Abstract
Objectives
To determine the inter-study reproducibility of left ventricular (LV) mechanical dyssynchrony measures based on standard cardiovascular magnetic resonance (CMR) cine images.
Design
Steady-state free precession (SSFP) LV short-axis stacks and three long-axes were acquired on the same day at three time points. Circumferential strain systolic dyssynchrony indexes (SDI), area-SDI as well as circumferential and radial uniformity ratio estimates (CURE and RURE, respectively) were derived from CMR myocardial feature-tracking (CMR-FT) based on the tracking of three SSFP short-axis planes. Furthermore, 4D-LV-analysis based on SSFP short-axis stacks and longitudinal planes was performed to quantify 4D-volume-SDI.
Setting
A single-centre London teaching hospital.
Participants
16 healthy volunteers.
Main outcome measures
Inter-study reproducibility between the repeated exams.
Results
CURE and RURE as well as 4D-volume-SDI showed good inter-study reproducibility (coefficient of variation [CoV] 6.4%–12.9%). Circumferential strain and area-SDI showed higher variability between the repeated measurements (CoV 24.9%–37.5%). Uniformity ratio estimates showed the lowest inter-study variability (CoV 6.4%–8.5%).
Conclusions
Derivation of LV mechanical dyssynchrony measures from standard cine images is feasible using CMR-FT and 4D-LV-analysis tools. Uniformity ratio estimates and 4D-volume-SDI showed good inter-study reproducibility. Their clinical value should next be explored in patients who potentially benefit from cardiac resynchronization therapy.
Keywords: Mechanical dyssynchrony, CMR feature-tracking, strain, systolic dyssynchrony index, uniformity ratio estimate
Background
Cardiac resynchronization therapy (CRT) improves quality of life and survival in patients with refractory heart failure due to systolic dysfunction and mechanical dyssynchrony.1 To select optimal patient collectives for CRT, considerable efforts have been directed towards imaging-based identification of dyssynchrony considering correction of dyssynchronous myocardial contraction as the main therapeutic mechanism of CRT.2 However, there are substantial numbers of patients not responding to CRT. While numerous echocardiographic dyssynchrony parameters have been proposed,3 none of these succeeded in improving patient selection for CRT.2,4,5 More recently, dyssynchrony measures based on cardiovascular magnetic resonance (CMR) imaging have been developed, most of them based on CMR myocardial tagging6,7 or displacement encoding with stimulated echoes (DENSE).8 Studies applying these measures in smaller cohorts showed promising results regarding the prediction of CRT response.9,10 However, both, CMR tagging and DENSE require acquisition of additional sequences and are often associated with time-consuming post-processing, which is most likely the reason why initial promising results have not yet prompted multicentre trials with larger patient numbers to explore the additional clinical merit of these novel parameters. More recently, mechanical dyssynchrony parameters based on CMR myocardial feature tracking (CMR-FT)11–13 and 4D left ventricular (LV) analysis post-processing software have been introduced.14,15 These measures are directly derived from conventional steady state free precession (SSFP) cine images and therefore appear particularly applicable for clinical and research use since no additional sequence acquisition is necessary.16 High inter-study reproducibility is a key requirement for such applications, but has not been addressed yet. Consequently, the aim of the present study was to investigate the inter-study reproducibility of dyssynchrony measures based on conventional SSFP images with a focus on LV systolic dyssychrony indexes (SDI) and uniformity ratio estimates (URE).
Methods
Sixteen healthy participants were included in the study, which was approved by the St Thomas’ Hospital Research Ethics Committee. The study complies with the Declaration of Helsinki and its later amendments. All participants gave written informed consent. Exclusion criteria included known cardiac, respiratory or renal disease or an absolute contraindication to CMR.
