Abstract
Sister chromatid exchanges (SCE) are considered indicators of genetic damage and early chromosome changes. The SCE frequency from an infertile 27-year-old, white male with tall stature and a nondicentric Y/Y translocation consisting of one short arm and two long arms of the Y chromosome was determined. The SCE frequency was 7.9 ± SD 1.4 while the SCE frequency from ten control subjects was 8.4 ± SD 0.51. A two-tailed t-test was applied to the SCE data and no significant difference was found between the individual with the Y/Y translocation and control subjects. Apparently, this altered chromosome does not interfere with the total chromosome behavior and the number of SCEs produced when compared to ten control subjects of both sexes.
Introduction
Sister chromatid exchanges (SCE) have intrigued investigators for many years. Taylor (1958) used autoradiography to examine the pattern of incorporation of tritiated thymine into the DNA molecule of chromosomes. He described the apparent exchange of DNA between chromatids of metaphase chromosomes as sister chromatid exchanges. Later, investigators (Zakharov and Egolina, 1972; Latt, 1973; Korenberg and Freedlender, 1974; Perry and Wolff, 1974) developed methods without the use of autoradiography to score SCEs in cells grown two cycles in the presence of 5-bromodeoxyuridine (BrdU) and stained with Hoechst 33258. The dye stained the unifilar chromatids (BrdU substituted in one strand of DNA) while the bifilar chromatids (BrdU substituted in both strands of DNA) remained pale when viewed with fluorescence microscopy. This method was later improved by the use of Giemsa stain. It was found that Giemsa stain would combine with the Hoechst 33258 to make a permanently stained preparation not requiring the use of fluorescence microscopy (Fig. 1). This technique became known as the fluorescence plus Giemsa (FPG) technique (Korenberg and Freedlender, 1974; Perry and Wolff, 1974).
Figure 1.
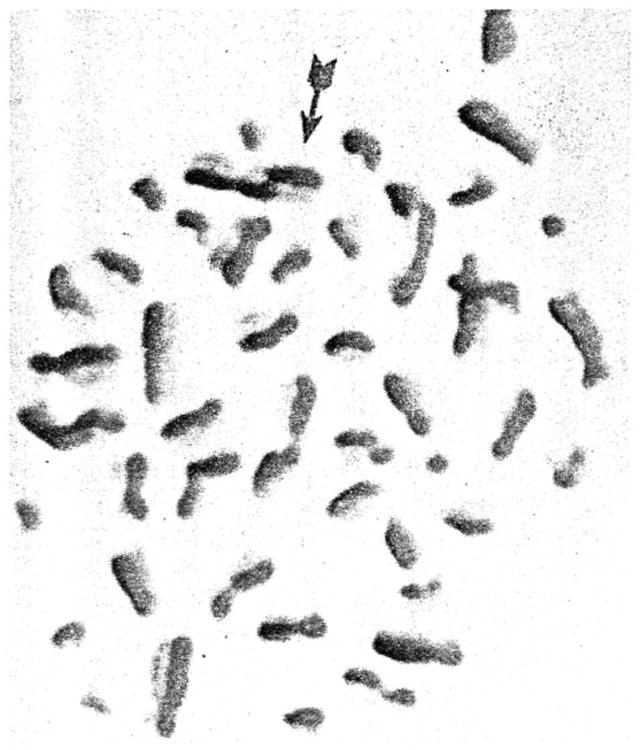
A normal human lymphocyte in metaphase stained with the FPG technique to show sister chromatid exchanges, one of which is indicated by the arrow (X1,000).
SCEs are considered indicators of genetic damage (Latt, 1974; Lambert, Lindblad, Nordenskjold, and Werelius, 1978) and have been used to investigate the effect of common abuses such as cigarette smoking (Hollander, Tockman, Liang, and Borgaonkar, 1978; Lambert, Lindblad, Nordenskjold, and Werelius, 1978) and alcohol consumption (Butler. Sanger, and Veomett, 1981) on the SCE frequency. SCEs have also been examined in human diseases, such as psoriasis (Lambert, Lindblad, Nordenskjold, and Werelius, 1978), xeroderma pigmentosum (Wolff, Bodycote, and Thomas, 1975), Down's syndrome (Yu and Borgaonkar, 1977), ataxia telangiectasia (Hatcher, Brinson, and Hook, 1976), Fanconi's anemia (Popescu, Turnbull, and Dipaola, 1977), and Bloom's syndrome (Shirashi, Freeman, and Sandberg, 1976; German, Schonberg, Louie, and Chaganti, 1977). Of these diseases only Bloom's syndrome showed an increase in SCEs, in which case they were approximately ten times greater than normal. Therefore, SCE analysis could be a useful tool in diagnosis of diseases as well as providing information on chromosome structure and function. SCE analysis may also be used to gain knowledge on the effects that altered genotypes may have on chromosome behavior, that is, a particular altered chromosome may interfere with the production of SCEs. Thus, an SCE analysis is presented for the first time from an individual with a rare, unbalanced chromosome translocation to examine differences in SCE frequency when compared with the SCE frequency found in ten control subjects of both sexes.
