Abstract
Purpose
This study examined the effects of chin-down swallowing on laryngeal vestibule closure. It also investigated the technique’s rehabilitative impact, by assessing the stability of effects across multiple trials and aftereffects in neutral swallows on cessation of the technique.
Method
Duration of laryngeal vestibule closure (dLVC) was measured with videofluoroscopy in 16 healthy participants (mean = 33.2 years, 9 men). Participants swallowed 40 times: 5 head-neutral swallows (N1), then 30 chin-down swallows, followed by 5 head-neutral swallows (N2). The first 5 chin-down swallows were categorized as early posture swallows (P1) and the last 5 as late posture swallows (P2). Within-participant comparisons determined the effects of the maneuver on dLVC during and after execution.
Results
The study found that dLVC increased during chin-down swallows (N1 to P1, p = .018). This increase remained stable throughout 30 repetitions (P1 to P2, p = .994). On return to neutral, dLVC returned to baseline (N1 to N2, p = .875).
Conclusions
This study demonstrated increased dLVC during chin-down swallowing, offering a possible mechanism responsible for previously reported reduced aspiration during the technique. As aftereffects were not evident after multiple chin-down swallows, the maneuver appears to offer more compensatory benefit than rehabilitative value for patients with dysphagia.
Keywords: dysphagia, physiology, response to intervention, stroke, swallowing
The chin-down posture is widely used to reduce aspiration and has been shown to be effective in various dysphagia populations (Ekberg, 1986; Lewin, Hebert, Putnam, & DuBrow, 2001; Logemann et al., 2008; Nagaya, Kachi, Yamada, & Sumi, 2004; Rasley et al., 1993; Solazzo et al., 2011; Terre & Mearin, 2012). However, the specific mechanisms responsible for improved airway protection remain unclear. The technique is hypothesized to widen the valleculae (Ekberg, 1986; Logemann, 1983) and place the epiglottis in a more protective position over the laryngeal inlet (Logemann, 1998). The theory behind improved airway protection is that the increase in vallecular capacity allows an increased window of time during which pharyngeal swallow response can be elicited (Logemann, 1998), thereby providing a “holding bay” for the bolus during the lapse of time between bolus entry into the pharynx and swallow initiation. However, the two studies that have looked at onset of the pharyngeal swallowing response with chin down have found that this measure remains unchanged (Bulow, Olsson, & Ekberg, 2001; Shanahan, Logemann, Rademaker, Pauloski, & Kahrilas, 1993). Moreover, the size of the valleculae (Welch, Logemann, Rademaker, & Kahrilas, 1993) is reportedly unaffected by the posture. In addition, there are discrepant findings regarding the changes in pharyngeal and laryngeal structures associated with the technique (Shanahan et al., 1993; Welch et al., 1993). These findings suggest that the theory of increased vallecular capacity and subsequent increased window of time for pharyngeal swallow initiation may not be an accurate explanation of the mechanism(s) by which airway protection is improved during chin-down swallowing.
The primary defense for prevention of airway invasion during swallowing is laryngeal vestibule closure (LVC), which is achieved when the hyoid and larynx approximate (Fink, Martin, & Rohrmann, 1979; Logemann et al., 1992). Specifically, Fink (1976) explains that approximation of these two structures results in compression of the median thyrohyoid tissue, which bulges backward as a result, closing the laryngeal entrance. Bulow and colleagues (Bulow, Olsson, & Ekberg, 1999, 2001) have shown that the hyoid bone and larynx are positioned closer together at rest as well as during swallowing, for both healthy subjects and those with dysphagia, during the chin-down position. Thus, reduced aspiration during chin-down swallowing (Ekberg, 1986; Lewin et al., 2001; Logemann et al., 2008; Nagaya et al., 2004; Rasley et al., 1993; Solazzo et al., 2011; Terre & Mearin, 2012) could occur as a result of anatomical alterations to the hyolaryngeal complex, that is, greater compression of the median thyrohyoid tissue while in the chin-down position. However, no studies have investigated LVC kinematics during chin-down swallowing. Knowledge of the specific physiologic components that are influenced by chin-down swallowing is crucial for appropriate clinical prescription of this intervention to physiologic abnormalities.
Another area of uncertainty is the stability of airway protection effects across multiple repetitions of chin-down swallowing. It has recently been shown that swallowing kinematics can gradually change over repeated trials of a novel swallowing task (Humbert et al., 2013; Humbert, Lokhande, Christopherson, German, & Stone, 2012). This suggests that such progressive changes may occur during other repetitive novel swallowing tasks, possibly related to some type of adaptation. Conversely, the kinematics of typical oropharyngeal swallowing behaviors do not exhibit gradual adaptation but rather remain stable across multiple executions (Humbert et al., 2012). It is unknown whether chin-down swallowing provides an experience novel enough to induce such gradual adaptations or whether the technique merely mimics typical swallowing behaviors. Existing investigations of chin-down swallowing have typically included only two (Nagaya et al., 2004; Shanahan et al., 1993; Welch et al., 1993) to six (Castell, Castell, Schultz, & Georgeson, 1993) repetitions of the posture, which may be insufficient to determine the presence of progressive kinematic changes, if any exist. Furthermore, trials are usually averaged to minimize variance in statistical analyses. Observing effects over multiple repetitions of chin-down swallowing will elucidate whether modifications are stable or adapt with continued execution.
