Abstract
Cadmium (Cd) toxicity affects numerous metabolic processes in plants. In the presence of Cd, plants accumulate specific amino acids which may be beneficial to developing Cd tolerance. Our study aimed to characterize the changes in the metabolism of selected free amino acids that are associated with Cd tolerance, and investigate the levels of selected microelements in order to relate these changes to the adaptation strategies of two metallophytes—Noccaea caerulescens (Redlschlag, Austria) and Noccaea praecox (Mežica, Slovenia). The plants were exposed to Cd contamination (90 mg Cd/kg soil) for 120 days in a pot experiment. Our results showed higher Cd accumulation in N. praecox compared to N. caerulescens. Cadmium contamination reduced the zinc and nickel levels in both species and a mixed effect was determined for copper and manganese content. Differences in free amino acid metabolism were observed between the two metallophytes growing under Cd-free and Cd-loaded conditions. Under Cd-free conditions, aromatic amino acids (phenylalanine, tryptophan and tyrosine) and branched-chain amino acids (leucine, isoleucine and valine) were accumulated more in the leaves of N. praecox than in N. caerulescens. Cd stress increased the content of these amino acids in both species but this increase was significant only in N. caerulescens leaves. Marked differences in the responses of the two species to Cd stress were shown for alanine, phenylalanine, threonine and sarcosine. Cadmium contamination also induced an increase of threonine as alanine and sarcosine decrease, which was larger in N. caerulescens than in N. praecox. All these factors contribute to the higher adaptation of N. praecox to Cd stress.
Introduction
Cadmium (Cd) is one of the most dangerous heavy metals for nearly all organisms due to its high solubility in water and toxicity even at very low concentrations [1]. Although Cd is toxic to plant growth, it is easily taken up by the roots and translocated to the shoots [2]. Uptake of Cd ions from the soil seems to occur mainly via non-selective transporters of calcium (Ca), iron (Fe), manganese (Mn) and zinc (Zn) [3]. Therefore, Cd may affect nutrient metabolism (uptake, transport and use) and interfere with essential mineral nutrients such as Ca, copper (Cu), Fe, potassium (K), nickel (Ni), magnesium (Mg), Mn and Zn [4–7]. These elements form part of important biomolecules—metalloenzymes and metalloproteins [8]. Copper, Mn, Ni and Zn were found in metalloenzymes as cofactors [8–10]. Metalloenzymes contain an essential metal ion cofactor in their catalytic active sites, forming biological metal complexes that perform a wide range of important functions such as the activation of small molecules, atom transfer chemistry, and the control of oxidation equivalents [8]. Metalloenzymes (superoxide dismutase, urease and cytochrome P450) can also significantly affect the resistance of plants against stress conditions [11, 12].
Many studies have examined the effect of Cd exposure on the physiology and metabolism of plants [1, 2, 6, 7, 13–18]. Cd affects metabolism of amino acids (AA) and organic acids in plants [2, 15]. Amino acids play significant roles in metal binding, antioxidant defence and signalling in plants during heavy metal stress [15, 16]. The metabolism of AA plays an important role in intracellular pH regulation, especially alanine (Ala) and γ-aminobutyrate (GABA) [19–22]. An important role in the growth of plant cell walls and stress adaptation is played by polypeptides and proteins with high contents of proline (Pro), hydroxyproline, glycine (Gly), cysteine, leucine (Leu) and methionine (Met) [23]. Branched-chain AA (BCAA), i.e. Leu, isoleucine (Ile) and valine (Val), play a role during osmotic stress and their accumulation points to the tight regulation of BCAA catabolism by environmental perturbations. Additionally, acetyl-CoA, propionyl-CoA, and acetoacetate, which are the breakdown products of BCAA, are potential energy sources for plants [24]. Phenylalanine (Phe), tyrosine (Tyr) and tryptophan (Trp) are aromatic AA derived from the shikimate pathway and are required for protein synthesis and production of aromatic secondary metabolites, e.g. anthocyanin [25–27], which are important for cell wall extensibility [28]. The AA sarcosine (Sar, N-methylglycine) is an intermediate of trimethylglycine (glycine betaine). Glycine betaine is known to be a metabolite that accumulates during oxidative stress [29]; therefore, Sar declines as a result of the methylation of Sar [30] or due to the lack of Gly caused by the reaction of Gly and choline [31]. Thus, a decline in Sar is related to the biosynthesis of glycine betaine and plant defence against oxidative stress. According to the literature, Sar complexation with metals is one of many functions of these specific AA; however, such complexation has not been confirmed in plants (increase of Sar with increasing toxic Cd content). We assume that one of the functions of Sar is its role as a methyl donor for antioxidative metabolites in plant stress metabolism (decrease of Sar with increasing Cd content).
Free AA have been shown to have functional roles in plant stress tolerance; therefore, our study focused on investigating the changes in the selected free AA of hyperaccumulating plants (Noccaea caerulescens and N. praecox) growing on an environmentally relevant substrate—soil—that occur under chronic stress caused by Cd. The main objective was to characterise changes in AA metabolism that are associated with plant tolerance, and in the contents of selected microelements, in order to relate these changes to the adaptation strategies of N. caerulescens and N. praecox growing under Cd stress. The experiment was aimed at AA that are important in: (i) stress responses associated with carbon (C) and nitrogen (N) metabolism (Ala, GABA and ornithine [Orn]); (ii) adaptive responses leading to pH cytosolic regulation (Ala and GABA); (iii) balancing the ratio between different AA pathways (Leu, Ile and Val) to the maintenance of homeostasis; (iv) catabolism of aromatic AA (Phe, Tyr and Trp), which is coupled with the phenylpropanoid pathway and generates antioxidant metabolites; and (v) complexation reactions with Cd and other metals (Sar).
Material and methods
Plant material and cultivation conditions
Noccaea caerulescens (formerly Thlaspi caerulescens J. & C. Presl, F. K. Mey) and Noccaea praecox (formerly Thlaspi praecox Wulfen, F.K. Mey) are metallophytes that are both able to grow on metal-contaminated soils [32] and accumulate certain trace metals in parts growing above ground [7, 33]. N. praecox from Mežica, Slovenia and N. caerulescens from Redlschlag, Austria were used in the pot experiments. Characteristics of the Mežica and Redlschlag sites were published previously [34, 35].
For the cultivation of Noccaea plants, 3 kg of soil from the non-polluted site, Prague-Demonstration Field of Czech University of Life Sciences-Výhledy II (see Table 1 for details), was mixed with a nutrient solution consisting of 0.3 g N as NH4NO3, 0.1 g P and 0.24 g K as K2HPO4. To the samples to be considered “contaminated”, a Cd solution with a concentration of 90 mg Cd/kg of soil as Cd(NO3)2·4H2O was added. Plants were cultivated in a greenhouse under controlled conditions with a 16 hour day/8 hour night cycle and a temperature cycle of 24°C day/18°C night. The water regime was controlled, and the soil moisture was maintained at 60% maximum water-holding capacity. Each treatment was performed in five replications. Plants were harvested 120 days after Cd application. Samples were kept frozen in liquid N for transport and then at –30°C until extraction.
Table 1. Characteristics and total initial Cd concentration in experimental soil.
| Soil type/subtype | pHKCl | CEC (mmol(+)/kg) | Corg (%) | CdT (mg/kg) |
|---|---|---|---|---|
| Chernozem/modal | 7.2 ± 0.1 | 258 ± 2.8 | 1.83 ± 0.01 | 0.42 ± 0.05 |
CEC, cation exchange capacity; Corg, organic carbon; CdT, total content of Cd.
Free amino acid analysis
For the analyses of AA, 0.5 ± 0.05 g samples of fresh biomass were extracted in 10.5 mL of methanol + double distilled H2O (7:3, v/v) for 24 hours. Derivatisation of free AA was performed using the EZ:faast set (Phenomenex, USA) following the manufacturer’s instructions. The content of free AA was determined by gas chromatography-mass spectrometry ([GC-MS] Hewlett Packard 6890N/5975 MSD, Agilent Technologies, USA) with a ZB-AAA 10 m x 0.25 mm AA analysis GC column and a constant carrier gas flow (He, 1.1 mL/min). The oven temperature program and MS conditions were the same as described by Pavlík et al. [36].