Materials and Methods
All subjects in this study were equally questioned about previous diseases, nutritional status, medical treatment, and x-ray investigations, as well as exposure to environmental and occupational hazards. Their smoking histories and alcohol consumption were also recorded.
Ten control subjects with no history of significant illness, and no medication or x-rays within the previous 6 mo. were selected. Two individuals smoked cigarettes (≤ 20 cigarettes/day) and denied ingesting alcohol on a regular basis.
A 27-year-old, white male with tall statute (6 ft 6 in) and a history of infertility was examined cytologically. An abnormal karyotype consisting of 46 chromosomes with a Y/Y translocation of a non-dicentric type was found on three separate occasions from blood lymphocytes, buccal cells, and skin fibroblasts. The Y/Y translocation consists of an abnormal chromosome with one centromere and the genetic information from one short arm and two long arms of the Y chromosome. No intertissue mosaicism was found. No other significant medical history was found and the remaining physical examination was normal.
Peripheral blood (0.4 ml) was added to Basal medium-Eagle's containing 20% fetal calf serum, antibiotics (penicillin and streptomycin), and 20μM 5-bromodeoxyuridine. Phytohemagglutinin-M was added and the cultures were incubated at 37 C in complete darkness to avoid photolysis. At 69 hr, 0.2 ml colcemid was added to arrest the cells at meta-phase and the cells were then harvested at 72 hr. This procedure consisted of placing the culture contents into a 15 ml, conical centrifuge tube and centrifuging 6 min at 1,000 rpm. The supernatant was removed and 6 ml of hypotonic 0.56% KC1 solution at 37 C was added. The contents were resuspended, allowed to incubate 6 min at 37 C, and then centrifuged at 1,000 rpm for an additional 6 min. During this centrifugation, the fixative was prepared by adding three parts methanol to one part glacial acetic acid. After centrifugation, the hypotonic KC1 was removed and the cells resuspended. Four ml of the fixative was added to the centrifuge tube, while gently dispersing the cells by agitation with a pipette. The cells were sedimented by centrifugation at 1,000 rpm for 6 min, the supernatant was aspirated, and the cells resuspended in 4 ml of fresh fixative. The cells were allowed to stand at room temperature for 15 min, and then were centrifuged at 1,000 rpm for 6 min. The supernatant was removed and fresh fixative was added in proportion to the size of the cell pellet, a smaller pellet requiring less fixative.
Cold, wet slides kept at 4 C prior to use were held at a 45° angle and three drops of the cell suspension were placed along the upper edge of the slide. The slides were immediately passed through a flame and allowed to air dry.
After 24 hr of storage in darkness, the slides were stained by a modification of the FPG technique developed by Perry and Wolff (1974). The slides were stained in 1 μgm/ml Hoechst 33258 for 12 min in darkness, washed in distilled water for 3 to 5 min, and then air dried and mounted in distilled water with a cover slip. The slide was then exposed to an ultraviolet lamp (wavelength of 366 nm, 115 volts, 60 Hz, 0.16 amps) for approximately 60 min at a distance of 3 cm, incubated in 10XSSC (standard saline citrate) at 60 C for 20 min, and then stained for 10 min in 3% Giemsa stain in phosphate buffer at pH 6.8. The stained slides were rinsed briefly in water, air dried, then viewed for SCEs by use of a 100× planar objective, on a standard Zeiss light microscope. A total of 252 cells from control subjects and 25 cells from the individual with the Y/Y translocation were analyzed.
Results and Discussion
The average SCE frequency in the ten control subjects was 8.4 ± SD 0.51 (Table I) while the SCE frequency of the individual with the Y/Y translocation was 7.9 ± SD 1.4. A two-tailed t-test was applied to the SCE data of this individual and the control subjects but no significant difference was found. Therefore, the abnormal Y/Y chromosome apparently did not alter the chromosome function or induce changes that could be detected by SCEs. It would be of interest to analyze SCE data from other individuals with abnormal karyotypes to examine differences in SCE frequency. The formation of SCEs remains in doubt as does the actual significance of SCEs with regard to diseases and various syndromes. The most popular theory for the origin of SCEs is the DNA repair process (Popescu, Turnbull, and Dipaola, 1977). The type of repair involved and the effect on mitosis or the progeny of mitosis remain obscure. By the examination of abnormal karyotypes in the future, more information may be obtained on the origin of SCEs and their relationship to specific chromosomes. It is possible that through SCE analysis a better understanding of sister chromatid exchanges may be attained.
