Abstract
Aim
Prospective study on 900 consecutive puerperae to assess normal values and range of the blood flow velocity in the middle cerebral artery in both hemispheres.
Material and method
M1 and M2 segments of both middle cerebral arteries were assessed in all subjects within 96 hours of delivery. Mean flow velocity was recorded after adjusting for insonation angle. Lindegaard index (LI = middle cerebral artery–Internal Carotid Artery mean flow velocity ratio) was calculated whenever the mean flow velocity exceeded 100 cm/second. Asymmetry indexes were calculated inter hemispherically for M1 and M2 segments separately.
Results
Mean flow velocities were 74 ± 17 and 72 ± 17 in right and 73 ± 17 and 72 ± 17 cm/second in the left M1 and M2, respectively. A total of 136 subjects (12.1%) exceeded the threshold of 100 cm/second, but LI was consistently <3 in all of them. Mean flow velocity was inversely and independently correlated to haemoglobin levels and to parity. Mean asymmetry indexes were 0.25 ± 23 in M1 and 0.45 ± 25 in M2.
Conclusion
Mean flow velocity in the middle cerebral artery of healthy subjects in early puerperium is higher than in age-matched non-puerperal women and may exceed the threshold of 100 cm/second with no evidence of intracranial spasm, because of blood loss during delivery. Mean flow velocity is independently correlated with parity. Right-to-left mean flow velocity asymmetry may reach 50% as a consequence of a transient imbalance in vascular tone regulation.
Keywords: Mean flow velocity, puerperium, asymmetry index, middle cerebral artery
Introduction
In the early days post delivery, brain circulation undergoes a profound adaptation to changing hemodynamic and biochemical conditions, including fluid shift, cardiac output, oestrogens and haemoglobin levels and arterial blood pressure.1,2
This process of gradual return to non-gravidic cerebral haemodynamics may be deranged in pre-eclampsia and eclampsia, in which a severe perturbation in cerebral autoregulation, as yet ill understood, may bring about cerebral oedema, infarction or haemorrhage.3–7
Transcranial Doppler sonography (TCD), due to its unique capability to assess in real time the fluctuations of the velocity of cerebral blood flow, has been extensively employed to study cerebral haemodynamics during pregnancy and in puerperium, but often with conflicting results.8,9
One of the main drawbacks in the interpretation of the existing literature is the lack of robust normative data on brain vessel velocimetry, as in the vast majority of reports the numerosity of the control groups with no recognised pathology, taken as reference standard, was less than 30 subjects.10 This has generated a great deal of uncertainty about the range of normal variations in blood flow velocity in intracranial vessels, thus hampering the establishment of sound cut-off values between normal and pathological findings.
To overcome this shortcoming, we undertook a prospective study on a large cohort of consecutive women who had given birth after a non complicated pregnancy and labour to assess normal values and range of the blood flow velocity in the middle cerebral artery (MCA) in both hemispheres.
Material and methods
The study protocol was approved by the S. Orsola Hospital Institutional Review Board (Ethics Committee). All patients provided informed consent before entering the study.
The study period extends from November 2011 to July 2013. We studied all female subjects having given birth after a non-complicated pregnancy and labour in the obstetrics department of the S. Orsola Hospital (first 86 cases, then Poliambulanza Hospital, as the former was incorporated in the latter) in Brescia, Italy.
The subjects underwent a basal visit within 96 hours of delivery, in which a chart review was performed and a structured interview was administered in person by one research assistant, with particular emphasis on headache. Information was collected on maternal demographics, labour and delivery details, anaesthesia during labour and delivery, and headache characteristics and management. The following data were also collected: age, ethnicity, height, weight gain, arterial blood pressure, history of primary headache disorders, history of arterial hypertension, smoking habits, alcohol intake, diabetes, dyslipidemia, family history of stroke and detailed characteristics of recent pregnancy and delivery, including medications and drugs taken during pregnancy. We also recorded in all subjects haemoglobin, uric acid and albuminuria levels. Thereafter, arterial blood pressure (ABP) was measured noninvasively. Patients fulfilling the criteria for pre-eclampsia (systolic BP > 140 Hg/mm or diastolic BP > 90 Hg/mm and albuminuria exceeding 0.3 g/l in a 24-hour collection) were excluded from the study.