In addition to the possibility of adaptation during chin-down swallowing, it is also unknown whether multiple repetitions of chin-down swallowing can influence neutral-position swallows that occur immediately after. Humbert and colleagues (2013) have raised new considerations for investigating aftereffects of swallowing treatments. They showed that compared with pretreatment swallowing, posttreatment regular swallows had different hyolaryngeal kinematics as a result of adaptations that occurred during the treatment task. These observed aftereffects cannot be not due to changes in strength given the short time frame in which they occur but rather reflect modifications that occur because of adaptations or learning processes. As it is unclear what characteristics are required to induce these changes, investigation of other therapies beyond the phase of execution is required.
The goals of this investigation were to examine the laryngeal closure kinematic mechanisms that are affected by chin-down swallowing (Question 1), to determine whether effects of chin-down swallowing are stable across multiple trials of the maneuver (Question 2), and to investigate after-effects in neutral swallows on cessation of the technique (Question 3). In response to Question 1, we hypothesized that the duration of laryngeal vestibule closure will increase during chin-down swallowing, while the time to laryngeal vestibule closure will decrease, on the basis of changes in hyolaryngeal position previously reported by Bulow et al. (1999, 2001) and the effect of these anatomical approximations on laryngeal vestibule closure (Fink, 1976). In response to Question 2, we hypothesized that the effects of chin-down swallowing on laryngeal vestibule closure kinematics will remain stable throughout multiple repetitions, on the basis of the fact that the hyoid and larynx will remain approximated throughout trials. On Question 3, we speculated that no aftereffects will be evident for LVC kinematics, on the basis of the assumption that effects of the maneuver would be a result of anatomic adjustments, which would not be evident once posture was returned to neutral.
Method
Participants
Sixteen healthy participants (mean = 33.2 years, range = 21–54 years; 9 men) were recruited for this study by responding to flyers advertising the study. Participants were from the general public and had no prior knowledge or experience of the chin-down maneuver. Participants reported a negative history of swallowing, speech, voice problems, and neurological disease. The age range of participants is appropriate for investigations of healthy swallowing, as indicated by previous research showing that age-related changes in swallowing physiology are not apparent until 60–70 years of age (Fei et al., 2013; Feng et al., 2013; Robbins et al., 2005; Robbins, Levine, Wood, Roecker, & Luschei, 1995). The local institutional review board approved procedures. All participants signed written informed consent. Data were collected by two of the authors.
Procedure
Participants were briefed on all procedures prior to initiation of the study. A flexible tube was taped to participants’ chins, with the tip of the tube positioned in the anterior oral cavity. Premeasured thin liquid barium (EZEM Varibar) boluses of 5 ml were delivered through this tube via syringes, which were manually connected to the other end of the tube by the researcher. The use of a flexible tube was considered necessary to eliminate the need for modifications of head position once the participant achieved both head-neutral and chin-down positions. It also alleviated the need for the researcher’s hand to be in the line of radiation from the fluoroscopy field of view. Participants were seated upright in view of a laptop monitor. A timed PowerPoint presentation was started at initiation of the study. The presentation visually counted down from 5, after which a visual and audio cue prompted the participant to swallow. All screens of the PowerPoint presentation were paired with audio cues so that when visual cues were inaccessible during chin-down swallowing, audio cues provided the swallow prompt. The “swallow” screen remained in view for 2 s, following which the countdown from 5 began again. The interswallow interval was approximately 7 s. Liquid barium was infused into the mouth over 2 s (between Screens 3 and 1). Participants were instructed to hold the bolus in their mouth until the “swallow” screen with the audio cue occurred. This allowed videofluoroscopic recordings to be timed with the swallow.
Before the start of the study, a practice run of two neutral swallows, two chin-down swallows, and two postposture neutral swallows was completed with water and without videofluoroscopy to ensure participants were familiar with the procedures. Neutral swallows were completed with the head in neutral position, as for typical swallowing. For the study, participants swallowed 5 ml of liquid barium a total of 40 times across three stages: Stage 1, neutral position (5 swallows); Stage 2, chin-down position (30 swallows); and Stage 3, neutral position (5 swallows). Thirty swallows was estimated to be a sufficient number to show gradual changes, if any were present, as previous studies showing such adaptations have included 26 (Humbert et al., 2013). A positioning audio cue occurred to indicate when to begin chin-down swallows (just before Stage 2), at which time the participant was instructed to tuck his or her chin to the chest (see Figure 1 for schematic of study design). The positioning audio cue was repeated to signal the return to neutral swallows (just before Stage 3). During the chin-down swallows (Stage 2), head angle was not controlled, as execution of the maneuver was intended to mimic clinical prescription. It has been shown that large participant variation occurs in the execution of chin-down posture with a simple instruction (Steele, Hung, Sejdic, Chau, & Fraser, 2011). This variance is important to consider when investigating the effects of the maneuver. In addition, more stringent methods were not considered necessary as Steele et al. (Steele et al., 2011) reported neck flexion of healthy participants to be around 19 degrees when provided with similar generic instructions, an angle larger than deemed necessary for observing clinical benefit (Welch et al., 1993). However, prior to the initiation of the practice run, participants were shown how to tuck their chins with minimal forward neck movement so as to achieve chin-down rather than head-forward posturing. Participants maintained the chin-down posture throughout the entire duration of Stage 2 so that no spontaneous swallows occurred in the neutral position. Participant posture was monitored by the investigators throughout data acquisition, and if movement from initial chin-down position was noted, the researcher instructed the participant to keep the chin tucked to their chest. The audio cue associated with the “swallow” screen was used to prompt swallowing during this time.
