Abstract
Background
Women with signs and symptoms of ischemia, no obstructive coronary artery disease (CAD) and preserved left ventricular ejection fraction (EF) often have diastolic dysfunction and experience elevated rates of major adverse cardiac events (MACE), including heart failure (HF) hospitalization with preserved ejection fraction (HFpEF). We evaluated the predictive value of inflammatory biomarkers for long-term HF hospitalization and all-cause mortality in these women.
Methods
We performed a cross-sectional analysis to investigate the relationships between inflammatory biomarkers [serum interleukin-6 (IL-6), C-reactive protein (hs-CRP) and serum amyloid A (SAA)] and median of 6 years follow-up for all-cause mortality and HF hospitalization among women with signs and symptoms of ischemia, non-obstructive CAD and preserved EF. Multivariable Cox regression analysis tested associations between biomarker levels and adverse outcomes.
Results
Among 390 women, mean age 56 ± 11 years, median follow up of 6 years, we observed that there is continuous association between IL-6 level and HF hospitalization (adjusted hazard ratio [AHR] 2.5 [1.2–5.0], p = 0.02). In addition, we found significant association between IL-6, SAA levels and all-cause mortality AHR (1.8 [1.1–3.0], p = 0.01) (1.5 [1.0–2.1], p = 0.04), respectively.
Conclusion
In women with signs and symptoms of ischemia, non-obstructive CAD and preserved EF, elevated IL-6 predicted HF hospitalization and all-cause mortality, while SAA level was only associated with all-cause mortality. These results suggest that inflammation plays a role in the pathogenesis of development of HFpEF, as well all-cause mortality.
Introduction
Signs and symptoms of ischemia in the absence of obstructive coronary artery disease (CAD) is highly prevalent in women and frequently associated with recurrent symptoms, repeated testing and coronary microvascular dysfunction (CMD) [1]. The pathophysiology and clinical determinants of these symptoms in such women have not been fully elucidated. However, release of vascular constricting factors and production of pro-inflammatory cytokines, cell adhesion molecules, and growth factors that, in turn, may induce inflammatory and proliferative changes in the vessel wall, has been suggested [2].
Women with signs and symptoms of ischemia and no obstructive CAD are at elevated risk for major adverse cardiac events (MACE), dominantly heart failure (HF) hospitalization [3] which we have documented to be heart failure with preserved ejection fraction (HFpEF) [4], and diastolic dysfunction [5, 6]. We have previously reported that inflammatory cytokines predict MACE but do not relate to angiographic CAD severity in women with preserved EF [7, 8]. Others have reported that elevated C-reactive protein levels correlate with symptoms and markers of myocardial ischemia in patients with chest pain and non-obstructive CAD [9]. While these observations suggest that systemic inflammation may be linked with CMD, relations to development of HFpEF have not been investigated.
Mechanistic pathways and treatment targets for HFpEF remain very poorly understood and evidence-based management strategies are lacking. Given the unexpected predominance of HFpEF hospitalization at 6-year follow-up in WISE [3, 4], we investigated the relationships between inflammatory markers [interleukin-6 (IL-6), C-reactive protein (hs-CRP), and serum amyloid A (SAA)] and longer-term adverse outcomes in this unique population of patients with signs and symptoms of ischemia with non-obstructive CAD and preserved EF as part of the Women’s Ischemia Syndrome Evaluation (WISE) study sponsored by the National Heart, Lung, and Blood Institute (NHLBI).
Methods
Study population and design
The WISE (ClinicalTrials.gov no. NCT00000554) design and protocol have been described in detail previously [10]. The study included 936 women referred for clinically indicated coronary angiography to further evaluate clinically stable symptoms/signs of myocardial ischemia at 4 sites (University of Alabama at Birmingham; University of Florida, Gainesville; University of Pittsburg; Allegheny General Hospital, Pittsburgh). Major exclusion criteria of the WISE study included cardiomyopathy, New York Heart Association class IV congestive heart failure, recent myocardial infarction, and significant valvular heart disease. For this analysis, participants with obstructive coronary artery disease (defined as >50% diameter stenosis in any vessel by QCA core lab) were excluded. The WISE study was approved by each institution’s review board and all participants gave written informed consent. Of the 936 women enrolled in the WISE study, a total of 456 women had non obstructive CAD and biomarker laboratory data, and 390 women had inflammatory biomarkers and follow-up information available for this analysis. Key demographic and laboratory variables were collected at baseline using standardized forms.