CMR imaging
Participants underwent three CMR examinations on the same day. All imaging was performed at 3 Tesla (Achieva, Philips Medical Systems, Best, The Netherlands) in the supine position using a 32-channel phased array receiver cardiac coil. On the study day, participants were encouraged to fast from midnight. The first CMR examination was performed at 9:00 (Exam A), immediately followed by a second exam at 9:30 (Exam B). Participants then left the department to eat and drink as normal. They returned at 14:00 for the third scan (Exam C). Exams A and B were compared to assess for the inherent inter-study variability associated with the respective CMR-FT-derived dyssynchrony indexes. Morning scans (Exams A and B) were compared to Exam C for the assessment of potential diurnal physiological alterations due to circadian rhythms or different states of hydration. The CMR protocol included initial survey and coil reference scans for all three examinations. Participants were removed from the scanner between different exams. Planning to define imaging planes was performed independently for all three CMR scans. Cine images were acquired using a standard ECG-gated balanced SSFP sequence in long-axis 2-, 3- and 4-chamber views and sequential short-axis planes covering the whole LV (in-plane resolution 1.8 × 2 mm; slice thickness 8 mm; 30 phases/cardiac cycle, corresponding to a temporal resolution of 25–35 ms at a heart rate of 60–80 bpm). The protocol was identically repeated for all three scans and for all volunteers.
CMR feature tracking
CMR-FT was performed in three short-axis planes (basal, mid-ventricular, apical) using dedicated software (TomTec Imaging Systems, 2D CPA MR, Cardiac Performance Analysis, Version 1.1.2, Unterschleissheim, Germany) (Figure 1(a)). LV endocardial and epicarial borders were tracked as previously described.17 The software automatically tracks 48 subendocardial and subepicardial tissue voxels throughout the cardiac cycle. Tracking was repeated for three times in each view. Results were based on the average of the three repeated measurements.18 The following dyssynchrony indexes were derived from CMR-FT.
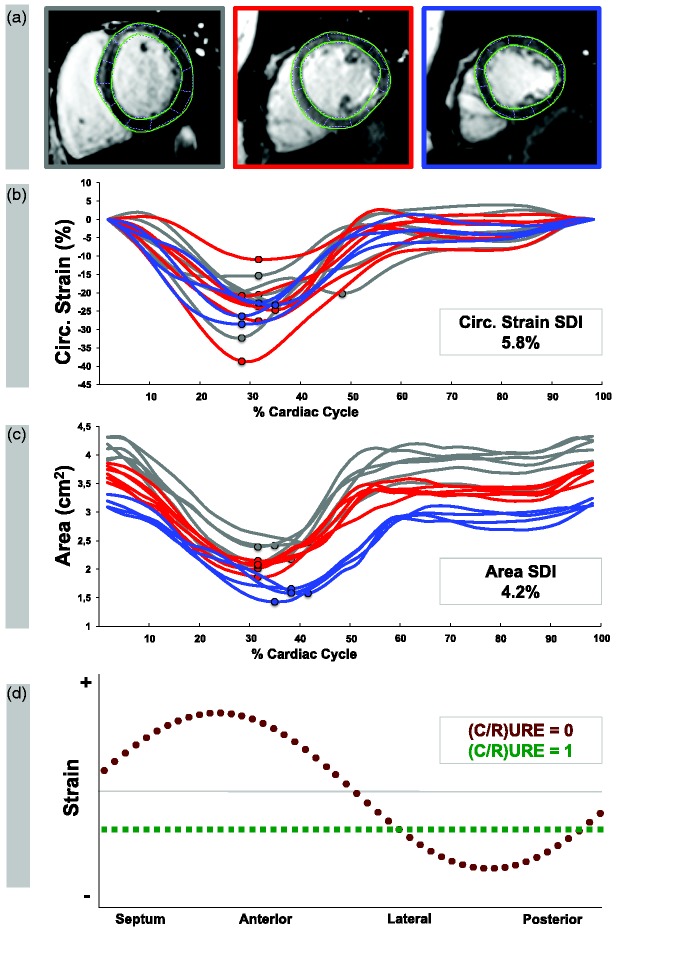
Figure 1.