Figure 1.
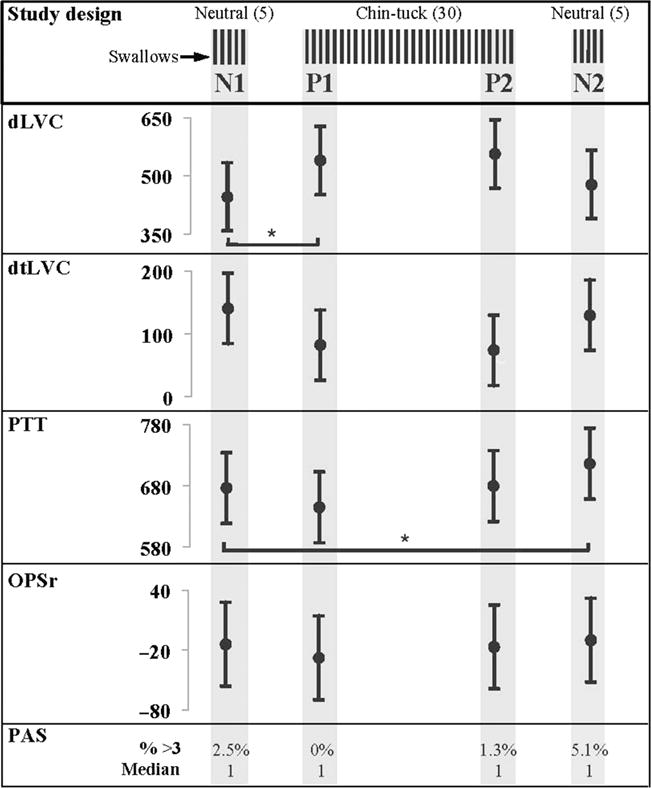
Study design includes 5 neutral swallows (N1), 30 chin-down swallows (first 5 representing P1, last 5 representing P2), and 5 post-chin-down neutral swallows (N2). Estimated means and 95% confidence intervals at each stage are provided for duration of laryngeal vestibule closure (dLVC), duration to laryngeal vestibule closure (dtLVC), pharyngeal transit time (PTT), and onset of the pharyngeal swallow response (OPSr). All values are in milliseconds. Statistical significance at the alpha level of .05 is indicated by an asterisk and based on Sidak-adjusted p values. Penetration–Aspiration Scale (PAS) scores are presented as a percentage of Penetration–Aspiration Scale scores in each stage of 3 and above, and median PAS scores at each stage are provided as well.
Videofluoroscopy
All swallows were recorded with continuous videofluoroscopy, acquired in the sagittal plane with a video capture rate of 30 frames per second. Image sequences were viewed in real time on a monitor and exported to an image processing system, where they were archived for off-line analysis. The field of view included the oral cavity, pharynx, laryngeal vestibule, subglottal air column, and upper esophagus, including the upper esophageal sphincter. A simultaneously recorded time code facilitated data analysis.
Data Analysis
Each swallowing clip was assigned a code for randomization during analysis by a laboratory technician. Two experienced investigators completed frame-by-frame blinded analysis of swallowing kinematics.
Measures
Duration of laryngeal vestibule closure and duration to laryngeal vestibule closure (described below) were used to investigate laryngeal vestibule closure kinematics. We also included bolus flow through the pharynx to supplement our measures of laryngeal vestibule closure (LVC) kinematics. This is based on the fact that changes in duration of bolus flow through the pharynx influence duration of LVC (Power et al., 2007). Finally, we measured the onset of the initiation of the pharyngeal swallow response, as chin-down swallowing is thought to provide increased time in which a pharyngeal swallow response can be safely initiated (Logemann, 1998). All measures are described below.
Duration of laryngeal vestibule closure (dLVC)
Airway protection can be quantified in a number of ways. Duration of LVC provides a direct assessment of laryngeal closure kinematics and is commonly used in investigations of airway protection (Logemann et al., 1992; Park, Kim, Ko, & McCullough, 2010; Power et al., 2007). Duration of LVC was defined as the duration of contact between the arytenoids and epiglottis base during the swallow (Bisch, Logemann, Rademaker, Kahrilas, & Lazarus, 1994; Logemann et al., 1992; Park et al., 2010; Power et al., 2007).
Duration to laryngeal vestibule closure (dtLVC)
The timing of LVC initiation is important for assessing the risk of airway compromise (Park et al., 2010). Therefore, we also measured the duration to laryngeal vestibule closure (dtLVC), defined as the time between initiation of the anterior-superior burst of the hyoid related to the pharyngeal swallow response and the first frame of LVC.