A transcranial ultrasound study was performed in all the remaining patients to detect any early sign of vasospasm. Sequential Transcranial Colour-Coded Sonography (TCCS) was conducted by trained examiners using a Philips IU 21 device. TCCS examinations were performed with a centre transmit frequency of 3–3.5 MHz in colour mode using established methods.11–13 The Doppler gate was set at 5–10 mm. The proximal (M1) and distal (M2) segments of the MCA were identified on colour representation through bilateral temporal skull windows from depths of 55 to 65 mm and 45 to 55 mm, respectively. When the spectral display of velocity reached a steady state, mean flow velocity (MFV) was recorded after adjusting for insonation angle (see Figures 1 and 2). Asymmetry indexes (AIs) according to the formula proposed by Zanette
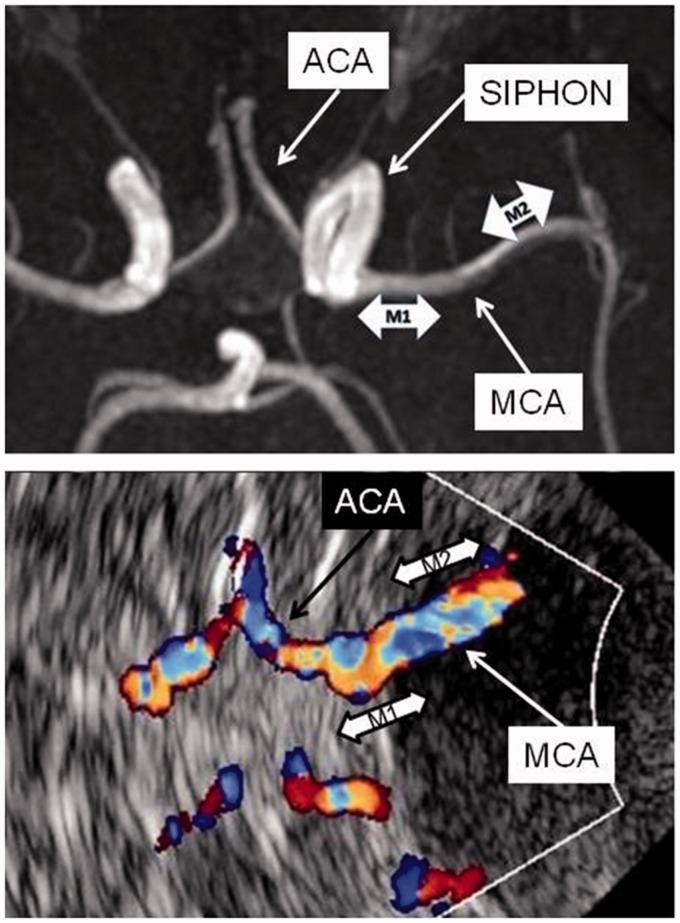
Figure 1.
Upper panel: MR angiography, axial view. Proximal and distal segments of MCA marked as M1 and M2, respectively. ACA: anterior cerebral artery; SIPHON: carotid siphon; MCA: middle cerebral artery. Lower panel: Transcranial colour coded sonography, axial view. M1 and M2 segments of MCA insonated at a depth of 55–65 mm and 45–55 mm, respectively, from the skull wall (marked by the white curved line). The carotid siphon is not visible as it courses orthogonal to the scanning plane.