Derivation of dyssynchrony indexes from CMR-FT. (a) CMR-FT was performed in basal, mid-ventricular and apical levels on standard cine images. (b) Circumferential strain systolic dyssynchrony indexes (SDI) were calculated from the standard deviation of the regional time to maximum circumferential subendocardial strain of 16 evenly distributed segments following a standard model.19 (c) Area SDI was calculated from the standard deviation of the regional time to minimum area from 16 evenly distributed segments. Grey lines indicate segments at basal levels, red lines indicate segments at mid-ventricular levels and blue lines indicate segments at apical levels. (d) Circumferential and radial uniformity ratio estimates (CURE and RURE, respectively) were calculated after plotting the circumferential and radial strain, respectively, at 48 evenly distributed locations for each time frame. The current example represents strain in one time frame. Each circle or square corresponds to one of the 48 spatial locations. The green-circled line represents perfect synchrony (corresponds URE = 1), while the red-squared line represents complete dyssynchrony (corresponds to URE = 0).
Circumferential strain SDI: calculated from the standard deviation of the regional time to maximum circumferential subendocardial strain (given as a time percentage of the length of the cardiac cycle) for 16 LV segments (according to the American Heart Association (AHA) LV model: six basal, six mid-ventricular and four apical segments) (Figure 1(b)).19
Area SDI: calculated from the standard deviation of the regional time to minimum area (given as a time percentage of the length of the cardiac cycle) for 16 LV AHA segments (Figure 1(c)).19
Uniformity ratio estimates: Circumferential (CURE) and radial uniformity ratio estimates (RURE) were calculated as previously described.20 In brief, CURE and RURE are ratios of the spatial uniformity of circumferential and radial strain averaged over time, respectively (Figure 1(d)).10 Circumferential and radial strain of 48 evenly distributed locations were analysed in basal, mid-ventricular and apical short-axis planes and plotted versus spatial-position for each time-frame. Corresponding plots were subjected to Fourier analysis. CURE and RURE were calculated using the formula proposed by Leclercq et al.20 CURE and RURE measures range between 0 (corresponding to complete dyssynchrony) and 1 (corresponding to perfect synchrony). In the present study, CURE and RURE were examined separately as well as the average of both (CURE:RUREAVG) as previously described.13
4D LV-analysis
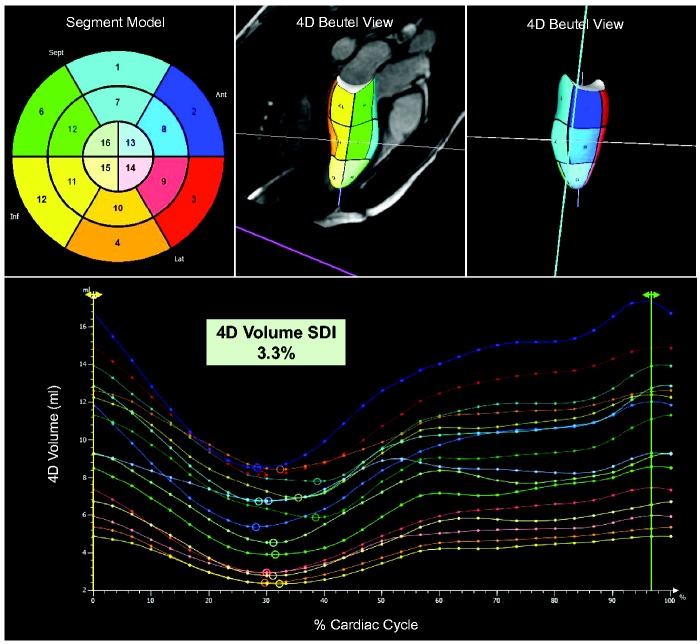
Prototype 4D LV-analysis software (TomTec Imaging Systems, Unterschleissheim, Germany) was applied to quantify regional volume changes over the cardiac cycle for 16 segments according to the AHA LV model.14,15 In brief, post-processing required the delineation of the LV endocardial border in two-, three- and four-chamber views at end-diastole and end-systole. Subsequent advanced algorithms were applied to track endocardial motion in long- and short-axis views over the cardiac cycle to produce a 3D shell of LV contraction (Figure 2, Video 1). 4D volume SDI was calculated from the standard deviation of the regional time to minimum volume (given as a time percentage of the length of the cardiac cycle) for the 16 segments (Figure 2).
