Abstract
Background
In several human cancers, overexpression of Skp2 (S-phase kinase associated protein 2), which targets p27 for degradation, portends a poorer prognosis. We examined whether Skp2 overexpression is associated with recurrence following radical prostatectomy (RP) for prostate cancer.
Methods
Immunohistochemical staining for Skp2, p27, and MIB-1 was performed on 109 men with node-negative prostate cancer surgically managed from 1985–1996. Associations between the stains were tested and Cox regression was used to determine the association between Skp2 expression and time to biochemical recurrence following RP.
Results
The 12 tumors (11%) with Skp2 overexpression all had correspondingly low p27 expression (P = 0.006), and a similar inverse Skp2/p27 relationship was seen in vitro in LNCap cells. Skp2 overexpression in tissue was associated with higher Gleason score (P = 0.002), more advanced pathological stage (P = 0.01), and higher MIB-1 index (P = 0.03), but a more favorable PSA profile (P = 0.04). Five men received a TURP. Among 104 who received RP, median follow-up was 67 months (range: 0.2–218). After adjusting for PSA, pathologic stage, and Gleason score, Skp2 overexpression remained significantly associated with a shorter time to biochemical recurrence (adjusted hazard ratio 4.8 (95% C.I. 1.6–14, P = 0.004)). The median time to recurrence with high vs. low Skp2 was 4 vs. 54 months.
Conclusions
Skp2 overexpression was seen in a significant minority of surgically-managed men and was independently associated with a higher risk of recurrence, raising the possibility that Skp2 could be useful as a prognostic biomarker and as a potential molecular target for novel systemic agents in prostate cancer.
Keywords: Skp2, Biomarker, Prostate cancer, Recurrence
1. Introduction
For patients with localized prostate cancer, the identification of molecular markers that are prognostic for outcome can be useful both to improve risk stratification beyond the current paradigm of prostate-specific antigen (PSA), Gleason score, and T-category, and to identify potential targets for novel therapeutic agents [1]. In several human cancers, overexpression of Skp2 (S-phase kinase associated protein 2) has been associated with poorer tumor differentiation, more advanced stage at presentation, and ultimately poorer clinical outcome [2]. Skp2 is an F-box protein that acts as the substrate recognition subunit of a complex that specifically targets p27 and their substrates for ubiquitylation and consequent degradation. Skp2-dependent degradation appears to be the major regulator of p27, which acts as a cell cycle inhibitor and a tumor suppressor by preventing Cdk1 and Cdk2 from driving the cell division cycle [3–5].
As Skp2 appears to be overexpressed only in tumor cells, it could represent an attractive candidate for targeting by novel anti-cancer agents. The purpose of this study is to examine the relationship between Skp2 expression and severity of disease in prostate cancer and to determine whether expression of Skp2 adds additional information beyond what is known from the PSA, T-stage, and Gleason score, to the prediction of outcome among men treated with radical prostatectomy.
2. Methods
2.1. Patient selection and clinical follow-up
Specimens were collected from 109 patients with localized prostate cancer who received a radical prostatectomy (n = 104) or transurethral resection of the prostate (TURP, n = 5) at Brigham and Women’s Hospital or Beth Israel Deaconess Medical Center from 1985–1996. Baseline patient characteristics are displayed in Table 1, obtained from a review of the medical records. Biochemical failure was defined as a post-prostatectomy PSA equal to or greater than 0.2 [6]. It was not the practice at our hospitals to give neoadjuvant treatment prior to prostatectomy, or to give adjuvant radiation or hormonal therapy prior to PSA failure. Follow-up time for the men who received RP was counted from the date of surgery. Median follow-up was 67 months (range: 0.2–218 months). This study was approved by the Dana-Farber/Harvard Cancer Center IRB.
Table 1.
