-order aberrations affects the accommodative response, we are using AO to determine the effect of higher-order aberrations on myopia onset and progression. As accommodation is linked to the convergence system we are also investigating the effect of aberrations on convergence using our binocular system shown in Figure 1(b).
Richard Legras (RL): AO devices are useful to simulate an optical correction such as multifocal optics aiming to compensate for presbyopia (Legras et al. 2010). As an example, one can rapidly observe the impact of keratoconic aberrations or the interaction of aberrations and through-focus quality of vision. You can simulate spherical aberration and add various magnitudes of defocus to understand how aberrations are balanced (Bernard et al. 2010). These simple experiments allow us to see and understand what theory can easily explain.
Linda Lundström (LL): In our lab, AO allows us to analyze the neural components of peripheral vision (resolution and detection in high and low contrast, moving and stationary targets) (Venkataraman et al. 2016; Rosén et al. 2012; Baskaran et al. 2012). The higher-order aberrations increase with increasing retinal eccentricity and thereby reduce retinal image quality. Also in situations where a spherocylindrical correction could have been sufficient, AO helps us reduce measurement time. This is because large aberrations give a poorly defined far-point and it is not always straight forward to find the optimum spherocylindrical correction off-axis. Eccentric subjective refraction is very time-consuming and although aberrometers can be useful, a separate refraction is not needed with the AO system.
Geungyoung Yoon (GY): My lab uses AO to investigate how the optical quality of the eye interacts with the neural system in determining perceived image quality and visual performance (Yoon et al. 2002, Sabesan et al. 2012, Zheleznyak et al. 2016). An AO vision simulator is a powerful tool to manipulate the eye’s aberration noninvasively and in real time. It simulates various optical conditions for presbyopia correction (Zheleznyak et al., 2013), binocular vision (Sabesan et al., 2012) and peripheral vision (Zheleznyak et al., 2016). With the full correction of the aberration, we are able to investigate neural function by bypassing the eye’s optics similar to laser interferometry. With the induction of the aberration, it is possible to present stimuli with the same optical conditions to individual visual systems, enabling study of how visual perception can differ due to various visual environments such as short- and long-term adaptation.
Susana Marcos (SM): We use AO to test the effects of manipulating the optical aberrations on visual perception and visual performance. In particular, we have addressed the visual benefit produced by the correction of high-order aberrations on visual acuity (Marcos et al. 2008), contrast sensitivity (de Gracia et al. 2011a), familiar face and facial expression recognition (Sawides et al. 2010a) and accommodation dynamics (Gambra et al. 2009). We have investigated the ability of the visual system to adapt to increased or decreased aberrations (Sawides et al. 2010b, Sawides et al. 2011a), and the correction of astigmatism (Vinas et al. 2012, Vinas et al. 2013), the extent to which subjects are adapted to the level and orientation of their native aberrations (Sawides et al. 2011b, Sawides et al. 2012, Sawides et al. 2013), and the internal code for blur (Radhakrishnan et al. 2015a, Radhakrishnan et al. 2015b). We have also investigated the effects of aberrations on subjective focus (Marcos et al. 2015) and of specific combinations of astigmatism and coma on increasing the depth-of-focus (de Gracia et al. 2011b). With our most recent polychromatic AO system (which is equipped with a supercontinuum laser source and both deformable mirror and a spatial light modulator) we have tested the impact of monochromatic aberrations on psychophysical and objective measurements of transverse chromatic aberration (Vinas et al. 2015a), as well as visual perception and visual performance with different multifocal corrections such as segmented lenses (rotationally symmetric and asymetric) with 2, 3 and 4 zones (Vinas et al. 2016).
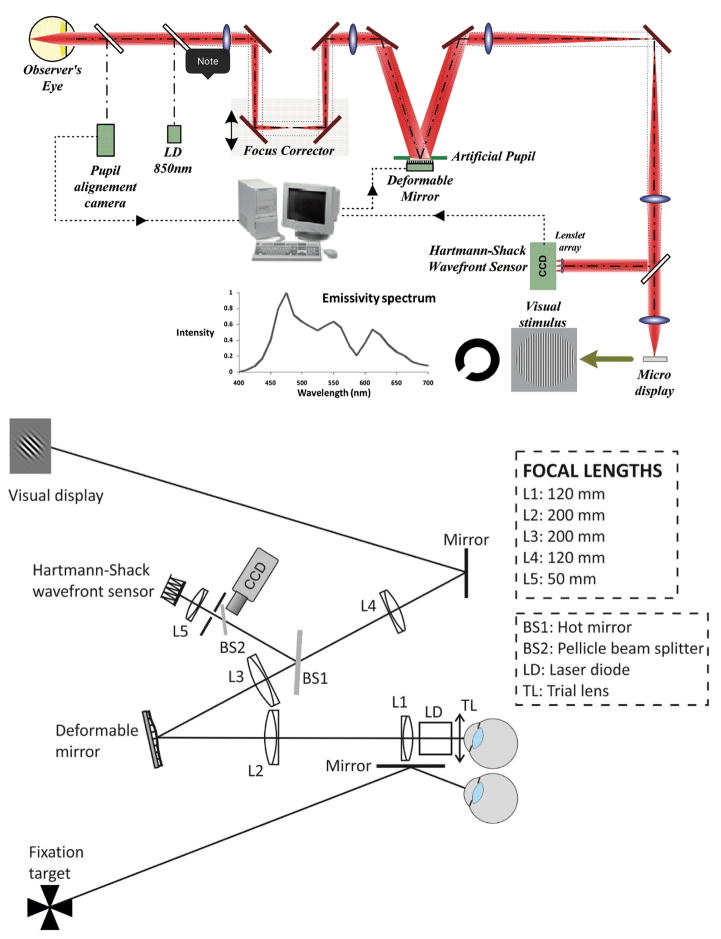
Figure 1.
Examples of implentations of Adaptive Optics systems for visual psychophysics. A. AO simulator at LOUM-Universidad de Murcia (courtesy of Pablo Artal). B. Schematic layout of binocular AO system at Bradford University (courtesy of Karen Hampson).
2.2 Adaptive optics and simulation of optical corrections
How is adaptive optics advancing development of new optical corrections and testing current refractive solutions?
PA: Simulation of optical corrections with AO is clearly an important application. We have used this technology to design and test different optical solutions (Piers et al. 2004, Manzanera et al. 2007, Artal et al. 2010, Schwarz et al. 2014b, Schwarz et al. 2014c, Leray et al. 2015) such as different types of IOLs. The ability to represent ophthalmic corrections provides benefits to the ophthalmic industry.
DA: AO provides a quick way of simulating different types of corrections and thus assessing their value, i.e. simulating contact lenses with asphericity to produce different amounts of primary and secondary aberrations, and simulating monovision in binocular AO systems.
KH: Aside from studying accommodation, AO allows for understanding the effect of aberrations on aspects of the visual system such as neural adaptation and visual acuity. This will result in the development of corrections that optimize vision. This will also impact the development of new corrections to halt myopia progression. For example, multifocal contact lenses may reduce myopia progression in children, and their mechanism could depend on the effect of peripheral refraction on axial elongation. Knowledge gained from studying the impact of higher-order aberrations on peripheral vision may lead to enhanced customized corrections. AO allows current and future optical corrections to be tested in situ before the corrective device is made.
LL: AO speeds up the testing of new optical designs significantly. For example, we have been able to evaluate the benefits of aberration correction in the remaining peripheral visual field for a person with central visual field loss (Baskaran et al. 2012).
RL: The main advantage of AO is the ability to test new optical designs without the need to manufacture them. In the field of presbyopic correction the combination of spherical and secondary spherical aberrations of opposite signs provide an enlarged depth-of-focus (Legras et al. 2010). Simulation of corrections performed by AO should be considered as a first step in the development of new optics that must finally be clinically tested in a population. AO permits testing optical corrections without the inherent difficulties of the manufacturing process.
GY: It is a very efficient way of testing some new ideas for vision correction without doing surgery or manufacturing actual ophthalmic lenses as AO can simulate those new designs optically (Zheleznyak et al. 2013) as well as to help understand what (e.g. ocular sensory dominance) makes a person a good or poor candidate for monovision presbyopia corrections (Zheleznyak et al. 2015).
SM: The ability to simulate optical corrections non-invasively allows testing new correction designs before manufacturing, therefore guiding the design and evaluating the need for customization of the solution for a patient. This is particularly relevant, as multiple IOL designs can be compared by the same subject (i.e., same brain) potentially significantly reducing costs of clinical trials (allowing only one IOL implanted per patient). In addition, the ability to perform measurements with and without the natural aberrations gives insight into the needs for the correction to be selected on an individual basis or alternatively whether the same correction works similarly across a larger population (Vinas et al. 2015b, Vinas et al. 2016). AO systems are able to predict both visual performance and short-term adaptation to new corrections before these are implanted in the eye or even manufactured (Radhakrishnan et al. 2014)
2.3 Technical aspects of adaptive optics visual simulators
What adaptive optics active element are you using in your adaptive optics simulator, and what is the reason for your choice?
PA: We previously used deformable mirrors but now use a Spatial Light Modulator LCOS-SLM by Hamamatsu Photonics in our recent instruments (Fernández et al. 2009a, Fernández et al. 2009b, Cánovas et al. 2010). The reasons are the flexibility and ease of use and their high resolution, which allows reproduction of complex wavefronts; the disadvantage is that they work best with narrow bands of light.
DA: We use the ImagineOptics Mirao D52 mirror. It has a large stroke which is beneficial in correcting large magnitudes of aberrations and avoids the shortcomings of LC-SLMs (including polarization and restricted spectral bandwidth). We would like to consider the Alpao mirror, but do not presently have the funds for this. We have spatial light modulators, but have not applied them.
KH: Our monocular system uses a 37-element piezoelectric deformable mirror (OKO Technologies, Netherlands; Hampson et al. 2009). Our binocular system uses two 52-actuator magnetic deformable mirrors (Mirao 52, Imagine Eyes, France). These choices were based on cost, stroke, and availablity at the time the systems were constructed.
LL: We have a deformable membrane mirror (Mirao 52 D from Imagine Eyes) (Rosén et al. 2012). It has the advantage of a relatively large stroke and being wavelength-independent, and at the same time it is affordable and can be bought together with software and a wavefront sensor for closed-loop operation.
RL: We use the Mirao™ 52-e deformable mirror which is implemented in the Crx1 device (Legras et al. 2010). The main advantage of this mirror is its ability to compensate for the wide variety of wavefront errors that affect living eyes.
GY: We have two AO vision simulators in the lab (Sabesan et al. 2007, Sabesan et al. 2009; Sabesan et al. 2010): (1) The binocular AO vision simulator uses two large stroke deformable mirror with 97 actuators (Alpao) and laboratory-developed wavefront sensors. Both provide the dynamic range for aberration measurement and correction necessary for our studies of highly aberrated eyes such as keratoconus. Also, these deformable mirrors have excellent linearity of the individual actuators and continuous wavefront profiles; (2) Liquid-crystal spatial light modulator (LCSLM) based AO vision simulator: The LCSLM provides phase control pixel by pixel, allowing us to simulate very complicated phase profiles such as diffractive optical elements and multifocal ophthalmic lenses (Sabesan et al. 2007).
SM. We use the 52-actuator MIRAO electromagnetic deformable mirror by Imagine Eyes (France) (Marcos et al. 2008). In our second AO system we use the MIRAO in combination with a Spatial Light Modulator by Holoeye, making it possible to simulate both smooth optical corrections and segmented and diffractive lenses, as well as correcting the subject’s high-order aberrations while simulating a wide range of static corrections (Vinas et al. 2016)
Do you perform a closed-loop operation (continous update of the deformable mirror during the vision evaluation) in your system?
PA: Usually, we perform a static correction of aberrations, although in some of our systems closed-loop versions are in operation. Some psychophysical experiments are lengthy and deem continous illumination with the probe light not feasible for a closed-loop correction. On the other hand, in our laboratory we use IR probe light at 1000 nm, which is invisible to the patient, not interfering with the visual stimulus in visible light.
DA: Open-loop operation for long vision stimulation tasks.
KH: Both the monocular and binocular systems (and imaging system) perform a closed-loop operation.
LL: Yes, we need this continuous closed-loop operation because of the amplitude of peripheral irregular aberrations; with a static aberration correction even small eye movements can cause the correction to be severely decentered. Small movements cannot be avoided since we have relatively long evaluation times and are using a head-rest (no bite-bar). The continuous closed loop is possible since the low intensity measurement light does not interfere with the peripheral vision evaluation (many subjects do not even notice whether the measurement light is turned on or not when using their peripheral vision).
RL: AO correction should be in a closed-loop configuration. Fluctuations of aberrations due to tears, eye movement and accommodation, if not neutralized by cyclopegia, would strongly impact the quality of the correction if performed statically.
GY: Yes, due to various factors such as eye movement and tear instability that could affect the visual performance, it is important to make sure that the optical condition under which vision is tested is reliable and consistent. We have developed our own AO closed-loop program.
SM: We reach the desired correction (i.e. correction of the subject’s high-order aberrations or a given aberration pattern) by a closed-loop operation. We then perform the psychophysical task with this as the static correction while the pupil position is continuously monitored. The loop is closed again before each psychophysical run to guarantee an efficient correction throughout the experiment.
What strategy do you use to present visual stimuli?
PA: We have mono and stereo stimuli (Manzanera el al. 2007, Fernandez et al. 2009b) that are presented with organic light emitting diodes (OLEDs) microdisplays.
DA: Stimuli have been presented on high-resolution screens in the past, but are now being presented on high quality OLEDs.
KH: A black Maltese cross printed on transparent plastic is used as an accommodative stimulus. The cross is back-illuminated by a light bulb with a diffuser to even out the brightness. Colored filters are used to select the wavelength. This method was chosen as it is cost effective
LL: We use an analogue cathode-ray-tube monitor (Nokia 446Xpro) seen through the AO system. It is driven by a Linux PC with a 10-bit NVIDIA graphics card and calibrated to give a linear response in luminance. To achieve more displayable low-contrast levels for contrast sensitivity measurements, we redefine the gamma curve of the monitor to display a narrower range of luminance values in smaller steps (utilizing the central part of the original color look up table interpolated to 10-bit resolution). The stimulus presentation is controlled through Matlab and the Psychophysics toolbox (Brainard 1997).
RL: Visual stimuli are displayed on eMagin’s OLED-XL™ Microdisplays subtending a visual angle of 114 × 86 arcmin with a resolution of 800 × 600 pixels (pixel size=0.143 arcmin). This pixel size enables the testing of visual acuity.
GY: We mainly use a DLP chip (TI) to directly display our visual stimulus. It can be controlled via Visual Psychophysics Toolbox (Matlab) (Brainard 1997). It is used for virtually any visual stimuli such as contrast grating, acuity letters, noise and natural images, etc.
SM: Visual stimuli are displayed on a CRT monitor, an OLED display or a DLP projector, illuminated by monochromatic light (with the coherence broken by holographic diffusers) from a supercontinuum laser source (Marcos et al. 2008, Vinas et al. 2015a, Vinas et al. 2016). The presentation of visual stimuli is driven by Psychophysics Toolbox and is synchronized with the AO system operation. Visual stimuli include high contrast visual targets and gray-scale natural images.
What do you believe are unique technical features in your system that you would recommend to investigators considering using adaptive optics?
PA: Binocular operation and high accuracy in reproducing complex wavefronts are perhaps our strongest points.
DA: We have a neat way of altering aberrations to explore subjective limits of blur, including being able to introduce astigmatism or cylinder at any orientation (Atchison et al. 2009a; Atchison et al. 2009b; Atchison & Guo 2010).
KH: Our monocular system: (1) has an extra wavefront sensing channel that does not pass via the deformable mirror. This allows direct measurement of fluctuations in the aberrations of the eye during the experiment and a greater variety of experiments to be carried out. An example is dynamically inverting the higher-order aberrations. This would not be possible in a traditional closed-loop AO system as it would not be possible to discern the ocular aberrations from those induced by the mirror. (The need for this can potentially be overcome with a carefully calibrated open-loop system.) (2) The light strikes the deformable mirror twice in order to effectively double the stroke of the mirror. Although current high-end deformable mirrors have sufficient stroke to compensate for aberrations in the majority of the population, this feature is particularly useful for lower cost mirrors and/or those with high-resolution but limited stroke. Our binocular system is able to dynamically manipulate and measure convergence in addition to being able to manipulate the aberrations. This will present a more complete picture of the effect of aberrations on binocular vision and visual function. In both systems: (1) A low-cost rotating diffuser reduces speckle in the Shack-Hartmann spots. This consists of a motor with a spinning piece of diffusion filter. This allows for cheaper laser diodes to be used as opposed to more expensive superluminescent diodes. (A more cost effective way is to use LEDs but it is more difficult to collect enough light.) (2) A single camera and a lenslet array is used for the sensing measurements to reduce cost and complexity.
LL: The “continuous closed-loop” operation is rare for vision evaluation. Most likely this is challenging for AO systems used to evaluate foveal vision because having the measurement light on can influence the measurement.
RL: The crx1™ (Imagine Eyes) device is compact, self calibrated and usable in a clinical environment.
GY: Total freedom of controlling the eye’s aberration in real time. Visual psychophysical tasks can be synchronized with AO in both time and space. Binocular AO opens up many new possibilities for investigating vision.
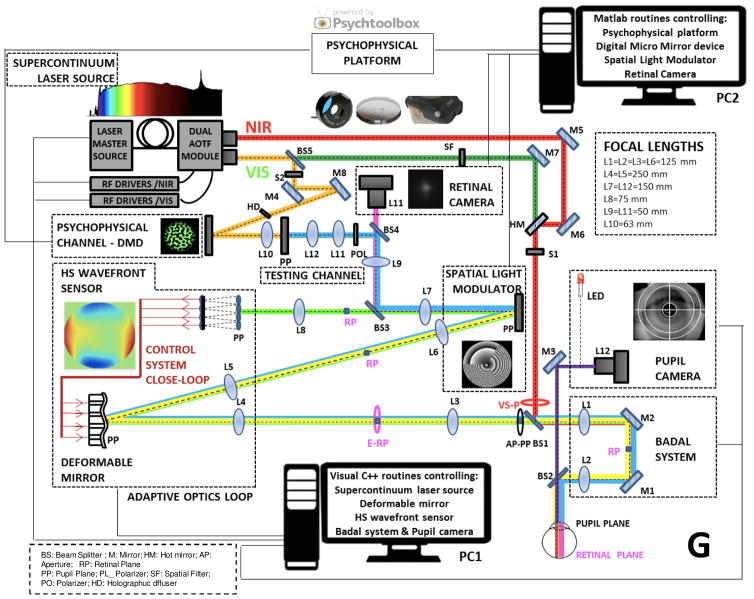
SM: Our second generation AO system includes a supercontinuum laser source, allowing measurements of aberrations at different wavelengths, measurement of longitudinal chromatic aberration and the presentation of both polychromatic and monochromatic stimuli (Vinas et al. 2015a, Vinas et al. 2016). Also, the system contains two AO elements (deformable mirror and spatial light modulator) expanding the range of possibilities for representing different refractive and diffractive corrections. An additional channel in the system contains a double-pass retinal imaging camera, which allows an independent measurement of retinal image quality (and its change upon a change in the aberration pattern).
What are the main technical limitations of current adaptive optics visual simulators?
PA: An important current limitation of AO visual simulators is related to the need for a careful alignment of the eye’s pupil and the correction. To ensure this, bite-bars are commonly used in laboratory settings. A future incorporation of eye tracker techniques could make the systems more user-friendly outside the lab for clinical applications. Another current limitation is that most corrector elements work in reflection mode. If future wavefront corrector devices operating in transmission are available, the instruments could be simpler and able to operate under natural viewing conditions.
DA: The main limitation is the accuracy in simulating real life scenarios. While AO corrects aberrations, it does so over the isoplanatic patch (1–2°). The displayed target can either be within that range, i.e. too small compared with real life targets, or several degrees across, i.e. only the central bit is corrected. Correcting over a larger field requires more complicated systems such as multiple beacons (Popovic et al, 2011), and becomes expensive.
KH: 1) Current systems are not multiconjugate and so the aberration manipulation of the stimulus is only effective over a small area (1–2°). In everyday life our field of vision is much greater than this. 2) Current systems are not open-view. 3) The majority of systems do not include convergence control. This is important even when carrying out experiments at fixed accommodation levels as the eyes converge in natural viewing conditions.
LL: The limited field of view. For applications in evaluating the neural components of vision, it would also be desirable to correct for chromatic aberrations.
RL: From my point of view, AO visual simulators using a deformable membrane are mainly limited by their inability to provide non continuous optical designs such as bifocal optics.
GY: Relatively narrow visual field due to limitations of optical components in AO; more actuators provide better performance.
SM: A general limitation of on-bench and desktop AO systems is a very narrow visual field (generally less than 2 deg). Deformable mirrors are limited to representing smooth surfaces. Spatial light modulators are subject to chromatic artifacts, as the phase map is only accurately represented for a single wavelength.
What limitations must current adaptive optics systems overcome to become widespread instruments in the ophthalmic industry and in the ophthalmologist/optometrist office?
PA: As with any disruptive and new technology, this is mostly a matter of time. Clinicians need to understand the potential for their patients. A potential perceived drawback by clinicians may arise from the inability to capture in the simulation all surgical aspects.
