Replacing petroleum by biomass can be economically feasible by generating revenue from the three primary biomass constituents.
Keywords: biomass, cellulose, hemi-cellulose, lignin, furfural, viscose, dissolving pulp
Abstract
The production of renewable chemicals and biofuels must be cost- and performance- competitive with petroleum-derived equivalents to be widely accepted by markets and society. We propose a biomass conversion strategy that maximizes the conversion of lignocellulosic biomass (up to 80% of the biomass to useful products) into high-value products that can be commercialized, providing the opportunity for successful translation to an economically viable commercial process. Our fractionation method preserves the value of all three primary components: (i) cellulose, which is converted into dissolving pulp for fibers and chemicals production; (ii) hemicellulose, which is converted into furfural (a building block chemical); and (iii) lignin, which is converted into carbon products (carbon foam, fibers, or battery anodes), together producing revenues of more than $500 per dry metric ton of biomass. Once de-risked, our technology can be extended to produce other renewable chemicals and biofuels.
INTRODUCTION
The conversion of lignocellulosic (nonedible) biomass to fuels and chemicals is a promising alternative to replace petroleum as a renewable source of carbon (1); however, most of the proposed processes are currently unable to compete economically with petroleum refineries due, in part, to incomplete utilization of the biomass feedstock. An approach to improve biomass utilization, and thus the economics of biomass-derived processes, involves the concept of an integrated biorefinery, analogous to a petroleum refinery, where multiple product streams are produced (2). This approach has been demonstrated to be challenging, because integration of streams in a biorefinery is more complex than in a petroleum refinery because of the different chemical structure of the biomass constituents (that is, cellulose, hemicellulose, and lignin) (1). In addition, implementation of new processes is associated with high technical risks, requires large capital investments, and, even if successful, may lead to the production of specialty chemicals that lack a developed market. All of these factors hinder the industrial application of technically successful biomass-derived technologies.
Herein, we propose a strategy that integrates biomass fractionation with simultaneous conversion of cellulose, hemicellulose, and lignin into products that have existing and established markets. Our biomass upgrading strategy (Fig. 1) produces (i) dissolving pulp, which is a high-purity cellulose pulp that is spun into textile fibers (3); (ii) furfural, which is a valuable platform chemical from hemicellulose (4); and (iii) carbon foams and battery anodes (5), which are two versatile renewable products from lignin (6, 7). These initial products have been targeted by pulp mills for many years, and we have thus selected them because they can be introduced directly into current markets, thereby minimizing market risk for the first commercial plant. Once the base technology is de-risked, it can be extended to produce intermediate platform molecules, such as fermentable sugars (8, 9), to manufacture renewable chemicals and fuels and to create new markets for furfural- and lignin-derived products, thereby expanding the opportunities of the biorefinery (10).
Fig. 1. TriVersa Process.
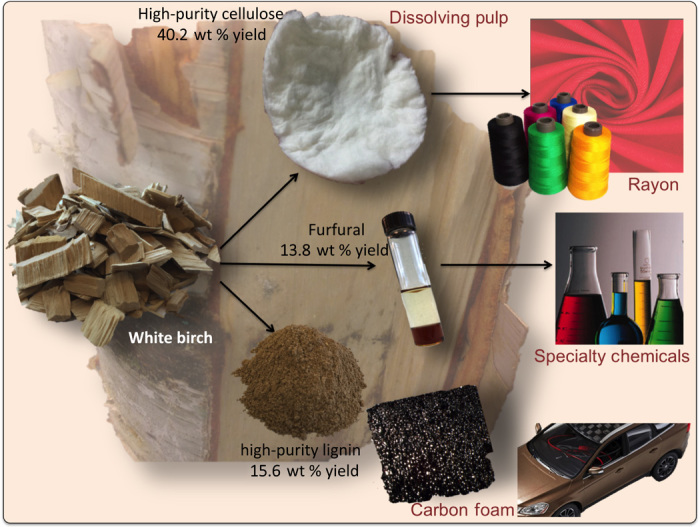
Lignocellulosic biomass can be fractionated into its main components to replace petroleum-derived chemicals used in daily life. Initial composition of white birch (dry weight): hexosan, 45.0 wt %; pentosan, 25.3 wt %; lignin, 21.7 wt %. The yields have been calculated on the basis of our techno-economic analysis.
RESULTS AND DISCUSSION
The key feature of our process is the efficient and high-yield fractionation of biomass into its individual components in such a way that it retains the value of each of these fractions. This fractionation is possible by using γ-valerolactone (GVL), a biomass-derived and sustainable renewable solvent (11) that has demonstrated advantageous properties to process lignocellulosic biomass (9, 12, 13). The use of GVL as a solvent offers unique advantages to the process by the virtue of its chemical and physical properties and solves typical problems associated with biomass fractionation, such as continuous biomass feeding (due to low vapor pressure of GVL), high biomass loading (due to the high solubility of lignin and sugars in GVL/water mixtures), clean component fractionation (due to mild process conditions), and the ability to effectively process the component streams downstream within the solvent, avoiding costly separations. Moreover, GVL can be produced from biomass within the process to make up for the losses, thus making for a closed-loop process. These features make the current technology highly cost-effective.
