Abstract
We previously developed a novel quantitative microsphere suspension hybridization (QMH) assay for high-throughput determination of genomic copy number by direct hybridization of unique sequence probes to genomic DNA followed by flow cytometric analysis. Herein, we describe the first clinical application of this assay examining the Prader-Willi syndrome (PWS) chromosome region at 15q11–13. We designed 30 unique sequence test probes (~60 nucleotides each) spanning 11.37 Mb of chromosome 15q11.2–q13.3 and a disomic reference probe (Actin Beta, chromosome 7p22.1), conjugated to spectrally distinct polystyrene microsphere levels. All probes were hybridized to biotin-labeled genomic DNA in multiplex QMH reactions, and hybridization was detected using phycoerythrin-labeled streptavidin and analyzed by dual-laser flow cytometry. Copy number differences were distinguished by comparing mean fluorescence intensities (MFI) of the test probes to the reference probe in 20 individuals with PWS and six controls. The mean MFI ratio for deleted loci was 0.56 ± 0.09 (n = 88) as compared to the MFI ratios for normal loci, 0.96 ± 0.06 (n = 236), and duplicated loci, 1.44 ± 0.10 (n = 22). A multiplex QMH assay could readily distinguish type I from type II deletions in PWS subjects, as well as small (~4.3 kb) imprinting center (IC) deletions, with no overlap in MFI values compared with normal loci. Using this diagnostic QMH assay, the precise deleted genomic interval could be ascertained in all PWS subjects examined in the present study.
Keywords: microsphere suspension assay, PWS, genetic testing, single copy probes
INTRODUCTION
Genomic rearrangements of chromosome 15q11–q13 cause diverse phenotypes including autism, Prader-Willi syndrome (PWS [OMIM176270]), and Angelman syndrome (AS [105830]). This chromosomal region is subject to genomic imprinting and characterized by complex combinations of low copy repeat elements [Nicholls et al., 1998]. PWS typically results from an ~6 Mb deletion in the paternal chromosome 15q11 to 13 region [Butler et al., 2008] with clustered breakpoints (BP) at either of two proximal sites (BP1 and BP2) and one distal site (BP3) which enables the classification of deletion subjects (type I and II) [Butler et al., 2004]. Additionally, defects within the bipartite imprinting center (IC) in this region can cause the disorder. One area within the IC is required for establishing and maintaining the paternal or maternal imprint and studies have ascertained the shortest region of overlap (SRO) which defines the PWS-IC (PWS-SRO) [Ohta et al., 1999; Mapendano et al., 2006]. Additionally, PWS can arise from chromosome 15 uniparental disomy (UPD) of maternal origin.
Diagnostic tests for identification of PWS include high resolution chromosomal analysis [Delach et al., 1994] for detection of the typical interstitial deletion of the chromosome 15q11–q13 region found in 70% of PWS subjects. However, false positive and false negative results have been demonstrated using chromosomal banding alone [Kuwano et al., 1992]. More recently developed techniques, such as microarray-based comparative genomic hybridization (array CGH), have been used to detect chromosome 15 deletions and duplications greater than about 10 kb in arrays using clones as probes [Sahoo et al., 2005], and the resolution is only limited by the size and distance between the arrayed interrogating probes in oligonucleotide-based array CGH [Stankiewicz and Beaudet, 2007]. However, an important limitation of at least some of the currently available commercial oligonucleotide arrays is the under-representation of probes in the IC region which could aid in defining different classes of IC deletions. While array CGH is becoming a more widely accepted molecular technique, fluorescence in situ hybridization (FISH) [Kuwano et al., 1992; Bettio et al., 1995] remains the most routinely used technique to confirm deletions or other chromosome abnormalities in cytogenetics. Conventional FISH techniques can detect the boundaries of deleted regions to a resolution of ~100 kb [Rapp, 1998]. A more recently developed FISH technique using unique sequence probe cocktails [Newkirk and Bi, patent pending, 2008] has an even smaller resolution (~4 kb) while maintaining the same signal intensity as conventional FISH probes (manuscript in preparation).
Polymerase chain reaction (PCR) methylation techniques and/or Southern hybridization are used to detect UPD and gene expression in PWS has been analyzed by gene expression microarrays [Bittel et al., 2003, 2005]. However, these techniques have their strengths and limitations such as the addition of competitor DNA which has been demonstrated to adversely affect both the reproducibility and accuracy of assays [Newkirk et al., 2005], large quantity of parental DNA or costs which may limit their use for routine clinical diagnostic tests. Other techniques for PWS analysis have been used including, multiplex ligation-dependent probe amplification (MLPA) [Nygren et al., 2005; Bittel et al., 2007], and multiplex amplifiable probe hybridization (MAPH) [Locke et al., 2004]. MLPA is a method to establish the copy number of up to 45 nucleic acid sequences in a single PCR reaction. It can be used to determine both copy number and methylation status in a sample of genomic DNA [see Bittel et al., 2007 for a summary]. However, MLPA is limited in probe flexibility due to the need for rigorous optimization required for the multiplex PCR reaction. MLPA also requires a costly capillary electrophoresis instrument.