Baseline patient characteristics stratified by Skp2 expression
| Total (N = 109) | Low Skp2 (N = 97) | High Skp2 (N = 12) | P value high vs. low Skp2 | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| N | % | N | % | N | % | ||
| Gleason | |||||||
| 6 or less | 33 | 30.3 | 32 | 33.0 | 1 | 8.3 | |
| 7 | 50 | 45.9 | 47 | 48.5 | 3 | 25.0 | |
| 8–10 | 26 | 23.9 | 18 | 18.6 | 8 | 66.7 | 0.001 |
| T-Category | |||||||
| pT2 | 55 | 53.4 | 54 | 57.4 | 1 | 11.1 | |
| pT3/T4 | 48 | 46.6 | 40 | 42.6 | 8 | 88.9 | 0.01 |
| TURP | 5 | — | 2 | — | 3 | — | |
| Missing | 1 | — | 1 | — | 0 | — | |
| PSA in ng/ml | |||||||
| 4 or less | 12 | 14.0 | 11 | 13.8 | 1 | 16.7 | |
| >4–10 | 37 | 43.0 | 36 | 45.0 | 1 | 16.7 | |
| >10–20 | 19 | 22.1 | 15 | 18.8 | 4 | 66.7 | |
| >20 | 18 | 20.9 | 18 | 57.4 | 0 | 0 | 0.042 |
| Missing | 24 | — | 18 | — | 6 | — | |
| Age at diagnosis | |||||||
| <60 | 19 | 17.4 | 16 | 16.5 | 3 | 25.0 | |
| 60–70 | 68 | 62.4 | 61 | 62.9 | 7 | 58.3 | |
| >70 | 22 | 20.2 | 20 | 20.6 | 2 | 16.7 | 0.755 |
2.2. Immunohistochemistry
Immunohistochemistry was performed on the paraffin-embedded material as previously described [7]. The following primary antibodies were used: anti-skp2 (generated by M.P.) at 1:100 dilution, anti-p27 (Transduction Laboratories, Lexington, KY) at 1:200 dilution, and anti-MIB1 (Immunotech, Westbrook, ME) at 1:50 dilution. Briefly, 5μ sections were deparaffinized and rehydrated. Antigen retrieval was performed in 10 mmol/l citrate buffer, pH6.0 (BioGenex, San Ramon, CA) in a 750 W microwave oven for 15 minutes. Slides were cooled down at room temperature for 30 minutes, then incubated with the primary antibody for 30 minutes at room temperature in the automated stainer (Optimax Plus 2.0bc, BioGenex, San Ramon, CA). The reaction was detected with the MultiLink-HRP kit (BioGenex) and revealed with 3,3-diaminobenzidine (DAB). Substitution of the primary antibody with phosphate buffered saline served as negative control.
P27, skp2, and MIB1 stains were scored as percent of cells showing nuclear staining, averaged from counting 100 tumor cells in 5 different areas under high (40×) power. For p27, only cells with intensity equal to or superior to that of infiltrating lymphocytes were counted as positive. A cutoff of 30% was chosen for high level of p27 expression as previously described [8]. A threshold of >4% vs. ≤4% was chosen for increased expression of Skp2, as this was the optimal cutpoint demonstrated in a prior study [9]. Finally, an identical cut-point of >4% vs ≤4% was also used for MIB-1, as has been done in prior studies [10].
2.3. Statistical methods
2.3.1. Correlations between Skp2 and other markers and clinical characteristics
For the 109 men in the study, Spearman’s rank correlation test was used to determine the relationship between Skp2 and p27 expression levels as continuous variables. Fisher’s exact test was used to determine the association between Skp2 and p27 expression levels dichotomized as high and low. The χ2 test was used to determine whether there was a difference in the distribution of prostatectomy Gleason score (categorized as 6 or less, 7, or 8–10), or pathologic T-category (T2 vs. T3), or PSA level (less then 4, 4–10, and >10), and MIB-1 levels (high vs. low) between those with high and low Skp2 expression.
2.3.2. Survival analysis among men who received RP
For the 104 men who received RP, Cox proportional hazards regression analysis was used to determine whether there was an association between Skp2 expression level and time to biochemical recurrence after adjusting for the known prognostic variables of pathologic stage (T3 vs. T2), prostatectomy Gleason score (8–10 vs. 7 vs. 6 or less), and baseline PSA (continuous). For the purpose of illustration, the method of Kaplan and Meier was used to graphically display biochemical failure-free survival stratified by level of Skp2 expression as well as by PSA, pathologic Gleason score, and pathologic stage.