Pharyngeal transit time (PTT)
PTT was defined as the time between entry of the bolus into the pharynx (past the ramus of the mandible) and clearance of the bolus past the lowest visible vertebra, similar to pharyngeal transit duration and “PTT” used in previous investigations (Lof & Robbins, 1990; Logemann et al., 1992; Power et al., 2007). The reference vertebra was kept consistent within participants.
Onset of the pharyngeal swallow response (OPSr)
We defined OPSr as the time between entry of the bolus into the pharynx (past the ramus of the mandible) and initiation of the anterior-superior hyoid burst (Lof & Robbins, 1990), similar to pharyngeal dissociation (Bulow et al., 2001) and pharyngeal swallow delay (Shanahan et al., 1993) reported in previous studies.
Functional airway protection measure
The Penetration–Aspiration Scale (PA; Rosenbek, Robbins, Roecker, Coyle, & Wood, 1996) was used to document instances of penetration and aspiration for all swallows. Each participant’s data were categorized into the following blocks: the first 5 neutral swallows (Stage 1) were used to reflect preposture neutral or baseline swallowing kinematics (N1). During Stage 2, the first 5 (of 30) chin-down swallows were used to show early posture kinematics (P1). The last 5 (of 30) chin-down swallows represented late posture kinematics (P2) (Figure 1). Question 1 (do LVC kinematics change with chin-down swallowing?) was assessed by comparing N1 and P1. Question 2 (do LVC kinematics change across multiple trials of chin-down swallowing?) was assessed by comparing P1 and P2. Question 3 (are aftereffects seen on return to neutral swallows after multiple chin-down swallows?) was tested by comparing N1 and N2.
Statistical Analysis
Using SPSS (Version 19), linear mixed-effects models (Gelman & Hill, 2007) were used to estimate the effects of chin-down posturing on each measure. To model each measure, there was a fixed effect of stage (N1, P1, P2, N2), with the intercept and effect of stage allowed to vary be-tween participants. Values from the five swallows at each stage were included from each participant. Confidence intervals were calculated for the estimated effects to indicate the degree of uncertainty in the estimation of the parameters. Pairwise comparisons generated by the software were used in the case of statistically significant main effects. The Sidak method was used to correct for multiple comparisons. The software outputs adjusted p values using this method, so that any p value less than .05 after adjustments can be considered statistically significant. Statistical analyses were performed on the raw data. Interrater reliability (20% of the data) and intrarater reliability (5% of the data) were analyzed using single-measure intraclass correlation coefficients (ICCs).
Results
A total of 315 (of a possible 320) swallows were included in the analyses: 80 swallows in N1, 79 swallows in P1, 78 swallows in P2, and 78 swallows in N2. Five swallows were excluded because of poor image contrast. All participants complete the study without adverse effects.
Inter- and intrarater reliability measured with ICCs showed excellent agreement between raters (≥.91) and within the same rater (≥.89) (see Table 1). There was a main effect of stage on dLVC, F(3, 44.34) = 6.04, p = .002; dtLVC, F(3, 45) = 3.86, p = .015; and PTT, F(3, 43.68) = 8.40, p = .000. There was no evidence of an effect of stage on OPSr, F(3, 45.11) = 0.66, p = .584; therefore, pairwise comparisons were not considered for this measure. Figure 1 shows estimated means and 95% confidence intervals for all measures at each stage. Table 2 provides estimated effects, 95% confidence intervals of the effects, and Sidak-adjusted p values for all measures. Note that OPSr is not included in Table 2 because of a nonsignificant main effect of stage.
Table 1.
Interrater reliability and intrarater reliability expressed as single-measure intraclass correlation coefficients.
| Measure | Interrater | Intrarater |
|---|---|---|
| dLVC | .97 | .98 |
| dtLVC | .93 | .89 |
| PTT | .93 | .90 |
| OPSr | .91 | .90 |
Table 2.
Estimated effects and 95% confidence intervals for N1 to P1, P1 to P2, and N1 to N2 comparisons for dLVC, dtLVC, and PTT.
| Measure and comparison | Lower (ms) |
Estimate (ms) |
Upper (ms) |
p |
|---|---|---|---|---|
| dLVC | ||||
| N1 to P1 | 11 | 94 | 176 | .018 |
| P1 to P2 | −65 | 17 | 99 | .994 |
| N1 to N2 | −50 | 32 | 114 | .875 |
| dtLVC | ||||
| N1 to P1 | −125 | −58 | 8 | .111 |
| P1 to P2 | −75 | −8 | 58 | 1.000 |
| N1 to N2 | −77 | −11 | 55 | .998 |
| PTT | ||||
| N1 to P1 | −70 | −32 | 7 | .166 |
| P1 to P2 | −4 | 35 | 74 | .099 |
| N1 to N2 | 0 | 40 | 79 | .046 |
Note. Pairwise comparisons were not considered for OPSr, as the main effect of stage was not significant. N1 to P1 comparisons correspond to Question 1 (effects of chin-down swallows); P1 to P2 comparisons correspond to Question 2 (stability of effects); and N1 to N2 comparisons correspond to Question 3 (aftereffects of chin-down swallows).