Reports have identified behavioral and cognitive differences in individuals with PWS having either typical Type I or Type II deletions [Butler et al., 2004; Zarcone et al., 2007] and maternal disomy 15, making distinction between the different PWS genetic subtypes useful for clinical management and educational programs. Further, FISH analysis cannot identify small IC deletions in PWS subjects due to the size of commercial probes (~150–200 kb) and the limit of resolution for the technique itself (~100–200 kb). Very few facilities offer specialized testing for IC defects and the turn-around time may be several months for analysis and cost can be considerable. If clinical features of PWS exist with a negative (normal) FISH result for a typical PWS deletion with abnormal methylation, and biparental inheritance, then an IC defect is assumed but the type is unknown (i.e., microdeletion or epimutation). Research data reported to date suggests that the percentage of IC deletion subjects with biparental inheritance of chromosome 15 is small [14%; Buiting et al., 1995]; however this could be an under representation if newer technology is used.
Previously, we described an assay for the detection of genomic copy number changes called quantitative microsphere hybridization [QMH; Newkirk, patent pending, 2008; Newkirk et al., 2005, 2006]. This assay utilizes unique sequence probes attached to spectrally distinct microspheres in multiplex hybridization to detect homologous target sequences in genomic DNA. Hybridized test and reference probes are detected using flow cytometry and geometric mean fluorescence intensity (MFI) ratios are calculated to determine copy number differences. QMH is an attractive option for a clinical diagnostic test since it offers a high-throughput platform which is highly reproducible and accurate in part due to the lack of competitor DNA in assays [Newkirk et al., 2005, 2006]. It utilizes a flow cytometer for data acquisition which is found in most clinical settings. Additionally, the per assay cost for QMH is less than $1 per multiplex assay with 10 probes which is far less than other diagnostic tests. This per sample calculation assumes a minimum of 100 samples will be run with each probe. The input sample requirement is minimal (10 ng), and capable of detecting deletions as small as 3 basepairs (BP) in the case of cystic fibrosis (manuscript in preparation). Examples of other previously developed QMH assays include those for chronic myelogenous leukemia [Newkirk et al., 2006], Charcot–Marie–Tooth syndrome, Alzheimer disease, velocardiofacial syndrome, and Duchenne muscular dystrophy (unpublished data). For the present study, we developed a series of single copy (sc) probes spanning the PWS chromosome region in an effort to delineate type I and type II deletion subjects and to identify deletions in the IC region.
MATERIALS AND METHODS
Quantitative Microsphere Hybridization
Probe selection, synthesis, and microsphere conjugation
A series of 30 different unique sequence [Newkirk and Bi, patent pending, 2008] test probes specific to chromosome 15q11–q13 were designed, as well as a disomic reference probe for beta actin (ACTB) on chromosome 7p22 (Table I, Fig. 1). The most proximal probe designed was PWS19.4 Mb, which was within range of the type I deletion BP currently defined as being between 18.683 and 20.220 Mb [Butler et al., 2008]. Probes were selected based on their unique sequence composition as indicated by only one BLAST (NCBI) and BLAT (UCSC Genome Browser) match per probe with no other homologous genomic regions elsewhere in the genome, length (~60 bases), and GC content (48–55%). Each probe was evaluated for potential stable secondary sequence conformations as predicted by MFold software (http://www.bioinfo.rpi/edu/applications/mfold/old/dna) and any probe not meeting defined criteria (ΔG < 50, ΔH < −1,000, ΔS < −3,500) was discarded from design of the PWS QMH multiplex assay (Table I). These precise parameters were determined by in vitro testing of probes with varying secondary structure characteristics and previous studies found that probes not meeting these criteria produced MFI ratios deviating from the predicted ratio of 1 for normal loci (two copies per diploid genome), 0.5 for deleted loci (one copy), and 1.5 for duplicated loci (three copies) [Newkirk et al., 2006]. Each probe was synthesized using a 5′-C6-amino modification (Integrated DNA Technologies, Coralville, IA) for coupling to carboxylated microspheres. Probes were conjugated to spectrally distinct microsphere levels of Luminex XMAP microspheres (Miraibio, San Francisco, CA) via a carbodiimide coupling reaction as described previously [Dunbar et al., 2003; Newkirk et al., 2006]. To minimize the initial expenditure on microspheres for assay development, only ten different levels were purchased, however previous studies have demonstrated successful multiplex analysis with up to 20 or more microsphere levels in a single reaction [Dunbar et al., 2003]. Quality control procedures for each microsphere-conjugated probe were performed to ensure proper attachment of probes to microspheres as defined earlier [Newkirk et al., 2006]. Additionally, microsphere-swap experiments were performed to verify that the MFI value was independent of the level of microsphere used for conjugation, the details for which were stated previously [Newkirk et al., 2006].