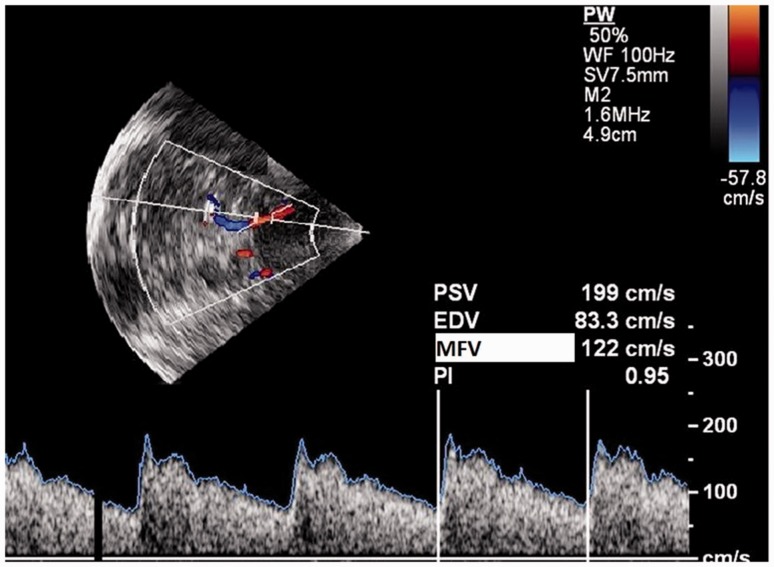
Figure 2.
Transcranial colour coded sonography: axial view. The sample volume is placed in the proximal segment of MCA. The spectral display of velocity is shown in the lower panel. Horizontal axis = time. Vertical axis velocity in cm/second. PSV: peak systolic velocity; EDV: end diastolic velocity; MFV: mean flow velocity; PI: pulsatility index.
were calculated inter hemispherically for M1 and M2 segments separately and intra hemispherically (by substituting in the formula proximal and distal to right and left velocities respectively) between M1 and M2 segments bilaterally.13,14 Asymmetry indexes are non-dimensional values that represent the percent difference in velocity between two arterial segments by taking as reference the mean of the velocities of the two segments under study. Submandibular windows were used to measure flow velocity in the distal internal carotid artery (ICA) proximal to its entry into the skull, at the depth of 40–60 mm. MFV of 100 cm/second was selected as the normal upper limit in this study, according to threshold criteria derived from the literature; any higher value was considered suggestive of MCA stenosis.15–17 In patients with MCA velocity exceeding the threshold, we also calculated Lindegaard Index (LI) by dividing the MFV in MCA by the mean flow velocity of ipsilateral distal extracranial ICA. Published studies have indicated that an LI of 3–6 can be considered as mild vasospasm and greater than 6 can be considered as moderate to severe vasospasm.18
At the end of the enrolment, we were thus able to assess normal values of MFV in both MCAs and to correlate the findings with variables potentially able to affect cerebral blood flow velocity.
Continuous variables were compared by means of two-tailed t-test if normally distributed or non-parametric tests if the distribution was skewed. Frequencies were compared by Chi square test and bivariate correlations with Pearson’ s correlation coefficient test. Regression analyses were conducted to assess independent predictors of MFV (SPSS version 22).
Results
During the inclusion period of 20 months, 900 women were enrolled in the study: it was found that 75.3% of them were of Caucasian ethnicity (the remaining being distributed between Chinese 2.2%, Indo-Pakistani 4.8%, Black 3.6%, Mediterranean African 3.9%, Slavic 4.3%, South American 2.1%, Romanian 3.6%, Unknown 0.2%), their mean age was 31 ± 5 (range 16–45), and overall 53% were at their first or second pregnancy, 32% at the third one, the remaining 15% being distributed to up to six previous pregnancies. They were evaluated at a mean of 1.5 ± 1 day post partum (range 0–11). Relevant demographic, anthropometric and biological variables are reported in Table 1.
Table 1.
Basal demographic, anthropometric and biological variables
| Variable | N./Available cohort | % | Mean | SD |
|---|---|---|---|---|
| History of migraine | 292/850 | 34.4 | ||
| History of hypertension | 35/863 | 4.1 | ||
| History of diabetes | 39/869 | 4.5 | ||
| Hystory of dyslipidaemia | 30/850 | 3.5 | ||
| Active smoking | 145/900 | 16.1 | ||
| Alcohol (≥2 drinks) | 9/900 | 1.0 | ||
| Ergot during delivery | 500/900 | 55.6 | ||
| BMI | 892/900 | 98.8 | 22 | 4 |
| Gestational age (weeks) | 892/900 | 98.8 | 39 | 2 |
| Systolic BP mmHg | 829/900 | 92.1 | 110 | 12 |
| Diastolic BP mmHg | 829/900 | 92.1 | 68 | 9 |
| Albuminuria (mg/dl) | 769/900 | 85.4 | 7.8 | 19.6 |
| Hb (g/L) | 758/900 | 85.4 | 11.3 | 1.3 |
N: number of women; Hb: haemoglobin.