Laboratory testing
Plasma sampled at baseline was frozen at -70 0C and stored in a central core lab (Cedars-Sinai, LA, CA) for subsequent measurement of inflammatory markers. Levels of IL-6 was measured using a commercially available enzyme-linked immunosorbent assay kit (Quantikine hs human IL-6, R&D Systems, Minneapolis, Minn.). Levels of high-sensitivity CRP (hsCRP) and SAA were measured by a high-sensitivity method on the Hitachi 911 analyzer using reagents from Denka Seiken (Niigata, Japan). All samples were assayed within 5 years of collection at a core laboratory (Ridker P, Brigham and Women’s Hospital, Boston, Mass) using previously validated techniques [8].
Follow-up procedures
The initial protocol-specified follow-up was conducted by experienced site nurses or physicians through direct, telephone, and/ or mail contact at 6 weeks, 1 year, and annually thereafter using a standardized scripted interview. Women were queried about symptoms, medication use, cardiovascular outcomes, hospitalizations, and diagnostic or revascularization procedures since last contact. For cases cared for at a WISE clinical center, patients’ medical records were also reviewed. The median follow-up time among surviving patients was 6 years. Subsequently, we conducted a National Death Index search for all women who were thought to be alive, and obtained additional death certificates. For this analysis, a major event (MACE) was defined as a first occurrence of all-cause mortality, nonfatal myocardial infarction (MI), nonfatal stroke, or HF-hospitalization.
Statistical methods
Demographic and patient characteristic variables were summarized using means and standard deviations or percentages where appropriate. Distributions of inflammatory markers are known to be positively skewed, therefore, medians were used to estimate central tendencies and Spearman correlation was used to estimate correlation between markers. For time to event data, the Kaplan-Meier method and log-rank test were used to estimate the incidence rates of all-cause mortality, cardiovascular mortality and HF hospitalization. The hazard ratio for all biomarkers is reported per log change. First, we conducted univariate model to adjust for confounders including age, BMI, history of diabetes, smoking, history of malignancy, history of autoimmune disease and concomitant medications [7]. A multivariable Cox Proportional Hazards model was used after removing variables that are not significant. As a result, each outcome has different multivariable model.
Results
Baseline characteristics
Pertinent baseline characteristics are summarized in Table 1. The mean age was 56±11 years, most were Caucasian and postmenopausal and about half had used hormonal replacement therapy (HRT). Most had multiple CVD risk factors, including diabetes (16%), dyslipidemia (45%), family history of premature CAD (66%), smoking history (20%), obesity (mean BMI 29.9±6.8) and hypertension (mean systolic blood pressure 136±21). Overall, 55% of the women had hs-CRP values >0.3 mg/dL, and 21% had SAA >1.0 mg/dL, values that are considered abnormally high [11, 12].
Table 1. Baseline characteristics (n = 390).
| Age (mean±SD) | 56±11 |
|---|---|
| CVD risk factors (%) | |
| • Diabetes • History of dyslipidemia • Family history of premature CAD • Current smoking • BMI (kg/m2) (mean±SD) • Systolic blood pressure (mmHg)(mean±SD) |
• 16 • 45 • 66 • 20 • 30±7 • 136±21 |
| Chronic kidney disease (%) | 3 |
| History of malignancy (%) | 11 |
| Depression (%) | 27 |
| History of autoimmune disease (%) | 9 |
| Medications (%) | |
| • ACEI • ARB • Beta-blockers • Diuretics • Vasodilators • Aspirin • Corticosteroid • HMG-Co reductase inhibitors |
• 21 • 3 • 33 • 25 • 7 • 51 • 4 • 20 |
| Inflammatory biomarkers level (median) | |
| • IL-6 • hs-CRP* • SAA |
• 2.8 pg/mL (Range: 0.43–33.4 pg/mL) • 0.37 mg/dL (Range: 0.02–16.4 mg/dL) • 0.54 mg/dL (Range: 0.08–73 mg/dL) |
*Level of <0.1 mg/dL is considered low risk for atherosclerosis.