Figure 2 outlines our biorefinery strategy to integrate product streams from biomass using GVL as a solvent. The first step, a key of our process, is the fractionation of the lignocellulosic biomass into its three primary constituents: hemicellulose, cellulose, and lignin. The fractionation allows the processing of each fraction separately, taking advantage of their different chemical structures and optimizing the target products. GVL is highly effective in this fractionation step (13–16) and is miscible with water at all concentrations; thus, the fractionation reactor can be loaded directly with wet wood chips, eliminating an expensive biomass preprocessing step, such as size reduction or drying. The goal of the fractionation step is to isolate the solid cellulose fraction from the dissolved hemicellulose and the lignin fractions. Although working at high biomass loading prevents the formation of furfural and degradation of lignin, the process is critical for retaining the value of all three fractions. Our fractionation step is carried out at low temperature (125°C) and low pressure (<3 bar) for 3 hours using a 70:30 GVL/water mass ratio and 0.1 M sulfuric acid as a catalyst, leading to the removal of >90% of the hemicellulose and >95% of the lignin even at a high biomass loading [>20 weight % (wt %)]. Depending on the processing conditions, the purity of the solid cellulose can reach up to 95% (see section S1 and table S1). Under our optimum conditions (table S1, entry 2), the conversion of xylose to furfural (<10%) and the biomass degradation is minimal (for example, >99% carbon balance), preserving the purity and structure of the lignin.
Fig. 2. Process flow diagram.
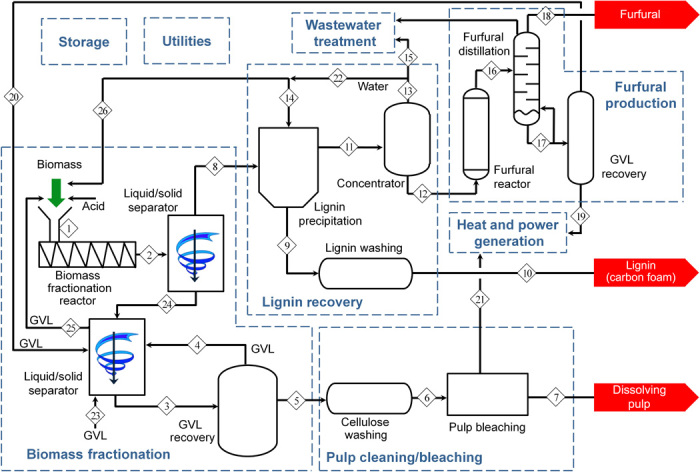
Overview of the process flow diagram to produce furfural, lignin, and dissolving pulp from lignocellulosic biomass.
Separation of the solid cellulose from the liquid solution is achieved using conventional liquid/solid separation techniques, such as centrifugation and filtration. This step must avoid the precipitation of soluble sugars or lignin over the solid cellulose stream, which would reduce the purity of the cellulose and the yield of the hemicellulose and lignin. Working at high biomass loading is challenging, and thus, two liquid/solid separation stages are combined to maximize the recovery of the soluble C5 sugars and the lignin (>95%) (see section S2 for details). The liquid fraction (stream 8) contains the soluble C5 sugars and lignin and is transferred to the lignin recovery step. The solid fraction (stream 24) is resuspended in recycled GVL solvent and then subjected to a second liquid/solid separation step to recover any soluble C5 sugars and lignin present in the remaining liquid. The solvent stream from the second separation step (stream 25) is reused directly to fractionate new lignocellulosic biomass upon the addition of sulfuric acid and water if necessary. This stream has low concentrations of dissolved lignin and C5 sugars that do not affect the fractionation of lignocellulosic biomass (fig. S1). Upon removal of the solvent by simple evaporation, the solid cellulose (stream 5) is washed with hot water to remove any precipitated sugars and other water-soluble products and then bleached using conventional techniques to increase brightness and purity (see section S3 for details). The final yield of cellulose after bleaching is approximately 40 wt % of the initial dry biomass (similar to other conventional technologies such as Kraft, and sulfite) (17). The easy removal of lignin and hemicellulose by the GVL solvent produces cellulose that is closer to a final dissolving pulp grade retaining the value of all fractions. The properties of this cellulose (hexosan, >96%; cupriethylenediamine (CED) viscosity, 5 to 15 centipoise; kappa number, <20) are similar to those of commercial dissolving pulps (3, 18), indicating that this material is suitable for commercial production of textiles and other high-end cellulose derivatives.
The next step in our integrated process is the recovery of soluble lignin from stream 8. GVL has an extraordinary ability to solubilize lignin (>10 wt %) (see table S3) (15), which allows operation at high biomass loading and still produces clean fractions (soluble lignin and hemicellulose and solid cellulose); however, the simple addition of water is sufficient to cause the precipitation of the lignin (19) while retaining the soluble C5 sugars in solution for further upgrading. The precipitation of lignin starts with a water/GVL mass ratio as low as ≈1.5 (see table S4). However, at this ratio, lignin retains considerable amounts of GVL, forming a viscous paste that is difficult to recover and may cause filter plugging. Similar solid handling issues are anticipated for those processes where lignin is recovered by evaporating the solvent. Increasing the water/GVL ratio to 14 reduces the retention of the solvent in the solid lignin, and the lignin precipitates as a fine light brown powder. For industrial applications, we consider an optimal water/GVL ratio of approximately 8 to minimize dilution of the C5 sugars. At this ratio, the lignin precipitates as a brown powder that is easily removed from a centrifuge or can be recovered by filtration (see sections S4 and S5 for details and fig. S3). The methods used to isolate the lignin produce a high-purity lignin with minimal ash and sugar content, which is necessary to make high-quality carbon products. In addition, the mild conditions used for the biomass fractionation step minimize lignin degradation reactions, producing a lignin product that retains most of its native β-O-4 bonds (fig. S5) and has high molecular weight (>7000), high glass transition temperature, and high melting point. As an example, we have used the GVL-derived lignin to produce battery anodes and a carbon foam material that can be used as lightweight structural foam cores, as high-temperature insulation, or for water filtration (see sections S6 and S7 for details). This lignin stream could also be used to produce other high–surface area carbon products that can be used in a variety of energy storage applications [that is, supercapacitors (20), battery anodes (5), and gas storage (21)], functional carbon products, or, as recently demonstrated, lignin monomers (22). The use of lignin as a carbon feedstock for battery anode material shows considerable promise because its nanographitic carbon structure is stable, with high theoretical and experimental stability and performance compared to natural flake graphite (5, 23). Carbons derived from GVL-fractionated lignin demonstrate similar nanographitic domains with a higher crystallinity (fig. S6) compared to previous anode materials, which should enhance battery stability and coulombic efficiency (5).
