Introduction
A telomere can be functionally defined as that region of DNA at the end of a linear chromosome that is required for replication and stability of the chromosome (3). Telomere replication poses special problems for the linear chromosomes of eukaryotes. The known biochemical properties of DNA polymerases (5 prime to 3 prime polymerization, requirement of a primer) would predict that a linear DNA would lose sequences from the ends of the molecule with each round of replication (28). Telomeres must utilize special mechanisms to ensure that all genetic information is transmitted with each cell division. Thus DNA ends have a special structure to avoid binding with the ends of DNA from other chromosomes, and thus to prevent end-to-end fusions and the formation of dicentric chromosomes in normal cells. Consequently, the non-pathologic telomere presents two biological problems with which to contend: 1) its normal replication, and 2) the prevention of aberrant binding.
End-to-end fusion of chromosomes is currently recognized more frequently in the cytogenetic analysis of human solid neoplastic tissue. Telomeric associations (fusions) are thought to represent a form of chromosome instability present in certain pathologic tissues such as giant cell tumor of bone (GCT). Herein we review the current understanding of telomere structure and function and describe telomeric associations in humans, particularly in GCT.
Normal Telomeres
Telomere Cytology
Telomeric regions of chromosomes are frequently heterochromatic in appearance (2). These heterochromatic regions are sometimes visualized as “knobs,” particularly in plant chromosomes (e.g., maize) which makes it possible to identify individual telomeric regions. Telomeres can also be specifically stained (T-banding).
Telomeres frequently associate with each other. During the leptotene stage of the first meiotic prophase, the telomeres of all chromosomes are attached to the same area of the nuclear membrane until chromosome pairing is completed (17). Telomeric associations are not confined to meiosis, but also occur during interphase and mitotic prophase in many systems (7) and during replication of DNA (6). These events provide an opportunity for telomeres to bind to one another to create endto-end fusions with complementary pairing of telomeric nucleotide sequences of different chromosomes.
Molecular Biology of Telomeres
Two classes of repetitive DNA elements have been implicated in the functional attribute of the telomere. The first is a simple, repeat sequence containing a G-rich nucleotide strand. This sequence has been found at the termini of linear chromosomes of representative plants, animals, protists, and fungi. This repeat sequence (TTAGGG)n, has also been isolated from the telomeres of human chromosomes (24). This simple terminal repeat sequence shows strong evolutionary conservation with no detectable non-telomeric tracks of this sequence in the human genome (25).
The second class of repetitive telomeric DNA is located just proximal to the terminal repeat and is species-specific. These subtelomeric repeats are not required for replication but are involved in the recombination often associated with telomeres. These subtelomeric repeats have been identified in a number of rye grass species, yeast, Drosophila melanogaster, and Ascaris (3).
Since all known cellular DNA polymerases require a primer, a special strategy is needed to prevent progressive loss of terminal nucleotides during replication. Therefore, a number of theoretical models have been proposed to account for the replication of telomeres (29). Small linear DNA genomes can replicate via circular (phageλ) or concatenated (T7) intermediates. A partially self-complementary terminal hairpin can act as its own primer. This occu rs in the mitochondria of Paramecium. Terminal proteins can prime DNA synthesis on the three prime end of the lagging strand and also replicate themselves, as found in adenovirus and bacteriophage Φ29.
An ingenious solution to telomeric replication is adopted by linear eukaryotic chromosomes. Telomeric replication in eukaryotes involves non-templated addition of telomeric repeats onto the ends of chromosomes. Non-templated addition of sequences allows a dynamic equil ibrium to be established in which chromosomes shorten by incomplete replication and are balanced by de novo sequence addition. A specific telomere terminal transferase, which is a ribonucleoprotein enzyme, is required for telomere elongation in vivo (14). Apparently, the problem of maintaining the integrity of the telomere during replication is solved by the terminal transferase.
In addition to their role in chromosome replication, functional telomeric DNA sequences are believed to confer stability to the chromosomes, preventing end-to-end fusions and DNA degradation. Simple terminal repeats are capable of forming stable hairpin structures via non-Watson-Crick base pairing (29). Specific association and disassociation of telomeric regions could occur without melting or destabilization of internal regions of the remaining DNA. The stability of the hairpin loop at the chromosome termini through non-Watson-Crick base pairing creates a chromosome cap that may help avoid end-to-end fusion of chromosomes. These selective base-pair bonding strengths allow differences in the association and disassociation of DNA in the telomere when compared to the remainder of the chromosome.
