Abstract
Recent work reported that Tomato yellow leaf curl virus (TYLCV) is seed-transmissible in tomato, contrary to previous belief. In this work, we explore whether TYLCV is also a seed-borne virus in another member of the Solanaceae family, the experimental host Nicotiana benthamiana.
Keywords: Tomato yellow leaf curl virus, Seed transmissibility, Nicotiana benthamiana
Recent work reported that Tomato yellow leaf curl virus (TYLCV) is seed-transmissible in tomato, contrary to previous belief. In this work, we explore whether TYLCV is also a seed-borne virus in another member of the Solanaceae family, the experimental host Nicotiana benthamiana.
Seed transmissibility of a plant virus is an important biological property that determines the design of effective management strategies to minimize virus-caused disease. Seed-borne viruses can be directly transmitted from one generation to the next, which in turn would act as a new source of inoculum. In the case of intermediate weeds that can act as alternative hosts growing in or in close proximity to croplands, the capability of the virus to persist in the seeds and infect the newly emerged plant can have an enormous impact on the spread of the disease, since it would allow the presence of the virus in the area independently of the insect vector and the choice of cultivated crop.
TYLCV (genus Begomovirus, family Geminiviridae) causes the most devastating viral disease of tomato, generating severe economic losses (Moriones and Navas-Castillo, 2000; http://www.cabi.org/isc/ datasheet/55402#). TYLCV is transmitted by the whitefly, Bemisia tabaci, and, in addition to tomato, can infect other cultivated crops (including bean, pepper, tobacco, and potato) as well as weeds. Since its first description in 1931, TYLCV has been considered non-seed-transmissible, like most geminiviruses (Kashina et al., 2003; http://www.cabi.org/ isc/datasheet/55402#). Importantly, a recent report has described that TYLCV-IL (hereafter referred to as TYLCV) is in fact seed-transmissible in tomato, and that the offspring of infected tomato plants can act as a source of inoculum for B. tabaci-mediated transmission (Kil et al., 2016). In the aforementioned work, Kil et al. (2016) detect the presence of TYLCV in floral tissues and seeds of TYLCV-infected tomato plants, as well as in the seedlings germinated from these seeds, by polymerase chain reaction (PCR). In addition, the authors performed a whitefly transmission assay by placing one donor tomato plant germinated from a TYLCV-infected seed together with three healthy receiver tomato plants in the presence of non-viruliferous whiteflies. Given that the healthy plants became infected with TYLCV in the course of this experiment, Kil et al. (2016) concluded that the seed-borne virus is transmissible by the insect vector.
The discovery of the seed transmissibility of TYLCV has come as a surprise to the scientific community working on plant-virus interactions, since it would mean that this relevant property of TYLCV, which would define a potential new transmission cycle, as proposed by Kil et al. (2016) has gone unnoticed by the multiple research groups working on TYLCV in both the public and private sectors since the description of the virus 75 years ago. The impact of these results, however, goes beyond basic science, since this newly uncovered property of TYLCV would demand a reassessment of the management and control strategies so far implemented for the viral disease.
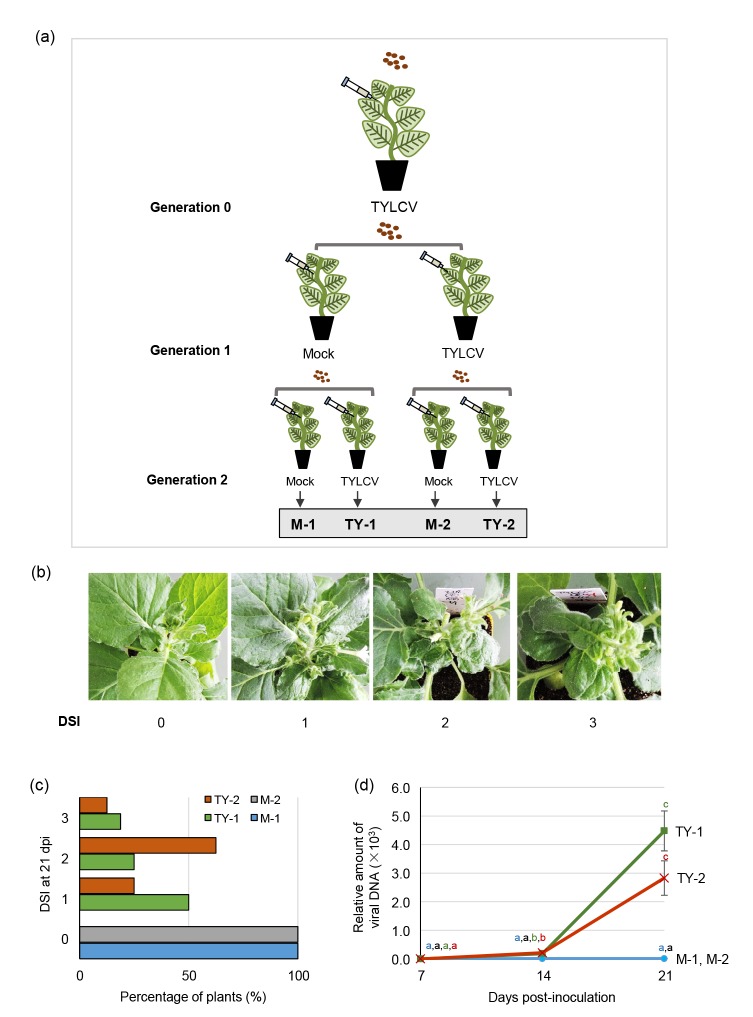
In order to determine whether seed transmissibility is a general property of TYLCV in different host species, we have tested the ability of this virus to be transmitted to the offspring via seeds in the experimental host N. benthamiana (family Solanaceae). For this purpose, we infected 4-week-old N. benthamiana plants (n=12; Generation 0 in Fig. 1) with a TYLCV infectious clone (GenBank AJ489258) using Agrobacterium tumefaciens-mediated inoculation (agroinoculation) in the stem. Plants were kept in a growth room under controlled conditions (25 °C, 16-h light cycle). All TYLCV-inoculated plants displayed the typical symptoms of the viral infection, including stunting and leaf