Figure 2.
Quantification of 4D systolic dyssynchrony indexes. 4D Beutel views were acquired from three left ventricular long-axis (two-, three- and four-chamber views) and a short-axis stack using dedicated prototype 4D LV-analysis software (TomTec, Unterschleissheim, Germany). Circles on the time volume graph correspond to regional minimum volumes. Systolic dyssychrony indexes were quantified from the standard deviation of the time to minimum volume for all 16 segments.19
Statistical analysis
Statistical analysis was performed using Microsoft Excel and IBM SPSS Statistics version 22 for Macintosh. Data from the repeated exams are expressed as mean ± standard deviation. The Shapiro–Wilk test was applied to test for normally distributed data. A one-way analysis of variance (ANOVA) for repeated measures was conducted to evaluate the null hypothesis that there is no change in dyssynchrony indexes between the repeated Exams A, B and C. All p values < 0.05 were considered statistically significant.
The inter-study variability was assessed by intraclass correlation coefficients (ICC) using a model of absolute agreement. Agreement was considered excellent when ICC > 0.74, good when ICC = 0.60–0.74, fair when ICC = 0.40–0.59, and poor when ICC < 0.4.21 The mean difference with 95% limits of agreement (± 2 standard deviations) between the repeated measurements was calculated according to the method of Bland and Altman.22 Coefficients of variation (CoV), defined as the standard deviation of the differences divided by the mean,23 were calculated. Furthermore, study sample sizes required to detect a relative 5%, 10%, 15% and 20% change in dyssynchrony parameters with a power of 90% and an α error of 0.05 were calculated as follows23
where n is the sample size, f = 10.5 for α 0.05 and P 0.9, σ the inter-study standard deviation and δ the magnitude of the differences to be detected.
Results
Sixteen healthy volunteers (eight male, eight female) aged 27.9 ± 5.7 with a body mass index of 26.2 ± 6.8 kg/m2 were included in the study. One participant did not attend Exam C. In total, 16 cases were compared to assess the inter-study reproducibility of CMR measures of LV dyssynchrony (Exam A vs. Exam B), 15 cases were compared for the assessment of diurnal variation (Exam A and B vs. Exam C), respectively. LV dyssynchrony indexes are summarised for all Exams in Table 1. There were no significant differences between the Exams A, B and C. Moreover, there was no measurable affection by diurnal variation.
Table 1.
Dyssynchrony indexes. Comparison of dyssychnrony indexes between the repeated measurements.
| Exam A | Exam B | Exam C | P * | |
|---|---|---|---|---|
| Circ. Strain SDI (%) | 5.9 ± 2.3 | 6.8 ± 2.5 | 7.1 ± 2.0 | 0.16 |
| Area SDI (%) | 3.5 ± 1.0 | 3.8 ± 1.1 | 4.0 ± 1.2 | 0.45 |
| CURE | 0.87 ± 0.07 | 0.84 ± 0.07 | 0.85 ± 0.08 | 0.20 |
| RURE | 0.84 ± 0.07 | 0.81 ± 0.11 | 0.83 ± 0.09 | 0.18 |
| CURE:RUREAVG | 0.86 ± 0.05 | 0.83 ± 0.08 | 0.84 ± 0.07 | 0.15 |
| 4D Volume SDI (%) | 3.0 ± 0.6 | 3.1 ± 0.5 | 2.9 ± 0.7 | 0.17 |
Note: Values are given as mean ± standard deviation.