The next step in our process is to increase the value of the hemicellulose stream (stream 11). GVL, a polar aprotic solvent, enables excellent yields of furfural by dehydration of xylose (24, 25). This approach eliminates the requirement for intermediate separation of the soluble C5 sugars and produces a commodity chemical with an established market that can be commercialized with low risk. Because the stream of C5 sugars was diluted with water to recover the lignin, a concentration step is necessary to reduce the amount of water in the GVL. Evaporating the water at low temperature (≈100°C) to a GVL/water mass ratio of 80:20 avoids degradation of the C5 sugars. After concentrating the solution, the C5 sugar concentration is approximately 10 wt % (stream 12). Furfural yields of 90% can be achieved in less than 1 min by increasing the temperature to 225°C, without the need of additional sulfuric acid for the reaction (see section S8 for details). Adding a small amount of Cl− anions to the system (for example, adding KCl) could be used to take advantage of the chloride ion effect (26) observed in furfural production. Using HCl as a catalyst, the furfural yield can be increased over >95% (fig. S9). After neutralization of the acid, furfural and water are evaporated from the high–boiling point solvent (GVL, 208°C), facilitated by the low boiling point of the furfural/water azeotrope (98°C). The final furfural purification step is similar to the distillation step currently used in the commercial production of furfural (4). The high concentration of furfural in water obtained after removal of the GVL (>25 wt % furfural in water) facilitates the distillation operation, because it exceeds the solubility limit of furfural in water (≈8.3 wt %), causing spontaneous separation of a furfural-rich organic layer.
After successfully recovering and using all three components, the final step in our process is the recovery and cleanup of the GVL solvent. At this point in the process (stream 17), the GVL contains small amounts (5 to 6 wt %) of heavy boiling point products (≈10 to 12 wt % of the original biomass). A GVL evaporation step is the most promising option for the cleanup of this solvent stream. By this method, more than 99.5% of the GVL can be recovered and recycled into the system (stream 20).
The main future challenge of our process is managing solvent losses. Although GVL is stable under the reaction conditions and minimal solvent losses are expected by solvent degradation or interactions between GVL and biomass-derived molecules (see section S9 for details), our experimental work has identified other solvent losses with the products and in the cleanup steps. These losses are quantified as 13 kg of GVL per metric ton (MT) of initial biomass in the cellulose stream, 20 kg of GVL per MT of initial biomass in the lignin stream, and 6 kg of GVL per MT of initial biomass in the furfural recovery and GVL cleanup step, for a total of 39 kg of GVL per MT of initial biomass (5.6% of the total products produced). A significant amount of the GVL can be recovered by washing the lignin with water. Although this step requires additional energy to recover the GVL, the cost of the wastewater treatment is reduced. In this scenario, the GVL losses can be reduced to 20 kg per MT of biomass or less than 3% of the final products. The amount of GVL makeup can be reduced if levulinic acid and hydroxymethylfurfural (HMF) produced in the process (≈10 kg per MT of biomass) are converted into GVL.
According to the process presented here, 1 MT of dry biomass can be converted to 402 kg of dissolving pulp grade cellulose, 156 kg of high-purity carbon foam lignin precursor, and 138 kg of furfural (Fig. 1). These yields correspond to conversion of almost 70% of the initial mass and >75% of the carbon content into high-value products (Fig. 3). The amount of biomass converted into products increases to 80 wt % if acetic acid (≈66 kg per MT of dry biomass), formic acid (≈27 kg per MT of dry biomass), and levulinic acid/HMF (≈10 kg per MT of dry biomass) are recovered as products. These yields are significantly higher than the yields obtained by other technologies that deliver final products from biomass, such as the production of cellulosic ethanol (228 kg of ethanol per MT or 22.8 wt % yield) (27) or the operation of a typical paper mill (400 to 500 kg per MT of biomass), and similar to those processes that stop at intermediate products, such as sugars (28).
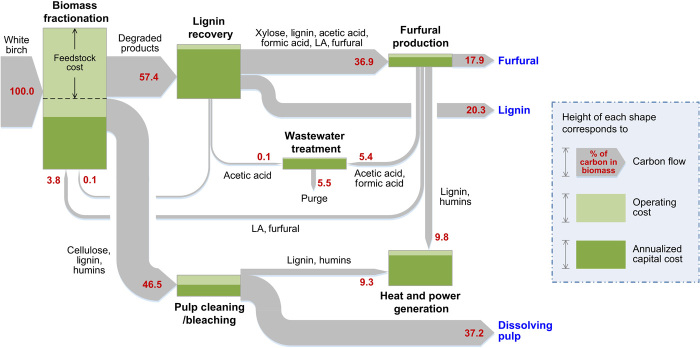
Fig. 3. Sankey diagram.
Sankey diagram for the integrated process to produce furfural, lignin, and dissolving pulp from lignocellulosic biomass. LA, levulinic acid.