2.3.3. Correlative cell line experiment
As a correlative experiment to confirm the relationship between Skp2 and p27 expression as well as their relative expression in actively cycling vs. noncycling cells, we examined Skp2 and p27 levels in LNCaP cells that were arrested by androgen depletion and then stimulated to divide by increasing concentrations of androgens. In order to deplete androgens from the media, LNCaP cells were grown in 10% charcoal-dextran stripped fetal bovine serum (FBS) for 48 hours. Cells were then stimulated with varying concentrations of dihydrotestosterone (DHT) for another 48 hours (0 nM, 0.1 nM, 1 nM, and 10 nM). Cells were subsequently harvested for direct western analysis of Skp2 and p27 expression. Cells grown in regular FBS were included as a control.
3. Results
3.1. Patterns of expression of Skp2 and p27 from tumor samples
Expression of p27 varied greatly in the 109 cases, ranging from 2% to 85% (mean = 27%). On the contrary, expression of skp2 showed a much narrower variation, ranging from 0% to 15% (mean = 2%). Skp2 antibody showed strong nuclear uptake, most often with cytoplasmic staining in tumor cells with a clean background. It rarely stained the epithelium of normal prostatic glands, and did not stain the basal cells.
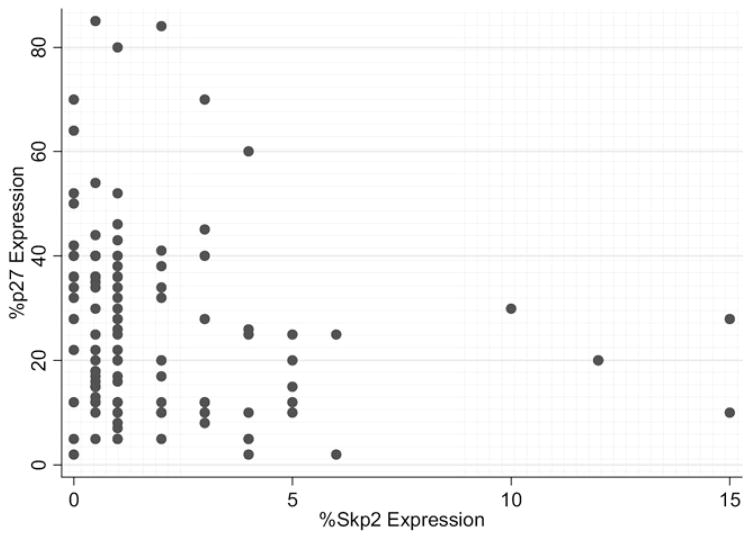
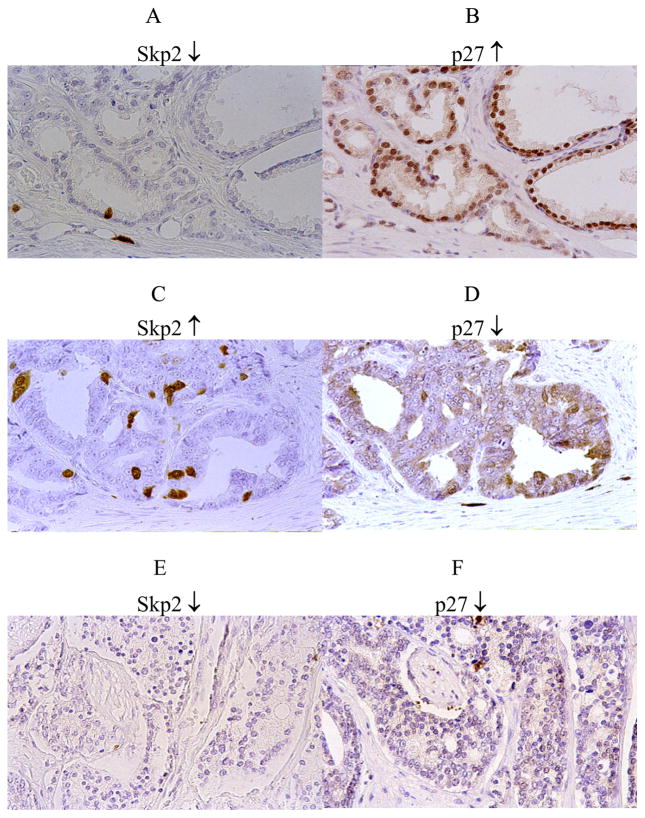
Overall, 12 tumors (11%) had high Skp2 expression. These 12 all had correspondingly low p27 expression (P = 0.006). The scatter plot of the Skp2 and p27 scores is displayed in Fig. 1 (χ = 0.005, Spearman’s ρ = −0.26). The three patterns of Skp2/p27 expression observed are displayed in Fig. 2. They included the 12 cases of high Skp2/ low p27 (11%), 40 cases of low Skp2 and high p27 (36%), and 58 cases of dual low expression for Skp2 and p27 (53%). There were no cases (0%) with high Skp2 and high p27.