DA: AO systems remain expensive, typically hard to run, and require very accurate alignment and expert monitoring at all times.
KH: I do not believe there is a problem with the instruments per se as there are commercially available compact systems.
LL: Reduced price and complexity. But also larger awareness among ophthalmologists and optometrists on how to use the information provided by these systems.
RL: To be considered as a tool in clinical practice, AO devices should be less expensive. Indeed, the usefulness for ophthalmologists/optometrists of present systems is limited. Most systems are big, complex and difficult to use for a non engineer, which restricts their use mostly to an academic lab or R&D environment. However, AO systems are currently used in the ophthalmic industry to develop new optical designs.
GY: High cost; Usability in clinical environment; Software development (closed-loop algorithm).
SM: The optimal clinical device should be see-through (thereby providing images of the real world and a large field of view), binocular, light and easy to use by the clinician. Besides, clinical evidence is needed to show that the simulated corrections are truly representing real lenses and that the simulated performance is equivalent to that of the patient post-operatively.
2.4 The future of adaptive optics simulators
Do you think there will be major advances in adaptive optics systems for vision? If so, what are the features you expect to be developed in the future?
PA: I anticipate that open-view systems will represent the next generation of AO devices.
DA: Combined AO systems that allow for visual testing as well as retinal imaging at a microscopic level. Such systems will allow better understanding of retinal circuitry and visual processing by single cones.
KH: (1) Polychromatic AO systems that can dynamically manipulate chromatic aberration as well as monochromatic aberrations. (2) Multi-conjugate systems to manipulate aberrations over a wide field of view.
RL: AO appears a rather mature technology, although new technological developments will allow for new capabilities, and very likely more compact systems. Today, AO remains primarily a research tool. To become widely used, a large-scale application needs to be found.
GY: Yes, it has been improving for the past two decades already and I expect to see more advances. Having low cost AO will allow for developing a commercially available clinical AO system.
SM: Laboratory systems will incorporate expanded capabilities, including multi-wavelength, multi-conjugate AO, and multi-modal operation (combining visual psychophysics and retinal imaging). Systems for clinical applications (simulations of optical corrections) will be binocular, wearable and see-through. Specific static corrections may not need a closed-loop operation or even wavefront sensing, largely decreasing complexity. We have developed a system, specifically targeted to the simulation of simultaneous vision corrections that operates on a different principle called temporal multiplexing (Dorronsoro et al. 2016b) which verifies those specifications, making it easy for the subject to experience the real world with various multifocal designs (Dorronsoro et al. 2016a) and be used with a specific purpose in the clinic.
How can adaptive optics vision systems help in clinical research?
PA: Key to understand the limitation of procedures and to optimize surgeries.
DA: From a stimulus and performance point of view, simulations of ophthalmic corrections.
KH: AO systems will play a pivotal role in determining the effect of higher-order aberrations on accommodation control in myopia onset and progression and neural adaptation.
LL: We use AO to better understand (1) how the remaining peripheral vision for people with central visual field loss can be facilitated through optical correction and (2) how the image quality on the peripheral retina may be used by the emmetropization process, which is important for understanding how to halt myopia.
RL: Clinical studies of new lens designs are time consuming, and another disadvantage is the variability between individuals that often conceals some effects. An alternative to the clinical testing of new designs could be the numerical simulation of their on-eye performance. However, numerical simulation should be compared to real optics in order to determine its accuracy. These comparisons should be done in highly controlled conditions that can only be achieved thanks to AO. Various experiments have shown that numerical simulation can be considered as an alternative to AO systems to simulate an optical design (Legras et al. 2010).
GY: To understand factors that affect clinical outcome by comparing to AO-simulated outcome.
SM: AO systems help understanding of the relative contribution of optical and neural factors on vision, the effect of neural adaptation in acceptance of new optical corrections and to optimize the correction given to a patient on optical and perceptual bases.
How can adaptive optics vision systems help in clinical practice?
PA: Key devices to optimize visual outcomes in refractive and cataract surgeries.
DA: It could be used to design contact lens presbyopic corrections (rather like corneal topographic software is used to design the back curve of rigid contact lenses with good fitting properties).
KH: The knowledge gained from research will inform customized vision corrections such as for myopia correction and control and presbyopia corrections.
LL: Help understanding complaints about poor night vision (whether contact lenses with certain aberration profiles will be beneficial). Help choosing an appropriate optical design (multifocal lenses, eccentric refraction for macular degeneration).
RL: AO systems should be very useful in clinical practice. The main application will be presbyopic correction performed by surgery (LASIK, IOL). Patients could view stimuli through their future correction prior to surgery. Since a large part of the acceptability of such corrections is dependent on the patient’s tolerance to blur, it would be useful to evaluate the quality of vision of the patient prior to surgery.
GY: Patients can experience the potential visual benefit from clinical interventions for refractive error and presbyopia correction
SM: The AO system should help the clinician choose the best correction (i.e., IOL design, refractive surgery) for a patient. Proof that including an AO test improves clinical outcomes and that it is cost-effective is needed for acceptance and routine use of the system in the clinic.
3. Adaptive optics for retinal imaging
Since the invention of the ophthalmoscope, imaging the fundus has become an essential part of the retinal exam and historically the most valuable tool for studying retinal and optic nerve diseases. A wide range of clinical instruments for retinal imaging has guided thinking about retinal disease, including direct and indirect handheld ophthalmoscopes, slit lamp, fundus camera, confocal scanning laser ophthalmoscope (cSLO), optic nerve head analyzer, ultrasonography, and optical coherence tomography (OCT). More complex retinal instruments currently reside in the research domain and are the likely progenitors of future clinical systems. Each instrument has its advantage(s) over the others in terms of what they can see and measure in the retina and collectively they provide a powerful arsenal for early diagnosis, monitoring progression of retinal disease, and studying the normal visual system.
Today, a major effort is underway for developing retinal imaging systems that are directed at identifying the earliest stages of disease. Pathogenesis begins at the level of individual cells, and understanding abnormal structure and function requires visualization of changes in the microscopic realm. The design of cameras for cellular-level imaging of the retina is truly challenging since the instrument must satisfy demanding criteria in the areas of (1) lateral resolution, (2) penetration through absorbers and scatterers in the eye, (3) optical sectioning, (4) speed, (5) sensitivity with a limited light budget for safety, and (6) contrast generation through selected imaging modalities. Successful visualization of individual retinal cells requires that the camera meet specific criteria in each of these areas. Resolving neighboring cells within the same focal plane requires a lateral resolution approaching the cell size. Foveal cone photoreceptors and ganglion cell bodies, for example, have lateral dimensions of around 3 and 10 μm, respectively, and require a retina camera that has sufficient resolution and contrast to permit detection in situ.
The handheld ophthalmoscope has a lateral resolution approaching 60 μm, and is generally limited to imaging with visible wavelengths. The fundus camera can achieve theoretical lateral resolution near 20 μm, but the optical resolution rarely reaches this precision. These clinical instruments are designed for observing gross anatomical features of the retina over large areas and do not permit observations of clinically relevant details at the cellular level. The clinical cSLO and OCT have an operational resolution better than that of the fundus camera. Both typically raster scan a focused light spot (typically a laser or similar source) across the retina in tandem with a small confocal aperture and a sensitive point or line detector, which records the reflected light. cSLOs in research laboratories can offer lateral resolution approaching the limit imposed by diffraction and aberrations (Wade & Fitzke 1998). Such instruments often operate with a pupil diameter in which the collective impact of diffraction by the pupil and aberrations is minimal and subsequent resolution is maximized (Bartsch et al. 1989). Larger pupils reduce diffraction, but this benefit is lost by the aberrations unleashed by dilatation.
Technical advances, notably in AO (Babcock 1953, Tyson 1998), have provided solutions to the long-standing problem of correcting the aberrations of the human eye and have enabled us to reap the resolution benefits afforded by ophthalmoscopes with large pupils. AO was first applied to retinal imaging using a custom fundus camera constructed on an optical bench (Liang, Williams & Miller 1997). The lateral resolution was made smaller than the width of a single foveal cone (<3 microns), permitting observations of cone photoreceptors in eyes with normal optics and retina. A wavefront-corrected cSLO (Burns et al. 2002, Roorda et al. 2002) or OCT (Zawadzki et al. 2005, Zhang et al. 2005) also provides increased contrast of retinal structures that improve this even further. A number of retinal imaging systems have since reached the diffraction-limit (<2 microns), and these have been incorporated into a wide variety of instruments for in vivo imaging.
3.1 Adaptive optics for understanding normal vision
How is adaptive optics retinal imaging helping you to investigate normal vision?
Stephen Burns (SB): By allowing us to measure variations in cone sampling with age and location (Song et al. 2011, Sawides et al. 2016) and properties of the vascular system (Chui et al. 2012a, Hillard et al. 2016), including how visual stimulation alters blood flow (Zhong et al. 2012).
Joseph Carroll (JC): Helping understand foveal development in normal and deseased retinas.
Adam Dubis (AD): One of my current projects is to understand blood vessel tone and control in normals and patients and the impact on neurovascular coupling. High resolution imaging allows measurement of capillary size, geometry, composition (wall/lumen ratio) as well as blood cell velocity.
Stacey Choi & Nathan Doble (SC & ND): Recent work has centered on the objective measurement of the Stiles-Crawford Effect of the first kind by imaging individual cone photoreceptors and their orientation (Morris et al. 2015). Another project has been extending the range of retinal eccentricities over which we can apply AO imaging, and we have imaged rods and cones out to 30° in the temporal and nasal retina (Wells-Gray et al. 2015), which is important in the study of diseases such as retinitis pigmentosa (RP). Examination of photoreceptor (rod and cone) densities and packing geometry parameters from control subjects can then be used as normative data against which other ocular conditions can be compared.
Ann Elsner (AE): There are wide variations among normal subjects in cone density and the distribution of cones depends not only upon total number (Elsner et al. 2016), but also foveal development. Older eyes have lower cone densities (Song et al. 2011), but it is not known if this is due to loss of cones, migration back to the periphery, or both. The outer nuclear layer does not adequately quantify small changes in cone density, since older subjects have, on average, fewer cones but thicker outer nuclear and Henle layers (Chui et al. 2012b). AO quantification of cones in an en face manner allows investigation of these factors, their interaction, and the neuroplasticity of the retina.
Ravi Jonnal (RJ): First, much of my work depends on measuring the phase of light returned from the retina, which enables measurement of structure and function at axial resolutions significantly smaller than afforded by OCT (Jonnal et al. 2007, 2010, 2012). AO uniquely permits this by isolating light from single cells. Second, AO uniquely allows me to investigate its cellular origins (Jonnal et al. 2014). This work, which is ongoing, is a guide to clinical OCT images, allowing more accurate interpretation of the latter even as the cost and complexity of AO currently hinder its clinical deployment.
Donald Miller (DM): AO in conjunction with OCT has given us the capability to observe “living histology” where we can track structural changes in cells over time, enabling us to reconstruct physiological processes actively occurring in the retina. Using AO-OCT and AO-flood, we have conducted quantitative studies of individual cone photoreceptors: measuring their antennae and waveguide properties (Gao et al. 2009, Liu et al. 2015), phototransduction (Jonnal et al. 2007), outer segment renewal (Jonnal et al. 2010, 2012), outer segment disc shedding, and cellular origins of their reflectance bands (Jonnal et al. 2014). We have also used it to study, in 3D, the optical properties of the tiniest of retinal vessels that form the foveal avasular zone (Wang et al. 2011, Miller et al. 2011), and retinal nerve fiber bundles from the optic disc to the fovea (Kocoaglu et al. 2011). More recently, we have mapped the spatial pattern of retinal pigment epithelial cells to cones (Liu et al. 2016) and now to capillaries of the choriocapillaris, all in the living human eye.
Hannah Smithson and Laura Young (HS & LY): With AOSLO, there is the potential to not only measure eye movements at high speed and high resolution but also to reference those motions to the retinal cone mosaic. We are using this to investigate the spatial sampling of images formed on the retina and how this interacts with eye motion. Our emphasis is on psychophysical experiments, linking performance in detection and discrimination tasks to retinal stimulation.
Yuhua Zhang (YZ): We have conducted a comprehensive assessment of macular cone distribution and variation within and between subjects (Zhang et al. 2015). Our study confirmed that in the living human eye, though cone densities vary significantly in the fovea, the total number of cones within the cone-dominated foveola is less variable than total number. Our study establishes a baseline for assessing aging, disease, and treatment effects on the cone photoreceptors.
3.2 Adaptive optics for understanding clinical conditions
How is adaptive optics retinal imaging advancing your understanding of clinical problems?
SB: It allows us to study remodeling of small blood vessels in both diabetes (Burns et al. 2014) and hypertension and to compare and contrast across diseases (Hillard et al. 2016). It also allows us to investigate the relation between sensitivity loss and nerve fiber defects in glaucoma (Huang et al. 2015). Finally, with visual stimulation we can investigate neurovascular coupling in normal (Zhong et al. 2012) and diseased retinae.
JC: It allows us to examine remnant cone structure in patients with inherited retinal degenerations (Genead et al. 2011), which is helping inform the therapeutic potential on a patient-by-patient basis.
AD: In addition to the investigations of neurovascular coupling, we are looking at photoreceptor topography in aging and inherited eye disease. We have used AO imaging to understand what structure remains at the edge of lesions (Dubis et al. 2014). Because photoreceptor cell replacement therapies are still a long way from human trials, determining what cellular structures exist in a given area, and targeting therapy to a specific region with abundant cells will be critical, and no tool other than AO aided imaging can provide this information.
SC & ND: Obtaining retinal cellular information allows one to detect subtle changes that would otherwise be missed by conventional clinical diagnostic imaging systems. Furthermore, quantitative analyses of various subtle changes in the retina over time will enhance our understanding of disease mechanisms and the development of the pathophysiology at the cellular level in both humans and animal models.
Alf Dubra (AlfD): I work with clinical colleagues to learn about microscopic anatomical changes in diseased retinas through the dissemination of instrumentation that we develop. When we study inherited retinal degenerations as AD said, ophthalmic AO allows identification of candidates for gene therapy trials as well as, hopefully, study of the efficacy of the treatment. We also try to establish the sequence in which cells are affected and/or die in diseases. A successful example was the discovery that in Oguchi’s disease the source of the increased retinal reflectivity with light exposure, a notable characteristic of this condition, was due to increased rod photoreceptor reflectivity. In collaboration with Toco Chui and Richard Rosen, we study retinal micro-vasculature and pathology (e.g., microaneurysms) in systemic and retinal conditions (Dubow et al. 2014). The use of multiple scattered light as a source of contrast reveals non-invasively individual blood cells and capillaries, even if they are not perfused. This visualization of blood flow at, literally, the quantum level, is fun every single time, and we believe it will be useful for early diagnosis as in many cases it does not require baseline imaging to detect abnormalities. Importantly, Drs. Rosen and Chui have demonstrated vascular changes in response to medication in less than two weeks, which could allow unprecedented personalized treatment.
AE: The retinal vasculature changes dramatically in some, but not all, individuals with only mild to moderate nonproliferative diabetic retinopathy: lengthened and tangled capillaries are seen, along with a variety of other types of vessel remodeling and changes in perfusion (Burns et al. 2014). Motion mapping shows that there can be slowed or non-existent flow through some retinal capillaries. Microcysts are imaged, with multiply scattered light imaging. There are extensive hard exudates, with wide variation in sizes including exudates too small to be seen on clinical exam, fundus photography, or even in OCT. The exudates may cover more area than is seen with these other methods. We see that there are cones in the blind spot and in geographic atrophy, and that these survive without adjacent RPE (King et al. 2017). This has tremendous impact on understanding how to preserve vision, but having the normal amount of RPE may not be as important to saving cones as previously thought.
Melanie Campbell (MC): We have studied changes in retinal structure in type I diabetes before the onset of diabetic retinopathy. We found that cone photoreceptor densities in the parafoveal retina are not reduced in adolescents with type 1 diabetes but participants with diabetes did not show the expected radial asymmetry observed in control participants (Tan et al. 2015). We have shown that the macular cone photoreceptor mosaic is markedly disrupted in young children with the KCNV2 cone dystrophy (Vincent et al. 2013).
RJ: We use AO-OCT to study microscopic changes in retinal structure associated with optic neuropathies (Werner et al. 2011) and age-related macular degeneration (AMD). We have shown changes in the reflectivity of photoreceptors in and adjacent to disease-affected retina (Panorgias et al. 2013), but we have yet to acquire complete photoreceptor mosaics in disease-affected portions of the retina, even when we believe photoreceptors to be present and functioning. Many groups, using AO in two- and three-dimensional modalities, have reported similar difficulties, and this has led to active investigation into the underlying reasons for poor image quality below drusen and/or at the margins of geographic atrophy. Even in the absence of high-quality images of AMD-affected retinae, the reasons for diminished image quality are likely to inform our understanding of disease progression in the photoreceptors.
DM: We are using AO-OCT to evaluate cell loss dynamics in the retinal transition zone of patients with retinitis pigmentosa, and disruption kinetics in the cellular surround of drusen, an early indicator of AMD.
Michel Paques (MP): In dry AMD, AO imaging provides detection of very small atrophic spots, an exquisite view of the border of atrophy, and the observation of the motility of pigmented cells (Gocho et al. 2013). These elements contribute to categorizing patients according to the presence or absence of atrophic spots, according to the speed of progression (roughly said, slow vs fast progressors), and also according to the presence of melanin-loaded motile cells. Fluorescence imaging AO is also somewhat more precise than blue autofluorescence imaging to document the extent of atrophy, especially in the case of foveal sparing. During arterial hypertension, en face AO provides a measure of the parietal thickness of arteries (Koch et al. 2014) and allows identification of chronic hypertension rather than an acute high blood pressure. AO is also useful to detect minute changes in vessel morphology (Paques et al. 2015). Analysis of photoreceptor pointing on off-axis images are of interest in macular diseases, in which the visualization of the cone mosaic can be dramatically modified by multiangle observation (Miloudi et al. 2015). In uveitis patients, we observed that paravascular inflammation (perivenous sheathing) may be more easily detected by AO than by other means (Errera et al. 2014), providing us with a tool for mapping the extent of perivenous sheathing in cases of uveitis.
HS & LY: We are studying the effects of inherited retinal diseases, such as Stargardt disease, on the photoreceptor mosaic. Using metrics of cone arrangement we look for early-stage changes in the retina and are embarking on a longitudinal study to track these during disease progression. We aim to link specific genotypes to phenotypical data obtained from AOSLO images. Our long-term goal is to identify pre-symptomatic changes, potentially allowing degeneration to be detected earlier, and to monitor the treated retina non-invasively on a cellular level.
YZ: Pseudodrusen appear different from typical drusen, and they were originally thought to lie below the retinal pigment epithelium (RPE) (Mimoun et al. 1990). By comparing OCT findings to histologic examination of one donor retina with extracellular material between the RPE and photoreceptors called subretinal drusenoid deposits, Zweifel et al. (2010) attributed pseudodrusen to these lesions, though debate remains. Using AO-SLO-OCT, we showed that defined stages of subretinal drusenoid deposits are associated with reflectivity changes consistent with perturbed surrounding photoreceptors (Zhang et al. 2014). AOSLO revealed a distinct en face structure of stage 3 subretinal drusenoid deposits: a hyporeflective annular zone containing indistinct photoreceptors surrounding a reflective central area. AOSLO imaging in conjunction with SD-OCT suggested that the hyporeflective annulus likely consists of photoreceptors with deflected, degenerated, or missing inner or outer segments, and the reflective core of stage 3 lesions is the subretinal drusenoid deposit material itself. AOSLO and multimodal imaging of subretinal drusenoid deposits indicate solid, space filling lesions in the subretinal space. Associated retinal reflectivity changes are related to lesion stages and are consistent with perturbations to photoreceptors, as suggested by histology (Zweifel et al. 2010).
3.3 Technical Aspects in adaptive optics retinal imaging
What adaptive optics active elements are you using, and what is the reason for your choice?