Question 1: Are LVC Kinematics Affected by Chin-Down Swallowing?
Duration of LVC increased during chin-down swallowing by an estimated 94 ms (95% CI [11, 176], p = .018, Cohen’s d = −0.55). Chin-down swallowing did not affect dtLVC (p = .111) or PTT (p = .166).
Question 2: Are Effects of Chin-Down Swallowing Stable Over Multiple Repetitions?
The comparison of P1 and P2 shows no evidence of change for dLVC across multiple trials of chin-down swallowing (p = .994). Therefore, the increase in dLVC remains stable throughout 30 chin-down swallows. Duration to LVC and PTT also do not change across multiple chin-down swallows (dtLVC, p = 1.000; PTT, p = .099).
Question 3: Is There Evidence of Aftereffects in Neutral Swallows After Chin-Down Swallowing?
There was no difference between N1 and N2 for dLVC (p = .875), or dtLVC (p = .998). Conversely, PTT increased by an estimated 40 ms from N1 to N2 (95% CI [0, 79], p = .046, Cohen’s d = −0.33).
Penetration–Aspiration Scores
The percentage of swallows with a PA score of 3 or higher was calculated for each stage. The rationale for a PA score of 3 as the cutoff is based on previous research that reports that PA scores of 1 and 2 are typical in healthy participants (Martin-Harris, Brodsky, Michel, Lee, & Walters, 2007; McCullough, Rosenbek, Wertz, Suiter, & McCoy, 2007; Robbins, Coyle, Rosenbek, Roecker, & Wood, 1999). To provide additional information on the spread of PA scores within each stage, median, 75% quartile, and range were also calculated. The highest PA score noted was 5, which occurred twice across all stages. The percentages were as follows: N1 = 2.5% (median: 1; 75% quartile: 1; range: 4), P1 = 0% (median: 1; 75% quartile: 1; range: 1), P2 = 1.3% (median: 1; 75% quartile: 1; range: 2), and N2 = 5.1% (median: 1; 75% quartile: 1; range: 2).
Discussion
Kinematic Effects of Chin-Down Swallowing
This study investigated laryngeal closure kinematics during chin-down swallowing. We have shown that LVC duration increased by approximately 100 ms (Cohen’s d of −0.55) during chin-down swallows, a moderate (Cohen, 1988) increase of 20% compared with neutral swallows. Furthermore, LVC closure time remained stable throughout 30 repetitions of the posture and decreased to baseline values upon return to swallow with the head in a neutral position. This finding suggests that increased LVC duration could be the primary mechanism responsible for improved airway protection associated with the technique, thus contributing to our knowledge about how chin-down swallowing decreases aspiration (Bulow et al., 1999, 2001; Shanahan et al., 1993; Welch et al., 1993). LVC duration likely increased as a result of the decreased distance between the hyoid bone and larynx when the chin is down (Bulow et al., 1999, 2001) and the resulting structural changes that facilitate LVC in this position (Fink, 1976; Fink et al., 1979).
Conversely, the duration to LVC closure (dtLVC) was not reduced during chin-down swallowing. The lack of change in dtLVC could be explained by the fact that the effects of chin-down posture on dtLVC are more variable compared with LVC duration (see 95% CI around estimated effects for both measures; Table 2). The reason for the increased variability of this measure is unknown. Future studies aiming to document changes in dtLVC with chin-down swallowing should consider the variance documented in this study.
We found no change in our measures relating to bolus flow during chin-down swallowing: the initiation of the pharyngeal swallow response (OPSr) and pharyngeal transit time (PTT). We included these measures, previously examined in studies of chin-down swallowing, to assess whether changes in LVC kinematic are related to bolus flow measures (Power et al., 2007). Our findings support reports by Bulow and colleagues, who also showed unchanged PTT during chin-down swallowing (Bulow et al., 1999, 2001). Furthermore, the finding that OPSr remained unchanged during the maneuver is consistent with two previous studies (Bulow et al., 2001; Shanahan et al., 1993). Thus, it appears that laryngeal closure abnormalities may benefit from chin-down swallowing to a greater extent than bolus flow-related abnormalities. However, future studies in individuals with dysphagia are needed to confirm this hypothesis. In this healthy cohort, the percentage of PA scores of 3 or more reduced during chin-down swallows compared with baseline (from 2.5% at baseline to 0% at P1 and 1.3% at P2). This suggests that even in a healthy population, the increase in dLVC during chin-down swallowing is associated with a reduction in the incidence of misdirected swallows.