Accumulation of cadmium and microelements
Plant samples (0.5 ± 0.05 g dry biomass) were decomposed using the dry ashing procedure. The ash was dissolved in 20 mL of 1.5% HNO3 (v/v) (Analytika Ltd., CZ) and the contents of Cd, Cu, Mn, Ni and Zn were determined by inductively coupled plasma optical emission spectroscopy with axial plasma configuration (Varian VistaPro, Varian, Australia). Certified reference material RM NCS DC 73350, poplar leaves (purchased from Analytika, CZ), were mineralised under the same conditions for quality assurance.
Statistical analysis
The statistical analysis was performed using Statistica 9.0 software (www.statsoft.com). Presented data are the mean values ± standard error (SE). All results were tested by one-way analysis of variance (ANOVA) considering interactions at the 95% significance level (p < 0.05) with subsequent Tukey’s honest significant difference (HSD) test and by correlation (R2, p < 0.05). A principal component analysis (PCA), in the CANOCO 4.5 program [37], was applied to all collected data as a single set. We used standardisation of species because data of different character were analysed together. PCA was used to make correlations between analysed data and similarity of different treatments visible from the complex data set. The results were visualised in the form of a bi-plot ordination diagram using the CanoDraw program.
Results
Effect of Cd on plant biomass and Cd accumulation
As reported in our previous paper [38], Cd induced a greater reduction of yield of above-ground biomass in N. caerulescens than in N. praecox. Noccaea species showed significant differences of Cd content in plant biomass. The results presented in Table 2 show that N. praecox accumulated significantly higher Cd content (7045 mg Cd/kg dry weight) than N. caerulescens (134 mg Cd/kg dry weight). The phytoextraction potential of Noccaea species is indicated by the remediation factor (RF), which reflects the amount of metal extracted by plants from the soil during vegetation period. For both plant species, the RF was calculated as follows (1):
| (1) |
where Cdplant is the content of Cd in plant dry biomass (mg/kg), DMplant the dry weight plant biomass yield (kg), Cdsoil the total concentration of Cd in the soil (mg/kg) and wsoil the amount of soil in the pot (kg), modified according to Zárubová et al. [39]. The RF of Cd for N. praecox and N. caerulescens was 12.6% and 0.26%, respectively.
Table 2. The Cd and microelements content (mg/kg DW) in the above-ground biomass of plants.
| Variable | Treatment | |||
|---|---|---|---|---|
| N. praecox | N. praecox-Cd | N. caerulescens | N. caerulescens-Cd | |
| Cd | 26.4aB ± 0.7 | 7045bB ± 66 | 2.4aA ± 0.05 | 134bA ± 6 |
| Zn | 543aA ± 46 | 539aB ± 38 | 495bA ± 29 | 201aA ± 16 |
| Cu | 5.4aA ± 0.5 | 6.0bA ± 0.3 | 6.8aB ± 0.7 | 6.2aA ± 0.6 |
| Mn | 70.1aA ± 11.0 | 76.2aA ± 9.2 | 110.8aB ± 8.5 | 108.4aB ± 7.8 |
| Ni | 71.6bA ± 6.7 | 42.8aB ± 3.2 | 104.5bB ± 6.7 | 9.2aA ± 0.9 |
Treatment abbreviations: N. praecox, Noccaea praecox control plants without cadmium; N. praecox-Cd, Noccaea praecox plants with Cd solution in soil with a concentration of 90 mg Cd/kg of soil; N. caerulescens, Noccaea caerulescens control plants without cadmium; N. caerulescens-Cd, Noccaea caerulescens plants with Cd solution in soil with a concentration of 90 mg Cd/kg of soil.
The values represent the means of data obtained in the experiment (n = 5). Different letters indicate values that are significantly different (p < 0.05):
A, B comparison between the two species with the same treatment (growing with or without Cd solution applied to the tested soil)
a, b comparison between treatments in each species.
Effect of Cd on microelements content
The microelement contents of N. praecox and N. caerulescens were significantly different (Table 2). The trends of Ni and Zn changes were similar in both plant species; however, the trends of Cu and Mn changes were opposite. High cation contents were seen in the control treatment of N. caerulescens. Manganese and Ni contents in the N. caerulescens control treatment were significantly higher than those in the N. praecox control treatment (58% and 46% higher, respectively). Cadmium contamination had an effect on the content of some microelements in the above-ground biomass. Cadmium reduced the contents of Zn and Ni but did not significantly alter Cu and Mn contents in any of the treatments. Zinc and Ni contents were significantly affected in Cd-exposed N. caerulescens (59% Zn reduction; 91% Ni reduction), while Cd-exposed N. praecox showed reductions of only 1% for Zn and 40% for Ni.
Effect of Cd on free amino acids content
The contents of Ala varied according to species and according to the Cd content in the plants (Table 3 and Fig 1). Decreases of Ala contents were apparent in both plants species growing on the Cd treatments (N. praecox by 15% and N. caerulescens by 19% compared to control treatments). Lower Ala contents were confirmed for N. praecox treatments than in N. caerulescens treatments. A 22% lower content of Ala was determined in N. praecox than in N. caerulescens controls and a 19% lower content in N. praecox-Cd than N. caerulescens-Cd.
Table 3. The concentrations of selected free amino acids (free AA; μmol/kg FW) in the above-ground biomass of plants.
| Free AA | Treatment | |||
|---|---|---|---|---|
| N. praecox | N. praecox-Cd | N. caerulescens | N. caerulescens-Cd | |
| Ala | 772aA ± 2 | 652bA ± 65 | 994bB ± 39 | 805aB ± 59 |
| GABA | 368aA ± 6 | 382aA ± 49 | 333aA ± 39 | 392aA ± 54 |
| Phe | 609aB ± 31 | 625aA ± 38 | 561aA ± 39 | 770bB ± 41 |
| Tyr | 625aA ± 71 | 643aA ± 68 | 599aA ± 57 | 789bB ± 39 |
| Trp | 714aA ± 56 | 747aA ± 42 | 630aA ± 46 | 800bA ± 57 |
| Ile | 137aA ± 15 | 139aA ± 11 | 120aA ± 17 | 168bA ± 19 |
| Leu | 560aA ± 34 | 573aA ± 38 | 537aA ± 31 | 651bA ± 41 |
| Val | 808aB ± 75 | 728aA ± 59 | 601aA ± 54 | 568aA ± 50 |
| Gly | 471aA ± 42 | 446aA ± 35 | 578bB ± 41 | 449aA ± 58 |
| Orn | 180aA ± 1 | 312bA ± 23 | 287aB ± 25 | 336aA ± 31 |
| Thr | 822aA ± 9 | 875aA ± 35 | 1440aB ± 50 | 2348bB ± 92 |
Free amino acid abbreviations: Ala, alanine; GABA, γ-aminobutyric acid; Phe, phenylalanine; Tyr, tyrosine; Trp, tryptophan; Ile, isoleucine; Leu, leucine; Val, valine; Gly, glycine; Orn, ornithine; Thr, threonine.
Treatment abbreviations: N. praecox, Noccaea praecox control plants without cadmium; N. praecox-Cd, Noccaea praecox plants with Cd solution in soil with a concentration of 90 mg Cd/kg of soil; N. caerulescens, Noccaea caerulescens control plants without cadmium; N. caerulescens-Cd, Noccaea caerulescens plants with Cd solution in soil with a concentration of 90 mg Cd/kg of soil.
The values represent the means of data obtained in the experiment (n = 5). Different letters indicate values that are significantly different (p < 0.05):
A, B comparison between the two species with the same treatment (growing with or without Cd solution applied to the tested soil)
a, b comparison between treatments in each species.
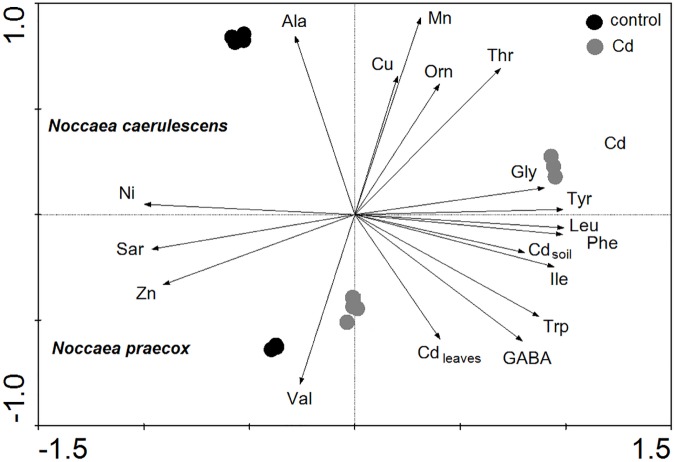
Fig 1. Ordination diagram showing the results of PCA analysis with content of Cd, microelements and selected free amino acids in above-ground biomass of plants.