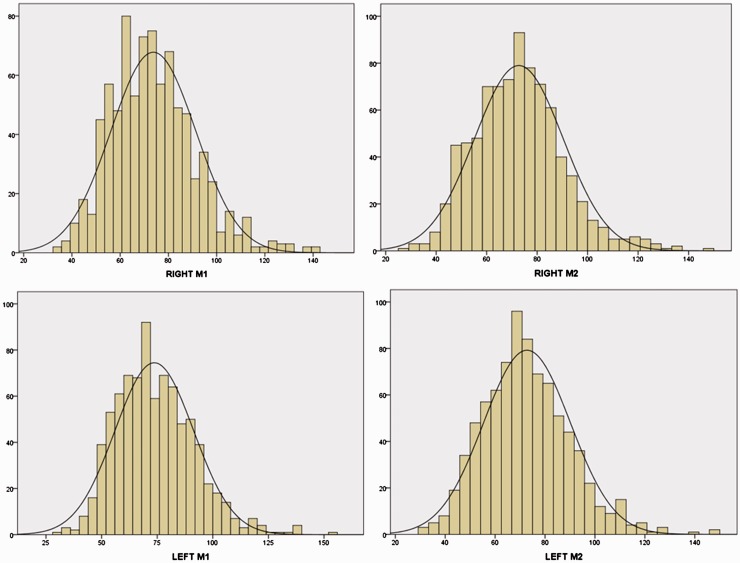
On basal TCCS assessment, flow velocities were overall symmetrically and evenly distributed (see Figure 3) and in the upper range of normal values (right M1 and M2 74 ± 17 and 72 ± 17 cm/second, respectively; left M1 and left M2 73 ± 17 and 72 ± 17 cm/second, respectively). Neither right vs. left M1 MFV nor right vs. left M2 MFV were statistically different from each other (p = 0 .935 and p = 0 .717 respectively), as well as M1 vs. M2 MFV on both right and left sides (p = 0 .092 and p = 0.117, respectively). A total of 136 subjects (12.1%) exceeded in at least one measurement the threshold of 100 cm/second, but LI was consistently < 3 in all of them, thus suggesting that velocity increase was not caused by intracranial vasospasm. As the four velocity values recorded for each patient were not statistically different from each other, we computed a single averaged MFV value for each patient as the mean of right and left M1 and M2. Averaged MFV negatively correlated with age (Pearson’s coefficient = −0.80, p = 0.020), parity (Pearson’s coefficient = −0.171, p < 0.0001) and with haemoglobin level (Pearson’s coefficient = −0.256, p < 0.0001). History of migraine did not influence averaged MFV (74 ± 14 cm/second in subjects with vs. 72 ± 14 in those without migraine, p = 0.076), nor did administration of ergometrine during labour (73 ± 14 cm/second when ergometrine was used vs. 74 ± 14 when it was not used, p = 0.182).
Figure 3.
Distribution of velocities in the right (upper panel) and left (lower panel) proximal (M1) and distal (M2) segments of middle cerebral artery. The black line depicts the normal distribution. Horizontal axis: velocity in cm/second. Vertical axis: frequency as number of subjects.
Looking at the raw data, we noticed that averaged MFV showed an abrupt drop in women of >1 parity (see supplementary Table 1), therefore we dichotomously regrouped parity in 0–1 and >1 (see Supplementary Table 2). Averaged MFV was significantly higher in 0–1 than in >1 parity women (76 ± 14 vs. 70 ± 12 cm/second respectively, p < 0.0001).
Comparing subjects with averaged MFV ≥ 100 cm/second with those below for anthropometric (age, height, weight, BMI, ABP) and biological (haemoglobin, albuminuria, uric acid) variables, haemoglobin (Hb) level was significantly lower in patients with MFV ≥ 100 cm/second (10.1 ± 2 vs. 11.4 ± 1.3 g/l, respectively – p < .0001 on t-test).