curling (Fig. 1), to a similar extent. Only four of twelve infected plants set seed, and in all four cases these were contained in the first capsule, which developed after inoculation, exclusively. Seeds coming from each TYLCV-infected plant were planted separately, and for each lineage (offspring of one given TYLCV-infected plant), four plants were inoculated with TYLCV, and four plants were mock-inoculated (Fig. 1; Generation 1). Whereas, as expected, the TYLCV-inoculated plants developed symptoms, the mock-inoculated plants remained symptomless until seeds were harvested from both TYLCV-and mock-inoculated plants (>12 weeks). All inoculated plants produced seeds. Again, seeds were planted separately, and infected with TYLCV or mock-inoculated (Fig. 1; Generation 2). The results obtained in the second generation were similar to those of the previous one: regardless of whether they came from TYLCV-infected or mock-inoculated plants, mock-inoculated plants in this generation remained symptomless throughout the duration of the experiment (>12 weeks) (Fig. 1). Symptom evaluation correlated with TYLCV accumulation, as measured by quantitative PCR (qPCR) with primers to quantify the TYLCV Rep gene (TGAGAACGTCGTGTCTT CCG and TGACGTTGTACCACGCATCA) and 25S ribosomal DNA interspacer (ITS) as the normalizer (ATAACCGCATCAGGTCTCCA and CCGAAGTT ACGGATCCATTT) (Lozano-Durán et al., 2011). As shown in Fig. 1, while TYLCV accumulated in infected plants as expected, the presence of the virus could not be detected in any of the mock-inoculated plants of Generation 2 at 12 weeks post-infection.
Fig. 1.
Tomato yellow leaf curl virus (TYLCV) is not seed-transmissible in Nicotiana benthamiana
(a) Graphical summary of the experimental design. Twelve four-week-old plants were inoculated with TYLCV (Generation 0). Seeds coming from TYLCV-infected plants were planted separately, and for each lineage four plants were inoculated with TYLCV and four plants were mock-inoculated (Generation 1). Seeds were harvested from TYLCV-and mock-inoculated plants coming from the first generation (four plants per lineage), planted separately, and infected with TYLCV or mock-inoculated as done previously (Generation 2). (b) Disease symptoms index (DSI) used in this work. A value of 0 represents symptomless plants, whereas a value of 1 to 3 represents plants showing increasing symptom severity, as illustrated in the pictures. (c) Average disease symptom severity in plants inoculated with TYLCV (TY-1, TY-2) or mock (M-1, M-2) at 21 days post-inoculation (dpi). (d) Relative viral DNA accumulation in plants inoculated with TYLCV (TY-1, TY-2) or mock (M-1, M-2) as determined by real-time PCR of total DNA extracted from apical leaves at 7, 14, and 21 dpi. Values represent the averages of 16 TYLCV-infected plants and 12 mock-inoculated plants, and are relative to that of TY-2 at 7 dpi. Bars represent standard errors. One-way analysis of variance (ANOVA) Tukey’s multiple comparison tests were used to distinguish differences among samples at P-value of <0.05. Different letters indicate statistically significant difference
Considering that a total of 48 mock-inoculated plants coming from TYLCV-inoculated plants were used in this experiment (16 in Generation 1 (4 lineages) and 32 in Generation 2 (8 lineages)), our results demonstrate that either TYLCV is not seed-transmissible in N. benthamiana, or the rate of transmission is too low to be detected in our experimental conditions (<4%), based on the absence of symptoms (Fig. 1c). Additionally, we could not detect the presence of viral DNA by qPCR in 12 mock-inoculated plants coming from TYLCV-infected plants (Fig. 1d). These data are in contrast to those presented by Kil et al. (2016) in tomato, where the infection rates in the offspring of agroinoculated TYLCV-infected plants were 80.77% in cotyledons and 73.33% in true leaves. TYLCV, therefore, seems to behave differently in different host species in terms of seed transmissibility. Another possibility that cannot be ruled out at the moment would be that differences in growth conditions, such as greenhouse cultivation versus growth chamber cultivation, drastically affect the efficiency of this process.