CAD: Coronary artery disease, CVD: cardiovascular disease, BMI: body mass index, ACEI: angiotensin converting enzyme inhibitors, ARB: angiotensin receptor blockers, IL-6: interlukin-6, hs-CRP: high sensitivity C-Reactive protein, SAA: serum amyloid A.
Adverse outcomes and inflammatory biomarkers
Among the 390 women with available follow-up data, 106 (23%) had a MACE over a median of 6 years follow up. These events included 45 deaths (11%), 28 of which (62%) were cardiovascular related causes. There was a total of 14 HF hospitalizations (3.5%), 19 nonfatal MI (4.9%), and 24 nonfatal stroke (6.2%). We observed a significant increase in all-cause mortality associated with IL-6 and SAA, while there was no significant association between hs-CRP and mortality (Table 2). There was also a significant association between IL-6 and HF hospitalization (Table 3).
Table 2. Adjusted relative risks (as continuous) for all-cause mortality from multivariable Cox regression analysis* (n = 390).
| Biomarker | HR (95% CI) | p-value |
|---|---|---|
| IL-6 | 1.8 (1.1–3.0) | 0.01 |
| hs-CRP | 1.1 (0.8–1.7) | NS |
| SAA | 1.5 (1.0–2.1) | 0.04 |
*adjusted for age, smoking history, diabetes, and the use of statins.
HR: hazard ratio per unit (log-scale) increase, CI: confidence interval, NS: non-significant, IL-6: interleukin-6, hs-CRP: high sensitivity C-reactive protein, SAA: Serum Amyloid A.
Table 3. Adjusted relative risks (as continuous) for HF hospitalization from multivariable Cox regression analysis* (n = 390).
| Biomarker | HR (95% CI) | p-value |
|---|---|---|
| IL-6 | 2.5 (1.2–5.0) | 0.04 |
| hs-CRP | 1.4 (0.9–2.3) | NS |
| SAA | 1.7 (1.0–3.0) | NS |
*adjusted for age, smoking history, diabetes, and the use of statins.
HR: hazard ratio per unit (log-scale) increase, CI: confidence interval, NS: non-significant, IL-6: interleukin-6, hs-CRP: high sensitivity C-reactive protein, SAA: Serum Amyloid A.
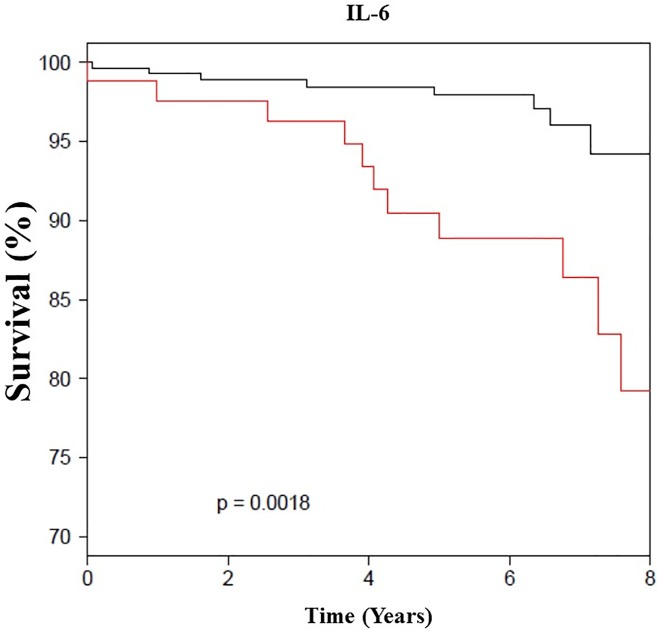
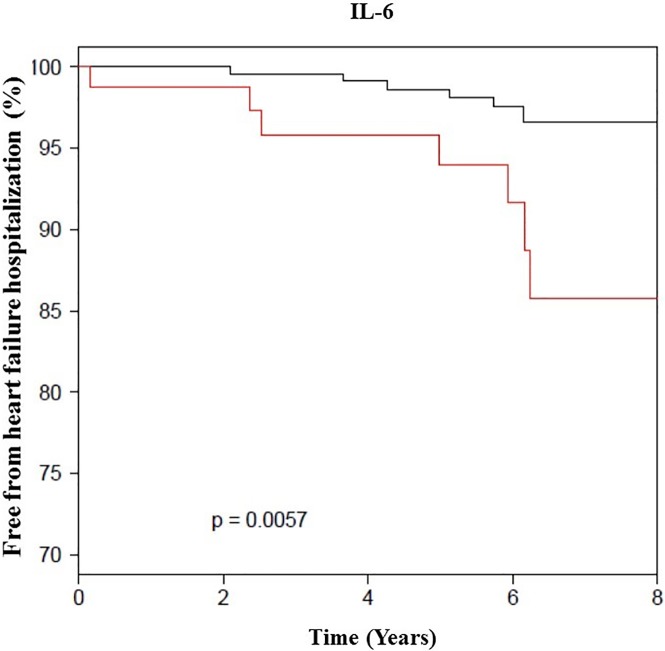
Each individual inflammatory biomarker was then grouped into quartiles and in combination to assess their relative contribution to mortality. When comparing IL-6 and SAA in their highest quartile (>4.79 pg/ml, >0.9 mg/dL, respectively) to the lowest quartile, there was significant increase in all-cause mortality AHR (4.2 [1.32–13.2], p = 0.02; 3.6 [1.3–10.3], 0.02), respectively (Fig 1). Among the 42 women who had IL-6, hs-CRP and SAA levels in their upper quartiles, 8 (20%) died compared to only one (3%) of 27 women who had all the three biomarkers in their lower quartile. Additionally, a level of IL-6 greater than 4.79 mg/dL was also highly predictive of HF hospitalization during the follow up period (Fig 2).
Fig 1. All-cause survival curves by IL-6 level (N = 390).