TABLE I.
Quantitative Microsphere Hybridization Probes Utilized in the Study of Prader-Willi Syndrome
| Probe name | Chromosome location (March 2006) | Secondary structure analysis |
|---|---|---|
| PWS19.4 Mb | chr15:19,412,113–19,412,168 | dG = −7.48,dH = −89.30,dS = −263.81,Tm = 65.4 |
| D15S541 | chr15:20,360,015–20,360,075 | dG = −4.19,dH = −80.40,dS = −235.83,Tm = 67.8 |
| GCP5 | chr15:20,402,365–20,402,429 | dG = −0.97,dH = −40.30,dS = −121.71,Tm = 58.0 |
| NIPA1 | chr15:20,637,672–20,637,733 | dG = −7.00,dH = −100.60,dS = −301.79,Tm = 60.2 |
| chr15:21,263,422 | chr15:21,263,422–21,263,482 | dG = −2.99,dH = −89.00,dS = −277.32,Tm = 47.8 |
| chr15:21,402,665 | chr15:21,402,665–21,402,725 | dG = −3.68,dH = −85.60,dS = −253.50,Tm = 64.5 |
| D15S11 | chr15:21,625,574–21,625,639 | dG = −5.20,dH = −105.00,dS = −308.83,Tm = 66.8 |
| D15S63 | chr15:22,581,253–22,581,317 | dG = −1.53,dH = −70.70,dS = −214.05,Tm = 57.1 |
| D15S128 | chr15:22,682,940–22,682,995 | dG = −6.81,dH = −99.30,dS = −298.21,Tm = 59.8 |
| AS-SRO | chr15:22,717,169–22,717,238 | dG = −0.37,dH = −25.00,dS = −76.22,Tm = 54.9 |
| IC3 | chr15:22,717,771–22,717,826 | dG = −5.40,dH = −63.90,dS = −188.62,Tm = 65.6 |
| IC2 | chr15:22,721,121–22,721,175 | dG = −5.75,dH = −63.20,dS = −185.23,Tm = 68.0 |
| IC1 | chr15:22,732,614–22,732,670 | dG = −2.45,dH = −55.10,dS = −169.76,Tm = 51.4 |
| IC | chr15:22,736,900–22,736,965 | dG = −1.45,dH = −50.30,dS = −151.17,Tm = 59.6 |
| SNRPN exon1 | chr15:22,751,254–22,751,319 | dG = −7.31,dH = −132.30,dS = −403.00,Tm = 55.1 |
| PWS-SRO | chr15:22,751,461–22,751,525 | dG = −4.82,dH = −62.40,dS = −178.18,Tm = 77.1 |
| SNRPN intron1 | chr15:22,752,948–22,753,011 | dG = −8.79,dH = −127.40,dS = −382.43,Tm = 60.0 |
| D15S12 | chr15:25,675,102–25,675,165 | dG = −1.24,dH = −38.40,dS = −114.99,Tm = 60.8 |
| OCA2 | chr15:26,017,987–26,018,049 | dG = −6.19,dH = −70.30,dS = −198.39,Tm = 81.2 |
| PWS26.04 Mb | chr15:26,048,967–26,041,021 | dG = −6.96,dH = −85.70,dS = −253.88,Tm = 64.4 |
| D15S1019 | chr15:27,355,543–27,355,605 | dG = −0.46,dH = −31.50,dS = −96.05,Tm = 54.8 |
| BP3distal | chr15:27,455,843–27,455,907 | dG = −0.43,dH = −22.40,dS = −67.99,Tm = 56.3 |
| D15S1048 | chr15:27,592,696–27,592,758 | dG = −4.37,dH = −119.50,dS = −356.27,Tm = 62.3 |
| D15S165 | chr15:28,985,442–28,985,500 | dG = −2.66,dH = −50.00,dS = −146.50,Tm = 68.2 |
| D15S1031 | chr15:29,809,165–29,809,225 | dG = −2.07,dH = −39.20,dS = −114.90,Tm = 68.0 |
| D15S1010 | chr15:30,784,010–30,784,069 | dG = −1.46,dH = −30.70,dS = −90.48,Tm = 66.1 |
| Beta actin | chr7:5,534,363–5,534,424 | dG = −8.82,dH = −112.70,dS = −334.93,Tm = 63.3 |
| D15S822 | chr15:24,873,458–24,873,521 | dG = −8.40,dH = −85.90,dS = −249.88,Tm = 70.6 |
| 15q14-5 | chr15:32,521,891–32,521,954 | dG = −2.10,dH = −70.60,dS = −220.86,Tm = 46.5 |
| 15q14-internal | chr15:32,568,078–32,568,140 | dG = −2.95,dH = −85.90,dS = −267.45,Tm = 48.0 |
Fig. 1.
