Abstract
Objectives
Poorly controlled hypertension (HTN) is extremely prevalent and, if left unchecked, subclinical hypertensive heart disease (SHHD) may ensue leading to conditions such as heart failure. To address this, we designed a multidisciplinary program to detect and treat SHHD in a high-risk, predominantly African American community. The primary objective of this study was to determine the cost-effectiveness of our program.
Methods
Study costs associated with identifying and treating patients with SHHD were calculated and a sensitivity analysis was performed comparing the effect of four parameters on cost estimates. These included prevalence of disease, effectiveness of treatment (regression of SHHD, reversal of left ventricular hypertrophy [LVH], or blood pressure [BP] control as separate measures), echocardiogram costs, and participant time/travel costs. The parent study for this analysis was a single-center, randomized controlled trial comparing cardiac effects of standard and intense (<120/80 mm Hg) BP goals at 1 year in patients with uncontrolled HTN and SHHD. A total of 149 patients (94% African American) were enrolled, 133 (89%) had SHHD, 123 (93%) of whom were randomized, with 88 (72%) completing the study. Patients were clinically evaluated and medically managed over the course of 1 year with repeated echocardiograms. Costs of these interventions were analyzed and, following standard practices, a cost per quality-adjusted life-year (QALY) less than $50,000 was defined as cost-effective.
Results
Total costs estimates for the program ranged from $117,044 to $119,319. Cost per QALY was dependent on SHHD prevalence and the measure of effectiveness but not input costs. Cost-effectiveness (cost per QALY less than $50,000) was achieved when SHHD prevalence exceeded 11.1% for regression of SHHD, 4.7% for reversal of LVH, and 2.9% for achievement of BP control.
Conclusions
In this cohort of predominantly African American patients with uncontrolled HTN, SHHD prevalence was high and screening with treatment was cost-effective across a range of assumptions. These data suggest that multidisciplinary programs such as this can be a cost-effective mechanism to mitigate the cardiovascular consequences of HTN in emergency department patients with uncontrolled BP.
Poorly controlled hypertension (HTN) is extremely prevalent in the United States,1–6 with a disproportionally high disease burden existing among African Americans, putting them at greater risk for poor cardiovascular outcomes such as stroke, myocardial infarction, chronic kidney disease, and heart failure (HF).1,7–11 Recommendations for therapy are well defined by the International Society of Hypertension in Blacks (ISHIB)12 and in the recent Joint National Committee reports on HTN.13,14 However, due to factors that are difficult to overcome (therapeutic inertia, poor adherence to therapy, socioeconomic barriers, and patient understanding of disease state), achieving blood pressure (BP) control remains a challenge.1,13,15–21
For patients with chronic but uncontrolled HTN, cardiac remodeling is a near-universal process that is associated with increased cardiovascular risk as it progresses. Despite this, underlying heart disease (HD) is typically not detected (or even screened for) until advanced remodeling is present and symptoms manifest,9,11,22–24 leaving clinicians with fewer options to prevent adverse events. Early identification of subclinical hypertensive heart disease (SHHD) and appropriate control of BP have become important steps in secondary cardiovascular disease prevention (especially for HF).11,25–28 Although some perceive that screening of asymptomatic patients provides no long-term benefit to morbidity,28–31 compelling arguments have been made for the utility of screening activities in communities that are predominately urban, African American and where limitations to healthcare access prompts heavy reliance on the emergency department (ED) for primary care.11,32–55 Increasing prevalence of the target condition is often tied to cost-effectiveness of such approaches and, in the case of SHHD, has direct implications for intensification of antihypertensive therapy.36
The development of preventable secondary complications of SHHD leads to substantial increases in morbidity, mortality, and health care costs.37 Multiple studies have demonstrated that uncontrolled HTN is a significant risk factor for the progression of SHHD and the onset of chronic HF and other clinically overt conditions.1,7–11 Given the disproportionate burden of hypertensive heart disease in urban, African American populations, the primary objective of this study was to assess the cost-effectiveness of a multidisciplinary screening program designed to identify and treat SHHD among ED patients with elevated BP.