Cdleaves, total content of Cd in leaves of plants; Cdsoil, content of Cd in soil; Cu, Mn, Ni and Zn, total content of elements; Ala, content of alanine; GABA, content of γ-aminobutyrate; Gly, content of glycine; Ile, content of isoleucine; Leu, content of leucine; Orn, content of ornithine; Phe, content of phenylalanine; Sar, content of sarcosine; Thr, content of threonine; Trp, content of tryptophan; Tyr, content of tyrosine and Val, content of valine.
GABA contents typically increase in response to various stresses; however, our results showed no significant differences in the GABA content between controls and Cd treatments or between species. The relationship between the contents of GABA and Ala in the above-ground biomass of plants was confirmed using linear correlation. A significant negative relationship was calculated for both N. praecox and N. caerulescens plants (R2 = 0.94); thus, GABA increased when the Ala content decreased.
The results indicate that two groups of AA: aromatic AA (such as Phe, Tyr and Trp, which are necessary for protein biosynthesis, for the biosynthesis of auxin from Trp, and for generation of antioxidant metabolites from Phe and Tyr); and branched-chain AA (such as Ile, Leu, and Val, which are the building blocks of proteins), accumulated more in the leaves of the control N. praecox treatment than in the leaves of the control N. caerulescens treatment (by 8% and 16%, respectively). Our analyses revealed that Cd stress increased the contents of these AA in both species, but this increase was significant only in Cd- exposed N. caerulescens (N. caerulescens-Cd, Table 3).
Phenylalanine was present in the two species in significantly different concentrations. Cadmium contamination significantly affected its content in N. caerulescens-Cd (increased by 37%) but had little effect on the phenylalanine content in Cd-exposed N. praecox (N. praecox-Cd, increased by 3%). The contents of Tyr and Trp were increased through Cd treatment, but a significant increase of Trp was only observed for N. caerulescens-Cd (by 27%). There was a strong correlation between the Cd content in plants and the content of these AA (R2 = 0.86 for Phe, 0.81 for Tyr, and 0.77 for Trp).
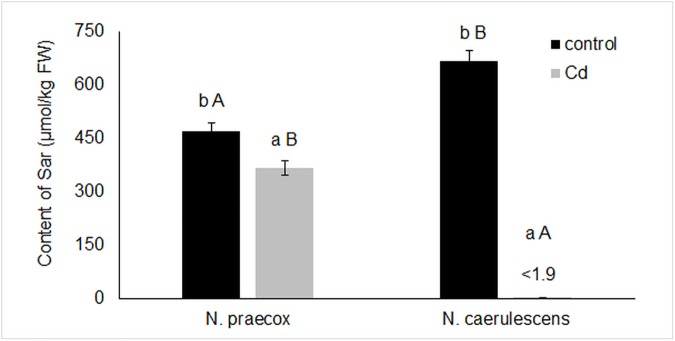
Results in Table 4 show that Phe, Trp, Tyr, Leu, Ile and Val correlated with Sar (R2 = 0.42–0.70). Sarcosine was only found in N. praecox, N. praecox-Cd and N. caerulescens; Sar was below the detection limit in N. caerulescens-Cd (Fig 2). As shown by the results in Table 4, Sar correlated with nitrogen-transport AA, i.e. aspartate (Asp), and also with AA in different metabolic pathways such as Ala, Gly, Trp, threonine (Thr) and Ile. The results in Table 4 confirm the relationships of these AA shown in the ordination diagram from the PCA analysis (Fig 1).
Table 4. Correlation (R2) of selected free amino acids in the above-ground biomass of plants.
| Variable | Ala | GABA | Phe | Tyr | Trp | Ile | Leu | Val | Gly | Orn | Thr | Sar | Asp |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ala | x | 0.94 | 0.62 | 0.61 | 0.59 | 0.26 | 0.80 | 0.34 | 0.03 | 0.05 | 0.06 | 0.54 | 0.34 |
| GABA | 0.43 | x | 0.82 | 0.81 | 0.76 | 0.66 | 0.80 | 0.13 | 0.55 | 0.04 | 0.27 | 0.33 | 0.13 |
| Phe | 0.25 | 0.99 | x | 0.99 | 0.61 | 0.78 | 0.80 | 0.17 | 0.94 | 0.17 | 0.92 | 0.42 | 0.21 |
| Tyr | 0.41 | 0.97 | 0.99 | x | 0.87 | 0.81 | 0.80 | 0.23 | 0.96 | 0.17 | 0.88 | 0.56 | 0.32 |
| Trp | 0.70 | 0.41 | 0.86 | 0.87 | x | 0.84 | 0.80 | 0.67 | 0.54 | 0.28 | 0.60 | 0.46 | 0.14 |
| Ile | 0.80 | 0.16 | 0.64 | 0.66 | 0.84 | x | 0.80 | 0.61 | 0.88 | 0.22 | 0.82 | 0.70 | 0.27 |
| Leu | 0.51 | 0.95 | 0.99 | 0.86 | 0.63 | 0.97 | x | 0.23 | 0.96 | 0.14 | 0.79 | 0.60 | 0.33 |
| Val | 0.91 | 0.16 | 0.24 | 0.12 | 0.17 | 0.17 | 0.29 | x | 0.01 | 0.94 | 0.30 | 0.61 | 0.54 |
| Gly | 0.46 | 0.59 | 0.77 | 0.82 | 0.50 | 0.84 | 0.80 | 0.75 | x | 0.36 | 0.84 | 0.57 | 0.32 |
| Orn | 0.29 | 0.92 | 0.92 | 0.94 | 0.39 | 0.23 | 0.80 | 0.76 | 0.11 | x | 0.32 | 0.13 | 0.20 |
| Thr | 0.05 | 0.79 | 0.90 | 0.87 | 0.72 | 0.75 | 0.78 | 0.17 | 0.83 | 0.33 | x | 0.26 | 0.19 |
| Sar | 0.64 | 0.27 | 0.02 | 0.61 | 0.66 | 0.06 | 0.06 | 0.21 | 0.52 | 0.22 | 0.58 | x | 0.83 |
| Asp | 0.37 | 0.13 | 0.10 | 0.11 | 0.35 | 0.73 | 0.11 | 0.04 | 0.91 | 0.08 | 0.61 | 0.92 | x |
Free amino acid abbreviations: Ala, alanine; GABA, γ-aminobutyric acid; Phe, phenylalanine; Tyr, tyrosine; Trp, tryptophan; Ile, isoleucine; Leu, leucine; Val, valine; Gly, glycine; Orn, ornithine; Thr, threonine; Sar, sarcosine; Asp, aspartate.
Fig 2. The content of Sar (μmol/kg FW) in the above-ground biomass of plants.
Limit of detection: 1.9 μmol/kg. The values represent the means of data obtained in the experiment (n = 5). Different letters indicate values that are significantly different (p < 0.05): A, B comparison between the two species with the same treatment (growing with or without Cd solution applied to the tested soil); a, b comparison between treatments in each species.
The difference in Gly content between the control treatments of both species was not statistically significant, but the content in N. caerulescens was higher (by 18%). A significant decrease in Gly was found for N. caerulescens-Cd relative to the control (22%). A not significant Cd-induced decrease of Gly was shown for N. praecox (6%). Significant differences were determined between the tested species for Orn (59% greater in N. caerulescens than in N. praecox) and between control treatment of N. praecox and N. praecox-Cd (73% increase).
Increases of free Leu and Ile concentrations under Cd stress were only observed for N. caerulescens (21% increase for Leu, and 40% increase for Ile). No significant changes in these AA were seen for N. praecox treatments. A different trend was observed for Val in both Cd treatments, but the changes were not significant. The correlations between Cd contents in plants and Leu and Ile contents were apparent (R2 = 0.65–0.67); however, this relationship was not confirmed for Val (R2 = 0.17). Threonine and Met serve as substrates for synthesis of Ile and their syntheses and catabolism also affect the availability of Ile. A significant correlation was found between Thr and Ile (R2 = 0.75); however, the Met concentrations were below the detection limit of GC-MS, so such a correlation could not be established. While both plant species tended to have higher concentrations of Thr in the Cd treatments, the difference between the Thr contents of the different species was significant. A significant increase in Thr was only observed in N. caerulescens-Cd, which increased by 63% relative to the control N. caerulescens plants.