Fig. 1.
Scatter plot of Skp2 Expression and p27 Expression.
Fig. 2.
Expression patterns of Skp2 and p27 in prostatic adenocarcinoma. (A/B) LowSkp2 and high p27; (C/D) high Skp2 and low p27; (E/F) low Skp2 and low p27. (Color version of figure is available online.)
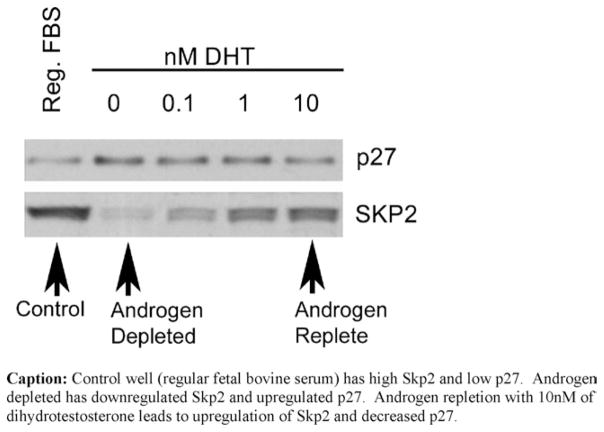
3.2. Expression of Skp2 and p27 in LNCaP cell lines
Fig. 3 shows the Western blot analysis of p27 and Skp2 expression in LNCaP cells with varying levels of androgen stimulation. The figure shows that Skp2 and p27 are inversely related. In the nutrient-rich control, Skp2 expression was high while p27 expression was low. In the androgen-depleted cells, Skp2 expression was low while p27 was high, suggesting that androgens play a role in decreasing Skp2 expression which leads to increased p27 and cell cycle arrest. Conversely, in the androgen repleted cells, Skp2 is up-regulated again and p27 is decreased as the cells re-enter the cell cycle.
Fig. 3.
Skp2 and p27 expression in LNCaP cells with varying degrees of androgen stimulation.
3.3. Association between Skp2 expression and markers of aggressive disease
Forty percent of tumors with high Skp2 also had high MIB-1 expression, while only 13% of tumors with low Skp2 had high MIB-1 (P = 0.058, Fisher’s exact test). As continuous variables, Skp2 and MIB-1 expression were significantly positively correlated (P = 0.03, Spearman’s ρ = 0.26). Skp2 overexpression was associated with higher Gleason score (P = 0.002), and more advanced pathological T-category (P = 0.011), as displayed in Table 1. There was also a significant difference in the distribution of PSA among those with high vs. low Skp2 expression, as there were no patients with high Skp2 and PSA greater than 20 (P = 0.04), but the difference in mean PSA between the two groups (12.1 for high Skp2 and 14 for low Skp2) was not significantly different.
3.4. Association between Skp2 and recurrence-free survival
Of the 109 men, 5 received a TURP (including 3 who had elevated Skp2), and 4 were missing follow-up information. The remaining 100 men were included in the survival analysis. On univariable analysis, elevated Skp2 expression was significantly associated with a shorter time to recurrence (hazard ratio = 3.7 [95% CI = 1.6 to 8.6]), P = 0.002, standard error 1.6. Because elevated Skp2 expression was also associated with other negative prognostic factors, a multivariable analysis was performed adjusting for the potential impact of Gleason score, pathologic T-category, and PSA on outcome. On multivariable analysis, elevated Skp2 expression remained significantly associated with a shorter time to recurrence, with an adjusted hazard ratio (AHR) of 4.8 (1.6–13.9), P = 0.004, standard error 2.6. The other factors significantly associated with outcome were pathologic T3 or T4 disease (AHR = 2.3, P = 0.028), and PSA (AHR = 1.04, P < 0.001). Gleason score was not significantly associated with outcome on multivariable analysis. Of note, p27 expression was also not found to be significantly associated with outcome.