The black line indicates IL-6 level <4.79 pg/mL and the red line is when IL-6 ≥4.79 pg/mL among women with signs and symptoms of ischemia, no obstructive CAD and preserved EF (median follow up of 6 years).
Fig 2. HF hospitalization by IL-6 level (N = 390).
The black line indicates IL-6 level <4.79 pg/mL and the red line is when IL-6 ≥4.79 pg/mL among women with signs and symptoms of ischemia, no obstructive CAD and preserved EF (median follow up of 6 years).
Discussion
We observed in this unique population of women with signs and symptoms of ischemia, non-obstructive CAD and preserved EF at enrollment, IL-6 was the strongest predictor of HF hospitalization in these women compared to hs-CRP and SAA level, especially in the highest quartile (>4.79 pg/mL). In addition to IL-6 was associated with increase mortality, SAA was linked to high mortality as well which we reported previously [7]. Notably, IL-6 predicted mortality and HF hospitalization during follow up, independent of cardiovascular risk factors and comorbidities, consistent with the epidemic of HFpEF which is now a major contributor to CVD mortality [3, 4]. Previously, we found IL-6, hsCRP and SAA are predictors of CVD in women, however the prior report included women with obstructive and non-obstructive CAD with shorter follow-up [7, 8]. Although plasma levels of these inflammatory markers are known to be elevated in proportion to the degree of functional disability and left ventricular dysfunction, to our knowledge, no prior study has evaluated whether inflammatory biomarkers predict the development of HF among women with normal ventricular function and non-obstructive CAD.
The measurement of inflammatory markers for risk stratification of both primary and secondary prevention of cardiovascular disease has been the focus of numerous prior investigations. These efforts have been motivated by observations that inflammation is a key process in the pathophysiology of atherosclerosis, the development of acute cardiovascular syndromes, and heart failure [7]. Not only have inflammatory markers been found to be significant predictors of cardiovascular disease in asymptomatic women [13], they also correlate with clinical activity in patients with ischemic symptoms and non-obstructive CAD [9]. In an analysis from Framingham Heart Study, the role of elevated levels of inflammatory cytokines, notably IL-6, was assessed and associated with an increased long-term risk of HF development in previously asymptomatic patients [14]. These data are congruent with our findings and our study extends this finding to symptomatic women. Furthermore, baseline LV function was not reported in the Framingham Heart Study analysis, so it is possible that the study may have identified patients with subclinical LV dysfunction. We believe this observed relationship between IL-6 and HF warrants further investigations.