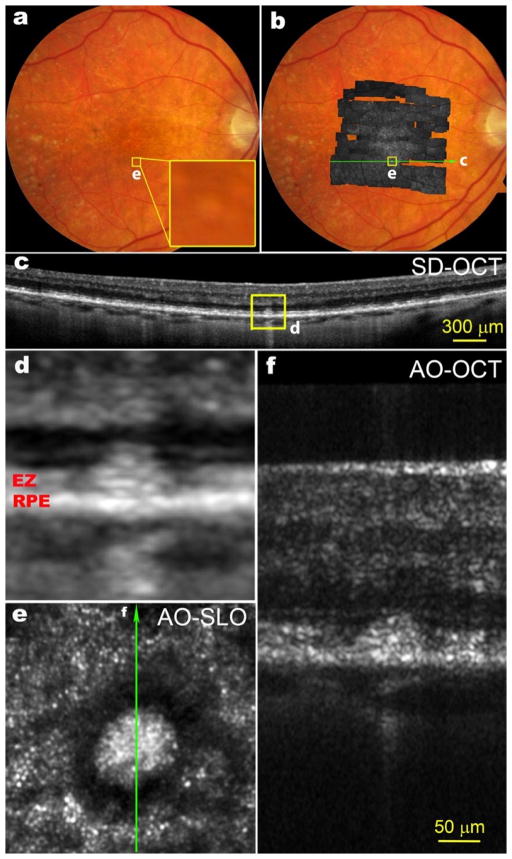
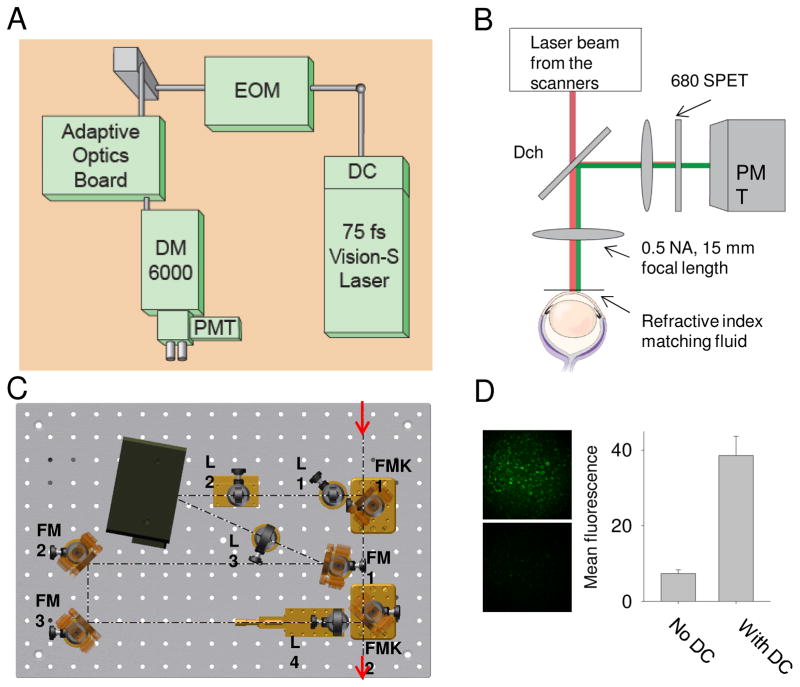
AE & SB: We use a woofer-tweeter arrangement with the Imagine Eyes Mirao 52e as the woofer and the 141 actuator BMC mirror as the tweeter (Ferguson et al. 2010, Zou 2008). This allows us to use the system without any additional refraction. We couple the system to a custom Shack-Hartmann wavefront sensor (SHWS) and our own software control algorithm. The combination is flexible and less expensive than an Alpao mirror, but has some added complexity in that an additional optical relay is required. We have also installed a clinical system that uses a BMC mirror in double pass mode (Webb et al. 2004) to save money and this has proven very reliable although the initial alignment was difficult.
AD & JC: We use an ALPAO 97-actuator deformable mirror (DM) and a custom-built SHWS. The mirror was selected because it performed better than others in our tests. The wavefront sensor is designed to match the actuator layout of our mirror.
SC & ND: Our DM is a 97-actuator ALPAO mirror, chosen because it has a large number of actuators- enough to provide good imaging for large human pupils coupled with large dynamic range to reduce dependence on trial lenses or a trombone (Doble et al. 2007). High stroke is useful in our AO-OCT systems as it removes the need to compensate for the extra dispersion when trial lenses are used. For AOSLO imaging we typically use a trial lens to remove second-order aberration preserving the DM for the high-order aberrations and for controlling temporal fluctuations along with using it for axial depth scanning. For wavefront sensing and control we use the ALPAO DM in conjunction with their WFS and Matlab control software. We find that it works very well with only a few modifications.
AlfD: A single voice-coil actuated continuous-membrane DM. This approach provides some of the best images to date, while minimizing complexity and maximizing light throughput (and thus light safety). Also, I strongly prefer these because their linearity, low-hysteresis and low cross-talk allow very simple control algorithms. In addition, their protected-silver coating, low scattering, 100% fill factor, smooth influence functions, large stroke and reliability are all pluses. The custom SHWS is matched to the DM (Dubra, 2007)
RJ: Our corrector is an ALPAO 97-actuator, high-speed mirror, and our wavefront sensor is a custom SHWS. We use a 20×20 lenslet array with a focal length of 30 mm and pitch of 0.5 mm, and we use 284 of the subapertures in a central, circular region of the array. The SHWS uses a Basler scientific CMOS sensor (acA2040-180kmNIR), which we selected for its high speed (180fps) and relatively low cost (< $3000, excluding CameraLink frame grabber). The AO system is controlled using custom software written in Python and C++.
DM: Currently using a single 97-actuator mirror from ALPAO. The large stroke and high fidelity of this mirror have worked well for correcting both low- and high-order aberrations of the eye. The mirror outperformed others we have used, including a 37-actuator Xinetics (Zhang et al. 2005), 140-actuator BMC (Cense et al. 2009), and 37-actuator AOptix (Zhang et al. 2006). We have been satisfied with SHWSs for measuring the aberrations of the eye, and while other sensor types may ultimately prove better, this has not happened.
HS & LY: We have chosen to use a BMC 140-element DM with a stroke of 3.5 μm. We initially chose this as a cost-effective solution for imaging in normal vision, where we expected the RMS wavefront aberration to be small. We find this deformable mirror to perform well in these conditions, provided that focus is corrected to within about 0.5 D. In normals we achieve this by natural accommodation to a target in a plane that is conjugate to the focal plane of the AOSLO. Any residual refractive error is corrected with trial lenses. Wavefront sensing is performed with a custom-built SHWS and real-time control is achieved using custom-written Python code.
Do you perform a closed-loop operation in your system?
SB, JC, SC & ND, AlfD, AD, DM, YZ: Yes.
AlfD: We operate in closed loop, with a very simple integrator algorithm having 90% gain. We have not explored improved controls, but we have on our list to deal with corneal reflection and pupil edges better, as well as to incorporate some prediction and deal with the dual set of spots in the SHWS (one mostly originating from the RPE and photoreceptor layers and one from the nerve fiber layer).
AE: We are investigating sensorless AO (Hofer et al. 2011) using the Digital Light Ophthalmoscope. The main advantage is decreased cost (simplicity). The difficulty is that most of the algorithms require many iterations and will need to be able to converge on the features of interest.
RJ: Generally we employ closed-loop correction at about 25 Hz. For a small fraction of subjects, e.g., those with extreme dry eye or excessive fixational eye movements, the AO system becomes unstable. In those cases we have opted to “freeze” the mirror after reaching a stable correction, but before acquiring retinal images. These images are worse than what we get during normal closed-loop operation, but better than if the AO system is unstable.
HS & LY: In general we run the AOSLO in closed-loop operation with a closed loop gain of 1. While a static correction works well over a short timescale, closed-loop operation provides better correction in general. This is because it not only corrects for changes in the ocular aberrations but also for apparent changes in these aberrations caused by movement of the eye’s pupil.
What types of detection strategy(ies) do you use to build up an image?
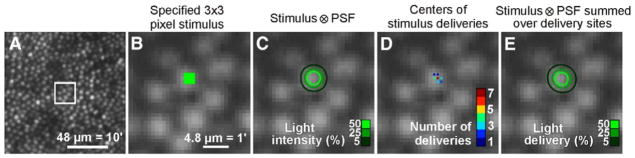
SB: We have used both line scanning (rolling shutter sCMOS) and point scanning. We select the light to build up the image using computer-controlled apertures (Burns et al. 2007) for confocal and multiply scattered light (Elsner et al. 1996, Chui et al. 2012a) imaging as well as using a DLP to allow dynamic aperture shaping. We collect up to four simultaneous channels, with one of them from a second light source with a precisely controled offset to control the temporal relation between the channels (de Castro et al. 2016).
AD: Currently we only use NIR imaging, with confocal and dual non-confocal channels. The non-confocal channels are split into left and right detection schemes. Averaging of the dual non-confocal channels produces a ‘dark-field’ scheme, which the difference equates to roughly differential phase or a ‘split-detection’ imaging scheme. The imaging scheme allows all three methods to be used simultaneously, for ease of co-registration, and imaging efficiency.
AlfD: Mostly point scanning, but we have also developed flood-illumination systems and we will explore line-scanning next.
AE: We are pseudoscanning by projecting lines with a DLP onto a 2D CMOS array with line-by-line readout that is synchronized to the illumination to serve as a confocal aperture. The goal is to decrease the cost of a system by moving to mass produced parts. The tradeoff is that we have to work within the capabilities of the commercially acquired subsystems.
RJ: Our imaging systems are all point-scanning OCT systems, both spectrometer-based (at around 850 nm) and swept-source (at around 1 μm). The first-generation system is a combined AO-OCT-SLO, which acquires an SLO frame with each OCT B-scan, in order to motion correct the OCT volumes (Zawadzki et al. 2011). The second-generation system’s sample channel is an out-of-plane design, with vertical folds in the system designed to offset the astigmatism and beam distortion caused by the horizontal folds (Lee et al. 2013). A majority of effort in the lab is directed toward angiography applications, which require custom scanning patterns. In post-processing, we employ a number of algorithms: speckle-, phase-, and complex-variance, along with bulk motion phase correction for angiography; automated layer segmentation; automated three-dimensional photoreceptor segmentation; Doppler and power Doppler analysis; and phase-sensitive imaging.
DM: Our system is fiber-based AO spectral-domain OCT and raster scans a point source in a symmetric sawtooth pattern across the retina. Novel is a high-speed detection channel that achieves A-scan rates of 1 MHz using a quad-spectrometer cascaded with three nanosecond optical switches, all tightly synchronized (Kocaoglu et al. 2014). The system is 25 times faster than clinical OCT.
HS & LY: We use a raster-scan system with off-the-shelf electronics to record the scan position and intensity measurements from an avalanche photodiode (APD), or for higher sensitivity, a photomultiplier tube (PMT). We capture the raw data from both scan directions over the entire scan range and reconstruct the images in post-processing.
YZ: I have developed a new generation AOSLO which addresses several major obstacles in imaging of older patients with AMD (Meadway et al. 2013, 2014). First, the pupil size decreases and wavefront aberration increases with aging. Second, many older patients have cataract, which significantly affects wavefront detection and impairs AO wavefront correction. Third, in patients with intraocular lenses, it is very common that proliferation and transformation of lens epithelial cell remnants lead to posterior capsular opacification or fibrosis over the intraocular lens. While capsulotomy can make an opening on the opacified posterior capsule, the clear pupil for imaging often has an irregular shape. This may not only reduce the useful pupil size but also cause complicated light scattering that impedes AO operation.
What do you believe are unique technical features in your system that you would recommend to investigators considering building an adaptive optics retinal imaging system?
SB and AE: (1) The use of high efficiency, low noise APDs allows long-term stability of imaging and eliminates detector damage from room lights. (2) The design minimizes astigmatism by controlling elevation and tilt of elements (Burns et al. 2007, Ferguson et al. 2010). (3) The focus adjusts from −11D to +4D and can be pushed through the whole retina, including over areas of major retinal elevation. (4) the configurable detection scheme can be dynamically adapted to the retinal features of interest (Chui et al. 2012a). Multiply scattered light imaging provides unique information and we use three channels of detection for one optical scan beam and a second channel to provide higher temporal resolution (de Castro et al. 2016). (5) The steerable front end allows the patient to look straight ahead while we direct the imaging field to areas of interest and provide a real time plot of the current AO field onto a static 30 deg SLO image. (6) a DLP projector for visual stimuli and fixation. (7) We dynamically evaluate SHWS spots to use the best parts of the pupil to adjust the AO mirror, helpful in patients with cataract, posterior capsule opacification, or IOLs. (8) Positioning of the subject’s eye is quick with the chin rest and pupil camera, plus added real time filtering of “good” SHWS spots and control of the pupil edges (Zou et al. 2011).
JC: Nothing unique, the system design has been replicated at many of our collaborators’ sites. Perhaps it is that – the open sharing of designs and construction assistance that is unique.
AD: The split detection, non-confocal imaging is essential to any scanning device. There is the additional cost and complexity, but it is zero cost in the photon budget. It completely changes what structures are visible, especially in degenerating retina where interpretation of images is difficult.
AlfD: I do not believe that we do anything unique in our AO ophthalmoscopes (Figure 8). The only thing I would recommend to others is adoption of the off-the-plane folding of each individual reflective telescope (when using reflective optics) to simultaneously mitigate monochromatic aberrations affecting pupil and image conjugate planes (Dubra et al., 2011). Also, I would suggest the adoption of a single DM as the wavefront correction strategy, for higher light throughput and lower instrument complexity (essential for maintenance and use by people lacking optics training).
RJ: The following features are certainly useful. (1) We invert the control matrix on the fly, and can change the number of SVD modes to optimize residual error and stability for individual subjects; 50 modes is a typically useful value. (2) We employ both modal and zonal reconstructors, and can switch between them as necessary. (3) Our system has the ability to stop down the “correction aperture” by partitioning the control matrix. When spots near the edge of the pupil have low quality, we can take them out of the control matrix and rely on modal correction (along with enforcing zero piston on the mirror) to supply the correct control values to actuators at the mirror’s edge. The same can be done for individual spots anywhere in the SHWS image. (4) We pick off a portion of the beam with a pellicle just upstream of the SHWS and focus it with a lens onto a 2D CMOS sensor (DCC1545M, Thorlabs). This gives us real-time evaluation of the system’s spot in sync with the SHWS measurements. This far-field camera allows us to verify that the PSF reconstructed from the wavefront, which itself is reconstructed from the SHWS slopes, bears at least qualitative resemblance to the real system spot and assurance that AO computations are correct. (5) A pupil camera is an extremely useful feature. On our wish-list is automatic pupil alignment. Imaging in the NIR, it is sometimes time-consuming to align the subject’s pupil to the beam. We plan to incorporate automatic beam and pupil tracking. Such a sub-system could be used to do initial alignment of the subject. Even better, because we have observed that the quality of correction can depend strongly on the beam’s position within the underfilled pupil, this system could be used to optimize AO correction as well.
-
DM: Our AO imaging system (Figure 9) shares many common design features but two that are (or were) unique to our system and others might find helpful: (1) Placement of the DM close to the eye. Former postdoc Yan Zhang discovered the importance of placing the DM on the same side of the scanners as the eye (Zhang et al. 2006). (2) Correction of system astigmatism using toroidal mirrors. Our in-the-plane solution provides an alternative to the off-the-plane design developed by Gómez-Vieyra et al. (2009). It has several key strengths in terms of calibration and performance that we believe are worth considering, but is expensive (Liu et al. 2013).
As an additional point, extensive AO controls are becoming increasingly common and offer improved flexibility in working with different types of eyes. While important, we have found that great AO performance is built on getting the details right in the hardware. This means rigorous calibration and precise alignment (Liu et al. 2013). These are well recognized by experienced AO users, but can come as a surprise to beginners.
-
HS & LY: Our AOSLO system uses a novel modular optical design that removes the need for either an astigmatic correction or a non-planar alignment. This configuration is compact, relatively simple to align and can be built with off-the-shelf optical components. Light detection for image reconstruction is achieved using an off-the-shelf APD and data capture is performed using off-the-shelf electronics. The use of such components makes replication of this system easy and relatively fast.
As a group that has developed an AOSLO recently, we have benefited substantially from open discussion with well-established labs. We are working with an excellent instrumentation group (Centre for Advanced Instrumentation, CfAI, Durham University, UK) and we benefit from being able to develop the instrument alongside evolving research questions, with a high degree of integration between technical expertise and vision research.
YZ: High speed (100 Hz) AO closed-loop frequency (Yu et al. 2015).
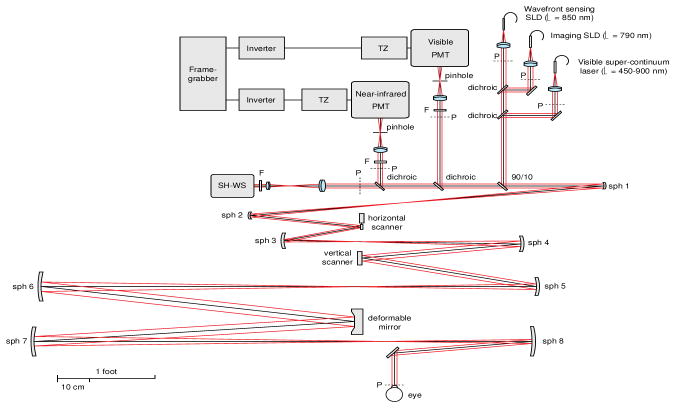
Figure 8.
Adaptive optics scanning ophthalmoscope sketch, flattened for clarity. PMT stands for photomultiplier, TZ for transimpedance amplifier, LD for laser diode, SLD for superluminescent diode, SH-WS for Shack-Hartmann wavefront sensor, sph for spherical mirror and F for interferometric band pass filter. The letter P indicates the pupil conjugate planes, in addition to the ones corresponding to the deformable mirror, the optical scanners and the SH-WS. (From Dubra & Sulai, 2011)
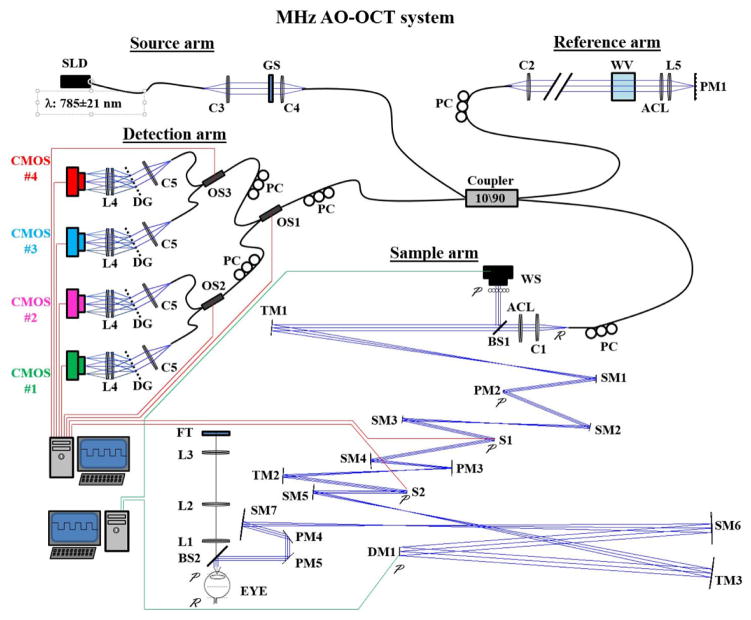
Figure 9.
Schematic of the Indiana MHz AO-OCT system. The in-the-plane sample arm (lower right) contains a 97-actuator ALPAO mirror (DM1) and Shack-Hartmann wavefront sensor (WS) for correction of ocular and system monochromatic aberrations; a custom achromatizing lens (ACL) for correcting ocular chromatic aberrations; and three custom toroidal mirrors (TM1, TM2, and TM3) for correction of astigmatism generated by the off-axis use of spherical mirrors. The detection arm (left) achieves megahertz imaging speed using a quadplex spectrometer design. Custom AO control software was developed in Matlab (Mathworks, Natick, MA) and incorporated the ALPAO Core Engine (ACE) Matlab libraries. See Kocaoglu et al. (2014) for details.
Do you believe that there are important differences between laboratory and commercial systems? If so, list the strengths and weaknesses as you see them
SB: Differences are not that large. Our lab system is easy to run and some commercial AOSLOs have very good performance. The main difference is size and flexibility. We build laboratory systems to be as reliable as clinical systems, but we do not make them small, because we often want to modify the optics. Another difference that is important, but is common to all research labs, is that we have access to all of the raw data, not just what unknown programmers have decided is appropriate. This second advantage however should be an essential part of clinical systems.
JC: Yes – lab systems are higher resolution, commercial are easier to use.
AD: The commercial device designers have a huge task in front of them; taking a field of bespoke instruments, often requiring operators with at least a working knowledge of various engineering fields and medical understanding, and trying to simplify it to make it clinically viable. Commercial design teams need to make design compromises to use lens or small diameter mirrors to fabricate devices small enough to fit in a clinical environment. The commercial systems have been much smaller and generally easier to operate, albeit with diminished quality in final images.
SC & ND: Two very different challenges in our opinion. Laboratory systems are typically state-of-the-art using the latest components and techniques. Usability is not a top concern along with other factors such as price and ease of maintenance. System specifics are changed as the research project develops. Commercial system performance is always going to be several years behind research systems given the development and implementation timeline. Commercial systems need to be cheaper and easier to maintain and calibrate in the field. Moreover, the patient interface and the time taken to acquire and process the data need to be much faster. They also need to be usable for every patient and wavefront sensor challenges such as media opacities need to be overcome.
AlfD: Laboratory instruments are usually larger, but that is not a major difference in terms of performance, as they have to be easily modifiable, while commercial instruments have to prioritize minimal maintenance.
AE: At least some commercial sytems have problems with too low spatial resolution in the instrument to allow for the measurement of foveal cones or small features. Sometimes you can see aliasing or image processing artifacts in the images, making it difficult to have confidence in quantification of images. Some commercial systems also provide software that is like a black box, and this can cause results to be difficult to interpret.
RJ: I have not worked with a commercial AO device, but as with commercial OCT devices, I suspect their proprietary inner workings will present challenges for investigators hoping to perform accurate measurements. I think a commercial AO imaging system that kept the AO details proprietary but published all the science imaging details could possibly be used to do sound science.
DM: This is difficult to answer because there are only a couple of commercial AO ophthalmoscopes. What I do know is that none of these systems have the combination of speed, 3D resolution, and fine spatial sampling needed to perform the imaging studies we are conducting. We also need access to the raw complex data, a general problem with most commerical systems regardless if they have AO.