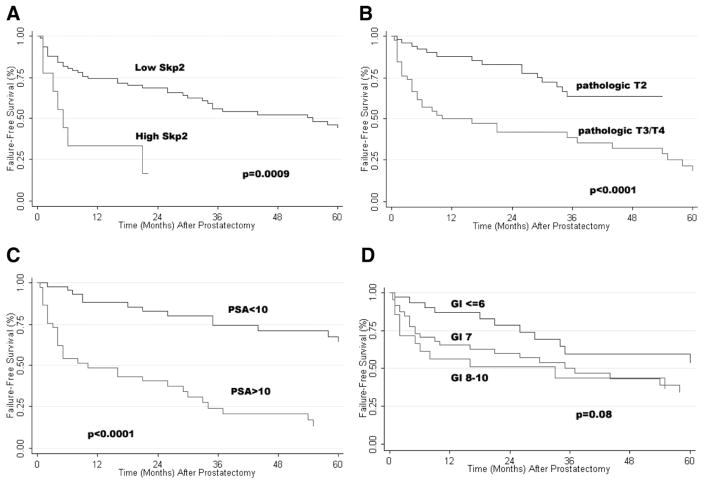
For illustrative purposes, recurrence-free survival stratified by low vs. high expression of Skp2 is displayed in Fig. 4. The median recurrence-free survival for those patients with low expression of Skp2 was 54 months, compared with 4 months for those with high expression of Skp2. At 1 year, the recurrence-free survival for those with low vs. high expression of Skp2 was 74% vs. 33% (P = 0.0009, Log rank).
Fig. 4.
Failure-free survival stratified by (A) Skp2 expression, (B) pathologic T-category, (C) baseline PSA, and (D) prostatectomy Gleason score.
4. Discussion
There is accumulating evidence that Skp2 is associated with more aggressive disease and poorer outcomes in several human cancers [2]. In this study, we examined the patterns of expression and clinical significance of Skp2 in men with node-negative prostate cancer who were treated surgically. We found that all cases with Skp2 overexpression also had correspondingly low p27 expression, which is consistent with the in vivo function of Skp2 in recognizing and targeting p27 for destruction through the ubiquitin-proteasome degradation system [3–5]. While high Skp2 was always associated with low p27 in this study, the converse was not necessarily true, as 53% of the cases had both low Skp2 and low p27, showing that p27 down-regulation may also occur by pathways unrelated to Skp2.
As a correlative study, we found that Skp2 and p27 expression were also inversely related in LNCaP cell lines and that stimulation of the cells with androgens led to an increase in Skp2 and decrease in p27 as the cells re-entered the cell cycle, suggesting that Skp2 regulation could be a mechanism by which the proliferation of prostate cancer cells is controlled.
Along the same lines, we found in the tumor samples that Skp2 overexpression was significantly associated with biological markers of more aggressive disease, including a higher MIB-1 index and higher Gleason scores. These results are similar to the findings of a study by Yang et al. and one by Ben-Izhak et al. who found that high Skp2 expression was associated with higher Ki-67 (i.e., MIB-1) index and Gleason score, although the Yang study found that the association with Ki-67 was only present when Skp2 was dichotomized into high vs. low, but not when Skp2 was analyzed as a continuous variable [9,11]. Interestingly, patients with high Skp2 were less likely to have high PSA levels in our study, while they were more likely to have higher PSAs in other the two studies. This seemingly paradoxical finding may partially reflect the phenomenon of high-grade cancers producing less PSA per quantity of tumor than low-grade tumors [12]. A more likely explanation for this finding may be related to the fact that patients who have the highest PSA, namely those with locally advanced and metastatic disease, were excluded from this study as such patients are typically not treated by radical prostatectomy. Had they been included, as they were in the Ben-Izhak study, it is possible that a similar unfavorable association between Skp2 expression and PSA might have been seen [9].