A potential mechanism to understand our observed association may be association between elevated levels of inflammatory cytokines and development of HF may include atherosclerosis, endothelial and microvascular dysfunction progression [15, 16]. Inflammatory cytokines cause modulation of matrix metalloproteinases, as well as platelet adhesion and aggregation, both of which have effect on plaque formation, plaque destabilization and rupture with proximal and distal vessel occlusion and embolization [17, 18]. IL-6 is the main inducer of hepatic production of acute-phase proteins, including hs-CRP and SAA. Both play a role in plaque formation [2] and are associated with biologic and environmental risk factors for cardiovascular events, including components of the metabolic syndrome (obesity, insulin resistance, diabetes, hypertension, and low HDL cholesterol levels), and lifestyle factors, such as smoking, abstinence from alcohol, and physical inactivity [19–21]. In addition, hs-CRP has a role in atherosclerosis by down-regulating nitric oxide release from endothelial cells [22–24] and stimulating endothelin-1 and IL-6 secretion, resulting in increased expression of adhesion molecules, stimulating monocyte chemoattractant protein-1, and facilitating macrophage low-density lipoprotein uptake [23, 24]. Others have also suggested that SAA modulates HDL metabolism and may be involved in diminishing its athero-protective effect [24].
A variety of inflammatory markers have been previously identified as having a potential role in the progression of HF. We found that in this unique population of women with symptomatic ischemia, non-obstructive CAD and preserved EF at baseline, serum IL-6 was associated with an increased risk of HF hospitalization in a continuous fashion without evidence of a threshold. The mechanism is not well understood in our cohort, however elevation of such markers may be a driver or a reaction to the pathophysiological processes of progressive ventricular remodeling. Experimental data have implicated a causative role of IL-6 in cardiomyocyte hypertrophy and apoptosis [25, 26], adverse LV remodeling [27], and progression to HF [28]. IL-6 also exerts a negative inotropic effect on cardiac myocytes by augmentation of nitric oxide and peroxynitrite formation [29] and by inhibition of sarcoplasmic reticulum Ca-ATPase (SERCA2). Overall, these data suggest that future prospective evaluation of inflammatory biomarkers for clinical prognostic utility and as a mechanistic pathway for HF progression would be important.
Strengths and limitations
Strengths of our study include a prospective, multicenter design and core laboratory masked assessments of inflammatory markers and coronary angiograms with long subject follow-up of 6 years. This allowed exclusion of obstructive CAD to investigate development of HF hospitalization in a unique population of women with signs and symptoms of ischemia, without obstructive CAD and preserved EF. A potential limitation is the highly selective study population consisting of middle age predominantly Caucasian women with suspected myocardial ischemia referred for specialized centers for clinically indicated coronary angiography. Thus our results may not be generalizable to other populations of women or to men. In addition, there were small number of events during the follow-up period. Furthermore, diagnosis of HF was defined as admission for HF, which was validated in a representative sample from one WISE site using patient hospital records [5]. Thus, data about patients with milder forms of HF, which could be managed in an outpatient basis, were not collected. Lastly, 7% of our cohort was lost to follow-up, although this is less than what had been reported to pose serious impact on the results [30]. We performed statistical comparison of baseline characteristics between patient with and without available follow-up data (lost to follow-up). There was no difference between the two groups.
Conclusions
In women with signs and symptoms of ischemia, non-obstructive CAD and preserved EF, the inflammatory biomarker IL-6 was predictive of HF-hospitalization and all-cause mortality, while SAA predicted all-cause mortality. These results suggest that vascular inflammation may be a mechanistic pathway and treatment target for HFpEF, which is a rising epidemic contributing to CVD morbidity and mortality which predominantly impacts women.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by contracts from the National Heart, Lung and Blood Institutes nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, K23HL105787, T32HL69751, R01 HL090957, 1R03AG032631 from the National Institute on Aging, GCRC grant MO1-RR00425 from the National Center for Research Resources, the National Center for Advancing Translational Sciences Grant UL1TR000124 and UL1TR001427, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, and QMED, Inc., Laurence Harbor, NJ, the Edythe L. Broad and the Constance Austin Women’s Heart Research Fellowships, Cedars-Sinai Medical Center, Los Angeles, California, the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, The Society for Women’s Health Research (SWHR), Washington, D.C., The Linda Joy Pollin Women’s Heart Health Program, and the Erika Glazer Women’s Heart Health Project, Cedars-Sinai Medical Center, Los Angeles, California. This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or National Institutes of Health.