HS & LY: Although we do not have experience with a commercial AOSLO system, previous experience has taught us that laboratory and commercial systems are, and should be, quite different. A commercial system should be robust and reliable so that images can be obtained easily, quickly, and with the least discomfort to the subject. Laboratory systems should be flexible, allowing changes to be implemented and tested as new requirements and ideas arise. The choice of one over the other depends on the main use of the system and the eyes being imaged. For standard imaging a commercial system may give the stability required for long-term, high-turnover imaging. They are also more patient friendly and less intimidating than an open system. However, for more challenging imaging, and particularly for technical development, it would be beneficial to have complete, unrestricted access to the system, as is afforded by a laboratory system.
What are the main technical limitations of current adaptive optics retinal imaging systems?
SB: Large eye movements, dry eye and dense media opacities.
JC: They do not work well in older eyes (media opacities) or patients with nystagmus.
AD: The limitations revolved around what structures are visible and the demand for more. I believe there is still a long way to go in our understanding of how photoreceptors are arranged and how neurovascular coupling works in the retina, the two largest fields. Many researchers want to have histology quality visualization of all retinal layers, not just photoreceptors and blood vessels. Overcoming the challenges around visualization of inner retinal neurons remains the largest limitation.
SC & ND: Not being able to get a large field of view comparable to fundus photographs prior to AO imaging for orientation purposes. Not being as tolerant as conventional ophthalmoscopy in seeing the retina through moderate amounts of ocular opacities from cornea to vitreous. Use of a large pupil in elderly populations is not always possible, indeed imaging over smaller pupils would relax alignment constraints for all populations. Coping with fast and large eye movements such as nystagmus.
AlfD: Complexity of maintenance, troubleshooting and achieving best possible image quality with all operators.
AE: Eye motion leading to intra-frame shearing. There are many alignment schemes, and none is perfect, leading to some data loss. This interacts with mapping the small fields onto a montage or bigger field with a high degree of precision. Small scale intraocular light scatter, such as that caused by tear film irregularities, can cause a substantial decrease in contrast. Comparing multi-modal data from different instruments is difficult, even when AO data are plotted onto the wide-field image.
RJ: The speed of our system is 30 Hz. The bottleneck in our AO loop is centroiding the spots, partly because we have more pixels in the sensor than we need. We have explored GPU-parallelization (combined with cross-correlation-based centroiding) to speed up the processing, but latencies in transferring data to and from the GPU were too high. We are investigating other avenues for parallelizing the problem, as well as testing other algorithms (e.g., using the brightest pixel as a proxy for the center of mass; or using cross-correlation with a template spot, which could better leverage the FFT capabilities of a GPU).
DM: Systems remain complex and require considerable technical support. An example that reflects this complexity at the operational level of AO is accuracy of the SHWS measurements. Complexities of the eye (e.g., retina is inaccessible) and system (e.g., non-common path aberrations), often leave doubt as to how close the SHWS measurement is to the actual aberrations, so much so that there is hesitation to report SHWS measurements, such as RMS wavefront error. Yet, these same measurements drive the wavefront corrector. This disconnect makes it difficult not only to assess AO performance, but also the gains that might accrue with further AO improvements.
HS & LY: For psychophysical assessment of visual function with normal and with damaged retinas, two attractive capabilities are real-time correction of eye movements and registration of multi-wavelength imaging and stimulation. These capabilities are being developed and further improvements will enable more sophisticated experimental designs. Improvements in resolution and sensitivity are also welcome. Clear imaging of foveal and parafoveal cones at low light levels (with minimal effects on visual response) will enable experiments that build on the wealth of existing data from psychophysical experiments with macroscopic stimuli.
YZ: Time-consuming image acquisition and processing.
3.4 Bringing AO-retinal imaging cameras to the clinic
What limitations must current adaptive optics systems overcome for adaptive optics to be widely used in the clinic?
SB: The systems are complex and this will always generate a cost differential. Decreasing cost requires a clinical application which has proven clinical utility and can thus be justified for patient treatment. This in turn requires accurate metrics for proven biomarkers that are currently lacking. More research is needed.
JC: Cost, ease of use.
AD: The limitations are largely in two areas. First, AO imaging can not be billed to US insurance companies; if AO becomes routinely useful for diagnosis and screening, it may be a profitable instrument for clinics. Second, while automated image analysis is improving, there is a great need for meaningful and repeatable analytics. We mainly use photoreceptor density or a derivative (center-center spacing, nearest neighbor distance and other Voronoi parameters), but even for these robust metrics, better normative databases are required.
SC & ND: In our view the biggest barrier is the cost of the AO elements. Wavefront sensorless approaches have the advantage of obviating the WFS but the deformable mirror (DM) remains expensive and may remain so. Low-order, low-cost (e.g., liquid crystal) correctors may work for large FOV imaging where contrast improvement is the main goal. Other challenges include the time to acquire data; the tradeoffs between dense spatial sampling, field of view, and data storage; the lack of robust microscopic metrics for retinal health.
AlfD: The major challenge is not the instrument, but rather the expertise and software required for image processing, interpretation and quantification. Things like cost, complexity, shorter imaging time and any other real or perceived challenges can be overcome within a company setting.
AE: Costs must be reduced and post-processing software must be improved, both in terms of usability and robustness.
RJ: Improving availability of AO in research could have two benefits: reduction of component costs via economy of scale; and development of collaborative, open-source software projects. These would mitigate two of the obstacles to commercial development.
DM: The generic answer is that current AO systems are too expensive, complex, and unreliable. However, these are solvable with directed engineering, mainly from industry. In academia, the focus has been on the scientific and clinical applications of these instruments rather than their technical limits. In the end, widespread use requires both. Much remains to be done.
MP: Interpretation of the AO images is challenging. A multimodal approach is often necessary for their correct interpretation. AO is exquisitely sensitive to any loss of transparency of the retina or ocular media. Better ergonomics (including wide field imaging, eye tracking, and automatic montaging) and multimodal imaging may be the keys to its widespread use in clinical practice, together with consensual interpretation schemes.
HS & LY: For widespread use in a clinic an AO retinal imaging system needs to work reliably with inexperienced subjects and with a high degree of automation. The most successful imaging sessions are those in which the subject can stay still, direct their fixation appropriately and maintain steady fixation. In the clinical population this can be a challenge and, as a result, it can be much more difficult to obtain good quality images. Developments that overcome these limitations, such as eye-movement compensation and targeting and tracking systems will be important for the proliferation of AO retinal imaging use in the clinic.
YZ: (1) Time-consuming image acquisition and processing. (2) Questions that cannot be addressed without AO high resolution imaging.
3.5 Future of AO for retinal imaging
Do you think there will continue to be major advances in adaptive optics systems for retinal imaging? If so, what features do you expect to be developed in the future?
SB: Yes, there will be an increasing ability to capture rapidly changing events, whether event related or ongoing physiological changes. Advances in using every photon (including multiply scattered ones) and what are now lost frames will allow detection of increasingly more anatomical substrates in the retina. Advances in computational imaging will also begin to play a major role in research systems.
JC: I think there will be superresolution techniques, expansion of non-confocal imaging, and imaging of non-photoreceptor structures. More importantly, I think advances in software for analyzing these images will be a major development.
AD: The development and modification of non-confocal methods yielded new inner retinal structures in animal models. Several groups are looking to develop real-time chromatic aberration correction to start doing spectroscopic analysis of the retina. Both of these modifications hold promise for the future.
SC & ND: The field requires advances in low cost wavefront correctors, and the ability to overcome media opacities and large eye movements. We need to generate ocular health measures based on whole retinal properties rather than the keyhole views we currently have.
AlfD: I expect advances to come in three categories. First, the imaging success rate has to increase dramatically and this will probably require: eye motion correction; better wavefront sensing and correction (to better cope with cataracts, corneal reflections, intraocular lenses, etc.); better fixation targets and/or beam steering to reduce imaging time; improved image registration algorithms (better and more automated registration); better and potentially automated focusing algorithms. Second, many existing imaging modalities need to be refined to reveal new retinal structures and cells. This includes, non-linear imaging, spectroscopic imaging and multiple-scattered imaging. Finally, we need to translate to the eye some of the super-resolving techniques that have transformed microscopy over the past decade, with the obvious example being structured illumination.
AE: There are several advances that are moving forward, such as the use of multiply scattered light, visual stimulation, and decreases in cost— in our labs and many others.
RJ: I do not know the next revolutionary change, but countless small problems persist, the solutions to which would greatly enhance AO imaging. In particular, we should pursue methods to improve reliability and robustness of these systems. This effort could be greatly aided by a real repository of open-source AO software (and perhaps hardware) tools, which would prevent the perpetual wheel-reinvention we currently observe and attendant bugs and lack of documentation.
DM: The holy grail of ophthalmic AO is to have the patient’s aberrations well corrected and stabilized within seconds of the subject sitting in front of the AO ophthalmoscope. This has to be done robustly and automatically in the presence of eye motion, ocular opacities, blinks, varying pupil sizes, IOLs, etc. As AO is inherently limited by software, realization of this goal will ultimately be through major advances in the control software.
HS & LY: There will continue to be developments in retinal imaging and we believe that changes in detection strategies (e.g., split detection and super resolution techniques) provide exciting opportunities for improved imaging of the different structures within the retina.
YZ: Yes. Multi-photon imaging. Cellular level retinal function biomarker and assessment.
What are the biggest challenges you face in using adaptive optics retinal imaging?
SB: The scientific issues of understanding the impact of disease on tissue at this scale and the resultant light-tissue interactions.
JC: Lack of adequately trained students and postdocs – too many go to industry.
AD: The challenges are in time required for acquisition and analysis of images. These features are a weakness as people not in the field do not appreciate the power of cellular imaging. Many clinicians in ‘imaging’ design trials with hundreds of patients take very crude OCT measurements and do very little analysis of these images. This is essentially a sledgehammer approach to understanding disease specific changes in retinal anatomy. AO-aided cellular imaging gives you a jeweler’s hammer. You are able to make very fine observation of the retinal changes and polish off what we learned with other imaging modalities.
SC & ND: Small pupil sizes and media opacities seen in elderly patients.
AlfD: Patients with strong involuntary eye motion, edema, multi-focal intraocular lenses and many other issues that we often do not understand that result in poor images (or even no images at all). The image processing currently requires heavy user input.
AE: Testing patients who have poor fixation and/or with disrupted tear film, which makes averaging difficult and sampling over time for blood flow even more difficult. Data analysis and interpretation is time consuming. Not every parameter can be explored, due to the fact that patients have a finite amount of time that they are available.
RJ: Our main challenge is in imaging older and/or disease-affected eyes. The reasons for this are not fully understood, but probably include the following: age-related changes in optical clarity and quality; exacerbation of fixational eye movements in those with compromised vision; and rapid variation in measured defocus due to elevation changes associated with drusen. We are undertaking a number of technical improvements to address these demands.
DM: The biggest challenge is to obtain sharp images across a wide range of eyes, with the most difficult being older and diseased. But these systems continue to require significant, extended funding to support the technical personnel and maintain equipment.
-
MP: Some are rather trivial: the field of observation is limited to the central 15°, which may leave out of reach more peripheral structures. The observation of structures with uneven surfaces such as the optic nerve is challenging; strong accommodation is also a problem when examining pediatric patients. Poor fixation is an issue. There is also a lack of software to extract information from multiangle imaging.
Some are related to remodeled tissue: Photoreceptor counting in diseased eyes is a challenge. This is probably because dystrophic retina combines several features, such as photoreceptor loss, decreased reflectance intensity, local variations of transparency in the extracellular milieu, changes in their optical properties due to cellular alterations, and photoreceptor disarray.
HS & LY: The largest challenge is in coping with the variation between subjects. When imaging eyes that are highly myopic or have a low retinal reflectance, as examples, it can take some time to set the system up.
4. Adaptive optics for biology and neurophysiology
The retina has been a favorite tissue for vascular biologists and neurophysiologists and the rapid advance of AO retinal imaging will further extend its value for research. AO brings near microscopic resolution to the study of retina, making it possible for the first time to examine thousands of cells simultaneously as well as longitudinally over weeks, months or even years. AO imaging is in its infancy, but even now it can be combined with other quickly evolving methods to give us unexpected insights. For example, when used with optogenetic tools it can offer new insight into developmental processes as well as degeneration or vision restoration efforts, limited only by our ingenuity in inserting different indicators and modulators into different classes of cells. If combined with fluorescence and additional imaging methods it can provide precise 3D location of labeled cells in the animal retina (Zawadzki et al. 2015).
One strength of AO is the ease with which it can be applied across species, permitting the investigator to choose the ideal model for examining particular issues. The mouse is a particularly apt species for many questions, given the ready availability of transgenics of all sorts and the many genetically matched human retinal diseases that are present in the mouse. Fortunately, the mouse eye has a large numerical aperture making both transverse and axial resolution of AO imaging superior to that found in larger species. However, larger species also have some merit. Dog retina provides models of retinal degeneration and AO imaging produces fine results (Campbell et al. 2016). The macaque is an especially valuable animal species to study, with a retinal structure and function that is virtually identical to humans. This is not true of other non-primate species, especially rodents, which share few of the specializations of the primate retina for visual acuity. An additional value of the macaque is that it is considered perhaps the best animal model of light damage to the retina, and phototoxicity is being studied in depth in macaques (Hunter et al. 2011, 2012; Masella et al. 2014a, 2014b). Even human retina may be available for both basic research and development of novel therapeutics if we can develop two-photon autofluorescence to examine the wide range of fluorophores that are available to measure the normal function and dysfunction of retinal processing. Currently two-photon imaging is being explored for imaging all retinal layers in living mice (Sharma et al 2013) and monkeys (Sharma et al 2016a, 2016b) and for ex vivo imaging of ocular tissue (Bueno et al. 2010, 2011; Skorsetz et al. 2016).
Retinal AO imaging has proven a powerful new approach for vascular and neurobiology and the range of currently ongoing experiments will seem very primitive in 10 years.
4.1 Adaptive optics and visual perception and function
How is adaptive optics retinal imaging helping you to investigate the physiology and structure of the eye?
PA: In the early days of AO for the eye, we built one of the first instruments to record retinal images with AO in humans in vivo (Vargas et al. 1998). Later, we focused on the visual applications of AO. In recent years, our imaging has focused on improving two-photon microscopy images obtained in ocular tissues in vitro (Gualda et al. 2010, Bueno et al. 2010). This includes retinal and corneal samples. In non-linear microscopy it is important to control the size of the spot on the sample. This is degraded by the system aberrations and the distortions created by the sample itself. AO allows us to improve images while reducing the light exposure on the samples.
MC: We use AO to look at the structure and location of amyloid deposits in the retina in an animal model of Alzheimer’s disease and to image these deposits in two-photon excited fluorescence (TPEF) (Campbell et al. 2016). We also image rodent models of diabetic retinopathy and other human diseases which manifest in the retina.
Jennifer Hunter (JH): We are investigating the kinetics of the macaque visual cycle. AO imaging helps us to see at the level of individual cells in small regions of the retina. Using TPEF of retinol in photoreceptors, we are tracking intensity changes presumably related to its production and clearance. TPEF also allowed us to image ganglion cells in vivo in macaque without the use of extrinsic labeling (Gray et al. 2006, 2008). This has been used to validate other new methods (multi-offset aperture) for visualising the ganglion cell layer. In vivo TPEF imaging has also produced images of many other retinal structures including Muller cells and blood vessel walls. In mouse, we have combined TPEF imaging of extrinsic fluorophores with AO assisted fluorescence lifetime ophthalmoscopy (AOFLIO).
-
Andrew Metha (AM): AO enables sufficient spatial resolution to study basic physiological processes in the living human eye at the level of single cells. There are several concurrent projects:
The first is an exploration of the reflectance characteristics of individual cone photoreceptors as the pigment held within their outer segments is bleached by light. Our areal (full-field) cameras can operate at frames rates of 1000 frames per second, gathering very high temporal resolution information regarding factors that change cone reflectance - the amount of photopigment bleached as well as interferometric contrast generated by ultra-small scale changes in optical path length between reflective (scattering) surfaces within cones.
Because these cameras operate in “global shutter mode”, wherein every pixel in an image array records light simultaneously, the images are not distorted by the inevitable small eye movements that always accompany fixation (tremors, drifts and micro-saccades). Combined with operating at frame rates in excess of several hundred per second, this enables us to track individual red and white blood cells as they traverse, single file, through retinal capillary networks. (Bedggood & Metha, 2012; Bedggood & Metha, 2014).
Even when not tracking single red blood cells, the minimal distortion allows measuring subtle changes in blood vessel diameter (for vessels 8–20 μm in diameter) in response to light stimulation or modified gas-breathing, and thus study neurovascular coupling (e.g., Duan et al. 2016).
Grazyna Palczewska (GP): Incorporation of AO helps with reducing laser power and increasing the dynamic range, both needed to obtain informative two-photon visualization of retinal cells in living animals (Palczewska et al. 2014).
Jesse Schallek: (JS): We study the neurovascular physiology of the retina. AO allows us to image microscopic capillaries 1/10th the thickness of a human hair. After correction, the lateral resolution in the mouse eye is approximately 1 μm which enables us to study neural cells throughout the retina, single blood cells, and their movement in the capillary network.
Lawrence Sincich (LS): Our primary goal for using AO retinal microscopy is to stimulate single photoreceptors in the living, intact eye. With this ability we can study perception at the cellular level and also conduct neurophysiology experiments where we can identify the photoreceptors that are providing input signals to downstream neurons recorded elsewhere in the brain. The hope is to learn how the signals from single and multiple photoreceptors lead to the often unique responses of individual visual neurons. The structure/function relationship in vision can be newly bridged: AO imaging provides the structure, stimulation with AO will give us the function (Sincich et al. 2016).
William Merigan (WM): It allows us to track neural functions over time in the living animal. It is excellent for tracking retinal disease, restoration, and other time dependent visual processes.
How is adaptive optics retinal imaging advancing your ability to bring new approaches to translational research?
PA: This is an important aspect. We are actually working on an AO microscope to be applied in vivo for the cornea. If it is successful, AO should play a significant role.
MC: A better understanding of the structure and location of amyloid deposits in the retina will give insight into retinal changes (thinning of the ONFL for example) and visual function in Alzheimer’s disease. It will also help in the development of noninvasive methods of imaging amyloid in the retina as a biomarker and early diagnostic of Alzheimer’s disease. We wish to extend these methods to develop biomarkers of other diseases.
JH: We are investigating phototoxicity with the most sensitive in vivo methods of light delivery and imaging of cellular structure. Imaging of lipofuscin autofluorescence in the RPE has provided a measure for establishing thresholds for photochemical disruption of the retina in response to long duration exposures to visible light. AO imaging has had a direct impact on the current ANSI Z136.1-2014 standard for the safe use of lasers. Our latest results are being considered for future revisions of the standard.
AM: Until AO came to intraocular imaging, we were not able to observe the passage of red and while blood cells through the capillaries of the retina (Bedggood & Metha 2012). This has limited our detailed understanding of this basic physiological process; how blood flow is regulated in response to neural activity (Duan et al. 2016), and how this is affected in diseases such as diabetes. Being able to track capillary blood flow means that we are better placed to understand retinal diseases in which blood flow is disrupted (such as by the formation of microaneurysms in diabetes, or the small red cells in thalassemia), as well as the impact of therapies aimed to ameliorate these effects.
GP: We investigate the mechanisms leading to retinal damage and prescreen drug candidates and their impact on preservation of the retina in living mouse models (Palczewska et al. 2014).
JS: Our study of microvascular perfusion in the living eye is first being investigated in animal models. This approach provides benefits during the developmental stages of new instrumentation. For example, refining strategies in animal models allow us to optimize system performance free of patient fatigue, low-light budget issues and wavefront correction optimization before it is deployed in the clinic. Our strategy is to optimize systems and validate imaging before deploying these to a clinical population.
LS: From the viewpoint of a neuroscientist, access to single sensory receptors, especially in vivo, is a tremendous advantage for studying both normal and disease states (Sincich et al. 2009, Bruce et al. 2015). Ordinarily such access is only available in dissected preparations, limiting what can be said about the naturalness of the cellular responses to stimuli. By doing our work in vivo, we can learn how signals from individual photoreceptors are used by the rest of the brain, with the potential of providing clearer distinctions between normal and early-stage disease. We can also be more confident that the responses from the receptors themselves are as natural as they can be (e.g. no concern about the bathing media, etc.).
WM: Our present focus is the study of visual function in individual cells, such as an exploration of the function of multiple RGC classes. Full application of our animal studies to human retina will require development of less invasive methods, such as two-photon imaging.
What adaptive optics system are you using, and what is the reason of your choice?
PA: We have been using different correcting devices during the years, both deformable mirrors and spatial light modulators. Each has advantages and disadvantages. Lately, we favor LCOS-SLM.
MC: We use systems with deformable mirrors that we ourselves have developed because of their flexibility. We have also used a PSI AO system with an ALPAO mirror.
JH: We use custom-built system and software. This provides greatest flexibility. We are using mirrors from ALPAO.
AM: We built our current AO system to allow maximum flexibility. Key features of the current system are the ALPAO 97-actuator 13.5mm mirror for wavefront correction, and the Andor Neo sCMOS camera for rapid imaging with minimal frame distortion. We also wanted flexibility in imaging wavelength both for differential cone stimulation as well as for multispectral imaging (for both cones and for blood oxygenation), so we invested in supercontinuum laser sources (Fianim 6 and 8 W).