The strong evolutionary conservation of functional telomeres in normal cell growth and division suggests a critical role for this structure. Molecular analysis of the telomere reveals it to be complex, possessing novel interactions and behavior. Therefore, it should not be surprising that in the pathologic state, aberrant telomere-telomere interactions occur.
Telomeric Association
Unlike associations seen among D and G group chromosomes where the satellites are in close proximity, telomeric associations involve a union of the ends of two or more chromosomes. Telomeric associations resemble dicentrics but are thought to represent two distinct chromosomes joined end-to-end without visible loss of genetic material from either chromosome end. Mitelman [1988] designated telomeric associations with the letters tas (22).
Although not defined by ISCN [1985] (18), telomeric associations or telomeric fusions have been described in the literature since the early 1970s. Telomeres were thought to have a mutual attraction, forming reversible or stable associations in Gryllus argentinus (9). They have also been observed in humans when unbroken chromosome ends join, leading to formation of chains and rings, which may involve all the chromosomes (10).
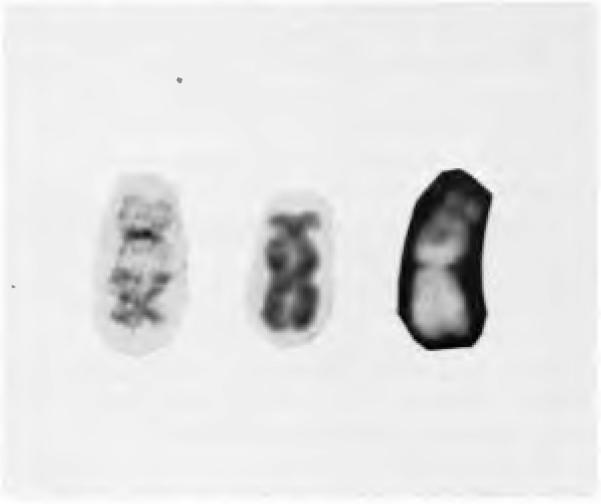
The nomenclature for telomeric associations has not been standardized. Much of this has to do with the fact that cytogeneticists cannot be certain if observed rearrangements between telomeres represent true attachments between the chromosome termini or if the abnormalities are the result of reciprocal translocations with the breakpoints in the telomeric regions. However, there is no evidence that true telomeric associations produce sniall, asymmetric fragments which may be found as a result of a chromosome translocation process. Many authors prefer to use the term “telomeric association” rather than “telomeric fusion,” since the latter descriptive term more specifically identifies the bond between the two chromosomes, which has not yet been completely elucidated. Traditionally, the karyotype nomenclature has referred to the telomeric association as a translocation dicentric, tdic. Other authors have labeled such telomeric associations as fus or ta. The authors concur with Mitelman and suggest that a true telomeric association be labeled as tas. The termini involved in the translocation are identified by whether or not they belong to the long or short arm of the chromosome. The nomenclature, therefore, uses the terminology qter and pter to represent the termini of the long and short arms, respectively. Figure 1 demonstrates examples of telomeric associations and the nomenclature which will be used throughout the remainder of the discussion.
Figure 1.
Examples of three telomeric associations involving chromosome 19 are presented. GTG and QFQ banding techniques are demonstrated. From left to right are shown: tas(19;20)(pter;qter), tas(10;19)(pter;qter), and tas (12;19)(pter;qter). All examples are from giant cell tumor of bone.
The Occurrence of Telomeric Associations in Human Cells
Until recently, telomeric associations rarely have been reported in the literature. Telomeric associations have been reported in human solid malignancies, hemopoietic malignancies, and benign solid tumors. Only rarely have telomeric associations been observed in senescent fibroblasts (1), SV40 transformed cells (30), and in patients with ataxia telangiectasia (15) and Thiberge-Weissanbach (11). True telomeric associations are distinguished from “Robertsonian” translocations or the fusions of the short or long arms of two acrocentric chromosomes to form new translocation chromosomes.