METHODS
Study Design and Setting
This cost-effectiveness analysis was an a priori aim included as part of a grant funded prospective, randomized controlled clinical trial (NCT00689819) designed to compare the effects of two BP targets (control, BP < 140/90 mm Hg [<130/80 mm Hg if diabetes or chronic kidney disease was present], and intervention, which had a singular target of <120/80 mm Hg) on reverse remodeling for patients with SHHD. Details of the study design, including SHHD determination and regression, have been previously published.38 A convenience sample of patients who presented to a single-center ED located in Detroit, Michigan, where the population is 83% African American was enrolled between November 2008 and April 2010. Enrolled patients were evaluated at 3-month intervals over the course of 1 year in a single HTN clinic. Care was delivered by a multidisciplinary team including an ED physician, a HTN specialist experienced with HTN in our patient population, a physician’s assistant (PA), a nurse practitioner (NP), research assistants, and clinic office staff. All care-related expenses, including transportation, telephone reminders, medications, and tests, were provided free of charge to all the participants. The institutional review board approved this study and all subjects provided written informed consent.
Selection of Participants
Study participants were recruited from a tertiary, academic medical center’s ED, which treats over 90,000 patients each year. Over a 17-month period, individuals 35 years of age and older who presented with an initial BP > 140/90 mm Hg were identified using the facility’s electronic medical record (FirstNet by Cerner Corp.). For inclusion, patients were required to have a repeat BP > 140/90 mm Hg and have normal exertional tolerance (defined as class 1 on Goldman Specific Activity Scale). Those with acute illness requiring hospitalization, history of previously diagnosed coronary artery disease or HF, and presenting symptoms (i.e. dyspnea, chest pain) potentially attributable to hypertensive heart disease and those being actively followed and/or treated by primary care physician were excluded.11,38,39 Patients who met these criteria were brought back for a follow-up screening echocardiogram in our outpatient HTN clinic.
In total, 160 individuals met initial inclusion criteria, 149 of whom returned for a subsequent screening echocardiogram. All echocardiograms were performed and detailed history obtained for screened participants within 1 week. All participants with SHHD, defined by presence of left ventricular (LV) hypertrophy (LV mass ≥ 48 g/m2.7 in males or ≥ 45 g/m2.7 in females), LV systolic dysfunction (ejection fraction < 50%), or diastolic dysfunction (combination of parameters based on validated criteria of LV stiffness and relaxation) were randomized into either control or intensive therapy arms.11
Study Procedures
Randomized participants were seen at baseline (initial visit) and 3-, 6-, 9, and 12-month intervals. A standard BP measurement protocol was utilized. A trained research assistant, using appropriately sized oscillometric brachial cuff, performed three BP measurements with the patient in a seating position and their arms resting comfortably at heart level. The average reading of the three measurements was used to determine the BP reading recorded for that clinic visit. A multidisciplinary group of PAs and NPs then titrated antihypertensive therapy as needed, according to study group assignment. During each clinic visit, participants were educated about the importance of medication adherence and all received telephone reminders for pending follow-up appointments. Additionally, to help ensure compliance, all medication costs were paid for using study funds. Echocardiograms, all interpreted by a single board-certified cardiologist blinded to patient information and study group, were repeated at the 12-month visit. SHHD regression was the absence of left ventricular hypertrophy (LVH) and systolic or diastolic dysfunction on repeat imaging. Because LVH is such an important consequence of HTN and contributor to important outcomes such as HF development, we also evaluated reversal of LVH (i.e., LV mass below threshold cut-points) as a separate outcome measure.
Cost Analysis
Total costs of the program (Table 1) were calculated by adding the cost of medications (based on purchase price from our outpatient pharmacy), laboratory tests (based on hospital research pricing), clinic activities (based on proportional space and personnel costs), echocardiograms (based on echocardiographic technician time), and participant time and travel. Time and travel costs were obtained through a survey and mean values of respondents were used to estimate a base case value of $22.72 ± $14.36 per person. When data were missing, values for time and travel costs were imputed three different ways, separated by participants who did and did not complete the study: as zero (i.e., assumption of no cost), as the cohort median, and as the cohort mean. Based on this, low, median, and high total costs were estimated.
Table 1.