Results of principal component analysis
The first axis of the PCA analysis explained 59%, the first two axes 86%, and the first four axes together 99.9% of the variability of all analysed data (Fig 1). The length and direction of the vectors indicate the strength of the vector effect and correlation between vectors. Long vectors for all our parameters indicate that the vector greatly affected the results of the analysis. Aromatic AA (Phe, Tyr and Trp) and BCAA (Ile, Leu and Val) were positively correlated with the content of Cd, as indicated by the angles smaller than 90° between the vectors for these parameters. On the other hand, Ala was clearly negatively related to the content of Cd. The first ordination axis divided individual pots into the N. caerulescens group on the top-side and N. praecox on the bottom-side of the diagram, indicating a large effect of plant species on the content of microelements and selected free AA. For N. caerulescens, marks for the control and Cd-exposed treatments were located in different parts of the diagram, which indicates a large effect of treatments on all the recorded data. In contrast, the data for N. praecox showed a minimal effect of treatments.
Discussion
Different accumulation of microelements in Noccaea plants is related to toxic Cd effect
Noccaea plants originating from heavy metal-contaminated regions are known to be metallicolous [40]. The two species under study differ depending on their adaptation to different soil contaminants. The N. caerulescens species from Redlschlag, Austria has adapted to growth on serpentinite (soil naturally rich in Ni, Co and Cr). This is in contrast to N. praecox, which originated from Mežica, Slovenia and has adapted to growth on soil with high Cd, lead and Zn contamination. N. caerulescens from serpentine soil containing an excess of Mg has been shown to adapt to different environments by increasing the uptake of cations and allocating more energy to cation absorption [32]. For this reason, Cu, Mn and Ni accumulation were significantly higher in the N. caerulescens control treatment compared to the N. praecox control treatment. The tested Noccaea plants also exhibited differences in the extent to which they reduced the concentration of various elements. Plants exposed to Cd showed decreased concentrations of Zn and Ni and the decrease in both these elements was significantly stronger in N. caerulescens-Cd than in N. praecox-Cd. These results confirmed the higher metal accumulation and tolerance in metallicolous N. praecox in contrast to serpentinite-grown N. caerulescens [17, 40, 41].
Based on the above facts we affirm that N. praecox plants are more efficient in their use of the accumulated cations which are important cofactors for metalloenzymes. The biogenic elements Zn, Cu, Mn, Fe and Ni are cofactors of metalloenzymes such as superoxide dismutase or cytochrome P450, a broad class of important biomolecules that form biological complexes with metals that perform a wide range of important functions including defence against oxidative stress [42]. Another metalloenzyme, Ni-urease, affects the regulation of N and C metabolism [43] by linking the urease cycle to the tricarboxylic acid (TCA) cycle. Regulation of the ratio of N to C is very important for overcoming environmental or oxidative stress. The formation of a bond between metals and enzymes is dependent on the metal concentration in the cell [44]. A decline of metal contents may thus result in a decrease in metalloenzyme activities. We propose that the increased uptake of Cu, Mn and Ni by N. caerulescens control treatment plants in contrast to N. praecox control treatment plants is the result of selection pressure from the serpentine area. The decrease in the uptake of some elements by N. caerulescens-Cd plants from Cd-contaminated soil in contrast to N. praecox-Cd is related to the more efficient use of these elements in metabolism. Metalloenzymes are therefore important for overcoming adverse conditions causing oxidative stress, which damages plant growth and development.
Difference in alanine and γ-aminobutyrate accumulation is related to pH regulation
It has been reported by several authors that Ala is markedly accumulated in response to various stresses and its role is discussed especially in relation to intracellular pH regulation [21, 45, 46]. Our results, however, demonstrate an opposite trend; Ala contents were decreased in both species growing on Cd-contaminated soil. Similar results were reported for non-hyperaccumulating plants growing on slightly arsenic (As)-contaminated soil [47] and for tobacco growing on Zn-contaminated soil [16]. The increased content of free Ala might be caused by a reduction in the rate of protein synthesis and an increased Ala synthesis due to disturbance of alanine aminotransferase reactions [48]. Our results showed that hyperaccumulating plants do not accumulate Ala in the cytosol as part of pH regulation, but they can use it for the biosynthesis of proline/alanine-rich protein kinases [49] or histidine- and alanine-rich proteins [50].
An increase in levels of GABA at the expense of Ala leads to a higher increased pH in the cytosol, which in turn alters the activities of enzymes as well as the activity and transport of plant hormones. We suggest that GABA may be more significant for effective pH regulation in the cytosol of both Noccaea species than Ala [51], this is also relevant when considering the isoelectric points of the two AA. One route for GABA synthesis is non-enzymatic conversion of Pro to GABA, which was recently reported to be an alternative route for providing adenosine triphosphate [52], to C and N under conditions of oxidative stress [53]. GABA plays different roles in plant metabolism, including N-C metabolism, energy balance, signalling and development, and stress defence [51]. The GABA shunt plays a key role in controlling the N/C ratio by linking AA metabolism and the TCA cycle, which is essential for higher plant species [22]. This role is confirmed by our finding of a significant correlation between GABA content and the total content of free AA (R2 = 0.99). Increased GABA levels in response to short exposures to different abiotic and biotic environmental stressors are commonly observed in several plants [20, 54]. A significant increase of GABA under Cd stress has been observed in tomato plants [6] while in our hyperaccumulator species GABA accumulation was not significant. This indicates that tomato plants are less well adapted to the toxic effect of Cd than Noccaea species.
Difference in accumulation of aromatic amino acids is related to antioxidative metabolism
Under Cd stress, the levels of Phe, Tyr and Trp were significantly increased only in N. caerulescens plants, which are less Cd-tolerant than N. praecox. These AA are necessary not only for protein biosynthesis but also for other important processes. For instance, Phe is also a substrate for the phenylpropanoid pathway, which produces a wide range of plant secondary products, especially antioxidative metabolites (flavonoids, anthocyanins, lignins and phenylpropanoic acids including salicylic acid) and phenolic compounds [55] that may act as growth promoters or growth inhibitors. The significant increase of Phe in N. caerulescens-Cd suggests a higher production of antioxidative metabolites. Tryptophan plays a major role in the regulation of plant development and defence responses [56] and is required for the biosynthesis of auxin (indole-3-acetic acid), a phytohormone that is necessary for the growth and development of plant cells [36], and plays an important role in the adaptation to environmental stress [57]. Exogenous Trp can be effectively used to improve plant growth in soil contaminated with Cd [56]. Biosynthesis of Trp is induced by stresses; an increased content of Trp has thus been found in plants under drought [58] and Cd stress [59]. Bivalent Trp side chains and metal ions were found to mutually interact [60]; this finding corresponds with our results. A significant increase in Trp was reported only in N. caerulescens-Cd plants, rather than in the more Cd-tolerant N. praecox plants.
Difference in glycine, sarcosine and ornithine accumulation is related to tolerance of studied hyperaccumulators
The presence and usability of AA (such as α-aminoadipic acid and Sar) occurring infrequently and/or at very low levels in plant stress metabolism, can be explained by epigenetic changes induced by stress in the tested Noccaea species [61].
The difference in Gly content between control treatments of both species was not significant. A significant decrease in Gly levels was only found for N. caerulescens-Cd. Glycine is a crucial amino acid for the biosynthesis of Sar and Cys via Ser. Glycine is involved in the biosynthesis of phytochelatines and antioxidant metabolites, and is also found in glycine-rich proteins that affect the growth and function of cell walls. Therefore, the decreased Gly contents in both species must be seen as the activation of adaptation processes to the toxic effect of Cd, as was the case with lettuce exposed to dust containing As and Cr [36]. Asp is decreased in less tolerant N. caerulescens as in lettuce [36], but is increased in tolerant N. praecox. Aspartate is an amino donor for the biosynthesis of Gly, as confirmed through the correlation of these AA (Table 4 and S1 Fig).