Stability of Kinematic Effects and Aftereffects
The results of our study show that increased laryngeal vestibule closure remained stable over 30 consecutive chin-down swallows and that no aftereffects were found for LVC kinematics. These findings suggest that chin-down effects may occur only during closer proximity of the hyoid and larynx, that is, while the chin is down. In other words, participants do not appear to alter swallowing kinematics in response to the novel experience of chin-down swallowing. This is not surprising, given that adaptation to a novel swallowing task has been found only in circumstances where swallowing function was obviously perturbed (Humbert et al., 2012, 2013). It is interesting that although PTT was not influenced during chin-down swallowing, aftereffects were evident in our participants, albeit relatively small aftereffects (PTT increased significantly upon return to neutral swallows by approximately 40 ms, Cohen’s d of −0.33; Cohen, 1988). This suggests that some other mechanisms, perhaps related to oral, pharyngeal, and/or upper esophageal pressure functions, were influenced during the course of consecutive chin-down swallowing, leading to alterations in postposture swallows. As none of our kinematic measures showed similar after-effects, we are unable to identify the specific cause of the change in PTT. Future studies should therefore incorporate further kinematic measures in an attempt to identify the cause of this increase. It is also possible that changes in PTT may result from repetitive swallowing alone. As we did not assess changes in PTT as a function of time across multiple neutral swallows, we are unable to confirm or disprove this possibility. Future efforts to document changes in these measures across neutral swallows would prove useful in answering such questions.
The incidence of PA scores of 3 or greater increased after consecutive chin-down swallows. This may have occurred because the bolus was in the pharynx longer while LVC duration simultaneously returned to baseline values, leaving the airway relatively vulnerable. However, this PA increase in healthy participants is relatively small. All instances of penetration or aspiration were trace amounts, a finding consistent with previous studies documenting penetration and aspiration in healthy participants (Martin-Harris et al., 2007; McCullough et al., 2007). Furthermore, the number of misdirected swallows overall was very small. Two swallows (of 80) had a PA score of 3 or more at N1, compared with 4 swallows (of 78) at N2. However, future studies should investigate whether such a risk exists in populations with dysphagia, for whom the threat of airway compromise is of greater concern. We also explored the relationship between dLVC and PA scores of 3 or more to determine whether these swallows were associated with shorter dLVC. Only neutral swallows were explored because of the small overall number of PA scores of 3 or more in the chin-tuck position. Average dLVC for these neutral swallows with a PA score of 3 or more was 428 ms (95% CI [362, 494]). These values are very similar to the overall means of the neutral swallows completed at N1 and N2 (see Figure 1), suggesting that the relationship between dLVC and penetration–aspiration is not straightforward. However, as mentioned above, the overall number of swallows during which penetration–aspiration occurred is very small compared with the number of swallows contributing to the overall mean, suggesting that these values should be interpreted with caution.
The relationship between dLVC and aspiration in neutral swallows has previously been investigated in patients by a small number of studies (Oommen, Kim, & McCullough, 2011; Park et al., 2010; Power et al., 2007, 2009). The lack of difference in dLVC values between aspirators and non-aspirators (Oommen et al., 2011; Park et al., 2010) are in line with our findings. Power and colleagues (2009) found that rather than dLVC in isolation being predictive of aspiration, when combined with other physiologic abnormalities (OPSr and PTT), the measure contributes a small but detectable improvement in predicting aspiration. This idea of a complex relationship between dLVC and aspiration is further supported by earlier work from the same research group (Power et al., 2007) that found that the relationship between dLVC and PTT they documented in healthy subjects was absent in stroke patients. One reason for this complex relationship is likely to be in the lack of consistency seen with occurrence of aspiration. As identified by Power and colleagues (2009), instances of aspiration are not consistent despite the constant presence of physiologic abnormalities. These results considered together suggest that although a direct relationship between dLVC and aspiration remains difficult to elucidate, facilitating dLVC will likely play a role in ameliorating instances of aspiration, based on the relationships identified between this measure and other physiologic abnormalities. However, investigating the relationship between dLVC and penetration–aspiration in patients during the chin-down maneuver is a necessary endeavor for future research.
Study Limitations
This study included healthy younger adults. Investigation of healthy participants is considered a necessary first step in a meticulous approach to validation of dysphagia management techniques (Logemann, 2005). Investigation of the kinematic mechanisms influenced by chin-down maneuver, stability of effects, and aftereffects is now required in patient populations. Despite the important role of laryngeal vestibule closure in protecting the airway during swallowing, measures of LVC are rarely used as outcome variables to document treatment effects. This study suggests that the physiologic abnormality of impaired laryngeal vestibule closure may benefit from chin-down swallowing to a greater degree than other physiologic abnormalities, such as delayed onset of the pharyngeal swallow or impaired pharyngeal transit time. This should be considered when designing studies to document the effects of the maneuver in patients.
Participants maintained the same chin-down posture throughout P1 and P2 in the current study, unlike in real-world situations in which participants move from neutral position to chin-down posture to allow for self-feeding. It is possible that variation in the chin-down posture associated with repositioning between boluses will result in some differences in the stability of effects noted here; therefore, this warrants further investigation. An additional consideration is that aftereffects may be evident following longer periods of chin-down swallowing (i.e., days to months).
Conclusion
This study provides empirical evidence of the underlying laryngeal closure mechanisms influenced by chindown swallowing as well as the stability and durability of effects. Although chin-down swallowing may not induce the learning processes considered important for neural and functional rehabilitation, its use as a compensatory treatment of aspiration in patients with dysphagia remains justified. Our findings suggest that chin-down swallowing may be more beneficial for patients with impaired laryngeal vestibule closure and potentially unsafe for patients with disordered PTT, especially when returning to the neutral position.