References
- 1.Marroquin OC, Kip KE, Mulukutla SR, Ridker PM, Pepine CJ, Tjandrawan T, et al. Inflammation, endothelial cell activation, and coronary microvascular dysfunction in women with chest pain and no obstructive coronary artery disease. American heart journal. 2005;150(1):109–15. Epub 2005/08/09. doi: 10.1016/j.ahj.2004.08.003 [DOI] [PubMed] [Google Scholar]
- 2.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, et al. Inflammatory markers and the risk of coronary heart disease in men and women. The New England journal of medicine. 2004;351(25):2599–610. Epub 2004/12/17. doi: 10.1056/NEJMoa040967 [DOI] [PubMed] [Google Scholar]
- 3.Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women's Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Archives of internal medicine. 2009;169(9):843–50. Epub 2009/05/13. PubMed Central PMCID: PMCPMC2782882. doi: 10.1001/archinternmed.2009.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakir M, Nelson MD, Jones E, Li Q, Wei J, Sharif B, et al. Heart failure hospitalization in women with signs and symptoms of ischemia: A report from the women's ischemia syndrome evaluation study. International journal of cardiology. 2016;223:936–9. Epub 2016/09/03. doi: 10.1016/j.ijcard.2016.07.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vilaro HT JR, cooper-DeHoff RM, HAndberg E, Sopko G, Kelsey S, Bairey Merz CN, Pepine CJ. Brachial Pulse Pressure Predicts Adverse Outcomes Including Heart Failure Hospitalizations in Women with Preserved Ejection Fraction and No Obstructive Coronary Artery Disease: A report from the Women’s Ischimia Syndrome Evaluation. Circulation. 2014;2014; 130(A11580). [Google Scholar]
- 6.Wei J, Nelson MD, Szczepaniak EW, Smith L, Mehta PK, Thomson LE, et al. Myocardial steatosis as a possible mechanistic link between diastolic dysfunction and coronary microvascular dysfunction in women. American journal of physiology Heart and circulatory physiology. 2016;310(1):H14–9. Epub 2015/11/01. doi: 10.1152/ajpheart.00612.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson BD, Kip KE, Marroquin OC, Ridker PM, Kelsey SF, Shaw LJ, et al. Serum amyloid A as a predictor of coronary artery disease and cardiovascular outcome in women: the National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE). Circulation. 2004;109(6):726–32. Epub 2004/02/19. doi: 10.1161/01.CIR.0000115516.54550.B1 [DOI] [PubMed] [Google Scholar]
- 8.Kip KE, Marroquin OC, Shaw LJ, Arant CB, Wessel TR, Olson MB, et al. Global inflammation predicts cardiovascular risk in women: a report from the Women's Ischemia Syndrome Evaluation (WISE) study. American heart journal. 2005;150(5):900–6. Epub 2005/11/18. doi: 10.1016/j.ahj.2005.02.002 [DOI] [PubMed] [Google Scholar]
- 9.Cosin-Sales J, Pizzi C, Brown S, Kaski JC. C-reactive protein, clinical presentation, and ischemic activity in patients with chest pain and normal coronary angiograms. Journal of the American College of Cardiology. 2003;41(9):1468–74. Epub 2003/05/14. [DOI] [PubMed] [Google Scholar]
- 10.Merz CN, Kelsey SF, Pepine CJ, Reichek N, Reis SE, Rogers WJ, et al. The Women's Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. Journal of the American College of Cardiology. 1999;33(6):1453–61. Epub 1999/05/20. [DOI] [PubMed] [Google Scholar]
- 11.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. Epub 2003/01/29. [DOI] [PubMed] [Google Scholar]
- 12.Gillmore JD, Lovat LB, Persey MR, Pepys MB, Hawkins PN. Amyloid load and clinical outcome in AA amyloidosis in relation to circulating concentration of serum amyloid A protein. Lancet (London, England). 2001;358(9275):24–9. Epub 2001/07/17. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. The New England journal of medicine. 2000;342(12):836–43. Epub 2000/03/25. doi: 10.1056/NEJM200003233421202 [DOI] [PubMed] [Google Scholar]
- 14.Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, et al. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107(11):1486–91. Epub 2003/03/26. [DOI] [PubMed] [Google Scholar]