As derived from one-way ANOVA for repeated measures across the Exams A, B and C
Inter-study reproducibility
Inter-study reproducibility was within acceptable limits for all LV dyssynchrony indexes. Table 2 summarises Bland–Altman analysis (mean differences ± 2 SD), ICC and CoV. Reproducibility was good for the circumferential strain SDI (ICC 0.67) and excellent for all other indexes (ICC 0.76 to 0.85), with CMR-FT derived uniformity ratio estimates showing the overall lowest variability (CoV 6.4%–8.5%). Circumferential strain and area SDI showed considerable inter-study variability as expressed by CoV (37.5% and 24.9%, respectively).
Table 2.
Inter-study reproducibility. Inter-study reproducibility for dyssynchrony indexes as determined by Bland–Altman analysis (mean difference ± 2SD), coefficients of variation (CoV) and intra-class correlation coefficients (ICC).
| Mean difference ± 2 SD | CoV (%) | ICC (95% CI) | |
|---|---|---|---|
| Circ. Strain SDI (%) | 0.8 ± 2.4 | 37.5 | 0.67 (0.10–0.88) |
| Area SDI (%) | 0.3 ± 0.9 | 24.9 | 0.76 (0.35–0.92) |
| CURE | 0.03 ± 0.06 | 6.5 | 0.79 (0.41–0.93) |
| RURE | 0.03 ± 0.07 | 8.5 | 0.80 (0.43–0.93) |
| CURE:RUREAVG | 0.03 ± 0.05 | 6.4 | 0.80 (0.40–0.93) |
| 4D Volume SDI (%) | 0.1 ± 0.4 | 12.9 | 0.85 (0.55–0.95) |
SD: standard deviation; CoV: coefficient of variation; ICC: intraclass correlation coefficient; CI: confidence interval.
Sample size calculations
Sample sizes required to detect a relative 5%, 10%, 15% and 20% change in dyssynchrony indexes are shown in Table 3. Required sample sizes increase with smaller differences to be detected. Sample sizes are ranging between n = 3 to detect a relative 20% change in CURE or CURE:RUREAVG (corresponds to a magnitude of 0.17 for both CURE and CURE:RUREAVG) and n = 1183 to detect a 5% change in circumferential strain SDI (corresponds to a magnitude of 0.32 % in the present study).
Table 3.
Sample sizes. Sample sizes required to detect a relative 5%, 10%, 15% or 20% change in dyssynchrony indexes (with a 90% power and an α error of 0.05).
| 5% | 10% | 15% | 20% | |
|---|---|---|---|---|
| Circ. Strain SDI (%) | 1183 | 296 | 132 | 74 |
| Area SDI (%) | 522 | 131 | 58 | 33 |
| CURE | 36 | 10 | 5 | 3 |
| RURE | 61 | 16 | 7 | 4 |
| CURE:RUREAVG | 35 | 9 | 4 | 3 |
| 4D Volume SDI (%) | 139 | 35 | 16 | 9 |
Discussion
The current study aimed to assess the inter-study reproducibility for the analysis of LV dyssynchrony indexes based on conventional CMR cine images. Firstly, it shows high inter-study reproducibility for CMR-FT-derived uniformity ratio estimates as well as for 4D LV-analysis derived volume SDI. Secondly, amongst the different methodologies, CMR-FT-derived circumferential strain SDI and area SDI show higher variability, which may limit their applicability in longitudinal studies with repeated measurements. Lastly, there was no measurable affection of LV dyssynchrony by diurnal variation studied with any methodology.