Total Costs of Program With Imputed Values for Missing Values of Time and Travel Costs
| Item | Completed Study Group (n = 88) |
Drop-out Group (n = 45) |
Total |
|---|---|---|---|
| Medications | $43,778 | ||
| Laboratory tests | $5,408 | $3,749 | $9,158 |
| Echocardiogram | $29,517 | ||
| Clinic | $32,380 | ||
| Time | |||
| Zero | $736 | $0 | $736 |
| Median | $1,448 | $891 | $2,339 |
| Mean | $1,476 | $1,157 | $2,633 |
| Travel | |||
| Zero | $1,159 | $317 | $1,476 |
| Median | $1,159 | $581 | $1,740 |
| Mean | $1,159 | $695 | $1,854 |
| Total | |||
| Low | $7,303 | $4,066 | $117,044 |
| Median | $8,015 | $5,221 | $118,912 |
| High | $8,044 | $5,600 | $119,319 |
To evaluate cost-effectiveness, costs per quality-adjusted life-year (QALY) were calculated. This approach is widely accepted and QALYs have long been used to guide healthcare resource allocation.40 A threshold cost of $50,000 per QALY is the traditional benchmark for determining the value of care. Therefore, values at or below this were adopted in our study as the measure of cost-effectiveness.41–43 The cost per QALY was modeled by the following mathematical equation, where 0.87 and 0.71 are used as standard utility values44 assigned to patients for whom chronic HF (the most likely adverse consequence of untreated SHHD) would or would not be prevented by treatment, respectively:
We based the probability of treatment effectiveness on preventing HF using three measures: regression of SHHD, reversal of LVH, and achievement of BP control. For purposes of this analysis, achievement of BP control was based on study randomization group targets rather than a singular BP goal. We varied the probability of successful outcome for each of these at 100, 50, and 25% for the low, median, and high end of the calculated program cost (Table 2) and then calculated the cost per case prevented, cost per QALY where HF would have been prevented and cost per QALY where HF would have developed. The variance levels for probabilities were selected to impart large, medium, and small impacts on the outcomes of interest.
Table 2.
Program Cost Estimates Based on Projected Disease Probability of Outcome
| Measure of Treatment Effectiveness | Program Cost Estimates and Projected Treatment Effectivenss
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Low, $117,044
|
Median, $118,912
|
High, $119,319
|
|||||||
| 100% | 50% | 25% | 100% | 50% | 25% | 100% | 50% | 25% | |
| Cost per case prevented | |||||||||
| BP control | $3,259 | $6,519 | $13,038 | $3,320 | $6,639 | $13,279 | $3,333 | $6,666 | $13,331 |
| SHHD regression | $10,104 | $20,209 | $40,418 | $10,291 | $20,582 | $41,165 | $10,332 | $20,664 | $41,238 |
| LVH regression | $5,052 | $10,104 | $23,409 | $5,146 | $10,291 | $23,782 | $5,166 | $10,332 | $23,864 |
| Cost per QALY-HF prevented | |||||||||
| BP control | $3,747 | $7,493 | $14,986 | $3,816 | $7,632 | $15,236 | $3,831 | $7,662 | $15,324 |
| SHHD regression | $11,614 | $23,228 | $46,457 | $11,829 | $23,658 | $47,316 | $11,876 | $23,751 | $47,503 |
| LVH regression | $5,807 | $11,614 | $26,907 | $5,914 | $11,829 | $27,336 | $5,938 | $11,876 | $27,430 |
| Cost per QALY-HF developed | |||||||||
| BP control | $4,591 | $9,182 | $18,363 | $4,676 | $9,351 | $18,703 | $4,694 | $9,388 | $18,777 |
| SHHD regression | $14,232 | $28,463 | $56,926 | $14,495 | $28,989 | $57,978 | $14,552 | $29,104 | $58,208 |
| LVH regression | $7,116 | $14,232 | $32,970 | $7,249 | $14,495 | $33,496 | $7,276 | $14,552 | $33,611 |
Cost per case prevented, cost per QALY where HF would have been prevented, and cost per QALY where HF would have developed were estimated using various probability of successful outcomes (100%, 50%, 25%) for the low, median, and high end of the calculated program cost.
BP = blood pressure; HF = heart failure; LVH = left ventricular hypertrophy; QALY = quality-adjusted life-year.
Sensitivity analyses were then performed for each of our three treatment effectiveness measures, comparing the effect of disease prevalence, echocardiogram costs, and time/travel costs on cost per QALY using a base case that included the prevalence of SHHD in our population, the proportion achieving the desired treatment effect for each measure, and a projected treatment effectiveness of 100% for SHHD and LVH regression and 50% for BP control. Medicare reimbursement rates were used to calculate adjusted total costs as influenced by changing echocardiogram costs, based on CPT code 93306 using national average global payments for 2013 and 2015, along with proposed future rates (Table 3).45 Tornado plots were constructed, representing the impact of these predefined parameters on the overall cost per QALY.
Table 3.