GP: We had developed systems and software based on an iterative algorithm to control the shape of the deformable mirror. We use image quality metrics as feedback without the use of the wavefront sensor. In this way we avoid non-common path aberrations.
JS: Our lab uses a three-channel AOSLO using PMT detection. Simultaneous channel acquisition allows us to study contrast from multiple light modalities at the same time. For example, we are using the split-detector configuration (Guevara-Torres et al. 2015) to measure the differential scatter of light in two channels, while simultaneously capturing visible fluorescence in a third channel. Simultaneous capture provides multimodality to examine contrast arising from independent physiological responses.
LS: Our system was custom built, in collaboration with Austin Roorda who developed scanning laser AO ophthalmoscopy. There were no commercial systems available at the time for human imaging (in 2010). But also, we wished to have 3–4 wavelength channels to perform both imaging and stimulation, which I believe no current commercial system has.
WM: We use custom-built AO systems designed specifically for monkey and mouse. Other species with similar eye size and numerical aperture can be imaged in the same systems.
Do you perform a closed loop operation in your system while imaging?
JH: In my opinion, closed loop is the best option. Some deformable mirrors will not maintain a constant shape for extended periods of time and hence open loop can result in drifts in image quality from changes in the eye’s aberrations and shifts in the mirror’s shape.
AM: Yes - given the (albeit rather slow) fluctuation of ocular aberration, we never considered not using a closed loop system. Although our frame rates are very fast (200–1000 Hz) we only correct as fast as we need to - around 15 Hz seems adequate.
JS: The majority of our imaging is in closed loop operation. However, there are conditions where open loop correction is beneficial. Examples of this are when correction needs to be applied for wavefront structures that are not planar (such as the optic disc). Aberrations can be corrected by correcting aberrations at a nearby retinal location (~50 micrometers away), freezing the correction, then repositioning the image field location to the target of interest. This approach comes with a set of assumptions and is dependent on constant centration of the eye-pupil, yet provides a solution for AO correction from highly irregular surfaces.
MC, LS, WM: Yes.
Do you use a wavefront sensing system? Why or why not?
PA: We have typically incorporated in our system a Shack-Hartmann sensor, but we are also using a sensorless approach. We modify the wavefront in a manner that we can optimize the signal accordingly.
MC, JH: Yes.
AM: Yes - we built our own from a lenslet array from AO associates (400 micron spacing, square array, focal length 24 mm; cost about $1000) and attached this to a good quality CCD camera from Pulnix. This was easy to do.
GP: Our method relies on optimizing the surface of deformable mirror based on the image quality metrics feedback without the use of wavefront sensor. With this approach subcellular images of retinal pigment epithelium were obtained.
JS: We use a wavefront system to provide rapid and correction based on the wavefront rather than image quality metrics to instruct best correction.
LS: We use a wavefront system because it is easy to pick off a small amount of light reflected back from the retina to measure aberrations with a Shack-Hartmann wavefront sensor. Aberration correction can be made up to a speed of 30 Hz, which is adequate for most of our imaging tasks. However, higher speeds would be better for those interested in studying motion perception and for neurophysiology.
WM: Yes, although we are enthusiastic about the promise of sensorless imaging.
What type of detection strategy do you use to build up an image?
PA: Second harmonic generation and non-linear fluorescence.
MC: We have used fluorescence and also intrinsic contrast. We are interested in further development of AO-corrected polarization contrast.
AM: We use full-field imaging with an areal arrays of detectors. We upgraded from a Megaplus CCD camera (which were what we could afford at the time - about 4Mpixel and 25 electron RMS read noise) to our current scientific CMOS camera (Andor Neo) and acheived massive increases in frame rate from about 20 frames per second to many hundreds of frames per second. The read noise of sCMOS cameras is wonderful - less than a few electrons per pixel RMS. We use global shutter mode to expose all pixels simultaneously. Although this limits the duty cycle to about 50%, this strategy ensures simultaneous exposure of all pixels resulting in very little (i.e. no) intra- and inter frame distortion at frame rates in excess of 100 frames per second. Such distortions are a major limitation to point scanning systems, which have the benefit of confocality to reject-out-of-image-plane scattered light and so enjoy much better contrast, especially if there is any intra-retinal scattering arising within diseased or older eyes. In our system, we gain great protection against eye movement-induced distortion, but our contrast is limited by light scatter from non-target structures. Line-scanning systems might be a good intermediate.
GP: To excite specific endogeneous and artificial fluorophores in the retina (Alexander et al, 2016), we apply tunable lasers with wavelengths appropriate for two-photon excitation of the intermediates and by-products of the retinoid cycle. The two-photon excited fluorescence is then collected in a non-descanned manner, to maximize the signal. In some cases, especially when working with high-quantum yield artificial fluorophores, we incorporate narrow bandpass filters to increase specificity of the detection. To improve signal to noise ratio of the RPE and retinal vasculature images in living mice we developed an algorithm for image registration and alignment.
-
JS: We use PMTs for high signal-to-noise acquisition and fast detector readout in low light configurations. High sensitivity detectors are needed to maximize signal from a limited light budget that must not impart phototoxic damage to the eye. After imaging, we use post processing registration to average images to further increase signal to noise. In recent work, we have applied computational strategies to optimize selective contrast within the image. For example, by computing the temporal variance in retinal structures over time, we can compute “motion-contrast” maps. Contrast is provided by structures that are constantly changing in time (e.g. blood flow). In this way, positive contrast arises from dynamic change; low contrast is provided by static structures (constant values). In the case of blood flow, this strategy provides perfused vascular contrast against the static neural parenchyma. A limitation of this technique is that it requires little or no residual motion or registration error.
In other work that examines single cell blood motion, we have found it beneficial to digitally oversample the spatial image. We capture many pixels within the space of the optical point spread function of the AOSLO system. This strategy allows us to spatially average N-pixels within the optical PSF without degradation in optical resolution. This strategy has helped increase SNR in images where every frame counts.
LS: We use a scanning laser system, in conjunction with PMTs, to create an image over time from the raster timing signals and the voltage output of the PMTs.
WM: Often we use slow accumulation of sparse photons (e.g., two-photon imaging), which is much more feasible in animal imaging due to the absence of eye movements and blinks. Use of functional measures in humans will require development of highly efficient, less time consuming approaches.
Do you believe fluorescence is important for biological imaging? If so at what wavelengths and to identify what molecular species?
MC: Yes, we use fluorescent markers of amyloid (including TPEF) as the gold standard for their identification and comparison with other methods of creating contrast.
JH: Definitely! There are immense possibilities in single-photon and two-photon excited fluorescence of intrinsic and extrinsic fluorescence.
AM: Yes, there is a reason that so many Nobel prizes have been awarded that are associated with GFP. In living human eyes the first use will be simple contrast enhancement of blood - not any particular molecular marker. I would worry about the safely of introducing other fluorescent molecules into living humans, but for animal studies I think this is just beginning.
GP: Yes fluorescence imaging is most important, because it tells us about the metabolic status of the tissue.
JS: Fluorescence imaging allows targeted contrast of the structure/protein of interest. In our work, we have used fluorescence to label proteins within cells that are transgenically expressed in the mouse (Schallek 2013). To date, there is a large catalog of available fluorophores in the 400–650nm range. Further improvements in the near infrared spectrum would have many additional benefits for light safety and mitigating tissue scatter.
LS: Fluorescence is undeniably important. Wavelengths and molecules of interest are going to vary tremendously, depending on the experiment at hand. In our work, current and future, wavelengths anywhere between 450 and 1400 nm will be usefully employed, some as excitation (including two-photon) and some as emission.
WM: Yes. A large number of distinguishable fluorescent fluorophores are now available that can be attached to function indicators, biomarkers, and biosensors. One type of indicator we have used is the genetically encoded calcium indicator G-CaMP (Yin et al. 2013; 2014). Multiple wavelengths are available, but future developments will see increasing use of red-shifted indicators, which provide better light safety.
What do you believe are unique technical features in your system that you would recommend to investigators considering building an adaptive optics system for in vivo or in vitro imagining?
PA: Our system also includes temporal compression. The combination with spatial AO can provide additional benefits.
MC: Our system is unique in having a treatment beam channel which is AO corrected. Generally, I would recommend including a fluorescence channel and the flexibility to add additional optical elements when needed.
JH: Many novel features have been developed: real time tracking, real-time stabilisation, real-time desinusoiding (without the need for reference grids), real-time registration and averaging to provide summed fluorescence images instantly (important for checking quality and focus). Computer control of almost all components and the ability to run automated image acquisition scripts like we used on the phototox protocol (2 degree reflectance and fluorescence pre-images, open and close shutter of exposure laser for specific amount of time, image reflectance during exposure, 2 degree reflectance and fluorescence immediately post-images, we could also change the field size if needed) (Zhang et al. 2015).
AM: This depends on the scientific questions being addressed. Our system is well suited to measuring with accurate spatial relationships (minimal eye movement-induced distortion), but this precludes studying the retina in some disease states with increased intraretinal scatter. Point (and line) scanning systems are more robust in this regard. The sCMOS cameras allow rapid frame rates, so the temporal resolution is exquisite.
GP: Two-photon-fluorescence imaging offers high resolution, low noise and can excite chromophores not accessible with traditional longer wavelengths used in SLO.
JS: In our research, we use fluorescence and split-detection approaches. These modalities provide new contrast and biomarker detection needed to disambiguate structurally and functionally relevant light signatures in the retina.
-
LS: Our system is multi-wavelength, currently with 3 channels whose wavelengths can be independently chosen, based off of light from a super-continuum laser. Having multiple channels offers a lot of flexibility. One does have to be concerned about the effects of chromatic dispersion when doing this, especially if trying to compare images of the same structure taken with different wavelengths. So this adds complexity, but once built into the system, handling it can be fairly straightforward (Harmening et al. 2012).
If you are interested in more than just imaging, a second feature we have is the ability to deliver visual stimuli, as movies or other constructs. This requires high-speed light modulation and control software, but allows experiments involving perception or some other type of response to a visual stimulus. High-speed switching will also be useful for fluorescence imaging, as the wavelength of interest can be turned on only when needed.
A third feature is real-time eye tracking, to compensate for eye motion which is present in behaving and (less so) in anesthetized animals. If one is interested in stimulating single photoreceptors in the eye, for instance, this type of eye tracking is essential (Harmening et al. 2014, Sincich et al 2016).
Image tracking would also be useful for AO imaging of any type of moving structure (e.g. wriggling embryos).
WM: Flexibility of switching between different laser lines, stimulation, self -aligning features, additional capabilities that can be switched between users.
Do you believe that there is a broader need for specialized systems for your area of research?
MC: Yes.
JH: The TPAOSLOs are specialised systems. Each system is designed for a specific pupil size. We had limited success with a single instrument for monkey and rat. The alignment always needed to be recalibrated and slowly drifted over time from the multiple users.
GP: To streamline work combining multiple modalities into a single imaging platform would offer a tremendous benefit.
JS: Specialized systems will always be at the forefront of developmental research. They will push the envelope forward for new modalities and applications. In particular, I believe new systems optimized for blood flow detection will have a large impact in the field.
-
LS: More robust systems with larger fields of view and better resolution will be needed to explore certain aspects of retinal function as well as retinal disease. Fields of view are currently on the order of one degree, which limits how natural a stimulus can be, especially for stimuli containing motion. It would be helpful for both neurophysiological and perceptual studies to have larger stimuli with well-controlled motion components.
The robustness of eye tracking is a current limit. Larger fields with stabilized stimulus delivery is computationally demanding. But work in that direction will need to be done, as eye/specimen motion is an inescapable problem, and such efforts will greatly improve patient imaging as their fixation capabilities are often quite compromised.
WM: Yes. We use systems tailored for both the species and questions to be asked. It is desirable, but not always possible, to build in flexibility so that multiple experiments can be conducted with a single system.
What are the main technical limitations of current adaptive optics retinal imaging systems for your work?
MC: Most are designed for human imaging and not small animals. Few have fluorescence or the flexibility to add additional corrections.
JH: Light detection. Timing for the AOFLIO to provide sufficient resolution.
AM: I would like a confocal point line-scanning system to allow improved contrast for studying blood flow, but detector sensitivity and speed are currently limiting. Another limit to point scanning speed is the speed of the fast scan: 15–17 kHz is not enough.
GP: A detector with higher quantum yield would be the most useful.
JS: Three advances would benefit current AO system performance: (1) Greater ability to montage and create AO images from large fields free of distortion. (2) Software refinement and ease of use would facilitate greater data collection and (3) Faster acquisition. Scanner speed appears to be a physically limiting factor in AOSLOs.
LS: Spatial resolution (although some systems have recently greatly improved this with better AO correction), frame rate for imaging (higher speeds will help), transverse chromatic aberration correction (short wavelengths are difficult to correct due to safety issues in living eyes), non-real time image processing of movie sequences, background light in imaging frame (light leaks through the light modulators make the imaging frame visible to the subject).
WM: Axial resolution for imaging stacked cells, such as macaque RGCs. Also, limited stimulus development, although that is advancing rapidly.
Do you think there will continue to be major advances in adaptive optics systems? If so, what are the features you expect to be developed in the future?
PA: I can anticipate that faster correcting devices will be available for real time imaging.
MC: Yes, I expect fluroscence systems and systems for small animals to be further developed and that the range of optical slicing in the small animal retina will increase. I also expect advances in polarization imaging.
JH: We are developing spectroscopy and expanding out AOFLIO capabilities. I expect that we will continue to FRET. There is significant interest in imaging Raman scattering. With advances in the components, there will be advances in the capabilities. For example, if a new detector could have sufficient sensitivity and detect polarisation, then that could open up new investigations. Our people are always interested in super-resolution. Any of the new advances that occur in microscopy could potentially be translated to retinal imaging.
AM: Yes. I think there are a range of tricks that have been employed in microscopy for a long time that are only now being entertained in the field of AO retinal imaging imaging (e.g. Zhou et al. 2014). Approaches like Alf Dubra’s split detector encourages me to think that phase contrast imaging might be a real option. We might also exploit polarization effectively to achieve phase contrast imaging. Imaging small animal eyes such as those of rodents holds promise due to the increased numerical aperture, but is frustrated by difficulties in wavefront sensing from a single retinal plane of interest (Zhou et al. 2012). To obviate this problem, it is good to see steady progress being made in the area of sensorless AO (Zhou et al. 2015, Wahl et al. 2016, Cua et al. 2016) and computational AO (Shimonski et al. 2015).
GP: Faster algorithms for mirror surface optimization.
JS: Future AO systems will allow users to select the layer that wavefront sensing is performed on. To date, most AOSLOs presume an isoplanatic patch which may not represent a flat surface. This is particularly true in the diseased eye and on structures that have strongly irregular surfaces such as the optic disc and large vessels. Another advance currently being pursued is that of wavefront sensorless correction, where features of the image drive the iterative control signal in the deformable mirror. These approaches can work without the aid of a Shack-Hartmann wavefront sensor, but can also piggy-back on top of already optimized correction. Image optimization on image features such as contrast and other image quality metrics may provide higher contrast images based on native contrast of the retina.
-
LS: Imaging based on scattered rather than reflected light will continue to improve with AO techniques and will provide much new information. two-photon fluorescence will also improve, as single-photon imaging is already edging up against safety limits for in vivo work.
As the diffraction limit is reached in most systems, having more robust control software to make operation of the systems more turnkey will be developed to make AO imaging more accessible by groups without extensive optics expertise.
WM: Yes, new methods for measuring the function of retinal neurons will continue to be developed: intensive fluorescence indicators, FRET indicators, two-photon autofluorescence, etc.
What are the biggest challenges you face in using adaptive optics retinal imaging?
MC: Maintaining the optical quality of the animal eye and alignment and tracking across the retina in the anaesthetized animal.
AM: In my system, poor contrast. Tear file stability also seems to be more problematic than I think it should be.
GP: Ideally there should be only one person needed to use the system throughout the entire imaging session from the placement of the animal to obtaining images.
JS: (1) Cost. (2) ease of use (software) (3) available post-processing strategies. (4) Limited availability of highly trained personnel to run, calibrate, refine and generally use the instrumentation.
LS: Cost and upkeep of systems are non-trivial. Keeping track of stabilized stimulus delivery is quite time consuming, though at first glance the problem seems mundane. Rapid (or even continuous!) transverse chromatic aberration measurement and correction is a challenge for multi-wavelength stimulation to be accurate at the cellular level in living eyes (Harmening et al. 2012).
WM: Insertion of genetically encoded indicators, especially in macaques. Also, the poor axial resolution of AO imaging system.
What is the functional readout you plan to use to examine retinal neuronal responses?
JH: Redox ratio is one of the things I will try to explore in the future using TPEF.
AM: We have done intensity-based analyses and made inferences about neuronal function from these.
GP: Visual sensitivity and changes in fluorescence.
JS: We are using metrics of blood flow, blood velocity, blood cell flux, vessel diameter and fluorescence change over time to assess the changes in blood flow in response to neural demand or visual stimulation.
LS: For perceptual studies it will be probability of seeing, or two-alternative forced- choice responses (contingent on type of experiment). For neurophysiology, responses are currently focused on extracellular neural recordings in primates, either single or multi-unit.
WM: Genetically encoded calcium and voltage indicators as well as two-photon autofluorescence signals.
What animal or human model will you use for this research?
PA: We use samples from rats, rabbit, chickens, pigs and humans. We are now looking for ethical approval for corneal imaging in vivo in humans.
MC: We use the dog, chicken and rat models and will use the mouse model in the future. We also image human eyes.
JH: Mouse and monkey are the ones we use now. There are other great models to look at depending on the application, such as ground squirrel, dog, marmoset, chicken, etc. The systems just need to be designed for the particular eye size.
-
AM: I have tried hard to get rodent AO imaging working in my lab, but there are limitations that are difficult to work around. For example, despite the increased NA for rodent eyes, the major problems is dealing with the dioptrically thick retina - i.e. using Shack-Hartman aberrometry might not be feasible, so we may need to invest more effort in “sensorless” AO methods.
These are not issues for human eye imaging. In humans, I want to study the neurovascular dysregulation leading to capillary angiopathy in young people with diabetes. Young diabetics can have clear media, so flood imaging is not impacted by out-of-plane scatter.
GP: Human, primate and various mouse models.
JS: My research uses mouse models, macaque models and human subjects in conditions of health and disease.
LS: Macaque and human.
WM: Mouse, monkey and marmoset for the foreseeable future. AO systems need to be designed for the particular eye size.
Which visual system neurons will you target (photoreceptors, RGCs, vascular system)
MC: We are excited at the possibility of imaging RGCs in Alzheimer’s models.
JH: A better questions might be which ones we won’t target. Even then, I think there is interest and much to be gained from studying all cell classes.
AM: Photoreceptors and blood cells.
GP: Photoreceptor cells and their interaction with RPE, RGC, vasculature.
JS: We are targeting ganglion cells, translucent cells of the inner retina, and the retinal circulation.
LS: Cone photoreceptors and vascular beds for imaging, the major classes of RGC, LGN, and V1 neurons for physiological recordings.
WM: We study retinal ganglion cells, bipolar cells and photoreceptors primarily.
Is your measurement method likely to alter neuronal responses?
MC: No.
JH: Response could be altered by anaesthesia. Alternatively, it could be intentionally pharmacologically altered.
AM: Yes, deliberately so. In one project, we stimulate the retina with light and look for changes in blood flow. This is to study neuro-vascular coupling (Duan et al. 2016). In other projects we deliberately bleach photoreceptors and study reflectance changes (Bedggood et al, 2013).
GP: Less than other modalities.
JS: Without using fluorescence, our imaging approach uses ~100–200 uW of NIR light. The mammalian eye is relatively insensitive to these wavelengths. In the mouse eye, which is substantially blue-shifted compared to primates, the impact of this light is even less. Using low level NIR light, the eye is either in the dark-adapted state, or is only weakly activating a population of long-wavelength sensitive cones.
LS: Not any more than when more traditional visual stimulation techniques are used.
WM: Insertion of genetically encoded calcium indicators into cells may alter the function or lifetime of these cells. Also, extensive imaging of individual cells over months may produce phototoxicity.
Figure 2.
Examples of implentations of Adaptive Optics systems for visual psychophysics. Top. Schematic layout of AO system at Laboratoire Aimé Cotton, Université Paris Sud 11 (courtesy of Richard Legras). Bottom: Schematic layout of AO system at KTH (courtesy of Linda Lundström).
Figure 3.
Examples of implentations of Adaptive Optics systems for visual psychophysics. Top:. Schematic layout of AO system at Center fof Visual Science, University of Rochester (courtesy of Geungyoung Yoon). Bottom: Schematic Layout of AO system at Queensland University of Technology (courtesy of David Atchison).
Figure 4.
Examples of. polychromatic AO system at VIOBIO Lab, Instituto de Optica (CSIC) (courtesy of Maria Vinas and Susana Marcos).
Figure 5.