Current recommendations for selecting patients for CRT include prolongation of the QRS duration on the electrocardiogram (ECG) representing an indirect marker of LV mechanical dyssynchrony.2 Numerous echocardiographic dyssynchrony parameters have been evaluated with the aim to directly quantify LV mechanical dyssynchrony;3 however, none led to a significant optimisation of CRT response.2,4,5 Since CRT specifically targets cardiac dyssynchrony, a direct, robust and reproducible quantification of LV mechanical dyssynchrony with superiority over current clinical parameters is crucial. Inter-study reproducibility is a key requirement when repeated examinations are required. Higher reproducibility means that smaller changes can be detected with increased reliability. On the other side, improved inter-study reproducibility also improves cost-effectiveness, as fewer subjects are required in clinical trials to detect equal magnitudes of change.24 This is going to be particularly interesting in future longitudinal studies on patients undergoing CMR prior and after the implantation of CMR-compatible CRT-devices, eventually allowing direct quantification of CRT response.2
Previous CMR-FT studies primarily focused on ventricular and atrial strain quantification.16,25–30 More recently, the feasibility of CMR-FT for the assessment of LV dyssynchrony has been demonstrated.12,13 Onishi et al.12 applied CMR-FT to quantify radial dyssynchrony as the time difference between the short-axis anteroseptal and posterior wall segmental peak strain and found reasonable agreement with speckle tracking echocardiography. Notwithstanding, it is important to note that previous validation studies demonstrated lower intra- and inter-observer reproducibility of CMR-FT-derived segmental radial strain compared to circumferential strain, which needs to be considered when interpreting the results of Onishi and co-workers.17,30 Furthermore, data comparing circumferential and longitudinal strain dyssynchrony measures highlight potential limitations of longitudinal strain analysis and support further efforts to develop dyssynchrony measures based on circumferential deformation.10 As a consequence, we applied segmental circumferential strain to calculate strain SDI rather than looking at the wall time delay based on segmental radial strain.
The analysis of 4D volume SDI as introduced by Sohal et al.15 offers quantification of dyssynchrony indexes based on segmental volume – rather than segmental strain – changes. In an initial study, 4D volume SDI accurately identified therapy responders in a patient collective receiving CRT with superiority over established parameters, for example QRS duration, presence of left bundle branch block and scar burden.14 More recently, Taylor et al.13 demonstrated CMR-FT-based acquisitions of uniformity ratio estimates, which had initially been validated using myocardial tissue tagging10,20 and DENSE.9 They demonstrated almost absolute discrimination between patients with non-ischemic cardiomyopathy and healthy controls applying these direct measures of dyssynchrony. In our study, both CMR-FT-derived uniformity ratio estimates and 4D LV-analysis-derived volume SDI demonstrated excellent reproducibility between repeated studies. Circumferential strain SDI demonstrated lower inter-study reproducibility, which is most likely a result of the aforementioned limited reproducibility of CMR-FT derived segmental strain.17 In contrast, 4D volume SDI is based on volumetric analyses, which previously demonstrated excellent inter-study reproducibility leading to a potential reduction of required sample sizes by up to 90% when compared to echocardiography.23 Considering the excellent inter-study reproducibility of both, CMR-FT-derived uniformity ratio estimates and 4D volume SDI as well as the promising initial results, there is high potential for clinical application, particularly for the prediction of potential CRT-derived functional benefits.
Limitations
The main limitation of the present study is the inclusion of healthy volunteers rather than patients. Reproducibility might vary between healthy volunteers and patients with different cardiovascular disorders. However, the reproducibility of CMR-FT derived measurements has been repeatedly shown to be similar between health and disease.17,30 The sample size of this study was relatively small. Ideally, a head-to-head comparison of the inter-study reproducibility between CMR tagging or DENSE derived dyssynchrony measurements would have been performed.
Conclusions
The inter-study reproducibility for LV dyssynchrony measures based on the analysis of conventional CMR cine images is good using CMR-FT-derived uniformity ratio estimates as well as 4D LV-Analysis derived volume SDI. Circumferential strain and area SDI are subject to larger inter-study variability, which needs to be considered for clinical and research use. The degree of inter-study reproducibility between the various techniques requires adequate adjustment of sample sizes in future longitudinal studies with repeated measurements. Future investigations will need to define the impact of these novel dyssynchrony parameters for clinical decision-making and patient management with a particular focus on prognostic implications in patients potentially benefiting from CRT implantation.