Adjusted Total Costs Based on Variable Echocardiogram Costs
| Echocardiogram Cost (Variable) | Adjusted Costs
|
||
|---|---|---|---|
| At $268 (proposed) | At $173 (2013) | At $229 (2015) | |
| Cost for 149 screened + 88 completed therapy | $63,516 | $41,001 | $54,273 |
| Difference* | $33,999 | $11,484 | $24,756 |
| Adjusted totals | |||
| Low | $151,043 | $128,528 | $141,800 |
| Median | $152,911 | $130,396 | $143,668 |
| High | $153,318 | $130,803 | $144,075 |
Difference = the added cost arising from using Medicare pricing as the input variable for echocardiogram cost rather than the research cost noted in Table 1, which was based primarily on echocardiographic technician time.
RESULTS
Of the 149 subjects enrolled, 133 (89.3%) had SHHD, 123 (control n = 65, intervention n = 58) were randomized, and 88 (control n = 45, intervention n = 43) completed the entire study protocol. The study population was mostly female (66%), and African-American (95%), with a mean (±SD) age of 49.2 (±8.3) years. The majority of patients (82.9%) had been previously diagnosed with HTN, only 28 (23%) of whom were on prescribed medication at baseline and had carried the diagnosis, on average, treated for 8.8 (SD ± 8.6) years. At initial screening in the ED, patients had a mean (±SD) systolic BP of 182.5 (±23.3) mm Hg and a mean (±SD) diastolic BP of 104.8 (±12.3) mm Hg. At randomization following the screening echocardiogram, mean (±SD) systolic BP was 151.2 (±24.1) mm Hg and mean (±SD) diastolic BP was 97.2 (±15.8) mm Hg. Of the 88 patients that completed therapy, 10 (11%) achieved SHHD regression, 20 (23%) experienced reversal of LVH, and 31 (35%) achieved BP control according to study specified goals.
Total estimated costs of the program (Table 1) ranged from $117,044 to $119,319. As shown in Table 2, the program was cost-effective (cost per QALY < $50,000) under all circumstances, except when the projected effectiveness of SHHD regression was < 25% (Table 3). That is to say, our approach would be cost-effective if the patient has a 25% or greater probability of preventing HF with any of our treatment effectiveness measures (SHHD regression, LVH regression, or BP control), across a range of cost assumptions. The cost per QALY for all three treatment effectiveness measures was consistently below the $50,000 mark when assessing cost per case prevented and cost per case where HF would have been prevented.
Sensitivity analyses demonstrated that for each of the three measureable treatment effects, only prevalence of SHHD moves the overall cost per QALY above the $50,000 mark. Specifically, overall cost per QALY exceeded $50,000 only at prevalence rates below 11.1% for SHHD regression, 4.7% for LVH reversal, and 2.9% for BP control (Figure 1).
Figure 1.
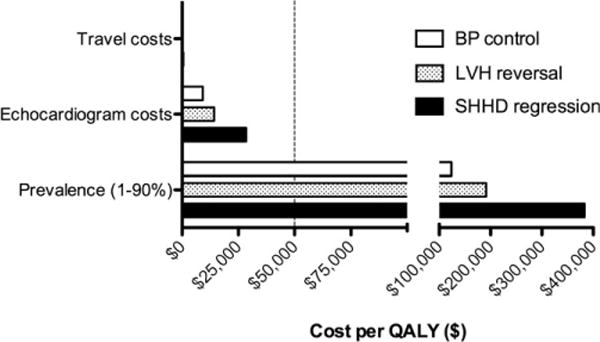
Cost per QALY for people achieving BP control, reversal of LVH, and regression of SHHD. Only prevalence decreases the cost per QALY below the $50K mark, which occurs at a prevalence of >2.9% for BP control, >4.7% for LVH regression, and >11.1% for SHHD regression. Note: Travel cost data are hidden by the Y-axis. BP = blood pressure; LVH = left ventricular hypertrophy; QALY = quality-adjusted life-year; SHHD = subclinical hypertensive heart disease.