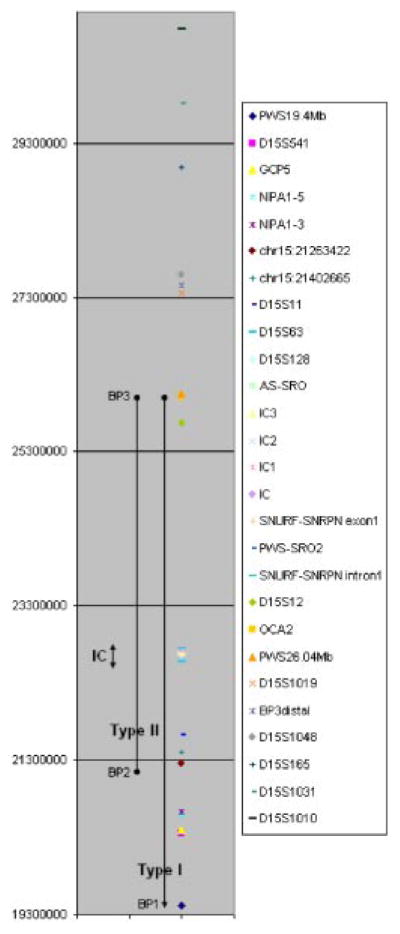
Map of PWS/AS region on chromosome 15q11–13. The chromosomal coordinates for QMH probes conjugated to microspheres are indicated to the left. The type I and type II deletion intervals as well as their breakpoint intervals (BP) are depicted, as well as the IC region. The locations of the various single copy probes used in the present study are indicated by the symbols as defined in the legend.
Subject samples and genomic target preparation
Genomic templates were prepared using 10 ng of each archived PWS DNA sample (IRB protocol 02 04-43). Genotypes for some samples were known from prior cytogenetic, array CGH, or microarray studies [Ohta et al., 1999; Butler et al., 2004; Bittel et al., 2006], while some were unknown (Table II). Genomic DNA was replicated in vitro using a modified protocol of the GenomiPhi kit (GE Healthcare, Piscataway, NJ) to allow for the direct incorporation of biotin into template DNA. First, 1 μl of each DNA sample (10 ng/μl) was mixed with 9 μl of Sample Buffer and heat denatured for 3 min at 95°C. Samples were snap-cooled on ice for 2 min followed by addition of 4 μl of 50 nmol Biotin-16-dUTP (Roche, Indianapolis, IN), 9 μl of Reaction Buffer, and 1 μl of Enzyme Mix. Reactions were incubated at 30°C for 24–36 hr and the Phi29 polymerase was inactivated by incubation at 65°C for 10 min. DNA samples were ethanol precipitated at room temperature by addition of 100 μl of water, 10 μl of 1.5 M NaOAc/0.5 M EDTA, pH 8.0 and 250 μl of ethanol. Successful replication of subject DNA was verified by PCR amplification of reference probes from three chromosomal locations (data not shown). Any DNA sample not yielding PCR product in this initial quality control step was not used for QMH. We previously found that samples that did not successfully amplify PCR product did not produce accurate QMH results [Newkirk et al., 2006]. In this study all DNA samples successfully yielded appropriately sized PCR products. DNA samples were sheared to ~1–2.5 kb by sonication (B-300), which was monitored by gel electrophoresis (Cambrex Bio Science, Rockland, ME).
TABLE II.