On the basis of our experimental data, we developed a process model and performed techno-economic analyses of our integrated process (see section S10 for details). The high yield of products obtained in our process resulted in a total cost (operating and annualized capital cost) of $0.71/kg of products, which is lower than those of a cellulosic ethanol facility [$1.06/kg of products (29)] or an advanced biofuel biorefinery [$1.38/kg of products (30)]. Among the process steps, the biomass fractionation step is the highest contributor toward both the operating and annualized capital costs. The high operating cost is primarily due to the feedstock cost ($125/ton), whereas the high annualized capital cost is due to the long residence time (3 hours), thus requiring a large volume biomass digestion reactor. We note that it should be possible to reduce the residence time by adjusting the parameters, such as temperature, pressure, and/or solvent-to-biomass mass ratio. Assuming 50% lower residence time and 20% lower feedstock cost, the total cost for the biomass fractionation step can be reduced by 21.1%. Because a large amount of water (water/GVL, 8:1) is used for the separation of lignin from the soluble sugars and GVL (to be recycled), the annualized capital cost for the lignin recovery step also becomes important, and further research is necessary to reduce the cost of this step.
The overall revenue of the process is about $500 per MT of dry biomass, which when combined with the low total cost results in an internal rate of return (IRR) of more than 30% (table S11), making our technology attractive for investment despite the initial high risk associated with the project. Once the technology is de-risked and a lower IRR is acceptable, this approach can be used to produce fermentable sugars (31), bioethanol (29), advanced biofuels (27, 30), and other specialty chemicals, enabling the concept of an integrated renewable biorefinery based on the utilization of lignocellulosic biomass that is cost-competitive with a current petroleum refinery.
CONCLUSIONS
Production of biorenewable chemicals and fuels that are competitive with their petroleum-derived equivalents requires the generation of revenue from each of the fractions present in lignocellulosic biomass, that is, cellulose, hemicellulose, and lignin. Because of the different structures and chemistries of these components, an effective fractionation step is critical to separate each component without diminishing the value of any component. GVL has proven to be an excellent solvent to fractionate lignocellulosic biomass. The hemicellulose and lignin fractions can be solubilized in a GVL/water/acid mixture, leaving behind a high-purity cellulose stream that can be used as dissolving pulp to produce fibers. Because of the mild processing conditions that can be used to solubilize the lignin and the hemicellulose, the lignin retains its native structure and can be isolated at high purity. The hemicellulose can be converted into furfural at high yields without requiring the purification of the sugars, thus reducing processing cost. By converting lignocellulosic biomass into dissolving pulp, furfural, and lignin, we can obtain revenues of about $500 per MT of dry biomass, which makes our technology economically competitive.
MATERIALS AND METHODS
Chemicals and materials
We acquired the GVL (>98% purity) from Shenzhen Nexconn Pharmatechs Ltd. All other reagents used were analytical grade, and we used them without further purification. We purchased sulfuric acid (>96%), glucose (>99%), xylose (>99%), mannose (>99%), galactose (>99%), fructose (>99%), arabinose (>99%), furfural (>98%), levulinic acid (>97%), formic acid (>99%), acetic acid (>99%), HMF (>98%), potassium iodide (ACS reagent), and sodium thiosulfate (1 N) from Sigma-Aldrich. We purchased potassium permanganate (0.100 N) and CED (1.0 M) from Ricca Chemical Company. We purchased Whatman filter paper (grade 42; ashless; diameter, 90 mm) from GE Healthcare. We acquired the white birch chips (approximately 2.54 cm × 2.54 cm × 0.65 cm) from Flambeau River Papers. We used white birch chips for the large-scale fractionation experiments. For the laboratory experiments, we used smaller chips created using a DR model CST1450-CHP knife mill fitted with a 1/8 screen. The chips were then screened with an 8-mm sieve screen to remove large pieces.
Biomass fractionation and dissolving pulp production
We performed the biomass fractionation experiments using 60-ml and 1- and 20-liter reactors (see sections S1 to S3 for a detailed description of each reaction setup and experimental procedure). Typically, we added the biomass into the reactor and the GVL/water/acid solution in the proportions indicated in each experiment. We heated the reactors externally. At the end of the experiment, we separated the liquid from the solids by filtration and washed the solids, first with a GVL/water mixture and later with hot water.
We determined the pulp moisture content by placing the sample in an oven at 105°C following TAPPI-T258 om-02. We measured kappa numbers using TAPPI Standard Test Method TAPPI-UM246 (Micro-Kappa number). We measured the cellulose chain lengths in the pulps by measuring CED viscosity using TAPPI-T230 om-04 [viscosity of pulp (capillary viscometer method)]. We measured the alpha-cellulose content of the bleached pulp by TAPPI-T203 (alpha-, beta-, and gamma-cellulose in pulp). We determined the chemical composition of the solid residue following the National Renewable Energy Laboratory (NREL)/TP-510-42618, “Determination of structural carbohydrates and lignin in biomass.” We analyzed the liquid composition by high-performance liquid chromatography (HPLC) (Waters 2695 system with a Bio-Rad Aminex HPX-87H column) equipped with a refractive index (RI) 410 detector (for carbohydrates, organic acids, and GVL) and an ultraviolet (UV) detector (for furfural and HMF). We used a protocol similar to NREL/TP-510-42618 to convert soluble oligomeric carbohydrates into monomers. We diluted the liquid 10 times with 4.4 wt % sulfuric acid and heated at 120°C for 1 hour before analyzing the samples. We bleached the samples using a modified version of the standard OD(EP)D sequence (see section S3 for further details).
Lignin production and characterization
We performed the lignin production, characterization, and upgrading studies using a GVL/liquid solution produced in a 20-liter twin digester reactor (see sections S3 and S4 for details). The reaction conditions to produce the lignin were 80:20 (w/w) GVL/water, 0.1 M sulfuric acid, 60 min at 130°C, and 0.21 to 0.24 MPa. We precipitated the lignin from the GVL/water solution by adding additional water. We recovered the lignin by centrifugation. We washed the lignin with water to remove the small amounts of GVL present and to minimize GVL interferences during the characterization.