The results showed no correlation between Met and Sar because the contents of Met were below the detection limit. It is generally known that Met participates in the N-methylation of Gly via S-adenosylmethionine by acting as a methyl donor [20, 62–64]. Oxidative stress of N. caerulescens-Cd was significantly stronger than that of N. praecox-Cd, as shown by a dramatic decrease of Sar content in N. caerulescens-Cd. In the Cd-treated plants Sar was only detected in N. praecox-Cd (the content of Sar in N. caerulescens-Cd was below the detection limit). We assume that the high Cd accumulation in N. praecox-Cd could be due to the chelation of Cd by Sar, because Sar can form complexes with Cd and other metals [65, 66] and can protect nucleic acids from oxidative stress [67]. This is in contrast with the fact that these chelates and free AA have a positive relationship with the toxic elements, such as with the chelation of Zn and histidine [67]. Sar-Ni complexes are probably degraded, as indicated by the decrease of Ni and Sar contents under Cd stress. The slow decline of Sar levels can be an advantage for N. praecox relative to N. caerulescens if the primary importance of Sar in studied hyperaccumulating plants is not in the formation of chelates with toxic Cd. The effect of Sar on changes of Cd content in plants was confirmed by the positive correlation coefficient (R2 = 0.52). A correlation coefficient of R2 = 0.29 was calculated for the effect of Cd contents on Sar. Based on these results we can speculate that complex of Sar and Cd is formed only under the effect of increased Cd content in tested plants. As is clear from our results, Sar chelates probably have only a secondary meaning for detoxification of Cd.
In Noccaea species, as shown in the diagram of Sar metabolism and the related AA (S1 Fig), Sar is used in metabolic pathways of other AA. Catabolism of 5-methyl cytosine to cytosine (a step in DNA methylation) is characterised as an epigenetic change [61]. In plants, this effect is related to an increase of methyl donors for biosynthesis of specific antioxidant metabolites [63, 68]. The catabolism of Sar therefore produces Gly and a methyl donor, which is transformed to homocysteine via formation of Met. Our results indicate a Gly decrease that is similar to our previous results [38] and is caused by Gly incorporation into serine, which is significantly enhanced in hyperaccumulating Noccaea species. The decrease of Sar is consistent with its role as a methyl donor for formation of specialised compounds, e.g. antioxidant metabolites [26, 27] which arise from AA, e.g. from Phe and Tyr. A significant correlation was therefore observed between the content of Gly and Sar including aromatic AA and mutual correlations of Sar and Tyr, which are the main substrates for the formation of antioxidant secondary metabolites such as polyamines [63], tocopherols [69, 70] phenylpropanoid metabolites including flavonoids, stilbenes etc. [62, 68, 71]. This is confirmed by the results of PCA (Fig 1) which imply that the content of Sar is inversely proportional to the content of Tyr and Gly. Simultaneously, Cd, which causes oxidative stress, is an important factor for production of Sar which is significantly connected with the content of Ni (Fig 1). Our results thus show that Sar content could be a significant factor (methyl donor) for the adaptability of the hyperaccumulators to oxidative stress, and the differences in adaptability between species which are directly proportional to the RF. Activation of the transmethylation cycle involves activation of O-, C- and/or N-methyltransferase and O-, C- and/or N-demethylase [62, 63, 68]. Based on these findings and with indirect evidence provided in this study we can infer that the tested hyperaccumulators, form methyl pools not only by choline and betaine [72, 73], but also by Sar, whose content decreases with increased Cd content and which originates from the metabolism of choline, betaine, N-dimethylglycine and glycine [30]. Sar catabolism to Gly then yields the methyl group used, via the transmethylation cycle of stress metabolism, for the biosynthesis of the above antioxidant compounds.
The activities of plant desaturases can be affected by Ni content. The effect of Ni on nitrogen metabolism in plants was described previously [43] and these results are supported by those reported here, which show a significant effect of decreased Ni on the AA levels. High plant Ni content is linked to the maximum efficiency of Ni incorporation into the active centre of ureases and glyoxalases. Ureases and glyoxalases play a significant role in cell catabolism [43, 74] and AA catabolism which are associated with plant senescence. The glyoxalase pathway and the two enzymes (glyoxalase I and glyoxalase II) have a significant effect on plant stress metabolism [12, 75]. Ureases are metalloenzymes that catalyse the hydrolysis of urea, an intermediate of plant arginine catabolism that is involved in the remobilisation of nitrogen from tissues [76]. Arginine degradation in the mitochondria via arginase activity leads to the formation of Orn and urea (urease-ornithine cycle). Ornithine can be used in glutamate synthesis, and urea is a source of N that can be sensed by plant cells and used for AA synthesis [77]. An unrelated study showed how urea and Orn accumulated in Arabidopsis plants, when Ni was a limiting factor for urease activity. Our results showed an increase of Orn in both N. praecox-Cd and N. caerulescens-Cd treatments; these increased Orn contents in both species may be related to decreased Ni contents in N. caerulescens-Cd and N. praecox-Cd. Ornithine contents are associated with N and C metabolism through a transfer of an AA, Asp, and with the TCA cycle via fumarate. An alternative pathway for AA regulation via Orn may be characteristic for these hyperaccumulators [78]. Nitrogen obtained from the urease-ornithine cycle may be used for the production of AA studied here, e.g. Ala, Sar and Val.
Urease and glyoxalase activity can be a source of energy during stress metabolism in hyperaccumulating plants [75]. For the above reasons, the use of Ni as a cofactor of urease and glyoxalases is important for the adaptation of hyperaccumulators to Cd contamination. A higher Ni content in N. praecox-Cd than in N. caerulescens-Cd was found in our experiments. This finding may explain the better ability of N. praecox from Mežica to use metabolite catabolism for the formation of compounds involved in the detoxification of toxic elements.
Difference in accumulation of branched-chain amino acids is related to amino acid homeostasis
Amino acid homeostasis is essential for growth, development and defence of plants against stress [79]. This homeostasis is regulated by de novo biosynthesis, uptake/translocation, and protein synthesis/degradation [80]. Leucine, Ile and Val catabolism play physiological roles beyond maintaining AA homeostasis [81]. These AA are pivotal in balancing the fluxes between different AA pathways [80]. The accumulation of Leu, Ile and Val may serve to promote stress-induced protein synthesis and may act as signalling molecules to regulate gene expression [24]. Only Leu and Ile showed significant increases for the N. caerulescens-Cd treatment. This finding confirmed that N. caerulescens plants are less adaptable to Cd stress and showed that in N. caerulescens plants stress activated enzymes associated with degradation proteins and senescence.
Leucine, together with Zn, is present in many enzymes that degrade proteins, such as leucine aminopeptidases [82]. These enzymes are involved in defence against stress and are connected to the transport of auxin, the formation of jasmonic acid and plant senescence induced by stress such as that caused by heavy metals. A higher content of Leu was determined in the Cd treatment of N. caerulescens plants compared to N. praecox plants. Our findings may be connected to the significantly lower contents of Zn (cofactor of superoxide dismutase) in N. caerulescens than in N. praecox. The reduction of Zn in N. caerulescens plants compared with N. praecox is one of the reasons for reducing the capacity of the plant antioxidant system [11, 42].
Plant pathways regulating Thr, Met and Ile metabolism are highly interconnected. Synthesis of Ile proceeds through Thr and Met; therefore, their synthesis and catabolism at different developmental plant phases and under different environmental conditions also influences the availability of Ile [24].
Differences in adaptability of the hyperaccumulators result not only from the regulation of metabolism of the studied AA but also the regulation of other AA [38] and fatty acids [17, 18]. The high adaptability of N. praecox to the toxic effect of Cd is the result of a complex regulation of AA and fatty acids metabolism.
Conclusion
Our results emphasise that N. praecox and N. caerulescens growing under the selective pressure of differently contaminated locations can differ in physiological performance. The results point to complex changes in the regulation of stress metabolism of plants exposed to selection pressure during their phylogenetic development in the specific polluted environment. Noccaea species showed contrasting responses to Cd contamination in the content of microelements and AA metabolism. Manganese and Ni contents in the sample of the N. caerulescens control treatment were significantly higher than in the N. praecox control treatment. The ability of N. caerulescens to take up cations is a result of the selective pressure of growth in a contaminated area. The contents of microelements in leaves of both Noccaea species were affected by Cd supply. Cadmium reduced Zn and Ni contents mainly in the N. caerulescens-Cd treatment, but did not significantly affect Cu and Mn in any of the treatments. Different responses to Cd contamination of Noccaea species were found for Ala, Phe, Thr and Sar. The more significant changes of contents of Thr (increase) and Sar (decrease) were determined from responses in N. caerulescens rather than in N. praecox under Cd stress. Plant growth is limited by these changes resulting from stress metabolism. The data on all tested parameters confirmed the higher adaption of N. praecox than N. caerulescens to Cd-induced stress.