Quantitative Microsphere Hybridiziation Results for All Subjects
| Probes/location (Hg18) |
19,412,113 |
20,360,015 |
20,402,365 |
20,594,853 |
21,263,422 |
21,402,665 |
21,625,574 |
22,581,253 |
22,682,940 |
22,717,169 |
22,736,900 |
22,751,254 |
22,751,461 |
25,675,102 |
26,017,987 |
26,048,967 |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjects | PWS19.4 Mb | D15S541 | GCP5 | NIPA1 | Chr15:21,263,422 | chr15:21,402,665 | D15S11 | D15S63 | D15S128 | AS-SRO | IC1 | SNRPN-exon1 | IC2 | D15S12 | OCA-2 | PWS26.04Mb | Genotype |
| PWS-6 | 0.92 | 0.87 | 1.03 | 0.86 | 0.75 | 0.62 | 0.53 | 0.62 | nt | 0.61 | 0.54 | 0.73 | 0.62 | 0.64 | 0.53 | 0.62 | Type II |
| PWS-10 | 1.00 | 0.97 | 0.80 | 0.80 | 0.40 | 0.59 | 0.46 | 0.67 | nt | 0.47 | 0.40 | 0.67 | 0.65 | nt | 0.56 | 0.71 | Type II |
| PWS-16 | 0.58 | 0.50 | 0.60 | 0.62 | 0.74 | 0.67 | 0.74 | 0.65 | nt | 0.64 | 0.58 | 0.73 | 0.64 | 0.72 | 0.58 | 0.63 | Type I |
| PWS-17 | 0.55 | 0.61 | 0.40 | 0.48 | 0.67 | 0.60 | 0.63 | 0.63 | nt | 0.70 | 0.46 | 0.46 | 0.54 | nt | 0.60 | 0.53 | Type I |
| PWS-18 | 0.47 | 0.45 | 0.62 | 0.52 | 0.41 | 0.49 | 0.40 | 0.56 | 0.58 | 0.47 | 0.50 | 0.62 | 0.48 | 0.91 | 1.02 | 0.98 | Atypical |
| PWS-21 | 0.69 | 0.54 | 1.00 | 1.04 | 0.99 | 1.04 | 0.63 | 0.65 | nt | 0.56 | 0.51 | 0.91 | 1.07 | nt | 0.93 | nt | Atypical |
| PWS-29 | nt | 1.00 | nt | 0.90 | 0.92 | 0.88 | 0.67 | 1.02 | nt | 0.56 | 0.55 | 0.91 | 0.93 | 0.68 | 0.80 | nt | Atypical |
| PWS-26 | nt | 1.30 | nt | 1.37 | 1.20 | 1.50 | nt | 1.57 | nt | 1.25 | 1.20 | 1.59 | 1.73 | nt | 1.31 | 1.52 | Duplication |
| PWS-27 | nt | 1.00 | nt | 1.40 | 1.32 | 1.40 | nt | 1.53 | 1.54 | 1.01 | 1.00 | 1.01 | 1.02 | 0.81 | 0.91 | nt | Duplication |
| PWS-36 | nt | 0.96 | nt | 0.77 | 0.98 | 1.01 | nt | 1.01 | nt | 1.04 | 0.97 | 0.92 | 0.96 | nt | 1.01 | nt | Obese subject |
| PWS-37 | nt | 1.06 | nt | 0.84 | 0.95 | 0.97 | nt | 1.08 | nt | 0.93 | 0.97 | 0.94 | 1.06 | nt | 1.03 | nt | Obese subject |
| PWS-46 | nt | 1.05 | nt | 0.95 | 1.08 | 1.02 | nt | 1.00 | nt | 0.96 | 1.03 | 0.95 | 0.95 | nt | 0.86 | nt | UPD |
| PWS-47 | nt | 0.86 | nt | 0.81 | 1.00 | 0.95 | nt | 1.03 | nt | 1.00 | 0.81 | 1.02 | 1.00 | nt | 0.98 | nt | UPD |
| Control-1 | 1.02 | 0.83 | 1.00 | 1.00 | 0.95 | 1.03 | 0.90 | 1.08 | 1.02 | 0.91 | 0.97 | 1.02 | 0.93 | 0.99 | 0.94 | 0.92 | Normal |
| Control-2 | 0.91 | 0.92 | 1.00 | 1.02 | 1.00 | 1.05 | 0.91 | 0.93 | 0.95 | 1.02 | 0.91 | 0.86 | 0.97 | 0.91 | 0.93 | 0.90 | Normal |
| Control-3 | 1.02 | 0.88 | 1.05 | 1.01 | 0.93 | 0.95 | 1.01 | 0.97 | 0.90 | 0.87 | 0.91 | 0.92 | 0.92 | 1.00 | 1.02 | 0.88 | Normal |
| Control-4 | 1.01 | 1.00 | 0.92 | 1.03 | 1.06 | 1.00 | 0.91 | 0.98 | 0.90 | 0.97 | 0.99 | 0.90 | 1.01 | 0.95 | 0.91 | 0.92 | Normal |
| Control-5 | 0.96 | 0.95 | 1.01 | 0.94 | 0.90 | 1.01 | 0.88 | 1.01 | 0.88 | 1.01 | 0.90 | 1.02 | 1.01 | 0.96 | 0.92 | 0.97 | Normal |
| Control-6 | 0.94 | 0.92 | 1.03 | 0.92 | 0.95 | 1.01 | 0.99 | 1.02 | 0.94 | 1.07 | 1.02 | 0.93 | 1.02 | 0.97 | 0.89 | 1.00 | Normal |
Those regions shaded in light gray represent deleted loci, and those in dark gray represent duplicated loci.