Acknowledgments
This research was conducted during the tenure of a post-graduate scholarship of the New Zealand Neurological Foundation awarded to Phoebe Macrae and was supported by National Institute on Deafness and Other Communication Disorders Grant 1K23DC010776-01, 2009–2014, awarded to Ianessa Humbert.
Footnotes
Editor: Jody Kreiman
Associate Editor: Caryn Easterling
Disclosure: The authors have declared that no competing interests existed at the time of publication.
References
- Bisch EM, Logemann JA, Rademaker AW, Kahrilas PJ, Lazarus CL. Pharyngeal effects of bolus volume, viscosity, and temperature in patients with dysphagia resulting from neurologic impairment and in normal subjects. Journal of Speech and Hearing Research. 1994;37:1041–1059. doi: 10.1044/jshr.3705.1041. [DOI] [PubMed] [Google Scholar]
- Bulow M, Olsson R, Ekberg O. Videomanometric analysis of supraglottic swallow, effortful swallow, and chin tuck in healthy volunteers. Dysphagia. 1999;14:67–72. doi: 10.1007/PL00009589. [DOI] [PubMed] [Google Scholar]
- Bulow M, Olsson R, Ekberg O. Videomanometric analysis of supraglottic swallow, effortful swallow, and chin tuck in patients with pharyngeal dysfunction. Dysphagia. 2001;16:190–195. doi: 10.1007/s00455-001-0065-9. [DOI] [PubMed] [Google Scholar]
- Castell JA, Castell DO, Schultz AR, Georgeson S. Effect of head position on the dynamics of the upper esophageal sphincter and pharynx. Dysphagia. 1993;8:1–6. doi: 10.1007/BF01351470. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Ekberg O. Posture of the head and pharyngeal swallowing. Acta Radiologica: Diagnosis. 1986;27:691–696. doi: 10.1177/028418518602700612. [DOI] [PubMed] [Google Scholar]
- Fei T, Polacco RC, Hori SE, Molfenter SM, Peladeau-Pigeon M, Tsang C, Steele CM. Age-related differences in tongue-palate pressures for strength and swallowing tasks. Dysphagia. 2013;28:575–581. doi: 10.1007/s00455-013-9469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Todd T, Lintzenich CR, Ding J, Carr JJ, Ge Y, Butler SG. Aging-related geniohyoid muscle atrophy is related to aspiration status in healthy older adults. Journals of Gerontology: Series A: Biological Sciences and Medical Sciences. 2013;68:M853–M860. doi: 10.1093/gerona/gls225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink BR. The median thyrohyoid “fold”: A nomenclatural suggestion. Journal of Anatomy. 1976;122:697–699. [PMC free article] [PubMed] [Google Scholar]
- Fink BR, Martin RW, Rohrmann CA. Biomechanics of the human epiglottis. Acta Oto-Laryngologica. 1979;87:554–559. doi: 10.3109/00016487909126464. [DOI] [PubMed] [Google Scholar]
- Gelman A, Hill J. Data analysis using regression and multilevel/hierarchical models. New York, NY: Cambridge University Press; 2007. [Google Scholar]
- Humbert IA, Christopherson H, Lokhande A, German R, Gonzalez-Fernandez M, Celnik P. Human hyolaryngeal movements show adaptive motor learning during swallowing. Dysphagia. 2013;28:139–145. doi: 10.1007/s00455-012-9422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert IA, Lokhande A, Christopherson H, German R, Stone A. Adaptation of swallowing hyo-laryngeal kinematics is distinct in oral vs. pharyngeal sensory processing. Journal of Applied Physiology. 2012;112:1698–1705. doi: 10.1152/japplphysiol.01534.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin JS, Hebert TM, Putnam JB, Jr, DuBrow RA. Experience with the chin tuck maneuver in postesophagectomy aspirators. Dysphagia. 2001;16:216–219. doi: 10.1007/s00455-001-0068-6. [DOI] [PubMed] [Google Scholar]
- Lof GL, Robbins J. Test–retest variability in normal swallowing. Dysphagia. 1990;4:236–242. doi: 10.1007/BF02407271. [DOI] [PubMed] [Google Scholar]
- Logemann JA. Evaluation and treatment of swallowing disorders. San Diego, CA: College-Hill Press; 1983. [Google Scholar]
- Logemann JA. Evaluation and treatment of swallowing disorders. 2nd. Austin, TX: Pro-Ed; 1998. [Google Scholar]
- Logemann JA. The role of exercise programs for dysphagia patients. Dysphagia. 2005;20:139–140. doi: 10.1007/s00455-005-0005-1. [DOI] [PubMed] [Google Scholar]
- Logemann JA, Gensler G, Robbins J, Lindblad AS, Brandt D, Hind JA, Miller Gardner PJ. A randomized study of three interventions for aspiration of thin liquids in patients with dementia or Parkinson’s disease. Journal of Speech, Language, and Hearing Research. 2008;51:173–183. doi: 10.1044/1092-4388(2008/013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann JA, Kahrilas PJ, Cheng J, Pauloski BR, Gibbons PJ, Rademaker AW, Lin S. Closure mechanisms of laryngeal vestibule during swallow. American Journal of Physiology. 1992;262:G338–G344. doi: 10.1152/ajpgi.1992.262.2.G338. [DOI] [PubMed] [Google Scholar]