We analyzed the chemical composition of the lignin following the NREL/TP-510-42618 protocol. We analyzed the molecular weight after an acetobromination derivatization procedure using a size exclusion chromatography system (Tosoh EcoSEC) equipped with a UV detector (265 nm) (see section S6A for complete details of the lignin characterization).
We determined the glass transition temperature (Tg) using a PerkinElmer Diamond differential scanning calorimeter. We conducted the experiments under nitrogen atmosphere using approximately 2 mg of lignin samples in triplicate. We first heated the lignin from 25° to 140°C at a heating rate of 100°C/min and held at that temperature until the change in thermal energy from the sample was zero to expel any moisture and to erase thermal history of the sample. We then cooled the sample and reheated it to 200°C at the same heating rate to calculate Tg. We measured the thermal decomposition of the lignin samples using a PerkinElmer Pyris 1 thermogravimetric analyzer. We conducted the experiments in duplicate using approximately 5 mg of specimens, heated from 20° to 105°C under a nitrogen atmosphere at a rate of 10°C/min, and held for 10 min to remove moisture. We then heated the sample to 950°C at the same heating rate.
We carried out nuclear magnetic resonance (NMR) analysis on a Varian 400-MR spectrometer. For 2D [heteronuclear single-quantum coherence (HSQC)] spectroscopy, we dissolved 100 mg of lignin in 0.75 ml of d6-DMSO; all samples were fully soluble. We recorded the NMR spectra at 25°C using the (HC)bsgHSQCAD pulse program. We used as internal reference the experiment, collecting 32 transients and 512 time increments in the 13C dimension. We performed the analysis of the data using Mnova version 10.0.1 (see section S6C for complete details of the NMR analysis and quantification).
Carbon foam production
We produced the carbon foam and nanographitic carbon-carbon composites using lignin produced in the 20-liter reactor (see sections S3 to S5 for detailed lignin production and section S6 for detailed lignin characterization). We exposed the dry GVL-lignin powder through a series of heat and processing treatments to make a highly graphitic structure. The heating steps included a combined thermal stabilization/pyrolysis step followed by passivation. We first exposed the GVL-lignin powder to nitrogen (N2) with water vapor (H2O) in a tube furnace for thermal stabilization and pyrolysis. We passed the inert gas, N2, through a bubbler at room temperature and fed into the furnace at a volumetric flow rate of 3 liter min−1. During the thermal stabilization and pyrolysis step, we heated the furnace from 30° to 1000°C at a rate of 10°C min−1 and then held at 1000°C for 1 hour, producing a carbon foam (see section S7 for complete details on carbon foam production).
Furfural production
We performed the initial experiments to study the production of furfural in the GVL/water solution in 10-ml high-pressure glass reactors (Alltech). We loaded the reactors with a solution of 80:20 (w/w) GVL/water, commercial xylose, and the catalyst. We mixed the reactors with a magnetic bar and heated to the reaction temperature in an oil bath. We analyzed the liquid by HPLC (Waters 2695 system with a Bio-Rad Aminex HPX-87H column), RI 410 detector (carbohydrates, organic acids, and GVL), and a UV detector (furfural and HMF). We studied the continuous production of furfural in an upflow reactor. We used the liquid solution recovered after the lignin precipitation to produce furfural. We concentrated the solution by evaporating the water at 100°C to a GVL/water ratio of 80:20 (w/w) before the experiment. We fed the liquid, which contained soluble C5 carbohydrates, through the flow reactor using an HPLC pump (LabAlliance Series I). The reaction conditions were 225°C for 1 min and 2.1 MPa. We used the residual sulfuric acid in the solution from the fractionation step as the catalyst for furfural production (see section S8 for a complete description of the reactor setup and experimental procedure).
Techno-economic model
We developed a process model, based on the experimental data, to study the techno-economic feasibility using the Aspen Plus process simulator (V7.3, Aspen Technology). We scaled the storage, utilities, and wastewater treatment based on the NREL model (30). We performed the equipment sizing and cost analysis using the Aspen Process Economic Analyzer (V7.3, Aspen Technology) based on the simulation results (see section S10 for a complete description of the economic parameters and assumptions).
Supplementary Material
Acknowledgments
Funding: This work has been funded in part by the NSF Small Business Innovation Research 1602713 and 1632394, and this material is based in part upon work supported by the U.S. Department of Energy (DOE) Great Lakes Bioenergy Research Center (DOE Office of Science Biological and Environmental Research; DE-FC02-07ER64494). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the NSF or DOE Great Lakes Bioenergy Research Center. D.M.A. and S.H.H. thank Glucan Biorenewables LLC for its funding support. Author contributions: All the authors contributed to the design and optimization of the process, discussion of the experimental results, and write-up of the article. S.Z., C.J.H., and T.R. performed the biomass fractionation and bleaching experiments. W.W., K.H., and C.T.M. performed the techno-economic analysis. O.H., J.T., V.G.-N., N.L., and D.P.H. performed the lignin characterization and carbon foam production. A.H.M., M.A.M., S.H.H., D.M.A., and J.A.D. performed the lignin separation and furfural production experiments. S.H.H., D.M.A., and J.A.D. performed the biomass fractionation and separation experiments, lignin production and separation studies, furfural production experiments, and GVL recovery and stability studies and did the overall process design. Competing interests: D.M.A. and S.H.H. are employees of Glucan Biorenewables LLC, which is commercializing the technology. J.A.D. is a co-founder of Glucan Biorenewables LLC. J.A.D. and D.M.A. are authors on several patents related to this work held by the Wisconsin Alumni Research Foundation and Glucan Biorenewables LLC (US9359650 B2, US8772515 B2, US8962867 B2, and US8399688 B2). J.A.D. is an author on an additional patent held by the Wisconsin Alumni Research Foundation (US9045804 B2). D.M.A. and S.H.H. are authors on a patent application filed by Glucan Biorenewables LLC (15/216,363, filed 21 July 2016). D.P.H. and V.G.-N. are authors on a provisional U.S. patent filed by the University of Tennessee Research Foundation (62/359,754, filed 8 July 2016). D.P.H., O.H., and N.L. are authors on a patent application filed by the University of Tennessee Research Foundation (PCT/US2015047423, filed 29 August 2015). Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/5/e1603301/DC1
Supplementary Materials and Methods
section S1. Biomass fractionation
section S2. Liquid/solid separation
section S3. High-purity cellulose production for dissolving pulp
section S4. Lignin recovery and precipitation experiments
section S5. Lignin recovery for carbon foam and battery anode production
section S6. Lignin characterization
section S7. Carbon foam production
section S8. Furfural production
section S9. Solvent stability study
section S10. Techno-economic model
fig. S1. Fractionation of white birch.