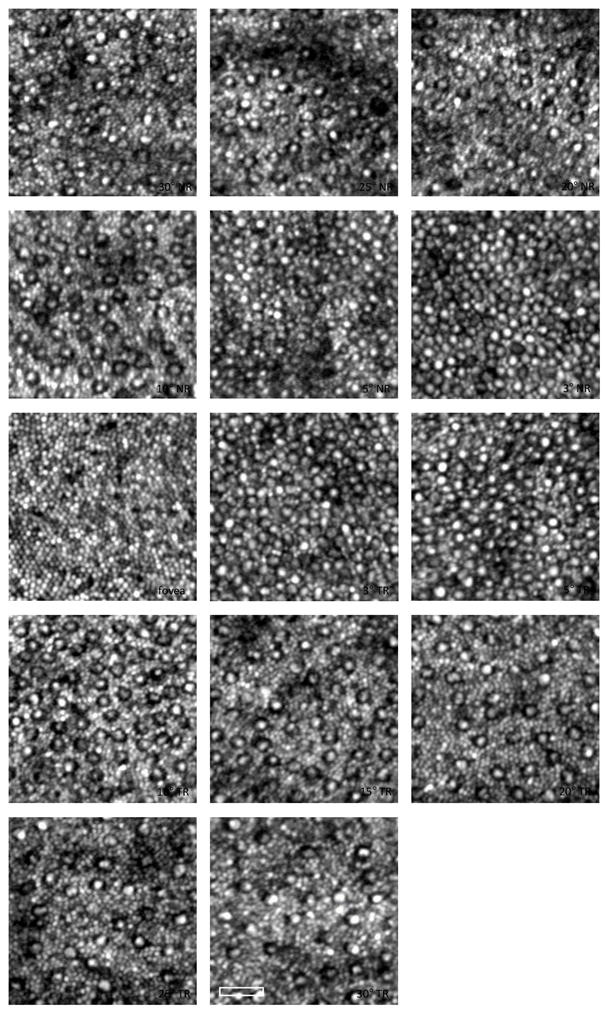
AO-SLO images of the cone and rod mosaic at locations spanning 30° NR to 30° TR for a normal human subject. Logarithmic intensity scaling to enhance the visualization of the rod photoreceptors. Each image is the registered average of ~50 frames. The scale bar is 25 μm. At large eccentricities an increase in the rod spacing was observed. (Wells-Gray et al., 2016)
Figure 6.
Figures show an example image of the cone mosaic overlaid in green with intensity patterns (retinal stimulation) that would result from stimuli presented to the retina. Panel a) shows sharp Landolt C, panel b) shows a Landolt C blurred by the optical components of the eye, panels c), d), and e) show three examples of the blurred Landolt C translated on the retina by fixational eye movement over a period of 100ms. Images have been scaled to represent a Landolt C with a gap equal to twice the resolution limit of the eye. From Young et al. (in prep.)
Figure 7.
Multimodal imaging of SDD. (a), Color fundus photograph. The yellow box (e) is of 300 μm on a side, (b), AOSLO montage overlaid on the fundus photograph. (c), A SD-OCT B-Scan taken along the green arrow-line in panel b shows that this SDD has broken the photoreceptor EZ band. (d), Magnification of boxed area in panel c. (e), The AOSLO image of the boxed retina in panels a and b. The bright spots outside the hyporeflective annuls are photoreceptors, mostly cones. (f), The AO-OCT scan of the SDD, as indicted by the green arrow line in panel e. The scale bar in panel f also applies to panels d and e. All SD-OCT images are in logarithmic grey scale. AO-OCT is in linear grey scale. The subject is an 83-year-old non-Hispanic man of European-descendant with intermediate stage non-neovascular AMD. (From Y. Zhang, unpublished)
Figure 10.
Images acquired with AO ultrahigh-resolution OCT. In a log-scale B-scan focused on the outer retina (A), the ELM, IS/OS, and COST bands are clearly visible, demarcating the IS and OS of the cones. In a linear-scale, magnified view (B), the IS/OS and COST reflections from individual cones are clearly visible, with red and yellow boxes outlining the relatively transparent individual inner and outer segments. The width of the bright reflections is consistent with known inner segment widths, while their height is comparable to the axial PSF height, which suggests origination at thin reflectors. Axial displacement of neighboring reflectors is apparent in both layers. When focus is shifted to the inner retina (C), individual nerve fiber bundles, up to 50 μm in diameter but separated as little as 5 μm, become visible. A magnified view of the latter (D) reveals capillaries (arrows) laying in multiple plexuses. These individual structures of the inner and outer retina appear as uniform bands in clinical OCT images. (From Jonnal et al., 2016).
Figure 11. Stimulus geometry and delivered light distribution.
A, AOSLO image of cone mosaic at 3.1° eccentricity, with outlined area scaled up in B–E. B, Cone reflectance profiles at this eccentricity span ~7 pixels, nearly 5 μm in diameter. Stimuli were specified in image pixels, a 3 × 3 pixel square stimulus in this example. C, Light intensity delivered to the retina is estimated by convolving the stimulus geometry with the diffraction-limited PSF of the eye (see Materials and Methods). Intensity contours show that the light spreads over a broader area than the 3 × 3 specification. D, Plot of actual delivery locations of the stimulus center relative to the targeted cone for a 22-trial psychophysical run. Positional delivery errors in eye motion correction caused stimulus deliveries to be jittered from trial to trial. E, Cumulative distribution of light delivery on the retina during the run in D, derived from the diffraction-limited stimulus integrated over the actual delivery locations. Transverse chromatic aberration was assumed to be constant for this analysis. from Figure 2 in Harmening et al. (2014) J. Neurosci.
Fig. 12.
Adaptive-optics two-photon microscope at the University of Murcia, Spain. Image examples of collagen fibers in the cornea.
Fig. 13.
Two-photon excitation imaging system (TPM) for mouse retina and RPE. (A) TPM system layout. DC stands for group velocity dispersion pre–compensation; EOM - electro–optic modulator; DM6000 - upright microscope; PMT - photomultiplier tube. (B) Dichroic mirror (DCh) and barrier filter 680 SPET separate fluorescence and excitation light. (C) Layout of the adaptive optics system. FMK1 and FMK2 stand for fold mirrors on kinematic magnetic bases; L1, L2, L3 and L4 - lenses; DM - deformable mirror; FM1, FM2 and FM3 - fold mirrors. (D) Left panel, RPE image in an ex vivo 1-month-old Abca4−/−Rdh8−/− mouse after exposure to bright light, obtained with (top image) and without (bottom image) DC; right panel, mean fluorescence measured with and without DC; error bars indicate S.D, n=3. (Palczewska et al, 2014)
Acknowledgments
Scientific Contributions.
John S. Werner: Scot Olivier, Sophie Laut, Don Miller, Steve Jones, Stacey Choi, Robert Zawadzki, Donald Miller, Sandra Balderas-Mata, Ravi Jonnal, Mehdi Azimipour; Susana Marcos: Lucie Sawides, Maria Vinas, Carlos Dorronsoro, Aiswaryah Radhakrishnan, Enrique Gambra, Miram Velasco, Clara Benedi, Sara Aissati, Ana M Gomez. Pablo Artal: Juan Bueno, Martin Skorsetz, Francisco Avila. Joseph Josua Fernandez, Pedro Prieto, Christina Schwarz and Silvestre Manzanera. David Atchison: Huanqing Guo, Andrew J. Lambert, Marwan Suheimat. Karen Hampson: Edward Mallen, Dr Matt Cufflin, Dr Alistair Curd, Niall Hynes Richard Legras: Hélène Rouger, Yohann Benard, David Rio. Linda Lundstrom: Peter Unsbo and Robert Rosén. Geungyoung Yoon: David Williams, Ramkumar Sabesan, Len Zheleznyak, Antoine Barbot, Rob Dowd, Keith Parkins. Stephen Burns and Ann Elsner: Toco Y P Chui, Weyao Zou, Alberto de Castro, Lucie Sawides, Ting Luo Joel A. Papay, Kirby D. Johnston, Bryan P. Haggerty and Matthew Muller, Weiyao Zou. Carroll: Robert F. Cooper and Yusufu Sulai. Stacey S. Choi and Nathan Doble: Cherry Greiner, Susanna Finn and Elaine Wells-Gray for the design and construction of our current combined AO-OCT-SLO system. And, John S. Werner and Robert J. Zawadzki, UC Davis for their help with the OCT implementation. Adam Dubis: Alfredo Dubra, Yusufu Sulai and Drew Scoles. Alfredo Dubra: Zachary Harvey, Yusufu Sulai, Drew Scoles, and Nripun Sredar. Ravi Jonnal: Don Miller, Yan Zhang, Jungtae Rha, Robert Zawadzki, Jack Werner, Barry Cense, Omer Kocaoglu, Susan Garcia, Justin Migacz, Iwona Gorczynskal. Donald Miller: Barry Cense, Ravi Jonnal, Omer Kocaoglu, Kazuhiro Kurokawa, Zhuolin Liu, Junie Qu, Jungtae Rha, Jack Werner, Robert Zawadzki, Yan Zhang. Michel Paques: Kiyoko Gocho, Céline Chaumette, Juilette Amaudruz, Edouard Koch, Jonathan Benesty, Serge Meimon, and Florence Rossant. Hannah Smithson and Laura K. Young: Timothy J. Morris and Christopher D. Saunter for instrument development, and Susan Downes for clinical collaboration. Yuhua Zhang: Austin Roorda, Jacque Duncan, Christine A Curcio, Cynthia Owsley, Alexander Meadway, Xiaolin Wang and Tianjiao Zhang. Jennifer Hunter: the ARIA group including staff, David and Bill. TPEF – Robin Sharma, Christina Schwarz, James Feeks, Sarah Walters, Kris Palczewski, Grazyna Palczewska. Phototoxicity – Jie Zhang, Tracy Bubel, Ranjani Sabarinathan. Andrew Metha: Dr Phillip Bedggood deserves special mention for any advancements that my lab may have contributed to AO technology and implemetation. Joe Zhou also made key advances. Grazyna Palczewska: Krzysztof Palczewski, Nathan Alexander, David Williams, Patrycjusz Stremplewski. Jessie Schallek: Keith Parkins, Andres Guevara, Aby Joseph. Lawrence Sincich: W.M. Harmening, W.S. Tuten, A.J. Roorda, K.S. Bruce, A.S. McKeown, P. Tiruveedhula. William H. Merigan: Vision restoration – Soon Keen Cheong, Sarah Walters, Christina Schwartz, Robin Sharma. Normal retinal physiology – Lu Yin, Juliette McGregor. Melanie Campbell: Laura Emptage, Marsha Kisilak, Carol Westall, Ajoy Vincent, Tom Wright, Jennifer Hunter, Elise Héon, Wylie Tan, Durgaa Rajendran, Yaiza Garcia-Sanchez, Laura Finkelberg, Christina Schwarz, Sarah Walters and Melissa Brooks.
Funding Sources: John S. Werner: NIH grants R01 EY 024239, P-30 EY012576 and Research to Prevent Blindness. Susana Marcos: European Research Council under the European Union’s Seventh Framework Program (FP/2007-2013)/ERC Grant Agreement [ERC-2011-AdC 294099]. Spanish Government grants FIS2011-25637 & FIS2014-56643-R, Spanish Government FPU and FPI Predoctoral Fellowships, CSIC JAE -Pre program. Pablo Artal: European Research Council Advanced Grant ERC-2013-AdG-339228 (SEECAT) and the Spanish SEIDI, grant FIS2013-41237-R. David Atchison: Australian Research Council Discovery Grants DP110102018 and DP140101480. Karen Hampson: UK College of Optometrists; EPSRC (Engineering and Physical Sciences Research Council), Grants EP/D036550/1and EP/G015473/1. Linda Lundstrom: Swedish Agency for Innovation Systems (VINNMER 2008-00992) and Swedish Research Council (621-2011-4094). Geungyoung Yoon: NIH/NEI, NYSTAR. Stephen Burns and Ann Elsner NIH grants EY007624, EY026105, EY024315 and EY004395. P30EY019008 and Foundation Fighting Blindness. Joseph Carroll: NIH R01EY017607, Foundation Fighting Blindness, and Research to Prevent Blindness. Stacey S. Choi and Nathan Doble: support by NIH EY020901 and Department of Defense (DoD) Telemedicine and Advanced Technology Research Center (TATRC) grant W81XWH-10-1-0738. Alfredo Dubra: the Burroughs Wellcome Fund, Research to Prevent Blindness, Glaucoma Research Foundation and National Eye Institute. Ravi Jonnal NEI K99 EY 026068. Donald Miller NEI R01-EY018339 and P30-EY019008. Michel Paques: Institut National de la Santé et de la Recherche Médicale (Contrat d’Interface 2011) the Agence Nationale de la Recherche (ANR-09-TECS-009, ANR-12-TECS-0015-03 and LabEx LifeSenses ANR-10-LABX-65). Hannah Smithson and Laura K. Young: the John Fell Fund (University of Oxford), Fight for Sight, and the Wellcome Trust Institutional Strategic Support Fund. Yuhua Zhang: NIH grants EY021903, EY024378, P30 EY003039) and NSF IIA-1539034. Melanie Campbell: Canadian Institutes of Health Research and Natural Sciences and Engineering Research Council of Canada Collaborative Health Research Program. Jennifer Hunter: NIH R44 AG043645, R01 EY022371, R01 EY004367, P30 EY001319, and BRP-EY014375. Also supported by an unrestricted grant to the University of Rochester Department of Ophthalmology from Research to Prevent Blindness, New York, New York. Andrew Metha: Supported by an Australia Research Council (ARC) Discovery Project Grant (DP0984649), an ARC Discovery Early Career Researcher Award (DE120101931), an A.E. Rowden White Foundation benevolent bequest, and the University of Melbourne Interdisciplinary Seed Fund. Grazyna Palczewska: NIH NEI R44 AG043645, European Private Foundation. Jesse Schallek: NIH Grants EY023496-01, EY014375,EY001319, The Schmitt Program on Integrative Brain Research, the Research to Prevent Blindness Stein Innovation award, Research to Prevent Blindness Career Development award (Schallek), an unrestricted grant to the University of Rochester Department of Ophthalmology from Research to Prevent Blindness, New York, New York, and Canon Inc. Lawrence Sincich: National Eye Institute, Eyesight Foundation of Alabama. William H. Merigan: NIH R44 AG043645, R01 EY021166, and P30 EY001319. Also by an unrestricted grant to the University of Rochester Department of Ophthalmology from Research to Prevent Blindness, New York, New York.
Footnotes
Commercial Disclosures
Susana Marcos: Co-founder of 2EyesVision SL, Spain. Essilor International, France, PhysIOL, Belgium, Oculentis, Germany, Staar, USA have funded research agreements where our custom AO systems have been used.
John S. Werner: None.
Stephen Burns: Aeon Imaging
William H. Merigan: None.
Pablo Artal: Founder of Voptica SL, Spain.
David Atchison: Research funding from Carl Zeiss Vision
Karen Hampson: None.
Richard Legras: Research funding from Vistakon (Johnson & Johnson) and Essilor.
Linda Lundström: None.
Geunyeung Yoon: Johnson & Johnson (research grant), Bausch and Lomb (research grant), Coopervision (research grant), TearLab (Research grant), Ovitz (consultant), Bausch and Lomb (consultant), Allotex (consultant).
Joseph Carroll: Athena Vision (Consultant), OptoVue (Research Support).
Stacey S. Choi: None.
Nathan Doble: Co-founder of Iris AO Inc., a manufacturer of MEMS deformable mirrors.
Adam Dubis: None.
Alfredo Dubra: Consultant for Boston Micromachines and Athena Vision (recently purchased by MeiraGTX), and past consultant for Canon USA.
Ann Elsner: Founder and principal owner of Aeon Imaging, LLC.
Ravi Jonnal: None.
Donald Miller: None.
Michel Paques: Former consultant to Imagine Eyes.
Hannah Smithson: None.
Laura K. Young: None.
Yuhua Zhang: None.
Melanie Campbell: Raytheon ELCAN Optical Technologies, P&P Optica, InterVivo Solutions (Collaboration and Research Support).
Jennifer Hunter: Patents held by UR and collaboration, including support, from Polgenix, Inc.
Andrew Metha: None:
Grazyna Palczewska: Director, Medical Devices Polgenix Inc.
Jesse Schallek: Canon Inc and Patents held by University of Rochester.