Mandahl observed telomeric associations in the soft tissue sarcoma, malignant fibrous histiocytoma (21). In that study, five telomeres were more frequently involved (6pter, 11 pter, 16qter, 20qter, and 21 pter). Similarly, three telomeres were more frequently involved in associations in capillary renal cell carcinoma reported by Kovacs (20). Finally, 8pter and 18pter telomeric associations were observed in a giant cell sarcoma (26). The hemopoietic malignancies in which telomeric associations have been identified include a B-cell lymphoid leukemia (12), T-cell lymphoid leukemia (23), and hairy cell leukemia (16).
Telomeric associations have also been reported in three benign solid neoplasms. These are giant cell tumor of bone (4, 27), atrial myxoma (8), and renal oncocytoma (20). Non-clonal telomere associations involving 2qter, 12pter, and Yqter were observed in the atrial myxomas (8).
Giant Cell Tumor of Bone
The study of giant cell tumor of bone has proved fruitful and will be discussed in order to gain a better understanding of telomeric associations in humans. The first fully reported cytogenetic investigation of GCT demonstrated non-clonal abnormalities involving telomeric associations (27), and has since been confirmed by other investigators (4). The telomeres most frequently involved were located on the long arms of chromosomes 19 and 20 and the short arm of chromosome 11. Other tas involving chromosomes 10pter, 16qter, 21 qter, and 22qter were observed.
Giant cell tumor of bone is a solid, primary bone neoplasia composed of stromal mononuclear cells and large multinucleate cells which resemble osteoclasis. These tumors occur most frequently in the ends of long tubular bones of skeletally mature individuals and constitute 5% of all primary bone tumors. GCT is usually considered benign and treated by surgical resection, however, a high local recurrence rate of up to 60% has been reported. There is a 2% rate for benign pulmonary metastases. This neoplasm has a proclivity to recur locally and demonstrates an unpredictable pattern of biologic aggressiveness manifest by its ability to develop tumor deposits in the lung which histologically look identical to the benign primary site.
The phenomenon of telomeric association in GCT is present not only in cells harvested within the first two to four weeks in the authors’ laboratories, but also in cells analyzed using direct harvest technique. The direct harvest technique involves cutting the tumors into small pieces, digesting with mixed collagenase solution, and centrifugation followed by incubation at 37°C for one hour. The tumor cells are harvested and examined for cytogenetic abnormalities after GTG banding. Telomeric associations were identified using this direct harvest technique, indicating that GCT telomeric associations are not a consequence of cell senescence (5). Recent data indicate that alterations of the telomere do occur in aging cells (13).
Co-cultivation with control fibroblasts from an opposite sex individual was performed to determine if cell-to-cell contact or a diffusible agent would induce chromosome changes in control cells or alter the frequency of chromosome aberrations in the tumor cells. GCT and control cells were cultured for approximately six weeks then analyzed using routine cell harvest and banding techniques. No telomeric associations were seen in the control fibroblasts while the percentage of GCT telomeric associations was unchanged; therefore, co-cultivation had no apparent effect on either control fibroblasts or the tumor cells.
To determine if a constitutional chromosome abnormality existed (e.g., telomeric associations or marker chromosomes), skin fibroblasts were cultured from one individual with GCT. No abnormal metaphases were identified, therefore, abnormal chromosome findings observed in the tumor cells of this patient were apparently acquired and not constitutional (27).
Culture time also affected the number of chromosome abnormalities observed in GCT cells. There were significantly more abnormalities (e.g., polyploidy) observed with longer culture times (e.g., 8-12 weeks) (5).
To determine whether or not intact terminal nucleotides of the chromosomes found in GCT telomeric association were present, a collaborative effort was undertaken in which labeled oligonucleotide molecular binding studies were performed. A simple TAG oligonucleotide DNA probe for the telomeric region was synthetically prepared and labeled. Preliminary in situ hybridization experiments with the radiolabeled telomeric DNA probe to metaphase GCT chromosomes indicated that silver grains were present in at least one telomeric association involving two chromosomes (e.g. 10qter; 13qter). Additional evidence that telomeric DNA is present at the fusion sites of a telomeric association was recently reported in the study of a patient with a constitutional chromosome anomaly interpreted as a dicentric X;22 translocation (19). Seven meta-phases showed one or more silver grains at the X;22 junction, indicating the presence of telomeric DNA at or near the fusion site. Thus, GCT preliminary data as well as the apparent constitutional X;22 telomeric association provide further evidence that intact telomeric DNA is probably present in telomeric associations. Additional research is underway with in situ hybridization to examine for differences in telomeric probe hybridization patterns between tas and other chromosome abnormalities which involve the fusion of two chromosomes, e.g. reciprocal translocations.