fig. S2. Lignin produced from white birch fractionation.
fig. S3. Lignin produced from white birch fractionation.
fig. S4. Thermal decomposition of lignin samples produced from white birch fractionation at 70:30 GVL/water, 125°C, 3 hours, and 0.1 M sulfuric acid and 80:20 GVL/water, 130°C, 1 hour, and 0.1 M sulfuric acid.
fig. S5. Aromatic (left) and side chain (right) region of the 13C-1H (HSQC) spectra of white birch, lignin samples produced from white birch fractionated at 70:30 GVL/water, 125°C, 3 hours, and 0.1 M sulfuric acid and 80:20 GVL/water, 130°C, 1 hour, and 0.1 M sulfuric acid.
fig. S6. X-ray diffraction patterns of carbonized and passivated GVL lignin compared to the original lignin (top).
fig. S7. Carbon foam produced from lignin (initial white birch fractionation conditions: 80:20 GVL/water, 130°C, 1 hour, and 0.1 M sulfuric acid).
fig. S8. Production of furfural in a batch reactor using 10 wt % xylose as feedstock.
fig. S9. Production of furfural in a continuous flow reactor using xylose as feedstock.
fig. S10. Gas chromatography–mass spectrometry (GC-MS) chromatogram of GVL (top), tetrahydrofuran (THF) (middle), and ethanol (bottom) after 12 hours at 130°C.
fig. S11. GC-MS chromatogram of GVL (top), THF (middle), and ethanol (bottom) after 12 hours at 130°C.
table S1. White birch fractionation optimization.
table S2. Properties of bleached high-purity cellulose sample from white birch wood chips to produce dissolving pulp.
table S3. Lignin solubility in GVL/water mixtures at room temperature.
table S4. Effect of water/GVL ratio on lignin precipitation.
table S5. Chemical composition and molecular weight of lignin samples.
table S6. Thermal properties of lignin samples.
table S7. Summary of NMR analysis of lignin samples.
table S8. Hydroxyl group content of lignin sample (70:30 GVL/water, 125°C, 3 hours, and 0.1 M sulfuric acid) obtained by quantitative 31P NMR spectroscopy (mmol/g).
table S9. Liquid composition after 12 hours at 130°C.
table S10. Liquid composition after 12 hours at 130°C.
table S11. List of economic parameters and assumptions.
table S12. Mass and energy balances (basis: 2000 tons of white birch per day).
table S13. Energy requirements before and after heat integration (basis: 2000 tons of white birch per day).
table S14. Capital and operating costs (basis: 2000 tons of white birch per day).
table S15. Process model development details.
REFERENCES AND NOTES
- 1.Alonso D. M., Bond J. Q., Dumesic J. A., Catalytic conversion of biomass to biofuels. Green Chem. 12, 1493–1513 (2010). [Google Scholar]
- 2.Ragauskas A. J., Williams C. K., Davison B. H., Britovsek G., Cairney J., Eckert C. A., Frederick W. J. Jr, Hallett J. P., Leak D. J., Liotta C. L., Mielenz J. R., Murphy R., Templer R., Tschaplinski T., The path forward for biofuels and biomaterials. Science 311, 484–489 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim A. A., Nada A. M. A., Hagemann U., El Seoud O. A., Preparation of dissolving pulp from sugar cane bagasse, and its acetylation under homogeneous solution condition. Holzforschung 50, 221–225 (1996). [Google Scholar]
- 4.K. J. Zeitsch, The Chemistry and Technology of Furfural and its Many By-Products (Elsevier, 2000). [Google Scholar]
- 5.García-Negrón V., Phillip N. D., Li J., Daniel C., Wood D., Keffer D. J., Rios O., Harper D. P., Processing-structure-property relationships for lignin-based carbonaceous materials used in energy storage applications. Energy Technol. 10.1002/ente.201600646 (2017). [Google Scholar]
- 6.Lora J. H., Glasser W. G., Recent industrial applications of lignin: A sustainable alternative to nonrenewable materials. J. Polym. Environ. 10, 39–48 (2002). [Google Scholar]
- 7.Wang S.-X., Yang L., Stubbs L. P., Li X., He C., Lignin-derived fused electrospun carbon fibrous mats as high performance anode materials for lithium ion batteries. ACS Appl. Mater. Interfaces 5, 12275–12282 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Shuai L., Questell-Santiago Y. M., Luterbacher J. S., A mild biomass pretreatment using γ-valerolactone for concentrated sugar production. Green Chem. 18, 937–943 (2016). [Google Scholar]
- 9.Luterbacher J. S., Rand J. M., Alonso D. M., Han J., Youngquist J. T., Maravelias C. T., Pfleger B. F., Dumesic J. A., Nonenzymatic sugar production from biomass using biomass-derived γ-valerolactone. Science 343, 277–280 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Tuck C. O., Pérez E., Horváth I. T., Sheldon R. A., Poliakoff M., Valorization of biomass: Deriving more value from waste. Science 337, 695–699 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Fegyverneki D., Orha L., Láng G., Horváth I. T., Gamma-valerolactone-based solvents. Tetrahedron 66, 1078–1081 (2010). [Google Scholar]