DISCUSSION
In this study of hypertensive ED patients, we found that a multidisciplinary program focused on detection and treatment of SHHD was cost-effective across a range of cost assumptions. This is important because SHHD is a meaningful point where intervention can prevent potential life-threatening conditions.12–14 However, for many with HTN, especially African Americans in underserved communities, heart disease will not be detected until advanced stages, limiting options for secondary or even tertiary prevention.9,11,22–24 While no one would suggest that ED physicians assume primary responsibility for the management of SHHD, in settings similar to ours where SHHD is likely to be prevalent, it is reasonable for the ED to play an active role in screening and referral. This process could include no more than BP readings and referral for a follow-up echocardiogram. Blood pressure readings are a routine measure taken as part of an ED patient visit and do not add to the total cost of care. Although echocardiograms are relatively expensive, not readily available in many EDs, and require (as of now) a dedicated technician and physician to read the results, they are superior to electrocardiograms when assessing for SHHD.46
Whether actual prevalence of disease in other communities will approach what we found in this study (89.3%) is unclear. Levy et al.11 previously showed that within a similar population—underserved African American, hypertensive ED patients—the prevalence of underlying SHHD is close to 90%. The prevalence of SHHD in other patient populations with HTN has not been well described; however, existing studies suggest it broadly ranges between 0.9 and 50%.10,11,47–51 Given that the highest calculated prevalence rate when cost per QALY exceeds the $50,000 mark in our data is 11%, it is likely that a similar program would remain cost-effective in the majority of other locations. It is important to note that for many disease states, the cost-effectiveness of screening is mutually exclusive from that of treatment. However, for the purposes of our study, no distinction was made between the cost of screening and the cost of treatment, as we sought to evaluate a program aimed at reducing the consequences of SHHD on an at-risk population—a process that involves both detection and ongoing management. To that end, we chose prevention of HF as the desired endpoint of treatment and constructed our cost-effectiveness models using HF as the outcome of interest. We did so because, of all the consequences associated with SHHD, HF is the most tightly linked overall and one that disproportionately affects African Americans.52 Moreover, prevention of HF through more comprehensive upstream screening for SHHD and intervention when present has become an area of increasing emphasis.53
Based on our sensitivity analysis, the true cost-effectiveness of our approach to diagnosis and treatment of SHHD likely sits between $20,000 and $30,000 per year—a figure that is similar to recently published data by Moise et al.43 that found more intensive BP control to be cost-effective at <$50,000 per QALY for management of HTN, particularly in patients with cardiovascular disease, chronic kidney disease, and a 10-year cardiovascular disease risk > 15%. In a recent perspective piece, Neumann et al.41 argue that the $50,000 per QALY measure may indeed be too low. As part of their analysis, they examined cost-effectiveness thresholds referenced by authors from 1990 to 2012. A majority of the studies reference $50,000 as the most widely used benchmark,43,54 with $100,000 being the second most popular value. Braithwaite et al.55 further suggest that a range between $95,000 and $264,000 per life-year saved should be considered when evaluating impact of care. In a study conducted by Shiroiwa et al.,56 willingness to pay for one additional QALY was measured to be $62,000. Although the usefulness of the $50,000 benchmark has been questioned, and many other benchmarks have been proposed, the fact remains that no one measure is appropriate in all decision contexts.41 We chose to adopt the $50,000 benchmark for our study because we thought it to be consistent with the economic reality of our study demographic. Using higher thresholds would only provide further evidence to support the cost-effectiveness of our approach, as there were few scenarios where sensitivity analysis exceeded the $50,000 mark and none that were greater than $62,000.
Problems with therapeutic inertia, poor adherence, socioeconomic challenges to self-care, and low disease-specific knowledge are known to affect HTN control.1,13,15–21 Accordingly, throughout the study, participants periodically filled out questionnaires aimed at accurately gauging, among other things, their time and travel costs. In analyzing responses, a majority indicated distance-to-travel to receive care as an important factor in their adherence and follow-up. As travel did not affect cost effectiveness in our study, paying for this and other potential barriers to follow-up for chronic HTN as part of a broad risk reduction program might be reasonable to consider.
LIMITATIONS
Several limitations to our study exist. This was a single-center trial with a limited number of subjects, over a relatively short time period, and this did not allow us to study cardiovascular outcomes over a longer period of time. The patient population was predominantly low-income and African American with poor access to primary medical care, making our results more difficult to apply across different demographic populations. However, this is a representative sample from a high-risk, underserved, and underrepresented population. As has been established previously, the prevalence of cardiovascular disease is exceedingly high within this demographic and SHHD is the only factor that determines the cost effectiveness of our treatment model, suggesting that our data are, at the least, applicable to similar high-risk populations. This study also had a high dropout rate in both control and intervention groups (28 and 24%, respectively) which was not unexpected considering the study population and how these at-risk communities typically interact with the health system.
