Abstract
During the past decades, prostate cancer (PCa), with an annual incident rate much higher than any other cancer, is the most commonly diagnosed cancer in American men. PCa has a relatively low progression rate yet the survival percentage decreases dramatically once the cancer has metastasized. Identifying aggressive from indolent PCa to prevent metastasis and death is critical to improve outcomes for patients with PCa. Standard procedure for assessing the aggressiveness of PCa involves the removal of tumor tissues by transrectal (TR) ultrasound (US) guided needle biopsy. The microscopic architecture of the biopsied tissue is visualized by histological or immunohistochemical staining procedures. The heterogeneity of the microscopic architecture is characterized by a Gleason score, a quantitative description of the aggressiveness of PCa. Due to the inability to identify the cancer cells, most noninvasive imaging modalities can only provide diagnosis of PCa at limited accuracy. This study investigates the feasibility of identifying PCa tumors and characterizing the aggressiveness of PCa by photoacoustic imaging assisted by cancer targeting polyacrylamide (PAA) nanoparticles (NPs). PAA is a biocompatible material used in clinics for the past 20 years. PAA NPs can protect capsulated optical contrast agents from interference by enzymes and enable prolonged systematic circulation in the living biological environment. The cancer targeting mechanism is achieved by conjugating the NPs to F3 peptides, which trace nucleolin overexpressed on the surface of cancer cells. Preliminary studies have shown that the NPs are capable of staining the PCa cells in vivo.
Keywords: Prostate cancer, nanoparticles, photoacoustic imaging
Introduction
During the past decades, Prostate cancer (PCa), with an annual incidence rate much higher than any other cancer, is the most commonly diagnosed cancer in American men [1]. PCa is ranked only next to lung and bronchus cancer in all cancer related deaths. PCa has a relatively low progression rate. Patients with early diagnosed PCa have a five year survival rate close to 100% yet the percentage decreases dramatically once the cancer has metastasized. Identifying the high grade cancer in prostate and precise therapy in prevention of metastasis is of particular benefit for PCa patients.
Blood test for the biomarkers is the primary screening approaches for PCa [2]. With preliminary diagnosis of PCa by blood test, the patients will be recommended for digital rectal examination, transrectal ultrasonography, and eventually the Ultrasound (US) guided biopsy, the gold standard procedure for evaluating the presence of PCa. The microscopic architectures of the biopsied tissues, stained and visualized by histology procedures, are observed by pathologists and assigned a Gleason grade [3], a highly prognostic factor for prostate cancer, as the conclusion for the diagnosis procedure. However, US imaging, with low sensitivity to PCa, could either miss cancer or lead to the undersampling of aggressive cancer. Non-guided and saturating biopsies following a predetermined pattern overlaid onto the prostate contour delineated by US are thereby frequently employed. More than 20 biopsy cores, each with a diameter of approximately 1mm and length of 15 mm, could be extracted [4]. Such procedure could cause anxiety, pain, potentially unrecoverable damage to the neurovascular bundle and consequently erectile dysfunction [5]. A strong need thereby exists for minimal invasive and cost-efficient technique that can 1) identify the malignancies in prostate to guide biopsy and/or 2) evaluate the aggressiveness of the malignancies without removing the tissue.
The recent development of hydrogel nanoparticle (NP) technology has allowed the targeted delivery of functional agents to the cancer cells by recognizing the specific proteins on the cancer cell surfaces [6]. A unique advantage of the polymer based hydrogel NPs is that their biocompatibility and chemically inertness assure a long circulation life and thereby minimize the toxicity of the functional loadings. Such NPs, when loaded with imaging contrast enhancement materials, facilitate in vivo tumor staining for the direct observation by noninvasive imaging modalities [7-10]. The NPs could also precisely deliver therapeutic drugs to the malignant tissues, enabling localized cancer treatment. In our previous studies, we have successfully developed cancer-targeting [6] and specifically prostate cancer targeting NPs [9] for tumor staining. Yet the major diagnostic challenge of prostate cancer, besides the delineation of the tumors, is the grading of the cancers by their microscopic architectures.
Laser (photo-) induced ultrasound (-acoustic) imaging, namely photoacoustic (PA) imaging (PAI), is a novel imaging modality combining the advantages of both optical and ultrasound imaging [11, 12]. A PAI system can be achieved by integrating a laser system to a clinical ultrasound system. PAI thereby possesses the intrinsic advantage in prostate imaging, over any other imaging modality, in that it is naturally coregistered with transrectal US for improved guidance for biopsy. Due to its multi-physics and multi-scale nature, PAI combines the excellent optical sensitivity for observing macroscopic, chemical contents and US resolution for observing the microscopic, physical architectures in biological tissue.