- 12.Alonso D. M., Wettstein S. G., Dumesic J. A., Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass. Green Chem. 15, 584–595 (2013). [Google Scholar]
- 13.Alonso D. M., Wettstein S. G., Mellmer M. A., Gurbuz E. I., Dumesic J. A., Integrated conversion of hemicellulose and cellulose from lignocellulosic biomass. Energy Environ. Sci. 6, 76–80 (2012). [Google Scholar]
- 14.Fang W., Sixta H., Advanced biorefinery based on the fractionation of biomass in γ-valerolactone and water. ChemSusChem 8, 73–76 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Lê H. Q., Zaitseva A., Pokki J.-P., Ståhl M., Alopaeus V., Sixta H., Solubility of organosolv lignin in γ-valerolactone/water binary mixtures. ChemSusChem 9, 2939–2947 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horváth I. T., Mehdi H., Fábos V., Boda L., Mika L. T., γ-Valerolactone—A sustainable liquid for energy and carbon-based chemicals. Green Chem. 10, 238–242 (2008). [Google Scholar]
- 17.G. A. Smook, Handbook for Pulp & Paper Technologists (Angus Wilde Publications, ed. 3, 2002). [Google Scholar]
- 18.H. Nanko, A. Button, Alan, D. Hillman, The World of Market Pulp (Tappi Press, 2010). [Google Scholar]
- 19.Wettstein S. G., Alonso D. M., Chong Y., Dumesic J. A., Production of levulinic acid and gamma-valerolactone (GVL) from cellulose using GVL as a solvent in biphasic systems. Energy Environ. Sci. 5, 8199–8203 (2012). [Google Scholar]
- 20.Hu S., Zhang S., Pan N., Hsieh Y.-L., High energy density supercapacitors from lignin derived submicron activated carbon fibers in aqueous electrolytes. J. Power Sources 270, 106–112 (2014). [Google Scholar]
- 21.Hayashi J.-i., Kazehaya A., Muroyama K., Watkinson A. P., Preparation of activated carbon from lignin by chemical activation. Carbon 38, 1873–1878 (2000). [Google Scholar]
- 22.Shuai L., Amiri M. T., Questell-Santiago Y. M., Heroguel F., Li Y., Kim H., Meilan R., Chapple C., Ralph J., Luterbacher J. S., Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science 354, 329–333 (2016). [DOI] [PubMed] [Google Scholar]
- 23.McNutt N. W., Rios O., Maroulas V., Keffer D. J., Interfacial Li-ion localization in hierarchical carbon anodes. Carbon 111, 828–834 (2017). [Google Scholar]
- 24.Gürbüz E. I., Gallo J. M. R., Alonso D. M., Wettstein S. G., Lim W. Y., Dumesic J. A., Conversion of hemicellulose into furfural using solid acid catalysts in γ-valerolactone. Angew. Chem. Int. Ed. 52, 1270–1274 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Mellmer M. A., Sener C., Gallo J. M. R., Luterbacher J. S., Alonso D. M., Dumesic J. A., Solvent effects in acid-catalyzed biomass conversion reactions. Angew. Chem. Int. Ed. 53, 11872–11875 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Marcotullio G., De Jong W., Chloride ions enhance furfural formation from D-xylose in dilute aqueous acidic solutions. Green Chem. 12, 1739–1746 (2010). [Google Scholar]
- 27.F. Kabir Kazi, J. Fortman, R. Anex, “Techno-economic analysis of biochemical scenarios for production of cellulosic ethanol” (Technical Report NREL/TP-6A2-46588, National Renewable Energy Laboratory, 2010).
- 28.Michels J., Wagemann K., The German lignocellulose feedstock biorefinery project. Biofuels Bioprod. Biorefin. 4, 263–267 (2010). [Google Scholar]
- 29.D. Humbird, R. Davis, L. Tao, C. Kinchin, D. Hsu, A. Aden, P. Schoen, J. Lukas, B. Olthof, M. Worley, D. Sexton, D. Dudgeon, “Process design and economics for biochemical conversion of lignocellulosic biomass to ethanol: Dilute-acid pretreatment and enzymatic hydrolysis of corn stover” (Technical Report NREL/TP-5100-47764, National Renewable Energy Laboratory, 2011).
- 30.R. Davis, L. Tao, C. Scarlata, E. C. D. Tan, J. Ross, J. Lukas, D. Sexton, “Process design and economics for the conversion of lignocellulosic biomass to hydrocarbons: Dilute-acid and enzymatic deconstruction of biomass to sugars and catalytic conversion of sugars to hydrocarbons” (Technical Report NREL/TP-5100-62498, National Renewable Energy Laboratory, 2015).
- 31.Motagamwala A. H., Won W., Maravelias C. T., Dumesic J. A., An engineered solvent system for sugar production from lignocellulosic biomass using biomass derived γ-valerolactone. Green Chem. 18, 5756–5763 (2016). [Google Scholar]
- 32.Mansfield S. D., Kim H., Lu F., Ralph J., Whole plant cell wall characterization using solution-state 2D NMR. Nat. Protoc. 7, 1579–1589 (2012). [DOI] [PubMed] [Google Scholar]
- 33.A. Sluiter, B. Hames, R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton, “Determination of sugars, byproducts, and degradation products in liquid fraction process samples: Laboratory analytical procedure (LAP)” (Technical Report NREL/TP-510-42623, National Renewable Energy Laboratory, 2008).