Another limitation is that the design of our mathematical model to determine QALY did not assign hazard ratios to measure regression versus nonregression of end-stage disease. We believe that this would have led to a more accurate assessment of HF progression. Additionally, the increased costs associated with developing HF—i.e., hospitalizations, interventions, loss of productivity, etc.—were not added to the equation except for in the adopted utility factors. It can be argued that by not including such data, the development of HF has little impact on the cost-effectiveness of our program. However, we believe that addressing specific outcomes is not essential in building a strong argument for intervention. Many different adverse consequences are expected as a result of uncontrolled HTN and utility measures adopted are the most productive way to represent the broad nature of possible outcomes. Finally, we assigned just one-life-year gained, which may be underrepresenting the actual benefits of disease regression. However, had we used a lengthier time measure, our results would have only been further validated.
Finally, this is a cost-effectiveness study, rather than one that measures (patient specific) willingness to pay for treatment. Such an investigation was beyond the scope of this study but may merit future research.
CONCLUSIONS
Our approach to screen for and treat subclinical hypertensive heart disease among urban ED patients with elevated blood pressure proved to be cost-effective across a range of cost- and treatment-effectiveness assumptions. The prevalence of subclinical hypertensive heart disease was particularly high in our predominantly African American cohort, many of whom utilize the ED for primary care, suggesting that multidisciplinary programs designed to prevent cardiovascular complications of hypertension could be beneficial in similar communities.
Acknowledgments
The authors thank all students and research technicians that were involved with the data collection and retention of participants enrolled in the study.
References
- 1.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–50. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 2.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52:818–27. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 3.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 4.Burt VL, Cutler JA, Higgins M, et al. Trends in the prevalence, awareness, treatment, and control of hypertension in the adult US population. Data from the health examination surveys, 1960 to 1991. Hypertension. 1995;26:60–9. doi: 10.1161/01.hyp.26.1.60. [DOI] [PubMed] [Google Scholar]
- 5.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 6.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 7.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics-2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360:1179–90. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agabiti-Rosei E, Muiesan ML, Salvetti M. Evaluation of subclinical target organ damage for risk assessment and treatment in the hypertensive patients: left ventricular hypertrophy. J Am Soc Nephrol. 2006;17:S104–8. doi: 10.1681/ASN.2005121336. [DOI] [PubMed] [Google Scholar]
- 10.Drazner MH, Dries DL, Peshock RM, et al. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension. 2005;46:124–9. doi: 10.1161/01.HYP.0000169972.96201.8e. [DOI] [PubMed] [Google Scholar]
- 11.Levy P, Ye H, Compton S, et al. Subclinical hypertensive heart disease in black patients with elevated blood pressure in an inner-city emergency department. Ann Emerg Med. 2012;60:467–74. doi: 10.1016/j.annemergmed.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Flack JM, Sica DA, Bakris G, et al. Management of high blood pressure in Blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension. 2010;56:780–800. doi: 10.1161/HYPERTENSIONAHA.110.152892. [DOI] [PubMed] [Google Scholar]
- 13.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 14.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 15.Bennett H, Laird K, Margolius D, Ngo V, Thom DH, Bodenheimer T. The effectiveness of health coaching, home blood pressure monitoring, and home-titration in controlling hypertension among low-income patients: protocol for a randomized controlled trial. BMC Public Health. 2009;9:456. doi: 10.1186/1471-2458-9-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berlowitz DR, Ash AS, Hickey EC, et al. Inadequate management of blood pressure in a hypertensive population. N Engl J Med. 1998;339:1957–63. doi: 10.1056/NEJM199812313392701. [DOI] [PubMed] [Google Scholar]