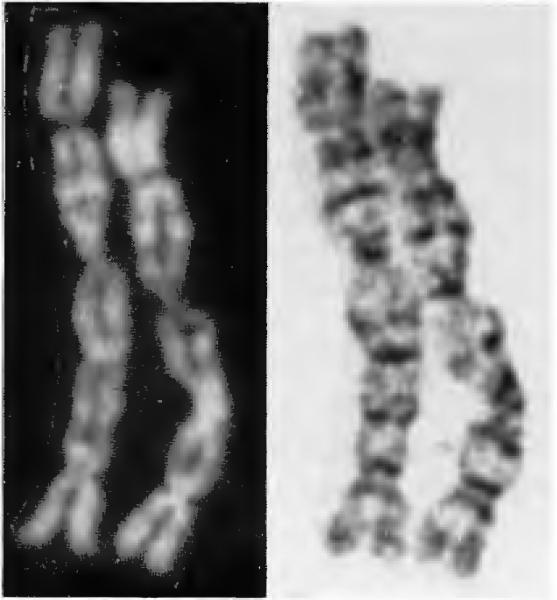
Unusual-appearing chromosomes have also been identified in some of the GCT cultures (27). Figure 2 shows an example of such a chromosome, which appears to represent four chromosomes involved in three tandem telomeric associations. The observations of such chromosomes as well as marker chromosomes in the karyotype lends credence to the belief that chromosome instability is a possible mechanism for telomeric associations.
Figure 2.
An unusual chromosome from giant cell tumor of bone demonstrating telomeric associations. QFQ and GTG banding of this quadracentric chromosome are shown.
Additional cytogenetic findings of interest were observed in a patient with a giant cell sarcoma. In this instance, this 19-year-old white female had a giant cell tumor of the distal femur resected five years previously. The initial diagnosis of GCT was confirmed histologically. Recently, the patient presented with pulmonary metastasis. A thoracotomy revealed the giant cell histology juxtaposed with osteosarcoma, indicating giant cell sarcoma as the correct diagnosis. Surgical resection was again undertaken of the primary tumor located in the distal femur. When the Jesional tissue from the femur was examined cytogenetically, multiple marker chromosomes were observed including a telomeric association (8pter; 18pter). The cytogenetic data from this interesting patient lends further evidence to support the role of telomeric associations in the oncogenesis of giant cell tumor of bone.
Comment
A primary molecular defect of the telomeres in GCT could explain the telomeric associations and possibly other cytogenetic anomalies (e.g. ring chromosomes) identified in patients with this bone neoplasm. While many telomeres in GCT appear to be involved in telomeric associations, there appears to be a non-random distribution of telomeric associations, particularly 10pter, 11 pter, 16qter, 19qter, and 20qter. It is possible that a primary structural or base alteration occurs in the termini of GCT, however, our prel iminary in situ hybridization studies indicate that intact telomeric DNA sequences are probably present at the telomeric association's junction site. If there is an alteration of the telomere, allowing for “sticky” ends, then the normal mitotic process and conceivably an alteration in biologic growth and cell differentiation may occur in GCT. It is not possible at this time to determine whether or not telomeric associations observed in GCT are the oncogenic transforming events in this neoplasm, or represent byproducts of uncontrolled neoplastic growth.
It does appear that the telomeric associations in GCT are the result of an association rather than a reciprocal translocation. The association appears to be stable and remains intact throughout cell division during prolonged culture time. There is evidence of duplicate telomeric associations in multinucleate cells (27). It is not clear why telomeric associations occur. In giant cell tumor of bone they occur with a frequency of approximately 10-30% of the tumor cells examined, but not all patients with GCT have telomeric associations (5, 27). Current data indicate that two-thirds to three-fourths of patients with GCT have telomeric associations with a non-random distribution. To date we have been unable to correlate a clinical outcome or prognosis with cytogenetic findings. Additional research is in progress.