This study also found that Skp2 overexpression was associated with a shorter time to biochemical recurrence, as Skp2 overexpressors had a median failure-free survival of 4 months, compared to nearly 54 months for those without elevated Skp2. This is consistent with the prior findings of Yang, et al, who found on univariable analysis that increased Skp2 was significantly associated with an increased risk of biochemical recurrence [11]. However, a unique observation in our study is that after adjusting for typical prognostic variables such as grade, stage, and PSA, over-expression of Skp2 remained significantly associated with a shorter time to biochemical recurrence. In the Yang study, the significance of Skp2 was lost in a model adjusting for stage, grade, PSA, margin status, and lymph node status. Differences in methodology could potentially explain some of this difference. The Yang study adjusted for pathologic margin status, while we did not have complete information on this and could not adjust for it. In addition, the Yang study examined Skp2 labeling index, which takes into account both frequency and intensity of staining, while we examined only frequency (as was done in the Ben-Izhak study). The Yang study also created tissue micro-arrays for analysis, and had more patients (622), which increases the reliability of their outcome estimates. Finally, while it is unlikely to significantly change the results, the Yang study used a PSA failure definition of PSA ≥ 0.4 ng/ml, while we used a slightly lower threshold of PSA ≥ 0.2, which could have led to failures being scored earlier in our study.
The clinical implications of these findings are two-fold. First, it raises the possibility that Skp2 overexpression could be used to help identify patients who are at the highest risk of failure after radical prostatectomy and therefore may need to be considered for adjuvant post-operative therapy. For example, among patients with pathologic T3 disease or positive margins, adjuvant postoperative radiotherapy has been shown to improve biochemical recurrence-free survival in a large European randomized trial, and has been shown to improve metastasis-free survival and overall survival in an American randomized trial with longer follow-up [13,14]. It is conceivable that patients who are Skp2 overexpressors could also benefit significantly from the addition of adjuvant radiation, even if they do not have T3 disease. In addition, the rapid time to biochemical recurrence among the Skp2 overexpressing cohort suggests that many of these patients may have harbored micrometastatic disease at the time of treatment and therefore could be considered for protocols evaluating the benefit of adjuvant systemic therapy in select high-risk patients.
The second clinical implication of this study is that it raises the possibility that specific targeting of Skp2 by a novel agent could improve outcomes in prostate cancer. While there is currently no drug available that can specifically target Skp2, there is in vitro evidence that the tamoxifen and the mTOR inhibitor rapamycin can decrease Skp2 expression in breast cancer cells [7,15,16]. Caveats to Skp2 targeting include the fact that only 11% of the patients in this cohort were Skp2 overexpressors, and also that it is not certain whether trying to lower Skp2 levels after diagnosis will be effective in altering outcome, or whether the deleterious effects of Skp2 on outcome have already occurred during the initial transformation prior to the time of diagnosis.
This study has potential limitations. First, the number of patients who were Skp2 overexpressors was relatively small, which limits the precision of the estimates of outcome for these patients, as well as the precision of the hazard ratios in the multivariable survival models. Another potential limitation of the study is the use of biochemical recurrence as the endpoint. As the median time between biochemical recurrence and clinically-evident metastases is 8 years, many men with biochemical recurrence may not live to experience distant metastases and may die of a competing cause beforehand [6,17]. Among our patients, there were not enough events to do an analysis with time to metastases or prostate cancer death, and thus far no study has examined the impact of Skp2 expression on either of these endpoints. It is possible that with further follow-up, such an analysis would be possible. In addition, the patients in the study were treated over a relatively long timeframe, and over such a timeframe, there may be subtle shifts in treatment techniques or even tissue fixation which can affect results. Also, while we attempted to assemble an unbiased sample based on the availability of tissue, it is possible that the sample selected does not perfectly reflect the population from which it is drawn. Additionally, the percent of skp2, p27, and MIB-1 staining was determined manually in this study, and it is possible that the use of automated optical analyzers could have generated a more precise score. Finally, high Skp2 in our sample was associated with higher T-stage, worse Gleason score, and lower PSAs than low Skp2, and these associations between input variables can make parameter estimates of multivariable models less stable, but we did not find a significant co-linearity effect in our model.
An unexpected finding in this study was that p27 expression was not significantly associated with outcome, as other studies have found that low p27 levels portend an increased risk of recurrence [8,18]. The reason for this discrepancy is unclear, but the fact that all patients with low p27 and correspondingly high Skp2 had a poor outcome raises the possibility declines in p27 due to Skp2 may lead to worse outcomes than p27 declines due to other processes.