- 17.Nelson SA, Dresser GK, Vandervoort MK, et al. Barriers to blood pressure control: a STITCH substudy. J Clin Hypertens (Greenwich) 2011;13:73–80. doi: 10.1111/j.1751-7176.2010.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis LM, Schoenthaler AM, Ogedegbe G. Patient factors, but not provider and health care system factors, predict medication adherence in hypertensive black men. J Clin Hypertens (Greenwich) 2012;14:250–5. doi: 10.1111/j.1751-7176.2012.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swain MA, Steckel SB. Influencing adherence among hypertensives. Res Nurs Health. 1981;4:213–22. doi: 10.1002/nur.4770040107. [DOI] [PubMed] [Google Scholar]
- 20.Nasser SA, Lai Z, O’Connor S, Liu X, Flack JM. Does earlier attainment of blood pressure goal translate into fewer cardiovascular events? Curr Hypertens Rep. 2008;10:398–404. doi: 10.1007/s11906-008-0074-2. [DOI] [PubMed] [Google Scholar]
- 21.Gil-Guillen V, Orozco-Beltran D, Perez RP, et al. Clinical inertia in diagnosis and treatment of hypertension in primary care: quantification and associated factors. Vol. 19. Blood Press; 2010. pp. 3–10. [DOI] [PubMed] [Google Scholar]
- 22.Chalmers J. Enhancing risk stratification in hypertensive subjects: how far should we go in routine screening for target organ damage? J Hypertens. 2002;20:1255–7. doi: 10.1097/00004872-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Struthers AD, Davies J. Should we add screening for and treating left ventricular hypertrophy to the management of all patients needing secondary prevention of cardiovascular disease? QJM. 2003;96:449–52. doi: 10.1093/qjmed/hcg067. [DOI] [PubMed] [Google Scholar]
- 24.Suarez C, Villar J, Martel N, et al. Should we perform an echocardiogram in hypertensive patients classified as having low and medium risk? Int J Cardiol. 2006;106:41–6. doi: 10.1016/j.ijcard.2004.12.076. [DOI] [PubMed] [Google Scholar]
- 25.Davis BR, Kostis JB, Simpson LM, et al. Heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Circulation. 2008;118:2259–67. doi: 10.1161/CIRCULATIONAHA.107.762229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okin PM, Devereux RB, Harris KE, et al. Regression of electrocardiographic left ventricular hypertrophy is associated with less hospitalization for heart failure in hypertensive patients. Ann Intern Med. 2007;147:311–9. doi: 10.7326/0003-4819-147-5-200709040-00006. [DOI] [PubMed] [Google Scholar]
- 27.Verdecchia P, Angeli F, Cavallini C, et al. Blood pressure reduction and renin-angiotensin system inhibition for prevention of congestive heart failure: a meta-analysis. Eur Heart J. 2009;30:679–88. doi: 10.1093/eurheartj/ehn575. [DOI] [PubMed] [Google Scholar]
- 28.Schocken DD, Benjamin EJ, Fonarow GC, et al. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117:2544–65. doi: 10.1161/CIRCULATIONAHA.107.188965. [DOI] [PubMed] [Google Scholar]
- 29.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC. Plasma brain natriuretic peptide to detect preclinical ventricular systolic or diastolic dysfunction: a community-based study. Circulation. 2004;109:3176–81. doi: 10.1161/01.CIR.0000130845.38133.8F. [DOI] [PubMed] [Google Scholar]
- 30.Vasan RS, Benjamin EJ, Larson MG, et al. Plasma natriuretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction: the Framingham heart study. JAMA. 2002;288:1252–9. doi: 10.1001/jama.288.10.1252. [DOI] [PubMed] [Google Scholar]
- 31.Wachtell K. Prevention of congestive heart failure in high risk patients. Eur Heart J. 2009;30:638–9. doi: 10.1093/eurheartj/ehp064. [DOI] [PubMed] [Google Scholar]
- 32.Karras DJ, Ufberg JW, Heilpern KL, et al. Elevated blood pressure in urban emergency department patients. Acad Emerg Med. 2005;12:835–43. doi: 10.1197/j.aem.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 33.Kramer H, Han C, Post W, et al. Racial/ethnic differences in hypertension and hypertension treatment and control in the multi-ethnic study of atherosclerosis (MESA) Am J Hypertens. 2004;17:963–70. doi: 10.1016/j.amjhyper.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Shea S, Misra D, Ehrlich MH, Field L, Francis CK. Predisposing factors for severe, uncontrolled hypertension in an inner-city minority population. N Engl J Med. 1992;327:776–81. doi: 10.1056/NEJM199209103271107. [DOI] [PubMed] [Google Scholar]
- 35.Umscheid CA, Maguire MG, Pines JM, et al. Untreated hypertension and the emergency department: a chance to intervene? Acad Emerg Med. 2008;15:529–36. doi: 10.1111/j.1553-2712.2008.00132.x. [DOI] [PubMed] [Google Scholar]