This study investigated the capability of F3 peptide conjugated NP for the characterization architectures in PCa tumors.
Approaches
In vitro test of the F3 peptide conjugated NPs with human PCa cells
The nanoparticles were incubated with human PCa cells (DU145) for 2 hours. NPs were labeled with FITC (green), while the cell nucleus was stained with DAPI (blue). NP concentration: 1.0 mg/mL; scale bar: 25 μm. These results demonstrated that the attachment of F3 peptide improved the uptake of PEG-PAA NPs into both DU145 cells significantly, which is related with the overexpression of nucleolin on the surface of both kinds of tumor cells.
In vivo test with xenograft tumor in rats
The NPs were tested with DU145 xenograft tumors in rats. Subcutaneous xenograft tumors were generated in nude rats (100-150 g). Approximately 5-10 million human derived tumor cells (DU145) in 0.2-0.5 ml of cultured media was implanted under manual restraint. Animals were physically monitored twice a week for tumor growth. Large tumors of around 1 cm3 were formed at the inoculation site by 7-14 days post-inoculation. When the tumors had grown to the expected volume, the rats were injected through tail vein with F3 conjugated PEG-PAA NPs loaded with Coomassie Blue CB at 60, 120 and 250 mg/Kg of body weight. The animals were anesthetized and PAI was performed in the experiment setup in Fig. 2.
Fig. 2.
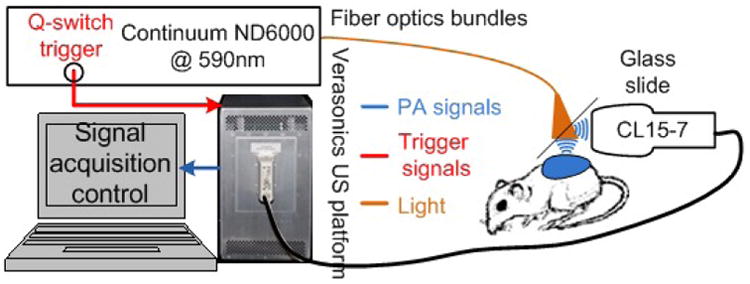
Experiment setup for xenograft tumor imaging in vivo. 590 nm laser targeting the optical absorption peak of Coomassie Blue encapsulated in the NPs were used. The laser was delivered through a fiber optics bundle. A US probe acquires US and PA measurements, which is processed and displayed by the Verasonics US platform [10].
Representative images of the tumors were shown in Fig. 3(a). The dynamics of the tumor in rats are shown Fig. 3(b)(c). Significant optical contrast enhancement can be observed.
Fig. 3.
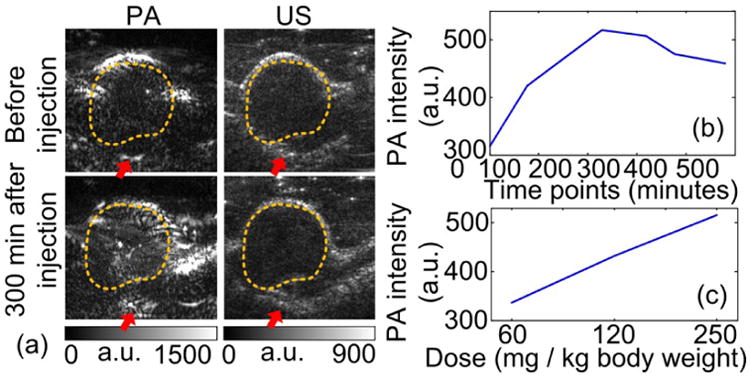
PA-US parallel imaging of a xenograft PCa tumor stained by CB F3 PAA PEBBLEs in vivo. (a) PA-US images before and after the PEBBLE injection. Although no prominent change is observed in US images, the pixel intensities within the tumors region (dashed contours) have significantly increased in the PA image. Red arrows mark the blood vessels connecting to the tumor. a.u.: arbitrary unit. (b) Averaged pixel intensities of the tumor in PA image with respect to time after PEBBLE injection. (c) PA intensity versus PEBBLE injection dose.
Imaging the a xenograft tumor from canine prostate
An optical contrast enhanced xenograft tumor was imaged thought a canine prostate to test the imaging depth. The tumors as deep as 2cm can be identified in the PA imaging.