Table I.
SCE data from the control subjects and the Y/Y translocation individual.
| Subject | Age | Sex | Mean of SCEs | Range of SCEs | Metaphase plates |
|---|---|---|---|---|---|
| PD | 28 | male | 8.3 | 6-12 | 26 |
| RIB | 27 | female | 8.2 | 5-13 | 20 |
| GD | 58 | male | 8.2 | 4-12 | 20 |
| JP | 41 | female | 7.7 | 5-12 | 20 |
| MAS | 28 | male | 8.1 | 5-11 | 21 |
| RD | 25 | female | 9.0 | 5-15 | 20 |
| MGB | 27 | male | 8.1 | 5-13 | 31 |
| AV | 28 | male | 8.5 | 5-13 | 26 |
| KKS | 28 | female | 8.3 | 4-14 | 37 |
| CW | 34 | female | 9.5 | 3-16 | 31 |
| RG* | 27 | male | 7.9 | 5-11 | 25 |
RG = individual with the Y/Y translocation.
Acknowledgments
This work was partially supported by the Nebraska Department of Health and the United States Department of Health, Education, and Welfare.
References
- Butler MG, Sanger WG, Veomett GE. Increased frequency of sister chromatid exchanges in alcoholics. Mutation Research. 1981 doi: 10.1016/0165-1161(81)90022-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German J, Schonberg S, Louie E, Chaganti RSK. Bloom's syndrome. IV. SCE in lymphocytes. American Journal of Human Genetics. 1977;29:248–255. [PMC free article] [PubMed] [Google Scholar]
- Hatcher NH, Brinson PS, Hook EB. SCE in ataxia telangiectasia. Mutation Research. 1976;35:333–336. doi: 10.1016/0027-5107(76)90197-4. [DOI] [PubMed] [Google Scholar]
- Hollander DH, Tockman MS, Liang YW, Borgaonkar DS. SCE in peripheral blood of cigarette smokers and in lung cancer patients and the effect of chemotherapy. Human Genetics. 1978;44:165–171. doi: 10.1007/BF00295409. [DOI] [PubMed] [Google Scholar]
- Korenberg JR, Freedlender EF. Giemsa technique for the detection of SCE. Chromosoma (Berlin) 1974;48:355–360. doi: 10.1007/BF00290992. [DOI] [PubMed] [Google Scholar]
- Lambert B, Lindblad A, Nordenskjold M, Werelius B. Increased frequency of sister chromatid exchanges in cigarette smokers. Hereditas. 1978;88:147–149. doi: 10.1111/j.1601-5223.1978.tb01617.x. [DOI] [PubMed] [Google Scholar]
- Latt SA. Microfluorometric detection of deoxyribonucleic acid replication in human metaphase chromosomes. Proceedings of the National Academy of Sciences (USA) 1973;70:3395–3399. doi: 10.1073/pnas.70.12.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latt SA. SCE, indices of human chromosome damage and repair: Detection by fluorescence and induction by mitomycin-C. Proceedings of the National Academy of Sciences (USA) 1974;71:3162–3166. doi: 10.1073/pnas.71.8.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry P, Wolff S. New Giemsa method for the differentiation staining of SCE. Nature (London) 1974;251:156–158. doi: 10.1038/251156a0. [DOI] [PubMed] [Google Scholar]
- Popescu NC, Turnbull D, Dipaola JA. SCE and chromosome aberration analysis with the use of several carcinogens: Brief communication. Journal of the National Cancer Institute. 1977;59:289–293. doi: 10.1093/jnci/59.1.289. [DOI] [PubMed] [Google Scholar]
- Shirashi Y, Freeman AI, Sandberg AA. Increased SCE in bone marrow and blood cells from Bloom's syndrome. Cytogenetics and Cell Genetics. 1976;17:162–174. doi: 10.1159/000130710. [DOI] [PubMed] [Google Scholar]
- Taylor JH. SCE in tritium labelled chromosomes. Genetics. 1958;43:515–529. doi: 10.1093/genetics/43.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff S, Bodycote J, Thomas GH. SCE in xeroderma pigmentosum cells that are defective in DNA excision repair or post-replication repair. Genetics. 1975;81:349–355. doi: 10.1093/genetics/81.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CW, Borgaonkar DS. Normal rate of SCE in Down's syndrome. Clinical Genetics. 1977;11:397–401. doi: 10.1111/j.1399-0004.1977.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Zakharov AF, Egolina NA. Differential spiralization along mammalian mitotic chromosomes. I. BudR revealed differentiation in Chinese hamster chromosomes. Chromosoma (Berlin) 1972;38:341–365. doi: 10.1007/BF00320156. [DOI] [PubMed] [Google Scholar]