Supporting information
Red colour, amino acids and their analogues. Blue colour, methyl and major metabolites, which are donors and/or acceptors of methyl. Bold font, metabolites, which are commented or mentioned in results and discussion. AAAs, aromatic amino acids; ALA, alanine; ASP, aspartic acid; Betaine, glycine betaine; CYSTA, cystathione; CYS, cysteine; Gly, glycine; HCY, homocysteine; HSE, homoserine; Me, methyl; MET, methionine; PHE, phenylalanine; SAHCY, S-adenosylhomocysteine; SAM, S-adenosylmethionine; SAR, sarcosine; SER, serine; TCA cycle, tricarboxylic cycle (citrate cycle); TRP, tryptophan; TYR, tyrosine; 5MeCytosine, 5-methylcytosine.
(TIF)
Acknowledgments
This research was supported by CIGA project No. 20142004 (Czech University of Life Sciences Prague).
Data Availability
All relevant data are within the paper.
Funding Statement
This research was supported by Czech University of Life Sciences - CIGA project No. 20142004. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Tamás L, Mistrík I, Alemayehu A, Zelinová V, Bočová B, Huttová J. Salicylic acid alleviates cadmium-induced stress responses through the inhibition of Cd-induced auxin-mediated reactive oxygen species production in barley root tips. J Plant Physiol. 2015; 173: 1–8. doi: 10.1016/j.jplph.2014.08.018 [DOI] [PubMed] [Google Scholar]
- 2.Xie Y, Hu L, Du Z, Sun X, Amombo E, Fan JB, et al. Effects of cadmium exposure on growth and metabolic profile of Bermudagrass [Cynodon dactylon (L.) Pers.]. PLOS ONE. 2014; 9(12): e115279 doi: 10.1371/journal.pone.0115279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verbruggen N, Hermans C, Schat H. Mechanisms to cope with arsenic or cadmium excess in plants. Curr Opin Plant Biol. 2009; 12(3): 364–372. doi: 10.1016/j.pbi.2009.05.001 [DOI] [PubMed] [Google Scholar]
- 4.Sanità di Toppi L, Gabbrielli R. Response to cadmium in higher plants. Environ Exp Bot. 1999; 41(2): 105–130. [Google Scholar]
- 5.DalCorso G, Farinati S, Maistri S, Furini A. How plants cope with cadmium: Straking all on metabolism and gene expression. J Integr Plant Biol. 2008; 50(10): 1268–1280. doi: 10.1111/j.1744-7909.2008.00737.x [DOI] [PubMed] [Google Scholar]
- 6.Zoghlami LB, Djebali W, Abbes Z, Hediji H, Maucourt M, Moing A, et al. Metabolite modifications in Solanum lycopersicum roots and leaves under cadmium stress. Afr J Biotechnol. 2011; 10(4): 567–579. [Google Scholar]
- 7.Martin SR, Llugany M, Barceló J, Poschenrieder C. Cadmium exclusion a key factor in differential Cd-resistance in Thlaspi arvense ecotypes. Biol Plantarum. 2012; 56(4): 729–734. [Google Scholar]
- 8.Whittaker JW. Molecular relaxation and metalloenzyme active site Modeling. Int J Quantum Chem. 2002; 90(4–5): 1529–1535. [Google Scholar]
- 9.Field LS, Luk E, Culotta VC. Copper chaperones: Personal escorts for metal ions. J Bioenerg Biomembr. 2002; 34(5): 373–379. [DOI] [PubMed] [Google Scholar]
- 10.Türkel N. Stability constants of mixed ligand complexes of nickel(II) with adenine and some amino acids. Bioinorg Chem Appl. 2015; article ID: 374782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cakmak I. Tansley review No. 111—Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000; 146(2): 185–205. [DOI] [PubMed] [Google Scholar]
- 12.Mustafiz A, Ghosh A, Tripathi AK, Kaur C, Ganguly AK, Bhavesh NS, et al. A unique Ni2+-dependent and methylglyoxal-inducible rice glyoxalase I possesses a single active site and functions in abiotic stress response. Plant J. 2014; 78(6): 951–963. doi: 10.1111/tpj.12521 [DOI] [PubMed] [Google Scholar]
- 13.Hernández LE, Cooke DT. Modification of the root plasma membrane lipid composition of cadmium-treated Pisum sativum. J Exp Bot. 1997; 48(312): 1375–1381. [Google Scholar]
- 14.Shah K, Kumar RG, Verma S, Dubey RS. Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci. 2001; 161(6): 1135–1144. [Google Scholar]
- 15.Xu J, Sun J, Du L, Liu X. Comparative transcriptome analysis of cadmium responses in Solanum nigrum and Solanum torvum. New Phytol. 2012a; 196(1): 110–124. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Zhu Y, Ge Q, Li Y, Sun J, Zhang Y, et al. Comparative physiological responses of Solanum nigrum and Solanum torvum to cadmium stress. New Phytol. 2012b; 196(1): 125–138. [DOI] [PubMed] [Google Scholar]
- 17.Zemanová V, Pavlík M, Kyjaková P, Pavlíková D. Fatty acid profiles of ecotypes of hyperaccumulator Noccaea caerulescens growing under cadmium stress. J Plant Physiol. 2015; 180: 27–34. doi: 10.1016/j.jplph.2015.02.012 [DOI] [PubMed] [Google Scholar]
- 18.Pavlík M, Zemanová V, Pavliková D, Kyjaková P, Hlavsa T. Regulation of odd-numbered fatty acid content plays an important part in the metabolism of the hyperaccumulator Noccaea spp. adapted to oxidative stress. J Plant Physiol. 2017; 208: 94–101. doi: 10.1016/j.jplph.2016.09.014 [DOI] [PubMed] [Google Scholar]
- 19.Pavlíková D, Pavlík M, Procházková D, Zemanová V, Hnilička F, Wilhelmová N. Nitrogen metabolism and gas exchange parameters associated with zinc stress in tobacco expressing an ipt gene for cytokinin synthesis. J Plant Physiol. 2014a; 171(7): 559–564. [DOI] [PubMed] [Google Scholar]
- 20.Pavlíková D, Zemanová V, Procházková D, Pavlík M, Száková J, Wilhelmová N. The long-term effect of zinc soil contamination on selected free amino acids playing an important role in plant adaptation to stress and senescence. Ecotox Environ Safe. 2014b; 100: 166–170. [DOI] [PubMed] [Google Scholar]
- 21.Miyashita Y, Dolferus R, Ismond KP, Good AG. Alanine aminotransferase catalyses the breakdown of alanine after hypoxia in Arabidopsis thaliana. Plant J. 2007; 49(6): 1108–1121. doi: 10.1111/j.1365-313X.2006.03023.x [DOI] [PubMed] [Google Scholar]
- 22.Seher Y, Filiz O, Melike B. Gamma-amino butyric acid, glutamate dehydrogenase and glutamate decarboxylase levels in phylogenetically divergent plants. Plant Syst Evol. 2013; 299(2): 403–412. [Google Scholar]
- 23.Pavlíková D, Pavlík M, Staszková L, Motyka V, Száková J, Tlustoš P, et al. Glutamate kinase as a potential biomarker of heavy metal stress in plants. Ecotox Environ Safe. 2008; 70(2): 223–230. [DOI] [PubMed] [Google Scholar]
- 24.Joshi V, Joung JG, Fei Z, Jander G. Interdependence of threonine, methionine and isoleucine metabolism in plants: accumulation and transcriptional regulation under abiotic stress. Amino Acids. 2010; 39(4): 933–947. doi: 10.1007/s00726-010-0505-7 [DOI] [PubMed] [Google Scholar]
- 25.Nikiforova VJ, Bielecka M, Gakière B, Krueger S, Rinder J, Kempa S, et al. Effect of sulfur availability on the integrity of amino acid biosynthesis in plants. Amino Acids. 2006; 30(2): 173–183. doi: 10.1007/s00726-005-0251-4 [DOI] [PubMed] [Google Scholar]
- 26.Tzin V, Galili G. The biosynthetic pathways for shikimate and aromatic amino acids in Arabidopsis thaliana. The Arabidopsis book/American Society of Plant Biologists. 2010; 8:e0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoo H, Widhalm JR, Qian Y, Maeda H, Cooper BR, Jannasch AS, et al. An alternative pathway contributes to phenylalanine biosynthesis in plants via a cytosolic tyrosine: phenylpyruvate aminotransferase. Nat Commun. 2013; 4: article number 2833. [DOI] [PubMed] [Google Scholar]