In vivo staining the architecture in prostate using the F3 conjugated nanoparticles
Figure 5 shows the capability of F3 PAA PEBBLEs in staining the microarchitectures in a transgenic mice species (TRAMP) in vivo. TRAMP mice spontaneously develop PCa with pathological similarity to human PCa. Tumors as large as 1 cm3 can be generated. PEBBLEs loaded with Methylene Blue (MB, excites at 643 nm and emits at 680 nm) were injected at the dose of 250 mg/Kg body weight for validation in fluorescence microscopy. The mice were euthanized 4 hours after injection. Two adjacent slides were cut from the prostate sample. One was H&E stained. The other was not stained for observing the fluorescence from MB. Representative microarchitecutre patterns (comparable to Gleason patterns in human [3]) specified in literature [13] are stained with strong optical contrast over non-cancerous supporting cells.
Fig. 5.
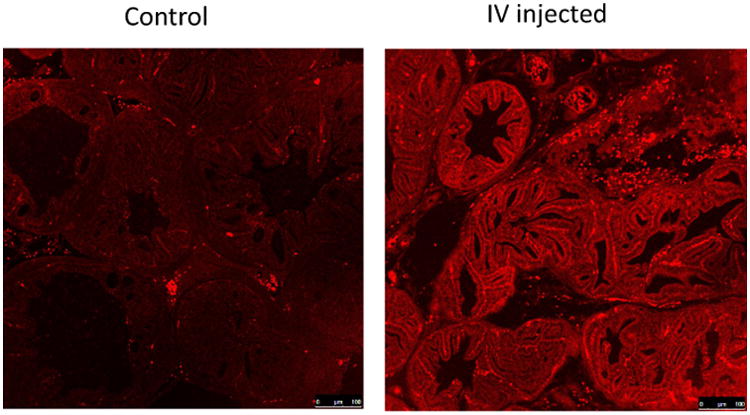
Microarchitectures of PCa in TRAMP mice stained in vivo by F3 PAA PEBBLEs. MB was encapsulated in the PEBBLES. Autofluorescence can be found in the control images. However, the MB introduced more fluorescence contrast in the IV injected case. Images were taken at 10× magnification.
Discussion and Conclusion
This study validated the F3 conjugated hydrogel NPs can enhance the optical contrast of PCa tumors and visualize the tissue architectures inside the tumors.
F3 peptide targets general cancer cells. Conjugating nanoparticles with antibodies of specific proteins expressed by metastatic PCa cells could further enrich the diagnostic information. The encapsulated optical contrast enhancing agents can also be replaced with those possessing strong optical absorption in the 700-900 nm range to further increase the achievable imaging depth.
Fig. 1.
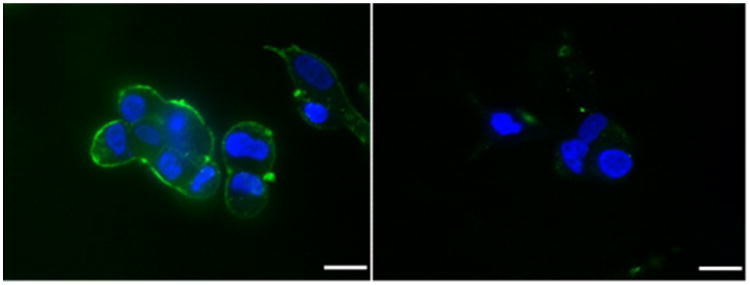
Confocal microscopy images of DU145 cells after incubation with F3-conjugated PEG-PAA NPs and nontargeted PEG-PAA NPs.
Fig. 4.
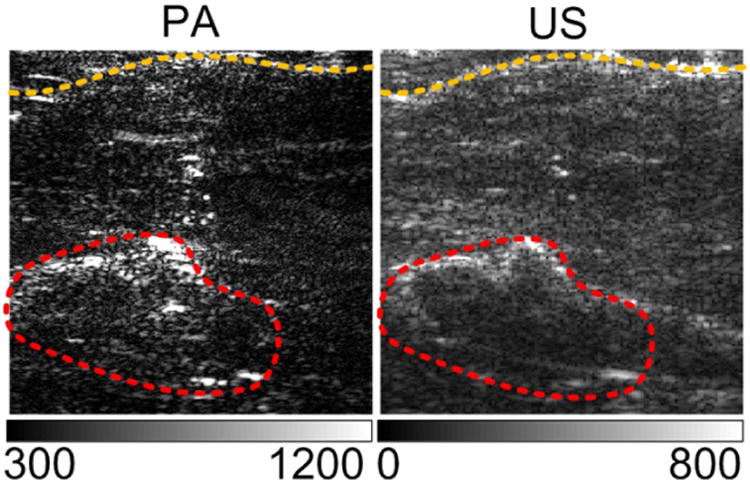
Imaging xenograft tumor through a canine prostate. A bagel prostate was cut in-half and covered the xenograft tumor in vivo. Compared to the dark tumor region in US image, the contrast of the tumor in PA image is enhanced. Dimensions of the images are 22 mm by 22 mm.