Our results have a potential impact at two different levels. On the one hand, it is important for researchers working on this or similar viruses to know how their objects of study behave in the model plant species used in their experiments—in this particular case, to know that TYLCV does not behave as an efficient seed-borne virus in the widely employed N. benthamiana in standard laboratory conditions. However, more importantly, this information can be relevant to provide a broader context for the impact of TYLCV’s seed transmissibility in tomato with regards to the design of control strategies in the field. One of the main risks of seed-borne viruses is their ability to persist in weed populations that will act as reservoirs, complicating eradication of the disease. Our work suggests that seed transmissibility may not be a general property of TYLCV: the fact that in the Solanaceae N. benthamiana, which is susceptible to the virus and supports high viral accumulation, TYLCV cannot be transmitted to the next generation via the seed suggests that this may also be the case in other alternative hosts. Additionally, TYLCV infection results in largely reduced seed production in N. benthamiana, which is completely abolished in many cases: this effect would counter-select viral propagation through seed, even in a scenario of granted seed transmissibility. However, as in the case of viral transmission through seeds, the TYLCV-induced reduction in seed production might be host-dependent, since this effect is not obvious in Arabidopsis thaliana, another experimental host for TYLCV (Cañizares et al., 2015), as also shown in our unpublished observations.
Whether TYLCV is efficiently transmitted by seeds in its several crop and wild host species described to date is a question that needs to be investigated and experimentally determined. It should be considered that multiple factors may influence the outcome of such experiments, including developmental stage of the inoculated plants, inoculation method, or presence of secondary pathogen infections. Given the practical implications of this research, rigorous, comprehensive experimentation will be essential to thoroughly define all possible ways of transmission of TYLCV and in turn ensure that appropriate policies and control strategies are efficiently implemented.
Acknowledgments
The authors would like to thank Xue DING and Li-ping WANG (Shanghai Center for Plant Stress Biology, Shanghai Institutes of Biological Sciences, University of Chinese Academy of Sciences, China) for technical assistance and Alberto P. MACHO (Shanghai Center for Plant Stress Biology), Tamara JIMENEZ-GONGORA (Shanghai Center for Plant Stress Biology), and Eduardo R. BEJARANO (University of Malaga, Spain) for critical reading of this manuscript. Tábata ROSAS-DÍAZ is the recipient of a President’s International Fellowship Initiative (PIFI) (No. 2016PB042) from the University of Chinese Academy of Sciences.
Footnotes
Project supported by the Shanghai Center for Plant Stress Biology of the University of Chinese Academy of Sciences and the 100 Talent Program of the University of Chinese Academy of Sciences, China
Compliance with ethics guidelines: Tábata ROSAS-DÍAZ, Dan ZHANG, and Rosa LOZANO-DURÁN declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Cañizares MC, Rosas-Díaz T, Rodríguez-Negrete E, et al. Arabidopsis thaliana, an experimental host for tomato yellow leaf curl disease-associated begomoviruses by agroinoculation and whitefly transmission. Plant Pathol. 2015;64(2):265–271. doi: 10.1111/ppa.12270. [DOI] [Google Scholar]
- 2.Kashina BD, Mabagala RB, Mpunami A. Biomolecular relationships among isolates of Tomato yellow leaf curl tanzania virus . Phytoparasitica. 2003;31(2):188–199. doi: 10.1007/BF02980789. [DOI] [Google Scholar]
- 3.Kil EJ, Kim S, Lee YJ, et al. Tomato yellow leaf curl virus (TYLCV-IL): a seed-transmissible geminivirus in tomatoes. Sci Rep. 2016;6:19013. doi: 10.1038/srep19013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lozano-Durán R, Rosas-Díaz T, Luna AP, et al. Identification of host genes involved in geminivirus infection using a reverse genetics approach. PLoS ONE. 2011;6(7):e22383. doi: 10.1371/journal.pone.0022383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moriones E, Navas-Castillo J. Tomato yellow leaf curl virus, an emerging virus complex causing epidemics worldwide. Virus Res. 2000;71(1-2):123–134. doi: 10.1016/S0168-1702(00)00193-3. [DOI] [PubMed] [Google Scholar]