Loci not tested are indicated by “nt.”
Hybridization reactions and flow cytometry
Fifty nanograms of each fragmented biotin-labeled genomic DNA sample was used per QMH reaction. Approximately 10,000 of each probe-coupled microsphere was combined and pelleted by centrifugation for 2 min at 16,500g. The pellets were diluted in 40 μl of 1.5× TMAC hybridization buffer (3-mol/L tetramethylammonium chloride, 50 mmol/L Tris–HCl, pH 8.0, 1 g/L Sarkosyl) per reaction for a master mix. Multiplex analysis of two to ten different levels of microspheres conjugated to probes for QMH was possible in a single reaction using the Luminex microspheres. Probes included in hybridization reactions were selected based on available genotypic evidence in each previously characterized subject, and unknown samples were initially hybridized with probes D15S541, NIPA1, chr15:21,26,3422, chr15:21,402,665, D15S63, AS-SRO, SNRPN-exon1, and OCA2 as well as ACTB (nine probes) in an effort to delineate type I and type II deletions, and IC defects (Table I). QMH probes used in subsequent hybridization reactions were selected adjacent to and within any deleted regions detected in the first QMH reaction in order to precisely characterize the deletion interval (Fig. 1). Reactions were heat denatured at 95°C for 5 min and allowed to hybridize for 3 hr at 52°C. Optimal reaction conditions were individually established for each probe conjugated to microspheres using hybridization gradients as described previously [Newkirk et al., 2006], and all probes shown in Table I yielded similar hybridization temperatures enabling multiplex analysis. TMAC stabilizes AT basepairs, thus minimizing the effect of base composition on hybridization conditions [Dunbar et al., 2003]. Reactions were washed using 150 μl of 1× TMAC and centrifuged for 2 min at 16,500g to pellet the microspheres. The supernatant containing all unhybridized DNA was removed and reactions were stained for 10 min in 12 μl of the reporter molecule, streptavidin-R-phycoerythrin (SPE; Molecular Probes, Eugene, OR), diluted 1:50 (10 μg/ml) in 1× TMAC. Reactions were washed using 150 μl of 1× TMAC, centrifuged for 2 min at 16,500g, supernatant was removed and pellets were resuspended in 70 μl of 1× TMAC. Samples were evaluated by flow cytometry analysis using a Luminex 200 (Applied Cytometry Systems, Plano, TX) and approximately 5,000 events per microsphere level were analyzed per reaction. Calibration of the Luminex 200 was performed using Luminex xMAP Calibration Classifier and Reporter Microspheres (Miraibio). After analysis of each sample by flow cytometry, the ratios of geometric mean fluorescence intensities (MFI) of test probe to reference probe were calculated and statistics (e.g., mean, 95% confidence intervals, and standard deviation) were generated using MS Excel. Geometric MFI values were previously shown to be the most accurate for assessing data from QMH assays since data are collected in logarithmic mode on the flow cytometer [Kirkwood, 1979; Coder et al., 1994; Newkirk et al., 2006].
RESULTS
Detection of Type I and Type II Deletions in PWS Subjects
A series of microsphere-conjugated sc probes spanning chromosome 15q11–q13 were hybridized to labeled genomic DNA from subjects with type I (n = 10; PWS-16 through PWS-25), and type II deletions (n = 10, PWS-6 through PWS-15) in the PWS region. In most instances deletions were determined by prior analysis (see Materials and Methods Section) and in other subjects the genotypes were unknown; however, the subjects had a clinical diagnosis of PWS (n = 2; Fig. 1). An initial multiplex hybridization reaction to delineate type I from type II deletions included test probes specific to PWS19.4 Mb, D15S541, NIPA1, chr15:21,263,422, chr15:21,402,665, D15S63, AS-SRO, SNRPN-exon1, OCA2, and PWS26.04 Mb as well as the ACTB reference probe. The copy number for all probes was readily distinguishable based on MFI ratios in each subject (Table II). All type I PWS subjects revealed a large deletion spanning from PWS19.4 to PWS26.04 Mb with all test probes deleted (PWS-16 and PW-17 as representative examples in Table II). Since PWS19.4 Mb was deleted in all type I subjects tested in the current study, this is in agreement with the most current definition of BP1 as between 18.683 and 20.220 Mb [Butler et al., 2008]. Type II subjects revealed a smaller deletion spanning from test probe chr15:21,263,422 to 26.04 Mb (PWS-6 and PWS-10 as representative examples in Table II). Normal control subjects and obese subjects (PWS-36 and PWS-37) tested using the same probes showed MFI ratios within normal range for all test loci (Table II).