- 34.Degen T., Sadki M., Bron E., König U., Nénert G., The HighScore suite. Powder Diffr. 29, S13–S18 (2014). [Google Scholar]
- 35.B. D. Cullity, S. R. Stock, Elements of X-ray Diffraction (Prentice Hall, 2001). [Google Scholar]
- 36.A. Dutta, A. Sahir, E. Tan, D. Humbird, L. J. Snowden-Swan, P. Meyer, J. Ross, D. Sexton, R. Yap, J. Lukas, “Process design and economics for the conversion of lignocellulosic biomass to hydrocarbon fuels: Thermochemical research pathways with in situ and ex situ upgrading of fast pyrolysis vapors” (Technical Report NREL/TP-5100-62455, National Renewable Energy Laboratory, 2015).
- 37.M. Stone, China’s antidumping investigations against cellulose pulp, (2017); www.paperadvance.com/blogs/michael-stone/2409-chinas-antidumping-investigations-against-cellulose-pulp.html.
- 38.R. Chen, Furfural price rises dramatically in China in Q4 2016, (2017); www.linkedin.com/pulse/furfural-price-rises-dramatically-china-q4-2016-rachel-chen.
- 39.China Chemicals Market (CCM), CCM: Furfural: Export price falls to record low in May 2016-China market news (2017); www.cnchemicals.com/Press/87387-CCM:%20Furfural:%20export%20price%20falls%20to%20record%20low%20in%20May%202016.html.
- 40.Han J., Sen S. M., Alonso D. M., Dumesic J. A., Maravelias C. T., A strategy for the simultaneous catalytic conversion of hemicellulose and cellulose from lignocellulosic biomass to liquid transportation fuels. Green Chem. 16, 653–661 (2014). [Google Scholar]
- 41.Han J., Sen S. M., Luterbacher J. S., Alonso D. M., Dumesic J. A., Maravelias C. T., Process systems engineering studies for the synthesis of catalytic biomass-to-fuels strategies. Comput. Chem. Eng. 81, 57–69 (2015). [Google Scholar]
- 42.Sen S. M., Alonso D. M., Wettstein S. G., Guerbuez E. I., Henao C. A., Dumesic J. A., Maravelias C. T., A sulfuric acid management strategy for the production of liquid hydrocarbon fuels via catalytic conversion of biomass-derived levulinic acid. Energy Environ. Sci. 5, 9690–9697 (2012). [Google Scholar]
- 43.U.S. Geological Survey (2016); http://minerals.usgs.gov.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/5/e1603301/DC1
Supplementary Materials and Methods
section S1. Biomass fractionation
section S2. Liquid/solid separation
section S3. High-purity cellulose production for dissolving pulp
section S4. Lignin recovery and precipitation experiments
section S5. Lignin recovery for carbon foam and battery anode production
section S6. Lignin characterization
section S7. Carbon foam production
section S8. Furfural production
section S9. Solvent stability study
section S10. Techno-economic model
fig. S1. Fractionation of white birch.
fig. S2. Lignin produced from white birch fractionation.
fig. S3. Lignin produced from white birch fractionation.
fig. S4. Thermal decomposition of lignin samples produced from white birch fractionation at 70:30 GVL/water, 125°C, 3 hours, and 0.1 M sulfuric acid and 80:20 GVL/water, 130°C, 1 hour, and 0.1 M sulfuric acid.
fig. S5. Aromatic (left) and side chain (right) region of the 13C-1H (HSQC) spectra of white birch, lignin samples produced from white birch fractionated at 70:30 GVL/water, 125°C, 3 hours, and 0.1 M sulfuric acid and 80:20 GVL/water, 130°C, 1 hour, and 0.1 M sulfuric acid.
fig. S6. X-ray diffraction patterns of carbonized and passivated GVL lignin compared to the original lignin (top).
fig. S7. Carbon foam produced from lignin (initial white birch fractionation conditions: 80:20 GVL/water, 130°C, 1 hour, and 0.1 M sulfuric acid).
fig. S8. Production of furfural in a batch reactor using 10 wt % xylose as feedstock.
fig. S9. Production of furfural in a continuous flow reactor using xylose as feedstock.
fig. S10. Gas chromatography–mass spectrometry (GC-MS) chromatogram of GVL (top), tetrahydrofuran (THF) (middle), and ethanol (bottom) after 12 hours at 130°C.
fig. S11. GC-MS chromatogram of GVL (top), THF (middle), and ethanol (bottom) after 12 hours at 130°C.
table S1. White birch fractionation optimization.
table S2. Properties of bleached high-purity cellulose sample from white birch wood chips to produce dissolving pulp.
table S3. Lignin solubility in GVL/water mixtures at room temperature.
table S4. Effect of water/GVL ratio on lignin precipitation.
table S5. Chemical composition and molecular weight of lignin samples.
table S6. Thermal properties of lignin samples.
table S7. Summary of NMR analysis of lignin samples.
table S8. Hydroxyl group content of lignin sample (70:30 GVL/water, 125°C, 3 hours, and 0.1 M sulfuric acid) obtained by quantitative 31P NMR spectroscopy (mmol/g).
table S9. Liquid composition after 12 hours at 130°C.
table S10. Liquid composition after 12 hours at 130°C.
table S11. List of economic parameters and assumptions.
table S12. Mass and energy balances (basis: 2000 tons of white birch per day).
table S13. Energy requirements before and after heat integration (basis: 2000 tons of white birch per day).
table S14. Capital and operating costs (basis: 2000 tons of white birch per day).
table S15. Process model development details.