- Martin-Harris B, Brodsky MB, Michel Y, Lee FS, Walters B. Delayed initiation of the pharyngeal swallow: Normal variability in adult swallows. Journal of Speech, Language, and Hearing Research. 2007;50:585–594. doi: 10.1044/1092-4388(2007/041). [DOI] [PubMed] [Google Scholar]
- McCullough G, Rosenbek J, Wertz R, Suiter D, McCoy S. Defining swallowing function by age. Topics in Geriatric Rehabilitation. 2007;23:290–307. [Google Scholar]
- Nagaya M, Kachi T, Yamada T, Sumi Y. Videofluorographic observations on swallowing in patients with dysphagia due to neurodegenerative diseases. Nagoya Journal of Medical Science. 2004;67:17–23. [PubMed] [Google Scholar]
- Oommen ER, Kim Y, McCullough G. Stage transition and laryngeal closure in poststroke patients with dysphagia. Dysphagia. 2011;26:318–323. doi: 10.1007/s00455-010-9314-0. [DOI] [PubMed] [Google Scholar]
- Park T, Kim Y, Ko DH, McCullough G. Initiation and duration of laryngeal closure during the pharyngeal swallow in post-stroke patients. Dysphagia. 2010;25:177–182. doi: 10.1007/s00455-009-9237-9. [DOI] [PubMed] [Google Scholar]
- Power ML, Hamdy S, Goulermas JY, Tyrrell PJ, Turnbull I, Thompson DG. Predicting aspiration after hemispheric stroke from timing measures of oropharyngeal bolus flow and laryngeal closure. Dysphagia. 2009;24:257–264. doi: 10.1007/s00455-008-9198-4. [DOI] [PubMed] [Google Scholar]
- Power M, Hamdy S, Singh S, Tyrrell P, Turnbull I, Thompson D. Deglutitive laryngeal closure in stroke patients. Journal of Neurology, Neurosurgery and Psychiatry. 2007;78:141–146. doi: 10.1136/jnnp.2006.101857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasley A, Logemann JA, Kahrilas PJ, Rademaker AW, Pauloski BR, Dodds WJ. Prevention of barium aspiration during videofluoroscopic swallowing studies: Value of change in posture. American Journal of Roentgenology. 1993;160:1005–1009. doi: 10.2214/ajr.160.5.8470567. [DOI] [PubMed] [Google Scholar]
- Robbins J, Coyle J, Rosenbek J, Roecker E, Wood J. Differentiation of normal and abnormal airway protection during swallowing using the Penetration–Aspiration Scale. Dysphagia. 1999;14:228–232. doi: 10.1007/PL00009610. [DOI] [PubMed] [Google Scholar]
- Robbins J, Gangnon RE, Theis SM, Kays SA, Hewitt AL, Hind JA. The effects of lingual exercise on swallowing in older adults. Journal of the American Geriatrics Society. 2005;53:1483–1489. doi: 10.1111/j.1532-5415.2005.53467.x. [DOI] [PubMed] [Google Scholar]
- Robbins J, Levine R, Wood J, Roecker EB, Luschei E. Age effects on lingual pressure generation as a risk factor for dysphagia. Journals of Gerontology: Series A: Biological Sciences and Medical Sciences. 1995;50:M257–M262. doi: 10.1093/gerona/50a.5.m257. [DOI] [PubMed] [Google Scholar]
- Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A Penetration–Aspiration Scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- Shanahan TK, Logemann JA, Rademaker AW, Pauloski BR, Kahrilas PJ. Chin-down posture effect on aspiration in dysphagic patients. Archives of Physical Medicine and Rehabilitation. 1993;74:736–739. doi: 10.1016/0003-9993(93)90035-9. [DOI] [PubMed] [Google Scholar]
- Solazzo A, Del Vecchio L, Reginelli A, Monaco L, Sagnelli A, Monsorro M, Grassi R. Search for compensation postures with videofluoromanometric investigation in dysphagic patients affected by amyotrophic lateral sclerosis. Radiologia Medica. 2011;116:1083–1094. doi: 10.1007/s11547-011-0698-1. [DOI] [PubMed] [Google Scholar]
- Steele CM, Hung D, Sejdic E, Chau T, Fraser S. Variability in execution of the chin-down maneuver by healthy adults. Folia Phoniatrica et Logopedica. 2011;63:36–42. doi: 10.1159/000319737. [DOI] [PubMed] [Google Scholar]
- Terre R, Mearin F. Effectiveness of chin-down posture to prevent tracheal aspiration in dysphagia secondary to acquired brain injury: A videofluoroscopy study. Neurogastroenterology and Motility. 2012;24:414–419. doi: 10.1111/j.1365-2982.2011.01869.x. [DOI] [PubMed] [Google Scholar]
- Welch MV, Logemann JA, Rademaker AW, Kahrilas PJ. Changes in pharyngeal dimensions effected by chin tuck. Archives of Physical Medicine and Rehabilitation. 1993;74:178–181. [PubMed] [Google Scholar]