Age, parity (dichotomously defined) and haemoglobin level were entered as predictors in a logistic regression analysis where averaged MFV > 100 cm/second was the dependent variable and in a linear regression analysis where MFV was the dependent variable. In both analyses, parity and haemoglobin levels remained significant predictors whereas age did not (Table 2).
Table 2.
Regression analysis
| Logistic regression analysis. Averaged MFV >100 cm/second as a dependent variable |
Linear regression analysis. Averaged MFV as a dependent variable |
|||||||
|---|---|---|---|---|---|---|---|---|
| 95% C.I. for O.R. |
95% C.I. for O.R. |
|||||||
| O.R. | Lower | Upper | Sign. | O.R. | Lower | Upper | Sign. | |
| Age | 1.011 | 0.968 | 1.055 | 0.622 | −0.025 | −0.264 | 0.129 | 0.498 |
| Haemoglobin | 0.728 | 0.627 | 0.845 | 0.0001 | −0.243 | −3.153 | −1.740 | 0.0001 |
| Parity | 0.443 | 0.279 | 0.705 | 0.001 | −0.178 | −6.913 | −2.892 | 0.0001 |
MFV: mean flow velocity; O.R: odds ratio; C.I.: confidence interval; Sign: level of significance.
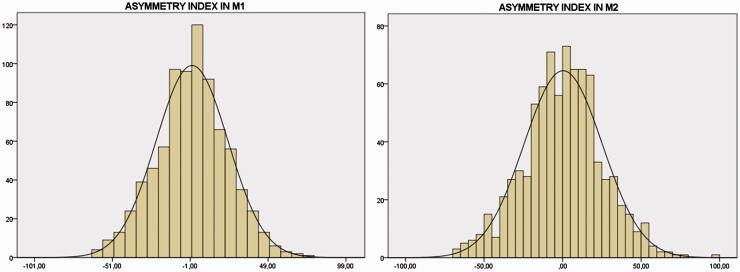
Interhemispheric asymmetry indexes were also distributed along a normal curve in both proximal and distal segments of MCA (see Figure 4). Mean values were 0.25 ± 23 in M1 and 0.45 ± 25 in M2 (p = 0.86). Likewise, on both sides proximal to distal asymmetry indexes were 1.6 ± 25 and 1.8 ± 25 in right and left MCAs, respectively (p = 0.85).
Figure 4.
Distribution of asymmetry indexes in proximal (M1) and distal (M2) segments of MCA. The black line depicts the normal distribution. Horizontal axis: non-dimensional index of asymmetry as calculated according to Zanette’s formula14: positive cases denote higher velocity in the right side MCA, negative cases denote higher velocity in the left side MCA. Vertical axis: frequency as number of subjects.
Discussion
The aim of the present study was to assess normal values and variability of the MFV of middle cerebral arteries in a large cohort of consecutive women in the first post-delivery week. We deliberately chose to limit the assessment to this artery because it carries almost 80% of the entire cerebral blood flow, its straight course allows prompt identification and assessment of the true blood flow velocity, and reproducibility of measurements is high.12,13 During pregnancy, MFV in MCA progressively decreases from the first to the third trimester.8–10 This change, assuming intact cerebral autoregulation, has been attributed to oestrogen-mediated vasodilatation of brain arteries.10 Following delivery, MFV progressively and quickly increases in the first post partum week, to decrease thereafter to pre-gestational levels by the 40th day post partum.10 Therefore, it is important to establish the upper limit of normality when MFV peaks at its maximum, as further increases may indicate a pathological vasoconstriction of brain vessels such as that occurring in pre-eclampsia or in the so-called post partum angiopathy manifesting as thunderclap headache.19–22
So far, studies assessing the variations of hemodynamic parameters in puerperium have relied on normative data derived from very small cohorts of control subjects, which partly explains the conflicting findings when comparing velocities in normal versus pre-eclamptic and eclamptic patients.3,8,9
To overcome this shortcoming, we studied 900 consecutive women at an average of 1.5 days post-partum after rigorously excluding all cases of suspected pre-eclampsia and were thus able to establish the mean and the range of velocity values in both the proximal and the distal segments of middle cerebral arteries of both sides, as well as the side-to- side asymmetry and proximal-to-distal asymmetry in each vessel.