In summary, this study found that Skp2 overexpression was found in a minority (11%) of men with nonmetastatic prostate cancer, and was associated with markers of aggressive disease and was independently associated with biochemical recurrence. These results provide further support that Skp2 expression may be useful as a prognostic marker in prostate cancer, and that Skp2 itself may be a good target for novel biological agents, but further confirmatory studies in large cohorts are needed.
Acknowledgments
The authors are grateful to Susanna Jacobus and Hajime Uno, both of the Dana-Farber Cancer Institute Department of Biostatistics and Computational Biology, for their advice on this manuscript.
Footnotes
Presented in abstract form at the ASCO 2008 Meeting.
M.L. has consulted for Novartis.
References
- 1.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 2.Hershko DD. Oncogenic properties and prognostic implications of the ubiquitin ligase Skp2 in cancer. Cancer. 2008;112:1415–24. doi: 10.1002/cncr.23317. [DOI] [PubMed] [Google Scholar]
- 3.Carrano AC, Eytan E, Hershko A, et al. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193–9. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 4.Sutterluty H, Chatelain E, Marti A, et al. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat Cell Biol. 1999;1:207–14. doi: 10.1038/12027. [DOI] [PubMed] [Google Scholar]
- 5.Tsvetkov LM, Yeh KH, Lee SJ, et al. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr Biol. 1999;9:661–4. doi: 10.1016/s0960-9822(99)80290-5. [DOI] [PubMed] [Google Scholar]
- 6.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 7.Signoretti S, Di Marcotullio L, Richardson A, et al. Oncogenic role of the ubiquitin ligase subunit Skp2 in human breast cancer. J Clin Invest. 2002;110:633–41. doi: 10.1172/JCI15795. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Yang RM, Naitoh J, Murphy M, et al. Low p27 expression predicts poor disease-free survival in patients with prostate cancer. J Urol. 1998;159:941–5. [PubMed] [Google Scholar]
- 9.Ben-Izhak O, Lahav-Baratz S, Meretyk S, et al. Inverse relationship between Skp2 ubiquitin ligase and the cyclin dependent kinase inhibitor p27Kip1 in prostate cancer. J Urol. 2003;170:241–5. doi: 10.1097/01.ju.0000072113.34524.a7. [DOI] [PubMed] [Google Scholar]
- 10.Ghanem MA, Van der Kwast TH, Sudaryo MK, et al. MIB-1 (KI-67) proliferation index and cyclin-dependent kinase inhibitor p27(Kip1) protein expression in nephroblastoma. Clin Cancer Res. 2004;10:591–7. doi: 10.1158/1078-0432.ccr-0884-02. [DOI] [PubMed] [Google Scholar]
- 11.Yang G, Ayala G, De Marzo A, et al. Elevated Skp2 protein expression in human prostate cancer: Association with loss of the cyclin-dependent kinase inhibitor p27 and PTEN and with reduced recurrence-free survival. Clin Cancer Res. 2002;8:3419–26. [PubMed] [Google Scholar]
- 12.Partin AW, Carter HB, Chan DW, et al. Prostate specific antigen in the staging of localized prostate cancer: Influence of tumor differentiation, tumor volume, and benign hyperplasia. J Urol. 1990;143:747–52. doi: 10.1016/s0022-5347(17)40079-6. [DOI] [PubMed] [Google Scholar]
- 13.Bolla M, van Poppel H, Collette L, et al. Postoperative radiotherapy after radical prostatectomy: A randomised controlled trial (EORTC trial 22911) Lancet. 2005;366:572–8. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 14.Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: Long-term follow-up of a randomized clinical trial. J Urol. 2009;181:956–62. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slotky M, Shapira M, Ben-Izhak O, et al. The expression of the ubiquitin ligase subunit Cks1 in human breast cancer. Breast Cancer Res. 2005;7:R737–44. doi: 10.1186/bcr1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapira M, Kakiashvili E, Rosenberg T, et al. The mTOR inhibitor rapamycin down-regulates the expression of the ubiquitin ligase subunit Skp2 in breast cancer cells. Breast Cancer Res. 2006;8:R46. doi: 10.1186/bcr1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–9. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 18.Freedland SJ, deGregorio F, Sacoolidge JC, et al. Preoperative p27 status is an independent predictor of prostate specific antigen failure following radical prostatectomy. J Urol. 2003;169:1325–30. doi: 10.1097/01.ju.0000054004.08958.f3. [DOI] [PubMed] [Google Scholar]