Conclusion
Telomeric associations have been reported in solid human neoplasms, leukemias, lymphomas, chromosome breakage disorders, and in senescent cells. Giant cell tumor of bone represents a tumor in which telomeric associations are consistently found in a non-random fashion. These associations are not thought to be translocations but represent bonding of the termini of two chromosomes. Molecular genetic studies with in situ hybridization indicate that telomeres are present. Telomeric associations are seen after direct harvest techniques and are not a by-product of cell senescence. The ability for termini to associate with one another does not appear to be transmissible to another cell line during co-cultivation. The association appears to be stable and remains intact through several cell divisions. Original research is underway to investigate the role of chromosome aberrations, particularly telomeric associations in the development of giant cell tumor of bone and other neoplasms. The increased awareness of telomeric associations on the part of the cytogeneticist and the cytogenetic technologist is important for the reporting of the occur-rences of these interesting chromosome anomalies and for the eventual elucidation of their role in neoplastic processes.
Contributor Information
H.S. Schwartz, Departments of Orthopaedics and Pathology, Vanderbilt University Medical Center, Nashville, TN 37232-2550, (615) 343-8612..
G.A. Allen, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, TN 37232-2578..
M.G. Butler, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, TN 37232-2578..
References
- 1.Benn PA. Specific chromosome aberrations in senescent fibroblast cell lines derived from human embryos. Am J Hum Genet. 1976;28:465–473. [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett MD. Heterochromatin, aberrant endosperm nuclei and grain shrivelling in wheat-rye genotypes. Heredity. 1977;39:411–419. [Google Scholar]
- 3.Blackburn EH, Szostak JW. The molecular structure of centromeres and telomeres. Ann Rev Biochem. 1984;53:163–194. doi: 10.1146/annurev.bi.53.070184.001115. [DOI] [PubMed] [Google Scholar]
- 4.Bridge JA, Neff JR, Bhatia PS, Sanger WG, Murphey MD. Cytogenetic findings and biologic behavior of giant cell tumors of bone. Cancer. 1990;65:2697–2703. doi: 10.1002/1097-0142(19900615)65:12<2697::aid-cncr2820651217>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Butler MG, Allen GA, Haynes JL, Schwartz HS. Cytogenetic abnormalities including telomeric associations in five giant cell tumors of bone. 1990 Submitted for publication. [Google Scholar]
- 6.Dancis BM, Holmquist GP. Fusion model of telomere replication and its implications for chromosomal rearrangements. Chromosomes Today. 1977;6:95–104. [Google Scholar]
- 7.Dancis BM, Holmquist GP. Telomere replication and fusion in eukaryotes. J Theor Bioi. 1979;78:211–224. doi: 10.1016/0022-5193(79)90265-0. [DOI] [PubMed] [Google Scholar]
- 8.DeWald GW, Dahl RJ, Spurbeck JL, Carney JA, Gordon H. Chromosomally abnormal clones and nonrandom telomeric translocations in cardiac myxomas. Mayo Clinic Proc. 1987;62:558–567. doi: 10.1016/s0025-6196(12)62293-9. [DOI] [PubMed] [Google Scholar]
- 9.Drets ME, Stoll M. C-banding and non-homologous associations in Gryl/us argentinus. Chromosomes. 1974;48:367–390. doi: 10.1007/BF00290994. [DOI] [PubMed] [Google Scholar]
- 10.Dutrillaux B, Aurias A, Couturier J, et al. Multiple telomeric fusions and chain configurations in human somatic chromosomes. In: Chapell A, de Ia Sorsa M, editors. Chromosomes Today. Vol. 6. Elsevier/North Holland; Amsterdam: pp. 37–44, 1977. [Google Scholar]