QMH results for two subjects studied revealed non-classical deletions as compared to the typical deleted segment for type I subjects (Table II). In subject PWS-21, probes for PWS19.4Mb, D15S541, D15S11, and D15S63 were present in one copy each, while GCP5, NIPA1, and chr15:21,263,422 were disomic. This intact region spans approximately 861 kb and could represent a sub-class of type I deletion, evidence for which has been documented by others [Bittel and Butler, 2005]. Comparison of array CGH and QMH analysis of PWS-21 further verified authenticity of QMH results for all loci found deleted by QMH (data not shown). Additionally, array CGH results for PWS-18 suggested a diploid copy number at microsatellite D15S822, which is approximately 1.3 Mb centromeric of BP3, which should be deleted in type I deletion subjects. By QMH analysis, PWS-18 did appear to have a type I deletion with a more proximal BP to BP3 since D15S12 was found to be intact, which could be suggestive of a sub-class of a type I deletion (Table II). A QMH probe specific to D15S822 was designed (Table I) and hybridized to labeled genomic DNA from PWS-18 with the reference ACTB probe. The MFI ratio for D15S822 in PWS-18 was 1.08 indicating that this locus is intact in this type I deletion subject, thus verifying the array CGH results (data not shown).
Detection of Deletions Extending to BP4, BP5 and 15q14 in Subjects With PWS
We examined several PWS subjects previously analyzed by array CGH which had suspected deletion BP extending to BP4 and BP5 and beyond to 15q14. One PWS female infant (PWS-28) with a cytogenetic deletion larger than the typical 15q11–q13 deletion was tested using QMH probes telomeric of BP3 (BP3distal, D15S1048, D15S165, D15S1031, D15S1010; Fig. 1, Table I). MFI ratios indicated that PWS-28 was deleted for BP3distal, D15S1048, D15S165, and D15S1031, but not D15S1010, which would classify the distal deletion BP as within range of BP5 (Table IV). Another PWS subject (PWS-32) with a type I deletion was also suspected to have a non-classical deletion by array CGH, telomeric of BP3 between chr15:32,523,241 and 32,590,702. Two QMH probes were designed with one probe potentially 5′ of the deletion and one probe within the suspected deletion interval, 15q14-5 and 15q14-internal, respectively (Table I). QMH results indicated that PWS-32 was intact for the internal deletion probe, 15q14-internal, however deleted for the probe suspected as being 5′ of the deletion by array CGH, 15q14-5 (Table III). Another subject with obesity and mental retardation but with normal methylation (PWS-30) was also thought to be deleted distal to BP3 (by parental recollection of previous genetic testing results elsewhere), however no MLPA probes were tested distal to OCA2. QMH was used to test PWS-30 for probes PWS26.04 Mb, D15S1019, BP3distal, D15S1048, D15S165, D15S1031, D15S1010, 15q14-5, and 15q14-internal. QMH results showed that PWS-30 was intact for all loci tested except for D15S165, thus suggesting a deletion at this locus (Table III). This locus was within regions known to be polymorphic and can show variation in normal individuals (http:///www.sanger.ac.uk/humgen.cnv). It is unknown whether alterations in copy number of loci in this region will lead to clinical outcome.
TABLE IV.
Quantitative Microsphere Hybridization Results Showing Mean Fluorescence Intensity Ratios for Prader-Willi Syndrome IC Defect Subjects
| Probes | PWS-4 | PWS-4mother | PWS-4father | PWS-4paternal grandfather | PWS-4paternal grandmother | PWS-5 | PWS-5mother | PWS-5father | PWS-5paternal grandmother |
|---|---|---|---|---|---|---|---|---|---|
| IC3 | 0.89 | 1.00 | 0.89 | 0.95 | 1.01 | 0.60 | 0.96 | 0.46 | 0.49 |
| IC2 | 0.61 | 0.86 | 0.54 | 0.96 | 0.52 | 0.49 | 0.87 | 0.42 | 0.42 |
| IC1 | 0.41 | 0.89 | 0.53 | 0.86 | 0.64 | 0.44 | 0.77 | 0.43 | 0.41 |
| IC | 0.89 | 1.01 | 0.85 | 0.96 | 1.03 | 0.44 | 0.95 | 0.63 | 0.65 |
| SNRPN-exon1 | 0.81 | 0.94 | 0.83 | 0.88 | 0.99 | 0.44 | 1.06 | 0.60 | 0.58 |
| SNRPN-intron1 | 0.87 | 0.92 | 0.92 | 0.97 | 0.97 | 0.87 | 0.97 | 0.90 | 0.89 |
| D15S63 | 1.00 | 0.91 | 0.93 | 0.93 | 0.99 | 0.97 | 0.98 | 0.97 | 0.87 |
| B-Actin | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
Shaded regions indicate those probes found deleted by QMH.