- 28.Cosgrove DJ. Enzymes and other agents that enhance cell wall extensibility. Annu Rev Plant Phys. 1999; 50: 391–417. [DOI] [PubMed] [Google Scholar]
- 29.Oda A, Shimizu M, Kuroha T, Satoh S. Induction of xylem sap methylglycine by a drought and rewatering treatment and its inhibitory effects on the growth and development of plant organs. Physiol Plantarum. 2005; 124(4): 515–523. [Google Scholar]
- 30.Niu X, Xiong F, Liu J, Sui Y, Zeng Z, Lu BR, et al. Co-expression of ApGSMT and ApDMT promotes biosynthesis of glycine betaine in rice (Oryza sativa L.) and enhances salt and cold tolerance. Environ Exp Bot. 2014; 104: 16–25. [Google Scholar]
- 31.Ashraf M, Foolad MR. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot. 2007; 59(2): 206–216. [Google Scholar]
- 32.Gonneau C, Genevois N, Frérot H, Sirguey C, Sterckeman T. Variation of trace metal accumulation, major nutrient uptake and growth parameters and their correlations in 22 populations of Noccaea caerulescens. Plant Soil. 2014; 384(1–2): 271–287. [Google Scholar]
- 33.Vogel-Mikuš K, Regvar M, Mesjasz-Przybyłowicz J, Przybyłowicz WJ, Simčič J, Pelicon P, et al. Spatial distribution of cadmium in leaves of metal hyperaccumulating Thlaspi praecox using micro-PIXE. New Phytol. 2008; 179(3): 712–721. doi: 10.1111/j.1469-8137.2008.02519.x [DOI] [PubMed] [Google Scholar]
- 34.Gosar M, Miler M. Anthropogenic metal loads and their sources in stream sediments of the Meža River catchment area (NE Slovenia). Appl Geochem. 2011; 26(11): 1855–1866. [Google Scholar]
- 35.Puschenreiter M, Schnepf A, Millán IM, Fitz WJ, Horak O, Klepp J, et al. Changes of Ni biogeochemistry in the rhizosphere of the hyperaccumulator Thlaspi goesingense. Plant Soil. 2005; 271(1–2): 205–218. [Google Scholar]
- 36.Pavlík M, Pavlíková D, Zemanová V, Hnilička F, Urbanová V, Száková J. Trace elements present in airborne particulate matter–Stressors of plant metabolism. Ecotox Environ Safe. 2012; 79: 101–107. [DOI] [PubMed] [Google Scholar]
- 37.ter Braak CJF, Smilauer P. CANOCO reference manual and CanoDraw for Windows user’s guide: software for canonical community ordination (version 4.5). Microcomputer Power; Ithaca, 2002. [Google Scholar]
- 38.Zemanová V, Pavlík M, Pavlíková D, Hnilička F, Vondráčková S. Responses to Cd stress in two Noccaea species (Noccaea praecox and Noccaea caerulescens) originating from two contaminated sites in Mežica, Slovenia and Redlschlag, Austria. Arch Environ Con Tox. 2016; 70(3): 464–474. [DOI] [PubMed] [Google Scholar]
- 39.Zárubová P, Hejcman M, Vondráčková S, Mrnka L, Száková J, Tlustoš P. Distribution of P, K, Ca, Mg, Cd, Cu, Fe, Mn, Pb and Zn in wood and bark age classes of willows and poplars used for phytoextraction on soils contaminated by risk elements. Environ Sci Pollut R. 2015; 22(23): 18801–18813. [DOI] [PubMed] [Google Scholar]
- 40.Visioli G, Gullì M, Marmiroli N. Noccaea caerulescens populations adapted to grow in metalliferous and non-metalliferous soils: Ni tolerance, accumulation and expression analysis of genes involved in metal homeostasis. Environ Exp Bot. 2014; 105: 10–17. [Google Scholar]
- 41.Reeves RD, Schwartz C, Morel JL, Edmondson J. Distribution and metal-accumulating behaviour of Thlaspi caerulescens and associated metallophytes in France. Int J Phytoremediat. 2001; 3(2): 145–172. [Google Scholar]
- 42.Fernández-Ocaña A, Chaki M, Luque F, Gómez-Rodríguez MV, Carreras A, Valderrama R, et al. Functional analysis of superoxide dismutases (SODs) in sunflower under biotic and abiotic stress conditions. Identification of two new genes of mitochondrial Mn-SOD. J Plant Physiol. 2011; 168(11): 1303–1308. doi: 10.1016/j.jplph.2011.01.020 [DOI] [PubMed] [Google Scholar]
- 43.Polacco JC, Mazzafera P, Tezotto T. Opinion—Nickel and urease in plants: Still many knowledge gaps. Plant Sci. 2013; 199: 79–90. doi: 10.1016/j.plantsci.2012.10.010 [DOI] [PubMed] [Google Scholar]
- 44.Egleston ES, Morel FMM. Nickel limitation and zinc toxicity in a urea-grown diatom. Limnol Oceanogr. 2008; 53(6): 2462–2471. [Google Scholar]
- 45.Limami AM, Glévarec G, Ricoult C, Cliquet JB, Planchet E. Concerted modulation of alanine and glutamate metabolism in young Medicago truncatula seedlings under hypoxic stress. J Exp Bot. 2008; 59(9): 2325–2335. doi: 10.1093/jxb/ern102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rocha M, Licausi F, Araújo WL, Nunes-Nesi A, Sodek L, Fernie AR, et al. Glycolysis and the tricarboxylic acid cycle are linked by alanine aminotransferase during hypoxia induced by waterlogging of Lotus japonicus. Plant Physiol. 2010a; 152(3): 1501–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pavlík M, Pavlíková D, Staszková L, Neuberg M, Kaliszová R, Száková J, et al. The effect of arsenic contamination on amino acids metabolism in Spinacia oleracea L. Ecotox Environ Safe. 2010; 73(6): 1309–1313. [DOI] [PubMed] [Google Scholar]
- 48.Hjorth M, Mathiassen SK, Kudsk P, Ravn HW. Amino acids in loose silky-bent (Apera spica-venti (L.) Beauv.) responding to prosulfocarb exposure and the correlation with physiological effects. Pestic Biochem Phys. 2006; 86(3): 138–145. [Google Scholar]
- 49.Mori T, Kikuchi E, Watanabe Y, Fujii S, Ishigami-Yuasa M, Kagechika H, et al. Chemical library screening for WNK signalling inhibitors using fluorescence correlation spectroscopy. Biochem J. 2013; 455: 339–345. doi: 10.1042/BJ20130597 [DOI] [PubMed] [Google Scholar]
- 50.Komatsu S, Jan A, Koga Y. Characterization of a histidine- and alanine-rich protein showing interaction with calreticulin in rice. Amino Acids. 2009; 36(1): 137–146. doi: 10.1007/s00726-008-0043-8 [DOI] [PubMed] [Google Scholar]
- 51.Bor M, Seckin B, Ozgur R, Yilmaz O, Ozdemir F, Turkan I. Comparative effects of drought, salt, heavy metal and heat stresses on gamma-aminobutryric acid levels of sesame (Sesamum indicum L.). Acta Physiol Plant. 2009; 31(3): 655–659. [Google Scholar]
- 52.Daş ZA, Dimlioğlu G, Bor M, Özdemir F. Zinc induced activation of GABA-shunt in tobacco (Nicotiana tabaccum L.). Environ Exp Bot. 2016; 122: 78–84. [Google Scholar]
- 53.Signorelli S, Dans PD, Coitiño EL, Borsani O, Monza J. Connecting proline and γ-aminobutyric acid in stressed plants through non-enzymatic reactions. PLOS ONE. 2015; 10(3): e0115349 doi: 10.1371/journal.pone.0115349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kinnersley AM, Turano FJ. Gamma aminobutyric acid (GABA) and plant responses to stress. Crit Rev Plant Sci. 2000; 19(6): 479–509. [Google Scholar]
- 55.Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Bio Med. 1996; 20(7): 933–956. [DOI] [PubMed] [Google Scholar]
- 56.Sanjaya Hsiao PY, Su RC Ko SS, Tong CG Yang RY, et al. Overexpression of Arabidopsis thaliana tryptophan synthase beta 1 (AtTSB1) in Arabidopsis and tomato confers tolerance to cadmium stress. Plant Cell Environ. 2008; 31(8): 1074–1085. doi: 10.1111/j.1365-3040.2008.01819.x [DOI] [PubMed] [Google Scholar]