- 11.Dutrillaux B, Croquette MF, Viegas-Pequignot E, Aurias A, et al. Human somatic chromosome chains and rings: A preliminary note on end-to-end fusions. Cytogenet Cell Genet. 1978;20:70–77. doi: 10.1159/000130841. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald PH, Morris CM. Telomeric association of chromosomes in B-cell lymphoid leukemia. Hum Genet. 1984;67:385–390. doi: 10.1007/BF00291396. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein S. Replicative senescence: The human fibroblast comes of age. Science. 1990;249:1129–1133. doi: 10.1126/science.2204114. [DOI] [PubMed] [Google Scholar]
- 14.Greider CW, Blackburn EH. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987;51:887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi K, Schmid W. Tandem duplication q14 and dicentric formation by end-to-end chromosome fusions in ataxia telangiectasia (AT). Humangenetik. 1975;30:135–141. doi: 10.1007/BF00291946. [DOI] [PubMed] [Google Scholar]
- 16.Heerema NA, Palmer CG, Harvey W, Jansen J, Srour E, et al. Telomeric association in a hairy cell leukemia line (GASH). Am J Hum Genet. 1987;41(3):A 122. [Google Scholar]
- 17.Holmquist GP, Dancis BM. Telomere replication, kineto-chore organizers, and satellite DNA evolution. Proc Natl Acad Sci USA. 1979;76:4566–4570. doi: 10.1073/pnas.76.9.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ISCN . In: An International System for Human Cytogenetic Nomenclature. 1. Harnden DG, Klinger HP, editors. Vol. 21. March of Dimes Birth Defects Foundation; New York: 1985. 1985. published in collaboration with Cytogenet Cell Genet (Karger, Basel 1985); also in Birth Defects: Original Article Series
- 19.Jedele KB, Michels VV, Thibodeau SN, DeWald GW, Wagner KV, Schad CR, Stesin M, Jenkins RB. Demonstration of telomeric DNA at the fusion site in a constitutional dicentric X; 22 chromosome translocation. Am J Hum Genet. 1989;45:A 102. [Google Scholar]
- 20.Kovacs G, Muller-Brechlin R, Szucs S. Telomeric association in two human renal tumors. Cancer Genet Cytogenet. 1987;28:363–366. doi: 10.1016/0165-4608(87)90225-1. [DOI] [PubMed] [Google Scholar]
- 21.Mandahl N, Heim S, Arheden K, Rydholm A, Willen H, Mitelman F. Rings, dicentrics, and telomeric association in histiocytomas. Cancer Genet Cytogenet. 1988;30:23–33. doi: 10.1016/0165-4608(88)90089-1. [DOI] [PubMed] [Google Scholar]
- 22.Mitelman F. Catalog of Chromosome Aberrations in Cancer. Third Edition Alan R. Liss, Inc.; 1988. [Google Scholar]
- 23.Morgan R, Jarzabek V, Jaffe JP, Hecht BK, Hecht F, Sandberg AA. Telomeric fusion in pre-T-Cell acute lymphoblastic leukemia. Hum Genet. 1986;73:260–263. doi: 10.1007/BF00401240. [DOI] [PubMed] [Google Scholar]
- 24.Moyzis RK, Buckingham JM, Cram LS, Dani M, et al. A highly conserved repetitive DNA sequence, {TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci USA. 1988;55:6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riethman HC, Moyzis RK, Meyne J, Burke DT, Olson MV. Cloning human telomeric DNA fragments into Saccharomyces cerevisiae using a yeast-artificial-chromosome vector. Proc Natl Acad Sci USA. 1989;86:6240–6244. doi: 10.1073/pnas.86.16.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz HS, Butler MG. Unpublished observation.
- 27.Schwartz HS, Jenkins RB, Dahl RJ, DeWald GW. Cyto-genetic analyses on giant cell tumors of bone. Clin Orthop Rei Res. 1989;240:250–260. [PubMed] [Google Scholar]
- 28.Watson JD. Origin of concatameric T4 DNA. Nature New Bioi. 1972;239:197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- 29.Weiner AM. Eukaryotic nuclear telomeres: Molecular fossils of the RNP world? Cell. 1988;52:155–157. doi: 10.1016/0092-8674(88)90501-6. [DOI] [PubMed] [Google Scholar]
- 30.Wolman SR, Steinberg ML, Defendi V. Simian virus 40-induced chromosome changes in human epidermal cultures. Cancer Genet Cytogenet. 1980;2:3946. [Google Scholar]