- 36.Levy PD, Flack JM. Should African-Americans with elevated blood pressure be routinely screened for hypertensive heart disease? Expert Rev Cardiovasc Ther. 2012;10:1201–4. doi: 10.1586/erc.12.130. [DOI] [PubMed] [Google Scholar]
- 37.Levy PD, Cline D. Asymptomatic hypertension in the emergency department: a matter of critical public health importance. Acad Emerg Med. 2009;16:1251–7. doi: 10.1111/j.1553-2712.2009.00512.x. [DOI] [PubMed] [Google Scholar]
- 38.Burla MJ, Brody AM, Ference BA, et al. Blood pressure control and perceived health status in African Americans with subclinical hypertensive heart disease. J Am Soc Hypertens. 2014;8:321–9. doi: 10.1016/j.jash.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 39.Lawrence WF, Fryback DG, Martin PA, Klein R, Klein BE. Health status and hypertension: a population-based study. J Clin Epidemiol. 1996;49:1239–45. doi: 10.1016/s0895-4356(96)00220-x. [DOI] [PubMed] [Google Scholar]
- 40.Gold M. Panel on cost-effectiveness in health and medicine. Med Care. 1996;34:DS197–9. [PubMed] [Google Scholar]
- 41.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness-the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–7. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 42.Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008;8:165–78. doi: 10.1586/14737167.8.2.165. [DOI] [PubMed] [Google Scholar]
- 43.Moise N, Huang C, Rodgers A, et al. Comparative cost-effectiveness of conservative or intensive blood pressure treatment guidelines in adults aged 35–74 years: The Cardiovascular Disease Policy Model. Hypertension. 2016;68:88–96. doi: 10.1161/HYPERTENSIONAHA.115.06814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heidenreich PA, Gubens MA, Fonarow GC, Konstam MA, Stevenson LW, Shekelle PG. Cost-effectiveness of screening with B-type natriuretic peptide to identify patients with reduced left ventricular ejection fraction. J Am Coll Cardiol. 2004;43:1019–26. doi: 10.1016/j.jacc.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 45.WPS Government Health Administrators. Medicare Physician Fee Schedule for Michigan Locality 0.1. 2015 Available at: http://www.wpsmedicare.com/index.shtml. Accessed Jun 06, 2016.
- 46.Mahn JJ, Dubey E, Brody A, et al. Test characteristics of electrocardiography for detection of left ventricular hypertrophy in asymptomatic emergency department patients with hypertension. Acad Emerg Med. 2014;21:996–1002. doi: 10.1111/acem.12462. [DOI] [PubMed] [Google Scholar]
- 47.Russo C, Jin Z, Elkind MS, et al. Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community-based cohort. Eur J Heart Fail. 2014;16:1301–9. doi: 10.1002/ejhf.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sera F, Jin Z, Russo C, et al. Ambulatory blood pressure control and subclinical left ventricular dysfunction in treated hypertensive subjects. J Am Coll Cardiol. 2015;66:1408–9. doi: 10.1016/j.jacc.2015.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ammar KA, Jacobsen SJ, Mahoney DW, et al. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation. 2007;115:1563–70. doi: 10.1161/CIRCULATIONAHA.106.666818. [DOI] [PubMed] [Google Scholar]
- 50.Betti I, Castelli G, Barchielli A, et al. The role of N-terminal PRO-brain natriuretic peptide and echocardiography for screening asymptomatic left ventricular dysfunction in a population at high risk for heart failure. The PROBEHF study J Card Fail. 2009;15:377–84. doi: 10.1016/j.cardfail.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Wang TJ, Levy D, Benjamin EJ, Vasan RS. The epidemiology of “asymptomatic” left ventricular systolic dysfunction: implications for screening. Ann Intern Med. 2003;138:907–16. doi: 10.7326/0003-4819-138-11-200306030-00012. [DOI] [PubMed] [Google Scholar]
- 52.Kishi S, Reis JP, Venkatesh BA, et al. Race-ethnic and sex differences in left ventricular structure and function: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. J Am Heart Assoc. 2015;4:e001264. doi: 10.1161/JAHA.114.001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wan SH, Vogel MW, Chen HH. Pre-clinical diastolic dysfunction. J Am Coll Cardiol. 2014;63:407–16. doi: 10.1016/j.jacc.2013.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 55.Braithwaite RS, Meltzer DO, King JT, Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46:349–56. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 56.Shiroiwa T, Sung YK, Fukuda T, Lang HC, Bae SC, Tsutani K. International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ. 2010;19:422–37. doi: 10.1002/hec.1481. [DOI] [PubMed] [Google Scholar]