Lawrence Sincich: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander NS, Palczewska G, Stremplewski P, Wojtkowski M, Kern TS, Palczewski K. Image registration and averaging of low laser power two-photon fluorescence images of mouse retina. Biomedical Optics Express. 2016;7:2671–2691. doi: 10.1364/BOE.7.002671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artal P, Chen L, Fernández EJ, Singer B, Manzanera S, Williams DR. Neural adaptation for the eye’s optical aberrations. Journal of Vision. 2004;4:281–287. doi: 10.1167/4.4.4. [DOI] [PubMed] [Google Scholar]
- Artal P, Manzanera S, Piers P, Weeber H. Visual effect of the combined correction of spherical and longitudinal chromatic aberrations. Optics Express. 2010;18:1637–1648. doi: 10.1364/OE.18.001637. [DOI] [PubMed] [Google Scholar]
- Artal P, Schwarz C, Cánovas C, Mira-Agudelo A. Night myopia studied with an adaptive optics visual analyzer. PLoS ONE. 2012;7(7):e40239. doi: 10.1371/journal.pone.0040239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison DA, Guo H. Subjective blur limits for higher order aberrations. Optometry and Vision Science. 2010;87:890–898. doi: 10.1097/OPX.0b013e3181f6fb99. [DOI] [PubMed] [Google Scholar]
- Atchison DA, Guo H, Fisher SW. Limits of spherical blur determined with an adaptive optics mirror. Ophthalmic and Physiological Optics. 2009;29:300–311. doi: 10.1111/j.1475-1313.2009.00637.x. [DOI] [PubMed] [Google Scholar]
- Atchison DA, Guo H, Charman WN, Fisher SW. Blur limits for defocus, astigmatism and trefoil. Vision Research. 2009;49:2393–2403. doi: 10.1016/j.visres.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Babcock HW. The possibility of compensating astronomical seeing. Publications of the Astronomical Society of the Pacific. 1953;65:229–236. doi: 10.1086/126606. [DOI] [Google Scholar]
- Bartsch DU, Intaglietta M, Bille JF, Dreher AW, Gharib M, Freeman WR. Confocal laser tomographic analysis of the retina in eyes with macular hole formation and other focal macular diseases. American Journal of Ophthalmology. 1989;108:277–287. doi: 10.1016/0002-9394(89)90118-9. [DOI] [PubMed] [Google Scholar]
- Baskaran K, Rosén R, Lewis P, Unsbo P, Gustafsson J. Benefit of adaptive optics aberration correction at preferred retinal locus. Optometry and Vision Science. 2012;89:1417–1423. doi: 10.1097/OPX.0b013e318264f2a7. [DOI] [PubMed] [Google Scholar]
- Bedggood P, Metha A. Direct visualization and characterization of erythrocyte flow in human retinal capillaries. Biomedical Optics Express. 2012;3:3264–3277. doi: 10.1364/BOE.3.003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedggood P, Metha A. Analysis of contrast and motion signals generated by human blood constituents in capillary flow. Optics Letters. 2014;39(3):610–613. doi: 10.1364/OL.39.000610. [DOI] [PubMed] [Google Scholar]
- Benard Y, Lopez-Gil N, Legras R. Subjective depth of field in presence of 4th-order and 6th-order Zernike spherical aberration using adaptive optics technology. Journal of Cataract and Refractive Surgery. 2010;36:2129–2138. doi: 10.1016/j.jcrs.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Bruce KS, Harmening WM, Langston BR, Tuten WS, Roorda A, Sincich LC. Normal perceptual sensitivity arising from weakly reflective cone photoreceptors. Investigative Ophthalmology & Visual Science. 2015;56(8):4431–4438. doi: 10.1167/iovs.15-16547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno JM, Gualda E, Artal P. Adaptive optics multiphoton microscopy to study ex vivo ocular tissues. Journal of Biomedical Optics. 2010;5(6):066004. doi: 10.1117/1.3505018. [DOI] [PubMed] [Google Scholar]
- Bueno JM, Gualda E, Artal P. Analysis of corneal stroma organization with wavefront optimized nonlinear microscopy. Cornea. 2011;30(6):692–701. doi: 10.1097/ICO.0b013e3182000f94. [DOI] [PubMed] [Google Scholar]
- Burns SA, Elsner AE, Chui TY, Vannasdale DA, Jr, Clark CA, Gast TJ, Malinovsky VE, Phan AD. In vivo adaptive optics microvascular imaging in diabetic patients without clinically severe diabetic retinopathy. Biomedical Optics Express. 2014;5:961–974. doi: 10.1364/BOE.5.000961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns SA, Marcos S, Elsner AE, Bara S. Contrast improvement of confocal retinal imaging by use of phase-correcting plates. Optics Letters. 2002;27:400–402. doi: 10.1364/OL.27.000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns SA, Tumbar R, Elsner AE, Ferguson D, Hammer DX. Large-field-of-view, modular, stabilized, adaptive-optics-based scanning laser ophthalmoscope. Journal of the Optical Society of America A. 2007;24:1313–1326. doi: 10.1364/JOSAA.24.001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell FW, Green DG. Optical and retinal factors affecting visual resolution. Journal of Physiology. 1965;181(3):576–593. doi: 10.1113/jphysiol.1965.sp007784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M, Emptage L, Schwarz C, Walters S, Kisilak M, Brooks M, Hunter J. In vivo and ex vivo multi-modal images in the canine model of Alzheimer’s disease. ARVO 2016 Annual Meeting Abstracts #2217.2016. [Google Scholar]
- Cánovas C, Prieto PM, Manzanera S, Mira A, Artal P. Hybrid adaptive-optics visual simulator. Optics Letters. 2010;35:196–198. doi: 10.1364/OL.35.000196. [DOI] [PubMed] [Google Scholar]
- Cense B, Gao W, Brown JM, Jones SM, Jonnal RS, Mujat M, Park BH, de Boer JF, Miller DT. Retinal imaging with polarization-sensitive optical coherence tomography and adaptive optics. Optics Express. 2009;17(24):21634–21651. doi: 10.1364/OE.17.021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Kruger PB, Hofer H, Singer B, Williams DR. Accommodation with higher-order monochromatic aberrations corrected with adaptive optics. Journal of the Optical Society of America A. 2006;23:1–8. doi: 10.1364/JOSAA.23.000001. [DOI] [PubMed] [Google Scholar]
- Cheng X, Bradley A, Ravikumar S, Thibos LN. Visual impact of Zernike and Seidel forms of monochromatic aberrations. Optometry and Vision Science. 2010;87(5):300–312. doi: 10.1097/OPX.0b013e3181d95217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin SS, Hampson KH, Mallen EAH. Role of ocular aberrations in dynamic accommodation control. Clinical and Experimental Optometry. 2009a;92:227–237. doi: 10.1111/j.1444-0938.2009.00361.x. [DOI] [PubMed] [Google Scholar]
- Chin SS, Hampson KM, Mallen EAH. Effect of correction of ocular aberration dynamics on the accommodation response to a sinusoidally moving stimulus. Optics Letters. 2009b;34:3274–3276. doi: 10.1364/OL.34.003274. [DOI] [PubMed] [Google Scholar]
- Chirre E, Prieto PM, Schwarz C, Artal P. Night myopia is reduced in binocular vision. Journal of Vision. 2016;16(8):16. doi: 10.1167/16.8.16. [DOI] [PubMed] [Google Scholar]
- Chui TYP, VanNasdale DA, Burns SA. The use of forward scatter to improve retinal vascular imaging with an adaptive optics scanning laser ophthalmoscope. Biomedical Optics Express. 2012a;3:2537–2549. doi: 10.1364/BOE.3.002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui TY, Song H, Clark CA, Papay JA, Burns SA, Elsner AE. Cone photoreceptor packing density and the outer nuclear layer thickness in healthy subjects. Investigative Ophthalmology & Visual Science. 2012b;53(7):3545–3553. doi: 10.1167/iovs.11-8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua M, Wahl DJ, Zhao Y, Lee S, Bonora S, Zawadzki RJ, Jian Y, Sarunic MV. Coherence-gated sensorless adaptive optics multiphoton retinal imaging. Scientific Reports. 2016;6:32223. doi: 10.1038/srep32223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalimier E, Dainty C, Barbur JL. Effects of higher-order aberrations on contrast acuity as a function of light level. Journal of Modern Optics. 2008;55:791–803. doi: 10.1080/09500340701469641. [DOI] [Google Scholar]
- de Castro A, Gang H, Sawides L, Luo T, Burns SA. Rapid high resolution imaging with a dual-channel scanning technique. Optics Letters. 2016;41:1881–1884. doi: 10.1364/OL.41.001881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gracia P, Dorronsoro C, Marin G, Hernandez M, Marcos S. Visual acuity under combined astigmatism and coma: optical and neural adaptation effects. Journal of Vision. 2011;11(2):5. doi: 10.1167/11.2.5. [DOI] [PubMed] [Google Scholar]
- de Gracia P, Marcos S, Mathur A, Atchison D. Contrast sensitivity benefit of Adaptive Optics correction of ocular aberrations. Journal of Vision. 2011;11(12):5. doi: 10.1167/11.12.5. [DOI] [PubMed] [Google Scholar]
- Doble N, Miller DT, Yoon G, Williams DR. Deformable mirror requirements for adaptive correction in a population of normal human eyes. Applied Optics. 2007;46:4501–4514. doi: 10.1364/ao.46.004501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorronsoro C, Radhkrishnan A, de Gracia P, Sawides L, Marcos S. Perceived image quality with simulated segmented bifocal corrections. Biomedical Optics Express. 2016;7(11):4388–4399. doi: 10.1364/BOE.7.004388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorronsoro C, Radhkrishnan A, Alonso-Sanz JR, Pascual D, Velasco-Ocana M, Perez-Merino P, Marcos S. Portable simultaneous vision device to simulate multifocal corrections. Optica. 2016;3(8):918–924. doi: 10.1364/OPTICA.3.000918. [DOI] [Google Scholar]
- Duan A, Bedggood PA, Bui BV, Metha AB. Evidence of flicker-induced functional hyperaemia in the smallest vessels of the human retinal blood supply. PloS ONE. 2016;11(9):e0162621. doi: 10.1371/journal.pone.0162621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubis AM, Cooper RF, Aboshiha J, Langlo C, Sundaram V, Liu B, Collison F, Fishman GA, Moore AT, Webster AR, Dubra A, Carroll J, Michaelides M. Genotype-dependent variability in residual cone structure in achromatopsia: Towards developing metrics for assessing cone health. Investigative Ophthalmology & Visual Science. 2014;55(11):7303–7311. doi: 10.1167/iovs.14-14225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubow M, Pinhas A, Shah N, Cooper R, Gan A, Gentile R, Hendrix V, Sulai Y, Carroll J, Chui T, Walsh J, Rosen R, Dubra A. Classification of human retinal microaneurysms using adaptive optics scanning light ophthalmoscope fluorescein angiography. Investigative Ophthalmology & Visual Science. 2014;55:1299–1309. doi: 10.1167/iovs.13-13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubra A. Wavefront sensor and wavefront corrector matching in adaptive optics. Optics Express. 2007;15:2762–2769. doi: 10.1364/OE.15.002762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubra A, Sulai Y. Reflective afocal broadband adaptive optics scanning ophthalmoscope. Biomedical Optics Express. 2011;2(6):1757–1768. doi: 10.1364/BOE.2.001757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubra A, Sulai Y, Norris JL, Cooper RF, Dubis AM, Williams DR, Carroll J. Noninvasive imaging of the human rod photoreceptor mosaic using a confocal adaptive optics scanning ophthalmoscope. Biomedical Optics Express. 2011;2(7):1864–1876. doi: 10.1364/BOE.2.001864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsner AE, Burns SA, Weiter JJ, Delori FC. Infra-red imaging of subretinal structures. Vision Research. 1996;36:191–205. doi: 10.1016/0042-6989(95)00100-e. [DOI] [PubMed] [Google Scholar]
- Errera MH, Coisy S, Fardeau C, Sahel JA, Kallel S, Westcott M, Bodaghi B, Paques M. Retinal vasculitis imaging by adaptive optics. Ophthalmology. 2014;121(6):1311–1312.e2. doi: 10.1016/j.ophtha.2013.12.036. [DOI] [PubMed] [Google Scholar]
- Ferguson RD, Zhong Z, Hammer DX, Mujat M, Patel AH, Deng C, Zou W, Burns SA. Adaptive optics SLO with integrated wide-field retinal imaging and tracking. Journal of the Optical Society of America A. 2010;27(11):A265–A277. doi: 10.1364/JOSAA.27.00A265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández EJ, Prieto PM, Artal P. Wave-aberration control with a liquid crystal on silicon (LCOS) spatial phase modulator. Optics Express. 2009a;17:11013–11025. doi: 10.1364/OE.17.011013. [DOI] [PubMed] [Google Scholar]
- Fernández EJ, Prieto PM, Artal P. Binocular adaptive optics visual simulator. Optics Letters. 2009b;34:2628–2630. doi: 10.1364/OL.34.002628. [DOI] [PubMed] [Google Scholar]
- Fernández EJ, Manzanera S, Piers P, Artal P. Adaptive optics visual simulator. Journal of Refractive Surgery. 2002;18(5):S634–S638. doi: 10.3928/1081-597X-20020901-27. [DOI] [PubMed] [Google Scholar]
- Fernández EJ, Prieto PM, Artal P. Adaptive optics binocular visual simulator to study stereopsis in the presence of aberrations. Journal of the Optical Society of America A. 2010;7(11):A48–A55. doi: 10.1364/JOSAA.27.000A48. [DOI] [PubMed] [Google Scholar]
- Gambra E, Sawides L, Dorronsoro C, Marcos S. Accommodative lag and fluctuations when optical aberrations are manipulated. Journal of Vision. 2009;9(6):4. doi: 10.1167/9.6.4. [DOI] [PubMed] [Google Scholar]
- Gao W, Jonnal RS, Cense B, Kocaoglu OP, Wang Q, Miller DT. Measuring directionality of the retinal reflection with a Shack-Hartmann wavefront sensor. Optics Express. 2009;17(25):23085–23097. doi: 10.1364/OE.17.023085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genead MA, Fishman GA, Rha J, Dubis AM, Bonci DMO, Dubra A, Stone EM, Neitz M, Carroll J. Photoreceptor structure and function in patients with congenital achromatopsia. Investigative Ophthalmology & Visual Science. 2011;52(1):7298–7308. doi: 10.1167/iovs.11-7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocho K, Sarda V, Falah S, Sahel JA, Sennlaub F, Benchaboune M, Ullern M, Paques M. Adaptive optics imaging of geographic atrophy. Investigative Ophthalmology & Visual Science. 2013;54:3673–3680. doi: 10.1167/iovs.12-10672. [DOI] [PubMed] [Google Scholar]
- Gómez-Vieyra A, Dubra A, Malacara-Hernández D, Williams DR. First-order design of off-axis reflective ophthalmic adaptive optics systems using afocal telescopes. Optics Express. 2009;17(21):18906–18919. doi: 10.1364/OE.17.018906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray DC, Merigan W, Wolfing JI, Gee B, Dubra A, Porter J, Twietmeyer T, Ahmad K, Tumbar R, Reinholz F, Williams DR. In vivo fluorescence imaging of primate retinal ganglion cells and retinal pigment epithelial cells. Optics Express. 2006;14:7144–7158. doi: 10.1167/iovs.12-10672. [DOI] [PubMed] [Google Scholar]
- Gray DC, Wolfe R, Gee B, Scoles D, Geng Y, Masella B, Dubra A, Luque S, Williams DS, Merigan WH. In vivo imaging of the fine structure of rhodamine labeled macaque retinal ganglion cells. Investigative Ophthalmology and Visual Science. 2008;49:467–473. doi: 10.1167/iovs.07-0605. [DOI] [PubMed] [Google Scholar]
- Gualda E, Bueno JM, Artal P. Wavefront optimized nonlinear microscopy of ex vivo human retinas. Journal of Biomedical Optics. 2010;15(2):026007. doi: 10.1117/1.3369001. [DOI] [PubMed] [Google Scholar]
- Guo Guevara-Torres A, Williams DR, Schallek JB. Imaging translucent cell bodies in the living mouse retina without contrast agents. Biomedical Optics Express. 2015;6:2106–2119. doi: 10.1364/BOE.6.002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Atchison DA. Subjective blur limits for cylinder. Optometry & Vision Science. 2010;87(8):E549–E559. doi: 10.1097/OPX.0b013e3181e61b8f. [DOI] [PubMed] [Google Scholar]
- Guo H, Atchison DA, Birt BJ. Changes in through-focus spatial visual performance with adaptive optics correction of monochromatic aberrations. Vision Research. 2008;48:1804–1811. doi: 10.1016/j.visres.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Hampson KH, Chin SS, Mallen EAH. Dual wavefront sensing channel monocular adaptive optics system for accommodation studies. Optics Express. 2009;17:18229–18240. doi: 10.1364/OE.17.018229. [DOI] [PubMed] [Google Scholar]
- Hampson KM, Chin SS, Mallen EAH. Effect of temporal location of correction of monochromatic aberrations on the dynamic accommodation response. Biomedical Optics Express. 2010;1:879–894. doi: 10.1364/BOE.1.000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson KM, Mallen EAH. Multifractality in steady-state accommodation control is robust to dynamic correction of aberrations using adaptive optics. Journal of Modern Optics. 2012;59:1056–1063. [Google Scholar]
- Hampson KM, Cufflin MP, Mallen EAH. Effect of correction of aberration dynamics on chaos in human ocular accommodation. Optics Letters. 2013;38:4747–4749. doi: 10.1364/OL.38.004747. [DOI] [PubMed] [Google Scholar]
- Harmening WM, Tiruveedhula P, Roorda A, Sincich LC. Measurement and correction of transverse chromatic offsets for multi-wavelength retinal microscopy in the living eye. Biomedical Optics Express. 2012;3(9):2066–2077. doi: 10.1364/BOE.3.002066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmening WM, Tuten WS, Roorda A, Sincich LC. Mapping the perceptual grain of the human retina. Journal of Neuroscience. 2014;34(16):5667–5677. doi: 10.1523/JNEUROSCI.5191-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard JG, Gast TJ, Chui TY, Sapir D, Burns SA. Retinal arterioles in hypo-, normo-, and hypertensive subjects measured using adaptive optics. Translational Vision Science & Technology. 2016;5(4):16. doi: 10.1167/tvst.5.4.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer H, Sredar N, Queener H, Li CH, Porter J. Wavefront sensorless adaptive optics ophthalmoscopy in the human eye. Optics Express. 2011;19:14160–14171. doi: 10.1364/OE.19.014160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Luo T, Gast TJ, Burns SA, Malinovsky VE, Swanson WH. Imaging glaucomatous damage across the temporal raphe. Investigative Ophthalmology & Visual Science. 2015;56:3496–3504. doi: 10.1167/iovs.15-16730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JJ, Masella B, Dubra A, Sharma R, Yin L, Merigan WH, Palczewska G, Palczewski K, Williams DR. Images of photoreceptors in living primate eyes using adaptive optics two-photon ophthalmoscopy. Biomedical Optics Express. 2011;2(1):139–148. doi: 10.1364/BOE.2.000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JJ, Morgan JIW, Merigan WH, Sliney DH, Sparrow JR, Williams DR. The susceptibility of the retina to photochemical damage from visible light. Progress in Retinal and Eye Research. 2012;31(1):28–42. doi: 10.1016/j.preteyeres.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonnal RS, Rha J, Zhang Y, Cense B, Gao W, Miller DT. In vivo functional imaging of human cone photoreceptors. Optics Express. 2007;15(24):16141–16160. doi: 10.1364/OE.15.016141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonnal RS, Besecker JR, Derby JC, Kocaoglu OP, Cense B, Gao W, Wang Q, Miller DT. Imaging outer segment renewal in living human cone photoreceptors. Optics Express. 2010;18(5):5257–5270. doi: 10.1364/OE.18.005257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonnal RS, Kocaoglu OP, Wang Q, Lee S, Miller DT. Phase-sensitive imaging of the outer retina using optical coherence tomography and adaptive optics. Biomedical Optics Express. 2012;3(1):104–124. doi: 10.1364/BOE.3.000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonnal RS, Kocaoglu OP, Zawadzki RJ, Lee S-H, Werner JS, Miller DT. The cellular origins of the outer retinal bands in optical coherence tomography images. Investigative Ophthalmology & Visual Science. 2014;55(12):7904–7918. doi: 10.1167/iovs.14-14907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonnal RS, Kocaoglu OP, Zawadzki RJ, Liu Z, Miller DT, Werner JS. A review of adaptive optics optical coherence tomography: Technical advances, scientific applications, and the future. Investigative Ophthalmology & Visual Science. 2016;57(9):OCT51-OCT68. doi: 10.1167/iovs.16-19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BJ, Sapoznik KA, Elsner AE, Gast TJ, Papay JA, Clark CA, Burns SA. SD-OCT and adaptive optics imaging of outer retinal tabulation. Optometry and Visual Science. 2017 doi: 10.1097/OPX.0000000000001031. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocaoglu OP, Cense B, Jonnal RS, Wang Q, Lee S, Gao W, Miller DT. Imaging retinal nerve fiber bundles using optical coherence tomography with adaptive optics. Vision Research. 2011;51(16):1835–1844. doi: 10.1016/j.visres.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocaoglu OP, Turner TL, Liu Z, Miller DT. Adaptive optics optical coherence tomography at 1 MHz. Biomedical Optics Express. 2014;5(12):4186–4200. doi: 10.1364/BOE.5.004186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch E, Rosenbaum D, Brolly A, Sahel JA, Chaumet-Riffaud P, Girerd X, Rossant F, Paques M. Morphometric analysis of small arteries in the human retina using adaptive optics imaging: relationship with blood pressure and focal vascular changes. Journal of Hypertension. 2014;32:890–898. doi: 10.1097/HJH.0000000000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert AJ, Birt BJ, Atchison DA, Guo H. Applying SLODAR to measure aberrations in the eye. Optics Express. 2008;16:7309–7322. doi: 10.1364/OE.16.007309. [DOI] [PubMed] [Google Scholar]
- Lammer J, Prager SG, Cheney MC, Ahmed A, Radwan SH, Burns SA, Silva PS, Sun JK. Cone photoreceptor irregularity on adaptive optics scanning laser ophthalmoscopy correlates with severity of diabetic retinopathy and macular edema. Investigative Ophthalmology & Visual Science. 2016;57(15):6624–6632. doi: 10.1167/iovs.16-19537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-H, Werner JS, Zawadzki RJ. Improved visualization of outer retinal morphology with aberration cancelling reflective optical design for adaptive optics – optical coherence tomography. Biomedical Optics Express. 2013;4:2508–2517. doi: 10.1364/BOE.4.002508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legras R, Benard Y, Rouger H. Through-focus visual performance measurements and predictions with multifocal contact lenses. Vision Research. 2010;50:1185–1193. doi: 10.1016/j.visres.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Legras R, Benard Y, Lopez-Gil N. Effect of coma and spherical aberration on depth-of-focus measured using adaptive optics and computationally blurred images. Journal of Cataract & Refractive Surgery. 2012;38:458–469. doi: 10.1016/j.jcrs.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Leray B, Cassagne BM, Soler V, Villegas EA, Triozon C, Perez GM, Letsch J, Chapotot E, Artal P, Malecaze F. Relationship between Induced Spherical Aberration and Depth of Focus after Hyperopic LASIK in Presbyopic Patients. Opthalmology. 2015;122:233–243. doi: 10.1016/j.ophtha.2014.08.021. [DOI] [PubMed] [Google Scholar]
- Liang J, Williams DR, Miller DT. Supernormal vision and high-resolution retinal imaging through adaptive optics. Journal of the Optical Society of America A. 1997;14(11):2884–2892. doi: 10.1364/JOSAA.14.002884. [DOI] [PubMed] [Google Scholar]
- Liu Z, Kocaoglu OP, Miller DT. In-the-plane design of an off-axis ophthalmic adaptive optics system using toroidal mirrors. Biomedical Optics Express. 2013;4(12):3007–3029. doi: 10.1364/BOE.4.003007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Kocaoglu OP, Miller DT. 3D Imaging of retinal pigment epithelial cells in the living human retina. Investigative Ophthalmology & Visual Science. 2016;57(9):533–543. doi: 10.1167/iovs.16-19106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Kocaoglu OP, Turner TL, Miller DT. Modal content of living human cone photoreceptors. Biomedical Optics Express. 2015;6(9):3378–3404. doi: 10.1364/BOE.6.003378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundström L, Manzanera S, Prieto PM, Ayala DB, Gorceix N, Gustafsson J, Unsbo P, Artal P. Effect of optical correction and remaining aberrations on peripheral resolution acuity in the human eye. Optics Express. 2007;15:12654–12661. doi: 10.1364/OE.15.012654. [DOI] [PubMed] [Google Scholar]
