Cardiac amyloidosis exists in 2 predominant forms: acquired monoclonal immunoglobulin light-chain (AL) and transthyretin-related (familial and wild-type/senile) amyloid (ATTR). Differentiation is important because they have different prognoses and are amenable to different treatment strategies. Cardiovascular magnetic resonance (CMR) is increasingly used to aid in the diagnosis of amyloid alongside histology on the basis of characteristic appearances on late gadolinium enhancement (LGE). CMR cannot distinguish between AL and ATTR amyloid; however, this is possible with single-photon emission computed tomography using bone tracers (99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid [DPD], 99mTc-Hydroxymethylene diphosphonate [HMDP], 99mTc-pyrophosphate [PYP]) that preferentially bind to ATTR versus AL deposits (1,2). We hypothesized that hybrid positron emission tomography/magnetic resonance (PET/MR) imaging using the PET bone tracer 18F-sodium fluoride might aid in both the diagnosis of cardiac amyloidosis and differentiation of ATTR and AL forms within a single, low-radiation scan. A previous case report had failed to demonstrate increased 18F-sodium fluoride uptake in ATTR but lacked a control group and accurate coregistration of PET activity to the myocardium (3).
Fourteen subjects (60.9 ± 11.7 years of age, 10 men) were prospectively recruited, comprising 4 patients with biopsy-proven ATTR (2 ILE122 mutated, 2 wild type), 3 with biopsy-proven AL amyloid, and 7 control subjects without clinical suspicion of amyloid. The Institutional Review Board approved the study (GCO#01-1032), and all patients gave written informed consent. Each patient underwent simultaneous 18F-sodium fluoride PET and CMR of the heart on a hybrid PET/MR system (Biograph mMR, Siemens, Erlangen, Germany). Dynamic PET data were acquired in list mode using a 90-min bed time, starting 5 min following administration of 386 ± 69 MBq of 18F-sodium fluoride. The last 30 min were binned to produce a static image for evaluation. The MR protocol included long- and short-axis cine imaging, LGE (10 min following administration of 0.02 mmol/kg; MultiHance, Siemens) and T1 mapping. Image analysis was performed on fused, coregistered PET and MR LGE images that allowed PET activity to be measured within the left ventricular myocardium and specific areas of amyloid deposition visualized on LGE. This is not possible with standard single-photon emission computed tomography or PET techniques. Myocardial PET uptake was quantified using standard uptake values and target-to-background ratio after correction for blood-pool activity in the right ventricle.
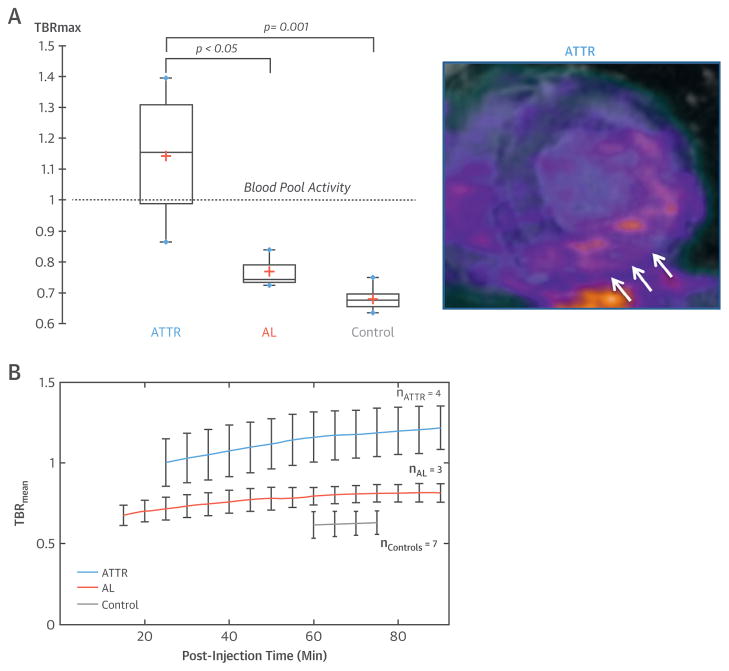
PET/MR imaging was well tolerated in all patients, and associated with an average radiation exposure of 7.3 mSv. All amyloid patients (67 ± 8 years of age, 6 men, ejection fraction 58 ± 7%) had characteristic LGE appearances with diffuse myocardial uptake. This was not observed in any of the control patients (55 ± 12 years, 4 men). The amyloid burden assessed by T1 mapping was similar in patients with AL and ATTR amyloid (native T1 1405 ± 26 ms vs. 1464 ± 160 ms, p = 0.57; post-contrast 311 ± 99 ms vs. 314 ± 23 ms, p = 0.94). Patchy areas of increased 18F-sodium fluoride uptake were observed in the myocardium of patients with ATTR but not the other 2 