MFV values were normally distributed and mean values symmetrical in both the proximal and distal segments. Absolute values compared well with the findings of the literature. Sanchez-Arjona et al. found a single value of 73.1 ± 14.33 cm/second and 75.68 ± 15.84 cm/second in right and left MCAs, respectively, in a sample of 100 women (aged 30 ± 6 years) assessed within five days (75% between 1 and 3) of delivery.23 Serra-Serra et al. reported an averaged value of 74.2 ± 10.5 cm/second on day 3 post delivery in 21 women aged 28.8 ± 4.5.10 Normative data in non-puerperal age-matched women are available in Liboni et al.: these authors found values of 72.15 ± 6.37 cm/second in the left and 70.68 ± 6.79 cm/second in the right MCA in a sample of 34 subjects aged 20–34.24
From the limited evidence available, it thus seems that the values in early post partum are slightly higher than in age-matched non-puerperal subjects. The reasons for this difference are probably multiple. A mild vasoconstriction caused by the abrupt drop in the oestrogen level as well as variations in haemodilution has been advocated as a putative mechanism.10,23 However, our findings indicate that the most important factor underlying the increased average MFV is haemoglobin level. In patients with at least one MFV value > 100 cm/second, as well as in those with averaged MFV > 100 cm/second, haemoglobin levels were significantly lower than in subjects with MFV ≤100 cm/second, and overall averaged MFV velocity was highly correlated with Hb. Since MFV as measured by transcranial Doppler is known to be negatively correlated with haematocrit,25 the most obvious explanation for the raised velocities is the blood loss related to delivery. It is important to have this finding in mind when using absolute values to classify normal vs. abnormal values, as the threshold of 100 cm/second is commonly held as the cut-off value for intracranial stenosis.15–17 In our cohort, 12.1% of patients exceeded that threshold in at least one measurement, and a similar proportion (10%) has been reported by Sanchez-Arjona et al.,23 but in our subjects Lindegaard Index, which compares mean flow velocities in ipsilateral MCA and ICA, was consistently <3 in all cases with MF >100 cm/second hereby excluding intracranial vasospasm as the underlying mechanism.
Age was the second variable which correlated with MFV in the univariate analysis. This relationship has been repeatedly reported but on a wider age range.24–27 In our cohort, the age range was quite narrow (16–45 years) with the 5th percentile at 23 and the 95th at 39, and age was no longer significant as independent predictor in the regression analysis. On the other hand, parity was strongly correlated with MFV (see Supplementary Figure 1) and remained a significant predictor of lower velocities in the regression analysis. Since parity was strongly correlated with age, the higher the parity the higher the age (see Supplementary Table 3 and Supplementary Figure 2), it appears that the rise in MFV following delivery is somehow related to previous gestational burden, being progressively dampened as the number of previous pregnancies increases. Whether this occurs because of a reduced sensitivity of brain vessels to hormonal changes or because of a reduced adrenergic discharge following delivery or for other reasons cannot be settled at present and could be matter for future studies.
One finding which is not available in the literature, to the best of our knowledge, is the degree of side-to-side and proximal-to-distal asymmetry in the examined vessels.
MFV showed a trend to decrease non-significantly from M1 to M2 on both sides as would be expected after the takeoff of penetrating arterioles between the proximal and distal portion of the main trunk. Our data show that up to about 50% reduced MFV in distal as compared to proximal segment may be normal, probably depending on the variable amount of blood flow diverted to basal penetrators.
Side-to-side asymmetry is currently being used to diagnose distal branch occlusion, following the seminal observations of Zanette et al. who established a cutoff threshold of 21%, based on the maximum asymmetry detected in a sample of 60 healthy people.14
Our findings in a much larger cohort of women indicate that this threshold has to be elevated to ± 48% for M1 and ± 50% for M2 segments (2 SD from the mean), as is shown in Figure 4, the plus or minus sign depending on whether MFV is higher in the right or left MCA, respectively. This, of course, applies only to the specific condition of early post-delivery state. The reason why in this particular condition interhemispheric asymmetries are so much expanded is unknown at the moment. One can only speculate that the sensitivity of brain vessels to the regulatory mechanism of vascular tone may become asymmetrical in some individuals in the early post-delivery period and hence a functional asymmetry in vessel diameter takes place. This effect is expected to progressively fade away and interhemispheric asymmetry returns within narrower limits in the following weeks.