- 15.Hartman J, Frishman WH. Inflammation and atherosclerosis: a review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiology in review. 2014;22(3):147–51. Epub 2014/03/13. doi: 10.1097/CRD.0000000000000021 [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr., Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. The New England journal of medicine. 2008;359(21):2195–207. Epub 2008/11/11. doi: 10.1056/NEJMoa0807646 [DOI] [PubMed] [Google Scholar]
- 17.Szmitko PE, Wang CH, Weisel RD, Jeffries GA, Anderson TJ, Verma S. Biomarkers of vascular disease linking inflammation to endothelial activation: Part II. Circulation. 2003;108(17):2041–8. Epub 2003/10/29. doi: 10.1161/01.CIR.0000089093.75585.98 [DOI] [PubMed] [Google Scholar]
- 18.Shainkin-Kestenbaum R, Zimlichman S, Lis M, Lidor C, Pomerantz M, Knyszynski A, et al. Effect of serum amyloid A, HDL-apolipoprotein, on endothelial cell proliferation. Implication of an enigmatic protein to atherosclerosis. Biomedical peptides, proteins & nucleic acids: structure, synthesis & biological activity. 1996;2(3):79–84. Epub 1996/01/01. [PubMed] [Google Scholar]
- 19.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107(3):391–7. Epub 2003/01/29. [DOI] [PubMed] [Google Scholar]
- 20.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Rimm EB. Leisure-time physical activity and reduced plasma levels of obesity-related inflammatory markers. Obesity research. 2003;11(9):1055–64. Epub 2003/09/16. doi: 10.1038/oby.2003.145 [DOI] [PubMed] [Google Scholar]
- 21.Imhof A, Froehlich M, Brenner H, Boeing H, Pepys MB, Koenig W. Effect of alcohol consumption on systemic markers of inflammation. Lancet (London, England). 2001;357(9258):763–7. Epub 2001/03/20. [DOI] [PubMed] [Google Scholar]
- 22.Verma S, Wang CH, Li SH, Dumont AS, Fedak PW, Badiwala MV, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106(8):913–9. Epub 2002/08/21. [DOI] [PubMed] [Google Scholar]
- 23.Verma S, Kuliszewski MA, Li SH, Szmitko PE, Zucco L, Wang CH, et al. C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation. 2004;109(17):2058–67. Epub 2004/04/14. doi: 10.1161/01.CIR.0000127577.63323.24 [DOI] [PubMed] [Google Scholar]
- 24.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148(2):209–14. Epub 2000/02/05. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Wang HY, Bassel-Duby R, Maass DL, Johnston WE, Horton JW, et al. Role of interleukin-6 in cardiac inflammation and dysfunction after burn complicated by sepsis. American journal of physiology Heart and circulatory physiology. 2007;292(5):H2408–16. Epub 2007/01/16. doi: 10.1152/ajpheart.01150.2006 [DOI] [PubMed] [Google Scholar]
- 26.Wollert KC, Chien KR. Cardiotrophin-1 and the role of gp130-dependent signaling pathways in cardiac growth and development. Journal of molecular medicine (Berlin, Germany). 1997;75(7):492–501. Epub 1997/07/01. [DOI] [PubMed] [Google Scholar]
- 27.Hirota H, Yoshida K, Kishimoto T, Taga T. Continuous activation of gp130, a signal-transducing receptor component for interleukin 6-related cytokines, causes myocardial hypertrophy in mice. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(11):4862–6. Epub 1995/05/23. PubMed Central PMCID: PMCPMC41807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janssen SP, Gayan-Ramirez G, Van den Bergh A, Herijgers P, Maes K, Verbeken E, et al. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation. 2005;111(8):996–1005. Epub 2005/02/16. doi: 10.1161/01.CIR.0000156469.96135.0D [DOI] [PubMed] [Google Scholar]
- 29.Yu XW, Liu MY, Kennedy RH, Liu SJ. Both cGMP and peroxynitrite mediate chronic interleukin-6-induced negative inotropy in adult rat ventricular myocytes. The Journal of physiology. 2005;566(Pt 2):341–53. Epub 2005/05/10. PubMed Central PMCID: PMCPMC1464742. doi: 10.1113/jphysiol.2005.087478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kristman V, Manno M, Cote P. Loss to follow-up in cohort studies: how much is too much? European journal of epidemiology. 2004;19(8):751–60. Epub 2004/10/08. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.