groups. In these subjects, a correlation between the burden of ATTR on native T1 mapping and 18F-sodium fluoride PET uptake was observed (r2 = 0.93; p = 0.04). In control subjects myocardial 18F-sodium fluoride standard uptake values were lower than blood pool (maximum target-to-background ratio [TBRmax] = 0.68 ± 0.04). Myocardial target-to-background ratio values in patients with ATTR (TBRmax = 1.14 ± 0.24) were 68% higher than control subjects and 48% higher than patients with AL amyloid (TBRmax = 0.77 ± 0.06). Notwithstanding the small cohort size, which might limit the representativeness of our statistical analysis, a TBRmax threshold of >0.84 appeared to differentiate all patients as having ATTR. Dynamic imaging revealed that the optimum contrast between blood pool and myocardial uptake in ATTR patients was observed ≥60 min, because of the faster, ongoing rise in myocardial PET activity observed (Figure 1).
FIGURE 1. 18F-Sodium Fluoride PET/MR for Cardiac Amyloidosis.
(A) Short-axis hybrid positron emission tomography/magnetic resonance (PET/MR) image demonstrating increased 18F-sodium fluoride uptake in a patient with transthyretin-related (familial) amyloid (ATTR) colocalizing to areas of late gadolinium enhancement (white arrows) in the inferolateral wall. Maximum target-to-background ratio (TBRmax) values in patients with ATTR were 48% higher than subjects with acquired monoclonal immunoglobulin light-chain (AL) amyloid and 68% higher than controls, both of whom demonstrated low myocardial uptake, below blood-pool levels (target-to-background ratio = 1.0). (B) Dynamic imaging assessing mean target-to-background ratio (TBRmean) values over time confirmed clear discrimination between the 3 groups, with greatest separation ≥60 min post-injection.
In conclusion, myocardial uptake of 18F-sodium fluoride is low, less than the blood pool, in control subjects and patients with AL amyloid. By contrast, increased 18F-sodium fluoride uptake is observed in areas of ATTR colocalizing to regions of LGE. Hybrid 18F-sodium fluoride PET/MR has the potential to aid in the diagnosis of cardiac amyloidosis, identify characteristic LGE, establish systolic function, and differentiate AL and ATTR all within a single accurately coregistered scan. Larger studies are now required for confirmation.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants NIH/NHLBI R01HL071021 (to Dr. Fayad) and NIH 5T32HL007824-18 (to Dr. Trivieri), and by British Heart Foundation grants SS/CH/09/002/26360, FS/13/77/30488, SS/CH/09/002/2636, FS/14/78/31020, CH/09/002 (to Dr. Dweck). Dr. Dweck was the recipient of the 2015 Sir Jules Thorn Award for Biomedical Research 2015. 15/JTA.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Rapezzi C, Quarta CC, Guidalotti PL, et al. Role of (99m)Tc-DPD scintigraphy in diagnosis and prognosis of hereditary transthyretin-related cardiac amyloidosis. J Am Coll Cardiol Img. 2011;4:659–70. doi: 10.1016/j.jcmg.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133:2404–12. doi: 10.1161/CIRCULATIONAHA.116.021612. [DOI] [PubMed] [Google Scholar]
- 3.Gagliardi C, Tabachhi E, Bonfiglioli R, et al. Does the etiology of cardiac amyloidosis determine the myocardial uptake of [18F]-NaF PET/CT? J Nucl Cardiol. 2016 Mar 14; doi: 10.1007/s12350-016-0457-8. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]