According to the protocol of the present study, a follow-up TCCS assessment was not routinely performed unless indicated on clinical grounds. Therefore, we were able to monitor the evolution of early interhemisheric asymmetry in only 12 subjects. In these patients, M1 asymmetry index was 0.8 ± 28 on the first assessment and 1.1 ± 5 on the follow-up assessment performed one month later (data not shown). These findings need to be taken very cautiously given the small sample size, but are consistent with the idea that early post-delivery asymmetry may represent a functional transient imbalance in the brain vessel sensitivity to regulatory stimuli.
Merits of the present study are the prospective nature, the large cohort size and the systematic investigation of all included subjects.
The main limit of the study was that it was based on a single centre experience.
In conclusion, our study indicates that in the early post partum state MFV in MCA of healthy subjects may exceed the threshold of 100 cm/second with no evidence of intracranial spasm, most probably as the result of the blood loss during delivery. Furthermore, MFV tends to be independently correlated with parity. Right-to-left MFV asymmetry may reach 50% as a consequence of a transient imbalance in vascular tone regulation.
Supplementary Material
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research authorship and/or publication of this article: GPA received a liberal grant from AFaR (Associazione Fatebenefratelli per la Ricerca).
Ethical Approval
The Ethics Committe of S. Orsola Hospital approved the study.
Guarantor
GPA
Contributors
GPA conceived the study, did the statistical calculations and wrote the first draft. RB provided the logistical setup for data collection; MC performed the neuroradiological investigations; AP and STR supervised the research assistants; AG, MPP, GT, SO, FR, EP collected the data.
Supplementary material
Supplementary material for this paper can be found at http://ult.sagepub.com/doi/suppl/10.1177/1742271X17690942
References
- 1.Akhter T, Larsson A, Larsson M, et al. Artery wall layer dimensions during normal pregnancy: a longitudinal study using noninvasive high-frequency ultrasound. Am J Physiol Heart Circ Physiol 2013; 304: H229–H234. [DOI] [PubMed] [Google Scholar]
- 2.Skeik N, Porten BR, Kadkhodayan Y, et al. Postpartum reversible cerebral vasoconstriction syndrome: review and analysis of the current data. Vasc Med 2015; 20: 256–265. [DOI] [PubMed] [Google Scholar]
- 3.Demarin V, Rundek T, Hodek B. Maternal cerebral circulation in normal and abnormal pregnancies. Acta Obstet Gynecol Scand 1997; 76: 619–624. [DOI] [PubMed] [Google Scholar]
- 4.Williams K, Wilson S. Persistance of cerebral hemodynamic changesin patients with eclampsia: a report of three cases. Am J Obstet Gynecol 1999; 181: 1162–1165. [DOI] [PubMed] [Google Scholar]
- 5.Del Zotto E, Giossi A, Volonghi I, et al. Ischemic stroke during pregnancy and puerperium. Stroke Res Treat 2011; 2011: 6060780–6060780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantu-Brito C, Arauz A, Aburto Y, et al. Cerebrovascular complications during pregnancy and postpartum: clinical and prognosis observations in 240 Hispanic women. Eur J Neurol 2011; 18: 819–825. [DOI] [PubMed] [Google Scholar]
- 7.Block HS. Neurological complications of pregnancy. Curr Neurol Neurosci Rep 2016; 16: 67–67. [DOI] [PubMed] [Google Scholar]
- 8.Sherman RW, Bowie RA, Henfrey MME, et al. Cerebral haemodynamics in pregnancy and pre-eclampsia as assessed by transcranial Doppler ultrasonography. Br J Anaesth 2002; 89: 687–692. [PubMed] [Google Scholar]