Acknowledgements
The authors are grateful to all those who have voluntarily accepted to take part in this study.
Contributorship
JTK, GM and AS designed the study protocol, performed data acquisition, performed statistical analysis and drafted the manuscript. JL, GH, EN and AC revised the manuscript and participated in the scientific discussion during the study. PL, RJ and SK revised the manuscript and performed data acquisition. All authors read and approved the final manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: JTK received a travel grant by the RWG Foundation. PL holds a Sir Henry Dale Fellowship funded jointly by the Wellcome Trust and the Royal Society (grant no. 099973/Z/12/Z). The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre and CRF based at Guy's and St Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. We are grateful for the financial support by the Research program of the Faculty of Medicine of the Georg-August-University Göttingen, Germany.
Ethical approval
The study was approved by the St Thomas’ Hospital Research Ethics Committee. The study complies with the Declaration of Helsinki and its later amendments. All participants gave written informed consent.
Guarantor
AS.
References
- 1.Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002; 346: 1845–1853. [DOI] [PubMed] [Google Scholar]
- 2.Brignole M, Auricchio A, Baron-Esquivias G, et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 2013; 34: 2281–2329. [DOI] [PubMed] [Google Scholar]
- 3.Anderson LJ, Miyazaki C, Sutherland GR, et al. Patient selection and echocardiographic assessment of dyssynchrony in cardiac resynchronization therapy. Circulation 2008; 117: 2009–2023. [DOI] [PubMed] [Google Scholar]
- 4.Chung ES, Leon AR, Tavazzi L, et al. Results of the predictors of response to CRT (PROSPECT) trial. Circulation 2008; 117: 2608–2616. [DOI] [PubMed] [Google Scholar]
- 5.Sung RK, Foster E. Assessment of systolic dyssynchrony for cardiac resynchronization therapy is not clinically useful. Circulation 2011; 123: 656–662. [DOI] [PubMed] [Google Scholar]
- 6.Bilchick KC, Dimaano V, Wu KC, et al. Cardiac magnetic resonance assessment of dyssynchrony and myocardial scar predicts function class improvement following cardiac resynchronization therapy. JACC Cardiovasc Imag 2008; 1: 561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrne MJ, Helm RH, Daya S, et al. Diminished left ventricular dyssynchrony and impact of resynchronization in failing hearts with right versus left bundle branch block. J Am Coll Cardiol 2007; 50: 1484–1490. [DOI] [PubMed] [Google Scholar]
- 8.Budge LP, Helms AS, Salerno M, et al. MR cine DENSE dyssynchrony parameters for the evaluation of heart failure: comparison with myocardial tissue tagging. JACC Cardiovasc Imag 2012; 5: 789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilchick KC, Kuruvilla S, Hamirani YS, et al. Impact of mechanical activation, scar, and electrical timing on cardiac resynchronization therapy response and clinical outcomes. J Am Coll Cardiol 2014; 63: 1657–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helm RH, Leclercq C, Faris OP, et al. Cardiac dyssynchrony analysis using circumferential versus longitudinal strain: implications for assessing cardiac resynchronization. Circulation 2005; 111: 2760–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jing L, Haggerty CM, Suever JD, et al. Patients with repaired tetralogy of Fallot suffer from intra- and inter-ventricular cardiac dyssynchrony: a cardiac magnetic resonance study. Eur Heart J Cardiovasc Imag 2014; 15: 1333–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onishi T, Saha SK, Ludwig DR, et al. Feature tracking measurement of dyssynchrony from cardiovascular magnetic resonance cine acquisitions: comparison with echocardiographic speckle tracking. J Cardiovasc Magn Reson 2013; 15: 95–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor RJ, Umar F, Moody WE, et al. Feature-tracking cardiovascular magnetic resonance as a novel technique for the assessment of mechanical dyssynchrony. Int J Cardiol 2014; 175: 120–125. [DOI] [PubMed] [Google Scholar]