- 57.Popko J, Hansch R, Mendel RR, Polle A, Teichmann T. The role of abscisic acid and auxin in the response of poplar to abiotic stress. Plant Biol. 2010; 12(2): 242–258. doi: 10.1111/j.1438-8677.2009.00305.x [DOI] [PubMed] [Google Scholar]
- 58.Witt S, Galicia L, Lisec J, Cairns J, Tiessen A, Araus JL, et al. Metabolic and phenotypic responses of greenhouse-grown maize hybrids to experimentally controlled drought stress. Mol Plant. 2012; 5(2): 401–417. doi: 10.1093/mp/ssr102 [DOI] [PubMed] [Google Scholar]
- 59.Liu X, Yang C, Zhang L, Li L, Liu S, Yu J, et al. Metabolic profiling of cadmium-induced effects in one pioneer intertidal halophyte Suaeda salsa by NMR-based metabolomics. Ecotoxicology. 2011; 20(6): 1422–1431. doi: 10.1007/s10646-011-0699-9 [DOI] [PubMed] [Google Scholar]
- 60.Li Y, Yang CM. A rationally designed novel receptor for probing cooperative interaction between metal ions and bivalent tryptophan side chain in solution. Chem Commun. 2003; 23: 2884–2885. [DOI] [PubMed] [Google Scholar]
- 61.Garg R, Bhattacharjee A, Jain M. Genome-scale transcriptomic insights into molecular aspects of abiotic stress responses in chickpea. Plant Mol Biol Rep. 2015, 33(3): 388–400. [Google Scholar]
- 62.Ibrahim RK, Bruneau A, Bantignies B. Plant O-methyltransferases: molecular analysis, common signature and classification. Plant Mol Biol. 1998; 36(1): 1–10. [DOI] [PubMed] [Google Scholar]
- 63.Roje S. S-adenosyl-L-methionine: Beyond the universal methyl group donor. Phytochemistry. 2006; 67(15): 1686–1698. doi: 10.1016/j.phytochem.2006.04.019 [DOI] [PubMed] [Google Scholar]
- 64.Zemanová V, Pavlík M, Pavlíková D, Tlustoš P. The significance of methionine, histidine and tryptophan in plant responses and adaptation to cadmium stress. Plant Soil Environ. 2014; 60(9): 426–432. [Google Scholar]
- 65.Krishnakumar RV, Rameela MP, Natarajan S. Crystal structure of sarcosine cadmium chloride. Cryst Res Technol. 1996; 31(2): 203–207. [Google Scholar]
- 66.Tewari BB. Studies on complexation in solution with a paper electrophoretic technique [The system mercury(II)/nickel(II)/lead(II)—sarcosine]. J Chil Chem Soc. 2012; 57(1): 995–998. [Google Scholar]
- 67.Krämer U, Cotter-Howells JD, Charnock JM, Baker AJM, Smith JAC. Free histidine as a metal chelator in plants that accumulate nickel. Nature. 1996; 379(6566): 635–638. [Google Scholar]
- 68.Moffatt BA, Weretilnyk EA. Sustaining S-adenosyl-L-methionine-dependent methyltransferase activity in plant cells. Physiol Plantarum. 2001; 113(4): 435–442. [Google Scholar]
- 69.Jin S, Daniell H. Expression of gamma-tocopherol methyltransferase in chloroplasts results in massive proliferation of the inner envelope membrane and decreases susceptibility to salt and metal-induced oxidative stresses by reducing reactive oxygen species. Plant Biotechnol. J. 2014; 12(9): 1274–1285. doi: 10.1111/pbi.12224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang J, Jia H, Feng G, Wang Z, Li J, Gao H, et al. Overexpression of Medicago sativa TMT elevates the alpha-tocopherol content in Arabidopsis seeds, alfalfa leaves, and delays dark-induced leaf senescence. Plant Sci. 2016; 249: 93–104. doi: 10.1016/j.plantsci.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 71.Yamaguchi M, Valliyodan B, Zhang J, Lenoble ME, Yu O, Rogers EE, et al. Regulation of growth response to water stress in the soybean primary root. I. Proteomic analysis reveals region-specific regulation of phenylpropanoid metabolism and control of free iron in the elongation zone. Plant Cell Environ. 2010; 33(2): 223–243. doi: 10.1111/j.1365-3040.2009.02073.x [DOI] [PubMed] [Google Scholar]
- 72.Yang WJ, Nadolskaorczyk A, Wood KV, Hahn DT, Rich PJ, Wood AJ, et al. Near-isogenic lines of maize differing for glycinebetaine. Plant Physiol. 1995; 107(2): 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nuccio ML, Russell BL, Nolte KD, Rathinasabapathi B, Gage DA, Hanson AD. The endogenous choline supply limits glycine betaine synthesis in transgenic tobacco expressing choline monooxygenase. Plant J. 1998; 16(4): 487–496. [DOI] [PubMed] [Google Scholar]
- 74.Thornalley PJ. Glutathione-dependent detoxification of alpha-oxoaldehydes by the glyoxalase system: involvement in disease mechanisms and anti-proliferative activity of glyoxalase I inhibitors. Chem-Biol Interact. 1998; 112: 137–151. [DOI] [PubMed] [Google Scholar]
- 75.Mustafiz A, Sahoo KK, Singla-Pareek SL, Sopory SK. Metabolic engineering of glyoxalase pathway for enhancing stress tolerance in plants. Plant Stress Tolerance: Methods and Protocols/Methods in Molecular Biology. 2010; 639: 95–118. [DOI] [PubMed] [Google Scholar]
- 76.Whitte CP. Urea metabolism in plants. Plant Sci. 2011; 180(3): 431–438. doi: 10.1016/j.plantsci.2010.11.010 [DOI] [PubMed] [Google Scholar]
- 77.Planchais S, Cabassa C, Toka I, Justin AM, Renou JP, Savouré A, et al. Basic amino acid carrier 2 gene expression modulates arginine and urea content and stress recovery in Arabidopsis leaves. Front Plant Sci. 2014; 5: article number 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Szabados L, Savoure A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010; 15(2): 89–97. doi: 10.1016/j.tplants.2009.11.009 [DOI] [PubMed] [Google Scholar]
- 79.Liu G, Ji Y, Bhuiyan NH, Pilot G, Selvaraj G, Zou J. Amino acid homeostasis modulates salicylic acid-associated redox status and defence responses in Arabidopsis. Plant Cell. 2010; 22(11): 3845–3863. doi: 10.1105/tpc.110.079392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.He Y, Dai SJ, Dufresne CP, Zhu N, Pang QY, Chen SX. Integrated proteomics and metabolomics of Arabidopsis acclimation to gene-dosage dependent perturbation of isopropylmalate dehydrogenases. PLOS ONE. 2013; 8(3): e57118 doi: 10.1371/journal.pone.0057118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Araújo WL, Ishizaki K, Nunes-Nesi A, Larson TR, Tohge T, Krahnert I, et al. Identification of the 2-hydroxyglutarate and isovaleryl-CoA dehydrogenases as alternative electron donors linking lysine catabolism to the electron transport chain of Arabidopsis mitochondria. Plant Cell. 2010; 22(5): 1549–1563. doi: 10.1105/tpc.110.075630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Taylor A. Aminopeptidases—Structure and function. FASEB J. 1993; 7(2): 290–298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Red colour, amino acids and their analogues. Blue colour, methyl and major metabolites, which are donors and/or acceptors of methyl. Bold font, metabolites, which are commented or mentioned in results and discussion. AAAs, aromatic amino acids; ALA, alanine; ASP, aspartic acid; Betaine, glycine betaine; CYSTA, cystathione; CYS, cysteine; Gly, glycine; HCY, homocysteine; HSE, homoserine; Me, methyl; MET, methionine; PHE, phenylalanine; SAHCY, S-adenosylhomocysteine; SAM, S-adenosylmethionine; SAR, sarcosine; SER, serine; TCA cycle, tricarboxylic cycle (citrate cycle); TRP, tryptophan; TYR, tyrosine; 5MeCytosine, 5-methylcytosine.
(TIF)
Data Availability Statement
All relevant data are within the paper.