Acknowledgments
This work was supported by National Institute of Health under grant numbers R01AR060350 and R01CA186769.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Lin PY, Cheng KL, McGuffin-Cawley JD, Shieu FS, Samia AC, Gupta S, Cooney M, Thompson CL, Liu CC. Detection of Alpha-Methylacyl-CoA Racemase (AMACR), a Biomarker of Prostate Cancer, in Patient Blood Samples Using a Nanoparticle Electrochemical Biosensor. Biosensors. 2012;2(4):377–387. doi: 10.3390/bios2040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gleason DF. Histologic grading of prostate cancer: A perspective. Human Pathology. 1992;23(3):273–279. doi: 10.1016/0046-8177(92)90108-f. [DOI] [PubMed] [Google Scholar]
- 4.Presti JC., Jr Prostate biopsy: how many cores are enough? Urologic Oncology: Seminars and Original Investigations. 2003;21(2):135–140. doi: 10.1016/s1078-1439(03)00006-1. [DOI] [PubMed] [Google Scholar]
- 5.Zisman A, Leibovici DAN, Kleinmann J, Siegel YI, Lindner A. The Impact of Prostate Biopsy on Patient Well-Being: : A Prospective Study of Pain, Anxiety and Erectile Dysfunction. The Journal of Urology. 2001;165(2):445–454. doi: 10.1097/00005392-200102000-00023. [DOI] [PubMed] [Google Scholar]
- 6.Ray A, Wang X, Lee YEK, Hah HJ, Kim G, Chen T, Orringer DA, Sagher O, Liu X, Kopelman R. Targeted blue nanoparticles as photoacoustic contrast agent for brain tumor delineation. Nano Research. 2011;4(11):1163–1173. doi: 10.1007/s12274-011-0166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang R, Xiong C, Huang M, Zhou M, Huang Q, Wen X, Liang D, Li C. Peptide-conjugated polymeric micellar nanoparticles for Dual SPECT and optical imaging of EphB4 receptors in prostate cancer xenografts. Biomaterials. 2011;32(25):5872–5879. doi: 10.1016/j.biomaterials.2011.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johannsen M, Gneveckow U, Thiesen B, Taymoorian K, Cho CH, Waldöfner N, Scholz R, Jordan A, Loening SA, Wust P. Thermotherapy of Prostate Cancer Using Magnetic Nanoparticles: Feasibility, Imaging, and Three-Dimensional Temperature Distribution. European Urology. 2007;52(6):1653–1662. doi: 10.1016/j.eururo.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Kim G, Huang SW, Day KC, O'Donnell M, Agayan RR, Day MA, Kopelman R, Ashkenazi S. Indocyanine-green-embedded PEBBLEs as a contrast agent for photoacoustic imaging. Journal of Biomedical Optics. 2007;12(4):044020–044028. doi: 10.1117/1.2771530. 044020. [DOI] [PubMed] [Google Scholar]
- 10.Popovtzer R, Agrawal A, Kotov NA, Popovtzer A, Balter J, Carey TE, Kopelman R. Targeted Gold Nanoparticles Enable Molecular CT Imaging of Cancer. Nano Letters. 2008;8(12):4593–4596. doi: 10.1021/nl8029114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan J, Xu G, Yu Y, Zhou Y, Carson PL, Wang X, Liu X. Real-time photoacoustic and ultrasound dual-modality imaging system facilitated with graphics processing unit and code parallel optimization. Journal of Biomedical Optics. 2013;86001:1. doi: 10.1117/1.JBO.18.8.086001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Pang Y, Ku G, Xie X, Stoica G, Wang LV. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nature biotechnology. 2003;21(7):803–806. doi: 10.1038/nbt839. [DOI] [PubMed] [Google Scholar]
- 13.Hurwitz AA, Foster BA, Allison JP, Greenberg NM, Kwon ED. Current Protocols in Immunology. John Wiley & Sons, Inc.; 2001. [DOI] [PubMed] [Google Scholar]