- Manzanera S, Prieto PM, Ayala DB, Lindacher JM, Artal P. Liquid crystal Adaptive Optics Visual Simulator: Application to testing and design of ophthalmic optical elements. Optics Express. 2007;15:16177–16188. doi: 10.1364/OE.15.016177. [DOI] [PubMed] [Google Scholar]
- Marcos S, Sawides L, Gambra E, Dorronsoro C. Influence of adaptive optics ocular aberration correction on visual acuity at different luminances and contrast polarities. Journal of Vision. 2008;8(13):1. doi: 10.1167/8.13.1. [DOI] [PubMed] [Google Scholar]
- Marcos S, Velasco-Ocana V, Dorronsoro C, Sawides L, Hernandez M, Marin G. Impact of astigmatism and high order aberrations on subjective best focus. Journal of Vision. 2015;15(11):4. doi: 10.1167/15.11.4. [DOI] [PubMed] [Google Scholar]
- Masella BD, Hunter JJ, Williams DR. Rod photopigment kinetics after photodisruption of the retinal pigment epithelium. Investigative Ophthalmology & Visual Science. 2014a;55(11):7535–7544. doi: 10.1167/iovs.13-13796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masella BD, Williams DR, Fischer WS, Rossi EA, Hunter JJ. Long-term reduction in infrared autofluorescence caused by infrared light below the maximum permissible exposure. Investigative Ophthalmology & Visual Science. 2014b;55:3929–3938. doi: 10.1167/iovs.13-12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadway A, Girkin CA, Zhang Y. A dual-modal retinal imaging system with adaptive optics. Optics Express. 2013;21:29792–29807. doi: 10.1364/OE.21.029792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadway A, Wang X, Curcio CA, Zhang Y. Microstructure of subretinal drusenoid deposits revealed by adaptive optics imaging. Biomedical Optics Express. 2014;5:713–727. doi: 10.1364/BOE.5.000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miloudi C, Rossant F, Bloch I, Chaumette C, Leseigneur A, Sahel JA, Meimon S, Mrejen S, Paques M. The negative cone mosaic: A new manifestation of the optical Stiles-Crawford effect in normal eyes. Investigative Ophthalmology & Visual Science. 2015;56:7043–7050. doi: 10.1167/iovs.15-17022. [DOI] [PubMed] [Google Scholar]
- Miller DT, Kocaoglu OP, Wang Q, Lee S. Adaptive optics and the eye (super resolution OCT) Eye. 2011;25(3):321–330. doi: 10.1038/eye.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimoun G, Soubrane G, Coscas G. Le drusen maculaires. Journal Français D’Ophtalmologie. 1990;13:511–530. [PubMed] [Google Scholar]
- Morris HJ, Blanco L, Codona JL, Li SL, Choi SS, Doble N. Directionality of individual cone photoreceptors in the parafoveal region. Vision Research. 2015;117:67–80. doi: 10.1016/j.visres.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewska G, Dong Z, Golczak M, Hunter JJ, Williams DR, Alexander NS, Palczewski K. Noninvasive two-photon microscopy imaging of mouse retina and retinal pigment epithelium through the pupil of the eye. Nature Medicine. 2014;20:785–789. doi: 10.1038/nm.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panorgias A, Zawadzki RJ, Capps AG, Hunter AA, Morse LS, Werner JS. Multimodal functional and structural assessment of geographic atrophy. Investigative Ophthalmology & Visual Science. 2013;54:4372–4384. doi: 10.1167/iovs.12-11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paques M, Brolly A, Benesty J, Lermé N, Koch E, Rossant F, Bloch I, Girmens JF. Venous nicking without arteriovenous contact: The role of the arteriolar microenvironment in arteriovenous nickings. JAMA Ophthalmology. 2015;133(8):947–950. doi: 10.1001/jamaophthalmol.2015.1132. [DOI] [PubMed] [Google Scholar]
- Popovic Z, Knutsson P, Thaung J, Owner-Petersen M, Sjostrand J. Noninvasive imaging of human foveal capillary network using dual-conjugate adaptive optics. Investigative Ophthalmology & Visual Science. 2011;52:2649–2655. doi: 10.1167/iovs.10-6054. [DOI] [PubMed] [Google Scholar]
- Piers P, Manzanera S, Prieto P, Gorceix N, Fernández EJ, Artal P. Use of adaptive optics to determine the optimal ocular spherical aberration. Journal of Cataract & Refractive Surgery. 2007;33:1721–1726. doi: 10.1016/j.jcrs.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Piers PA, Fernández EJ, Manzanera S, Norrby S, Artal P. Adaptive optics simulation of intraocular lenses with modified spherical aberration. Investigative Ophthalmology & Visual Science. 2004;45:4601–4610. doi: 10.1167/iovs.04-0234. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan A, Dorronsoro C, Sawides L, Marcos S. Short-term neural adaptation to bifocal images. PLoS ONE. 2014;9(3):e93089. doi: 10.1371/journal.pone.0093089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan A, Sawides L, Dorronsoro C, Peli E, Marcos S. Single neural code for blur in subjects with different interocular optical blur orientation. Journal of Vision. 2015a;15(8):15. doi: 10.1167/15.8.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan A, Dorronsoro C, Sawides L, Webster MA, Marcos S. A cyclopean neural mechanism compensating for optical differences between the eyes. Current Biology. 2015b;25(5):R188–R189. doi: 10.1016/j.cub.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar A, Sarver EJ, Applegate RA. Change in visual acuity is highly correlated with change in six image quality metrics independent of wavefront error and/or pupil diameter. Journal of Vision. 2012;12(10):11. doi: 10.1167/12.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roorda A, Romero-Borja F, Donnelly WJ, III, Queener H, Hebert TJ, Campbell MCW. Adaptive optics scanning laser ophthalmoscopy. Optics Express. 2002;10:405–412. doi: 10.1364/OE.10.000405. [DOI] [PubMed] [Google Scholar]
- Rosén R, Lundström L, Unsbo P. Adaptive optics for peripheral vision. Journal of Modern Optics. 2012;59:1064–1070. doi: 10.1080/09500340.2012.683827. [DOI] [Google Scholar]
- Rouger H, Benard Y, Gatinel D, Legras R. Visual tasks dependence of the neural compensation for the keratoconic eye’s optical aberrations. Journal of Optometry. 2010;3(1):60–65. doi: 10.3921/joptom.2010.60. [DOI] [Google Scholar]
- Sabesan R, Yoon G. Visual performance after correcting higher order aberrations in Keratoconic eyes. Journal of Vision. 2009;9:1–10. doi: 10.1167/9.5.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabesan R, Yoon G. Neural compensation for long-term asymmetric optical blur to improve visual performance in keratoconic eyes. Investigative Ophthalmology & Visual Science. 2010;51:3835–3839. doi: 10.1167/iovs.09-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabesan R, Ahmad K, Yoon G. Correcting highly aberrated eyes using large-stroke adaptive optics. Journal of Refractive Surgery. 2007;23:947–952. doi: 10.3928/1081-597X-20071101-16. [DOI] [PubMed] [Google Scholar]
- Sabesan R, Zheleznyak L, Ahmad K, Yoon G. Binocular visual performance and summation after correcting higher order aberrations. Biomedical Optics Express. 2012;3(12):3176–3189. doi: 10.1364/BOE.3.003176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawides L, De Castro A, Burns SA. The organization of the cone photoreceptor mosaic measured in the living human retina. Vision Res 2016. 2016 Aug 3; doi: 10.1016/j.visres.2016.06.006. pii: S0042-6989(16)30049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawides L, de Gracia P, Dorronsoro C, Webster M, Marcos S. Adapting to the blur produced by high order aberrations. Journal of Vision. 2011a;11(7):21. doi: 10.1167/11.7.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawides L, de Gracia P, Dorronsoro C, Webster M, Marcos S. Vision is adapted to the natural level of blur present in the retinal image. PLoS ONE. 2011b;6(11):e27031. doi: 10.1371/journal.pone.0027031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawides L, Dorronsoro C, de Gracia P, Vinas M, Webster M, Marcos S. Dependence of subjective image focus on the magnitude and pattern of High Order Aberrations. Journal of Vision. 2012;12(8):4. doi: 10.1167/12.8.4. [DOI] [PubMed] [Google Scholar]
- Sawides L, Dorronsoro C, Haun A, Peli E, Marcos S. Using pattern classification to measure adaptation to the orientation of high order aberrations. PLoS ONE. 2013;8(8):e70856. doi: 10.1371/journal.pone.0070856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawides L, Gambra E, Pascual P, Dorronsoro C, Marcos S. Visual performance with real-life tasks under adaptive-optics ocular aberration correction. Journal of Vision. 2010a;10(5):19. doi: 10.1167/10.5.19. [DOI] [PubMed] [Google Scholar]
- Sawides L, Marcos S, Ravikumar S, Thibos L, Bradley A, Webster M. Adaptation to astigmatic blur. Journal of Vision. 2010b;10(12):22. doi: 10.1167/10.12.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallek J, Geng Y, Nguyen H, Williams DR. Morphology and topography of retinal pericytes in the living mouse retina using in vivo adaptive optics imaging and ex vivo characterization. Investigative Ophthalmology & Visual Science. 2013;54:8237–8250. doi: 10.1167/iovs.13-12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz C, Canovas C, Manzanera S, Weeber H, Prieto PM, Piers P, Artal P. Binocular visual acuity for the correction of spherical aberration in polychromatic and monochromatic light. Journal of Vision. 2014a;14(2):8. doi: 10.1167/14.2.8. [DOI] [PubMed] [Google Scholar]
- Schwarz C, Manzanera S, Artal P. Binocular visual performance with aberration correction as a function of light level. Journal of Vision. 2014b;14(14):6. doi: 10.1167/14.14.6. [DOI] [PubMed] [Google Scholar]
- Schwarz C, Manzanera S, Prieto P, Fernández E, Artal P. Comparison of binocular through-focus visual acuity with monovision and a small aperture inlay. Biomedical Optics Express. 2014c;5:3355–3366. doi: 10.1364/BOE.5.003355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz C, Prieto PM, Fernández EJ, Artal P. Binocular adaptive optics vision analyzer with full control over the complex pupil functions. Optics Letters. 2011;36:4779–4781. doi: 10.1364/OL.36.004779. [DOI] [PubMed] [Google Scholar]
- Sharma R, Schwarz C, Williams DR, Palczewska G, Palczewski K, Hunter JJ. In vivo two-photon fluorescence kinetics of primate rods and cones. Investigative Ophthalmology & Visual Science. 2016a;57(2):647–657. doi: 10.1167/iovs.15-17946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Williams DR, Palczewska G, Palczewski K, Hunter JJ. Two-Photon Autofluorescence Imaging Reveals Cellular Structures Throughout the Retina of the Living Primate Eye. Investigative Ophthalmology & Visual Science. 2016b;57(2):632–646. doi: 10.1167/iovs.15-17961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Yin L, Geng Y, Merigan W, Palczewska G, Palczewski K, Williams D, Hunter J. In vivo two-photon imaging of the mouse retina. Biomedical Optics Express. 2013;4:1285–1293. doi: 10.1364/BOE.4.001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemonski ND, South FA, Liu Y-Z, Adie SG, Scott Carney P, Boppart SA. Computational high-resolution optical imaging of the living human retina. Nature Photonics. 2015;9:440–443. doi: 10.1038/NPHOTON.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sincich LC, Tuten WS, Sabesan R, Roorda A, Harmening WM. Functional imaging of cone photoreceptors. In: Kremers K, Barass R, Marshall J, editors. Human Color Vision. Springer International Publishing; New York: 2016. pp. 71–104. [Google Scholar]
- Sincich LC, Zhang Y, Tiruveedhula P, Horton JC, Roorda A. Resolving single cone inputs to visual receptive fields. Nature Neuroscience. 2009;12(8):967–969. doi: 10.1038/nn.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorsetz M, Artal P, Bueno JM. Performance evaluation of a sensorless adaptive optics multiphoton microscope. Journal of Microscopy. 2016;261:249–258. doi: 10.1111/jmi.12325. [DOI] [PubMed] [Google Scholar]
- Song H, Chui TY, Zhong Z, Elsner AE, Burns SA. Variation of cone photoreceptor packing density with retinal eccentricity and age. Investigative Ophthalmology & Visual Science. 2011;52(10):7376–7384. doi: 10.1167/iovs.11-7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Wright T, Rajendran D, Garcia-Sanchez Y, Finkelberg L, Kisilak M, Campbell M, Westall C. Cone–photoreceptor density in adolescents with type 1 diabetes. Investigative Ophthalmology & Visual Science. 2015;56(11):6339–6343. doi: 10.1167/iovs.15-16817. [DOI] [PubMed] [Google Scholar]
- Tyson RK. Principles of Adaptive Optics. 2. Academic Press; San Diego: 1998. [Google Scholar]
- Vargas-Martín F, Prieto P, Artal P. Correction of the aberrations in the human eye with a liquid crystal spatial light modulator: limits to the performance. Journal of the Optical Society of America A. 1998;15:2552–2562. doi: 10.1364/JOSAA.15.002552. [DOI] [PubMed] [Google Scholar]
- Venkataraman AP, Winter S, Rosén R, Lundström L. Choice of grating orientation for evaluation of peripheral vision. Optometry and Vision Science. 2016;93(6):567–574. doi: 10.1097/OPX.0000000000000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinas M, de Gracia P, Dorronsoro C, Sawides L, Marin G, Hernandez M, Marcos S. Astigmatism impact on visual performance: Meridional and adaptational effects. Optometry and Vision Science. 2013;90:1430–1442. doi: 10.1097/OPX.0000000000000063. [DOI] [PubMed] [Google Scholar]
- Vinas M, Dorronsoro C, Cortés D, Pascual D, Marcos S. Longitudinal chromatic aberration of the human eye in the visible and near infrared from wavefront sensing, double-pass and psychophysics. Biomedical Optics Express. 2015a;23:948–962. doi: 10.1364/BOE.6.000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinas M, Dorronsoro C, Garzón N, Poyales F, Marcos S. In vivo subjective and objective longitudinal chromatic aberration after bilateral implantation of the same design of hydrophobic and hydrophilic intraocular lenses. Journal of Cataract & Refractive Surgery. 2015;41(10):2115–2124. doi: 10.1016/j.jcrs.2015.11.009. [DOI] [PubMed] [Google Scholar]
- Vinas M, Sawides L, de Gracia P, Marcos S. Perceptual adaptation to the correction of natural astigmatism. PLoS ONE. 2012;7(9):e46361. doi: 10.1371/journal.pone.0046361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinas M, Dorronsoro C, Gonzalez V, Cortes D, Radhakrishnan A, Marcos S. Testing vision with angular and radial multifical designs using adaptive optics. Vision Research. 2016 doi: 10.1016/j.visres.2016.04.011. in press. [DOI] [PubMed] [Google Scholar]
- Vincent A, Wright T, Garcia-Sanchez Y, Kisilak M, Campbell M, Westall C, Héon E. Phenotypic characteristics including in vivo cone photoreceptor mosaic in KCNV2-related “cone dystrophy with supernormal rod electroretinogram”. Investigative Ophthalmology & Visual Science. 2013;54(1):898–908. doi: 10.1167/iovs.12-10971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade AR, Fitzke FW. A fast, robust pattern recognition system for low light level image registration and its application to retinal imaging. Optics Express. 1998;3:190–197. doi: 10.1364/OE.3.000190. [DOI] [PubMed] [Google Scholar]
- Wahl DJ, Bonora S, Mata OS, Haunerland BK, Zawadzki RJ, Sarunic MV, Jian Y. SPIE BiOS. International Society for Optics and Photonics; 2016. Wavefront sensorless approaches to adaptive optics for in vivo fluorescence imaging of mouse retina; pp. 97170A–97170A. [Google Scholar]
- Wang Q, Kocaoglu OP, Cense B, Bruestle J, Jonnal RS, Gao W, Miller DT. Imaging retinal capillaries using ultrahigh-resolution optical coherence tomography and adaptive optics. Investigative Ophthalmology & Visual Science. 2011;52(9):6292–6299. doi: 10.1167/iovs.10-6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb RH, Albanese MJ, Zhou Y, Bifano T, Burns SA. A stroke amplifier for deformable mirrors. Applied Optics. 2004;43(28):5330–5333. doi: 10.1364/AO.43.005330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells-Gray EM, Choi SS, Bries A, Doble N. Variation in rod and cone density from the fovea to the mid-periphery in healthy human retinas using adaptive optics scanning laser ophthalmoscopy. Eye. 2016 doi: 10.1038/eye.2016.107. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells-Gray EM, Zawadzki RJ, Finn SC, Greiner C, Werner JS, Choi SS, Doble N. Performance of a combined optical coherence tomography and scanning laser ophthalmoscope with adaptive optics for human retinal imaging applications. Proceedings of SPIE. 9335, Adaptive Optics and Wavefront Control for Biological Systems; 2015. p. 93350N. [DOI] [Google Scholar]
- Werner JS, Keltner JL, Zawadzki RJ, Choi SS. Outer retinal abnormalities associated with inner retinal pathology in nonglaucomatous and glaucomatous optic neuropathies. Eye. 2011;25(3):279–289. doi: 10.1038/eye.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Geng Y, Osakada F, Sharma R, Cetin AH, Callaway EM, Williams DR, Merigan WH. Imaging light responses of retinal ganglion cells in the living mouse eye. Journal of Neurophysiology. 2013;109(9):2415–2421. doi: 10.1152/jn.01043.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Masella B, Dalkara D, Zhang J, Flannery JG, Schaffer DV, Williams DR, Merigan WH. Imaging light responses of foveal ganglion cells in the living macaque eye. Journal of Neuroscience. 2014;34(19):6596–6605. doi: 10.1523/JNEUROSCI.4438-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon G, Williams DR. Visual performance after correcting the monochromatic and chromatic aberrations of the eye. Journal of the Optical Society of America A. 2002;19:266–275. doi: 10.1364/JOSAA.19.000266. [DOI] [PubMed] [Google Scholar]
- Young LK, Morris TJ, Saunter CD, Smithson HE. A compact, in-plane and modular AOSLO with off-the shelf electronics for high resolution retinal imaging. doi: 10.1364/BOE.9.004275. in prep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Zhang T, Meadway A, Wang X, Zhang Y. High-speed adaptive optics for imaging of the living human eye. Optics Express. 2015;23:23035–23052. doi: 10.1364/OE.23.023035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawadzki RJ, Jones SM, Olivier SS, Zhao M, Bower BA, Izatt JA, Choi S, Laut S, Werner JS. Adaptive-optics optical coherence tomography for high-resolution and high-speed 3D retinal in vivo imaging. Optics Express. 2005;13:8532–8546. doi: 10.1364/OPEX.13.008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawadzki RJ, Jones SM, Pilli S, Balderas-Mata S, Kim DY, Olivier SS, Werner JS. Integrated adaptive optics optical coherence tomography and adaptive optics scanning laser ophthalmoscope system for simultaneous cellular resolution in vivo retinal imaging. Biomedical Optics Express. 2011;2:1674–1686. doi: 10.1364/BOE.2.001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawadzki RJ, Zhang P, Zam A, Miller EB, Goswami M, Wang X, Jonnal RS, Lee SH, Kim DY, Flannery JG, Werner JS, Burns ME, Pugh EN., Jr Adaptive-optics SLO imaging combined with widefield OCT and SLO enables precise 3D localization of fluorescent cells in the mouse retina. Biomedical Optics Express. 2015;6(6):2191–210. doi: 10.1364/BOE.6.002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yang Q, Saito K, Nozato K, Williams DR, Rossi EA. An adaptive optics imaging system designed for clinical use. Biomedical Optics Express. 2015a;6:2120–2137. doi: 10.1364/BOE.6.002120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Godara P, Blanco ER, Griffin RL, Wang X, Curcio CA, Zhang Y. Variability in human cone topography assessed by adaptive optics scanning laser ophthalmoscopy. American Journal of Ophthalmology. 2015b;160:290–300. doi: 10.1016/j.ajo.2015.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Cense B, Rha J, Jonnal RS, Gao W, Zawadzki RJ, Werner JS, Jones S, Olivier S, Miller DT. High-speed volumetric imaging of cone photoreceptors with adaptive optics spectral-domain optical coherence tomography. Optics Express. 2006;14(10):4380–4394. doi: 10.1364/OE.14.004380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Rha J, Jonnal RS, Miller DT. Adaptive optics parallel spectral domain optical coherence tomography for imaging the living retina. Optics Express. 2005;13(12):4792–4811. doi: 10.1364/OPEX.13.004792. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang X, Rivero EB, Clark ME, Witherspoon CD, Spaide RF, Girkin CA, Owsley C, Curcio CA. Photoreceptor perturbation around subretinal drusenoid deposits as revealed by adaptive optics scanning laser ophthalmoscopy. American Journal of Ophthalmology. 2014;158:584–596 e1. doi: 10.1016/j.ajo.2014.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheleznyak L, Alarcon A, Dieter KC, Tadin D, Yoon G. The role of sensory ocular dominance on through-focus visual performance in monovision presbyopia corrections. Journal of Vision. 2015;15(6):17. doi: 10.1167/15.6.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheleznyak L, Ghosh A, Barbot A, Yoon G. Optical and neural anisotropy in peripheral vision. Journal of Vision. 2016;16(5):1. doi: 10.1167/16.5.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheleznyak L, Sabesan R, Oh JS, Macrae S, Yoon G. Modified monovision with spherical aberration to improve presbyopic through-focus visual performance. Investigative Ophthalmology & Visual Science. 2013;54:3157–3165. doi: 10.1167/iovs.12-11050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong ZY, Huang G, Chui TYP, Petrig BL, Burns SA. Local flicker stimulation evokes local retinal blood velocity changes. Journal of Vision. 2012;12(3):3410729. doi: 10.1167/12.6.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Bedggood P, Metha A. Limitations to adaptive optics image quality in highly powered eyes. Biomedical Optics Express. 2012;3(8):1811–1824. doi: 10.1364/BOE.3.001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Bedggood P, Bui B, Nguyen CT, He Z, Metha A. Contrast-based sensorless adaptive optics for retinal imaging. Biomedical Optics Express. 2015;6(9):3577–3595. doi: 10.1364/BOE.6.003577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Bedggood P, Metha A. Improving high-resolution retinal image quality using speckle illumination HiLo imaging. Biomedical Optics Express. 2014;5:2563–2579. doi: 10.1364/BOE.5.002563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Qi X, Burns SA. Woofer-tweeter adaptive optics scanning laser ophthalmoscopic imaging based on Lagrange-multiplier damped least-squares algorithm. Biomedical Optics Express. 2011;2(7):1986–2004. doi: 10.1364/BOE.2.001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, Qi X, Burns SA. Wavefront aberration sorting and correction for dual-deformable-mirror adaptive optics system. Optics Letters. 2008;33(22):2602–2604. doi: 10.1364/OL.33.002602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel SA, Spaide RF, Curcio CA, Malek G, Imamura Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010;117:303–312 e1. doi: 10.1016/j.ophtha.2009.07.014. [DOI] [PubMed] [Google Scholar]