- 14.Sohal M, Duckett SG, Zhuang X, et al. A prospective evaluation of cardiovascular magnetic resonance measures of dyssynchrony in the prediction of response to cardiac resynchronization therapy. J Cardiovasc Magn Reson 2014; 16: 58–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sohal M, Shetty A, Duckett S, et al. Noninvasive assessment of LV contraction patterns using CMR to identify responders to CRT. JACC Cardiovasc Imag 2013; 6: 864–873. [DOI] [PubMed] [Google Scholar]
- 16.Schuster A, Hor KN, Kowallick JT, et al. Cardiovascular magnetic resonance myocardial feature tracking: concepts and clinical applications. Circ Cardiovasc Imaging 2016; 9: e004077–e004077. [DOI] [PubMed] [Google Scholar]
- 17.Schuster A, Kutty S, Padiyath A, et al. Cardiovascular magnetic resonance myocardial feature tracking detects quantitative wall motion during dobutamine stress. J Cardiovasc Magn Reson 2011; 13: 58–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuster A, Stahnke VC, Unterberg-Buchwald C, et al. Cardiovascular magnetic resonance feature-tracking assessment of myocardial mechanics: intervendor agreement and considerations regarding reproducibility. Clin Radiol 2015; 70: 989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002; 105: 539–542. [DOI] [PubMed] [Google Scholar]
- 20.Leclercq C, Faris O, Tunin R, et al. Systolic improvement and mechanical resynchronization does not require electrical synchrony in the dilated failing heart with left bundle-branch block. Circulation 2002; 106: 1760–1763. [DOI] [PubMed] [Google Scholar]
- 21.Oppo K, Leen E, Angerson WJ, et al. Doppler perfusion index: an interobserver and intraobserver reproducibility study. Radiology 1998; 208: 453–457. [DOI] [PubMed] [Google Scholar]
- 22.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307–310. [PubMed] [Google Scholar]
- 23.Grothues F, Smith GC, Moon JC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol 2002; 90: 29–34. [DOI] [PubMed] [Google Scholar]
- 24.Addetia K, Bhave NM, Tabit CE, et al. Sample size and cost analysis for pulmonary arterial hypertension drug trials using various imaging modalities to assess right ventricular size and function end points. Circ Cardiovasc Imag 2014; 7: 115–124. [DOI] [PubMed] [Google Scholar]
- 25.Hor KN, Gottliebson WM, Carson C, et al. Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase imaging analysis. JACC Cardiovasc Imag 2010; 3: 144–151. [DOI] [PubMed] [Google Scholar]
- 26.Kowallick JT, Kutty S, Edelmann F, et al. Quantification of left atrial strain and strain rate using Cardiovascular Magnetic Resonance myocardial feature tracking: a feasibility study. J Cardiovasc Magn Reson 2014; 16: 60–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kowallick JT, Morton G, Lamata P, et al. Quantification of atrial dynamics using cardiovascular magnetic resonance: inter-study reproducibility. J Cardiovasc Magn Reson 2015; 17: 36–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kowallick JT, Morton G, Lamata P, et al. Inter-study reproducibility of left ventricular torsion and torsion rate quantification using MR myocardial feature tracking. J Magn Reson Imag 2016; 43: 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kowallick JT, Silva Vieira M, Kutty S, et al. Left Atrial performance in the course of hypertrophic cardiomyopathy: relation to left ventricular hypertrophy and fibrosis. Invest Radiol 2017; 52: 177–185. [DOI] [PubMed] [Google Scholar]
- 30.Schuster A, Paul M, Bettencourt N, et al. Cardiovascular magnetic resonance myocardial feature tracking for quantitative viability assessment in ischemic cardiomyopathy. Int J Cardiol 2013; 166: 413–420. [DOI] [PubMed] [Google Scholar]