- 9.Williams K, Galerneau F. Maternal transcranial Doppler in pre-eclampsia and eclampsia. Ultrasound Obstet Gynecol 2003; 21: 507–513. [DOI] [PubMed] [Google Scholar]
- 10.Serra-Serra V, Kyle PM, Chandran R, et al. Maternal middle cerebral artery velocimetry in normal pregnancy and postpartum. Br J Obstet Gynaecol 1997; 104: 904–909. [DOI] [PubMed] [Google Scholar]
- 11.von Reutern GM, Budingen HJ. Ultrasound diagnosis in cerebrovascular disease, Stuttgrat: Georg Thieme, 1993. [Google Scholar]
- 12.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg 1982; 57: 769–774. [DOI] [PubMed] [Google Scholar]
- 13.Bartels E. Color-coded duplex ultrasonosgraphy of the cerebral vessels – atlas and manual, Stuttgart: Schattauer, 1999. [Google Scholar]
- 14.Zanette EM, Fieschi C, Bozzao L, et al. Comparison of cerebral angiography and transcranial Doppler sonography in acute stroke. Stroke 1989; 20: 899–903. [DOI] [PubMed] [Google Scholar]
- 15.Felberg RA, Christou I, Demchuk AM, et al. Screening for intracranial stenosis with transcranial Doppler: the accuracy of mean flow velocity thresholds. J Neuroimag 2002; 12: 1–6. [DOI] [PubMed] [Google Scholar]
- 16.Navarro JC, Lao AY, Sharma VK, et al. The accuracy of transcranial Doppler in the diagnosis of middle cerebral artery stenosis. Cerebrovasc Dis 2007; 23: 325–330. [DOI] [PubMed] [Google Scholar]
- 17.Zhao L, Barlinn K, Sharma VK, et al. Velocity criteria for intracranial stenosis revisited an international multicenter study of transcranial Doppler and digital subtraction angiography. Stroke 2011; 42: 3429–3434. [DOI] [PubMed] [Google Scholar]
- 18.Lindegaard KF, Nornes H, Bakke SJ, et al. Cerebral vasospasm diagnosis by means of angiography and blood velocity measurements. Acta Neurochirurgica 1989; 100: 12–24. [DOI] [PubMed] [Google Scholar]
- 19.Headache classification subcommittee of the International Headache Society. The international classification of headache disorders. Cephalalgia 2004; 24: 1–160. [Google Scholar]
- 20.Calabrese LH, Dodick DW, Schwedt TJ, et al. Narrative review: reversible cerebral vasocontriction syndromes. Ann Intern Med 2007; 146: 34–44. [DOI] [PubMed] [Google Scholar]
- 21.Ducros A. Reversible cerebral vasoconstriction syndrome. Lancet Neurol 2012; 11: 906–917. [DOI] [PubMed] [Google Scholar]
- 22.Fugate JE, Amerisio SF, Ortiz G, et al. Variable presentations of postpartum angiopathy. Stroke 2012; 43: 670–676. [DOI] [PubMed] [Google Scholar]
- 23.Sánchez-Arjona MB, Franco-Macías E, Casado-Chacón JL, et al. Velocimetría Doppler transcraneal en puérperas normotensas. Rev Neurol 2003; 36: 101–104. [PubMed] [Google Scholar]
- 24.Liboni W, Allais G, Mana O, et al. Transcranial doppler for monitoring the cerebral blood flow dynamics: normal ranges in the Italian female population. Panminerva Med 2006; 48: 187–191. [PubMed] [Google Scholar]
- 25.Brass LM, Pavlakis SG, DeVivo D, et al. Transcranial Doppler measurements of the middle cerebral artery. Effect of hematocrit. Stroke 1988; 19: 1466–1469. [DOI] [PubMed] [Google Scholar]
- 26.Grolimund P, Seiler RW. Age dependence of the flow velocity in the basal cerebral arteries—a transcranial Doppler ultrasound study. Ultrasound Med Biol 1988; 14: 191–198. [DOI] [PubMed] [Google Scholar]
- 27.Tegeler CH, Crutchfield K, Katsnelson M, et al. Transcranial Doppler velocities in a large, healthy population. J Neuroimag 2013; 23: 466–472. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.