TABLE III.
Quantitative Microsphere Hybridization Results for chr15q11–q13 Deletions Distal to BP3
| Probe | PWS-28 | PWS-30 | PWS-32 | Control |
|---|---|---|---|---|
| OCA-2 | 0.53 | nt | nt | 0.96 |
| PWS-26.04 Mb | nt | 0.97 | 0.97 | 0.99 |
| D15S1019 | 0.59 | 0.94 | nt | 1.02 |
| BP3distal | 0.53 | 0.9 | nt | 1.01 |
| D15S1048 | 0.51 | 0.91 | nt | 1.01 |
| D15S165-60 | 0.46 | 0.54 | nt | 1.05 |
| D15S1031 (2nd test) | 0.54 | 1.09 | 0.9 | 1.05 |
| D15S1010 | 0.94 | 0.95 | 0.93 | 0.99 |
| 15q14-5 | nt | 0.95 | 0.50 | 0.98 |
| 15q14-internal | nt | 0.82 | 0.96 | 0.96 |
| ACTB | 1.00 | 1.00 | 1.00 | 1.00 |
Loci not tested are indicated by “nt.”
Detection of Differentially Sized IC Defects in Prader-Willi Syndrome Subjects
All PWS subjects in the present study were examined for possible IC deletion during the initial QMH screen using the SNRPN-exon1 probe. The subject showing a deletion at SNRPN-exon1 (PWS-5) as well as a suspected IC deletion subject (abnormal methylation, normal conventional FISH studies, biparental inheritance of chromosome 15) which showed no deletion at SNRPN-exon 1 (PWS-4) were then examined using three QMH probes specific to the PWS IC region that were initially designed (IC, SNRPN-exon1, SNRPN-intron1; Table I). Five test probes (IC3, IC, SNRPN-exon1, SNRPN-intron1, D15S63) and the reference probe, ACTB, were hybridized in a multiplex QMH reaction to labeled genomic DNA from each of the aforementioned PWS subjects and available family members. QMH results showed that PWS-5 had an IC deletion only at the SNRPN-exon1 target sequence, however PWS-4 showed no deletion for any of the initial test probes (Table IV). Another QMH probe was developed just proximal to the IC probe (IC1) and all subjects and family members were tested. QMH results showed that PWS-4 and PWS-5 were deleted for this probe, as well as the father of PWS-4 (PWS-4Father) and PWS-5 (PWS-5Father) and the paternal grandmothers of PWS-4 (PWS-4PatGrandmother) and PWS-5 (PWS-5PatGrandmother; Table IV). For confirmation, a second unique sequence QMH probe located distal to IC1, designated as IC2, was designed and hybridized to DNA from PWS-4 to 5 and parents and grandparents of the subjects. QMH results showed that this probe was also deleted in PWS-4 and PWS-5, as well as the fathers and paternal grandmothers of PWS-4 and PWS-5. PWS-4 was previously classified as a non-deletion IC status in the literature [Ohta et al., 1999]. Subsequently, we tested DNA from the paternal grandmother of subject PWS-5 as well as the paternal grandfather and paternal grandmother of subject PWS-4 using all seven PWS-IC QMH probes (Table IV). Results revealed a deletion of IC1 and IC2 in the paternal grandmother of PWS-4, which was the same deletion found in PWS-4 (Table IV). The paternal grandfather of PWS-4 was biallelic for all loci tested. This indicates that the deletion was passed from the paternal grandmother to the father to the proband. The paternal grandmother of PWS-5 tested positive for a deletion including SNRPN-1exon1, IC, IC1, IC2, and IC3 which are the same probes found deleted in PWS-5, indicating a similar inheritance of the IC deletion. These QMH results were confirmed using QPCR (data not shown).
Detection of Duplications in the PWS Region by QMH
The same QMH probes used for the detection of deletions were applied to detect duplications in one known duplicated 15q11-q13 subject, PWS-26, and one subject with a duplication of the proximal long arm of chromosome 15 of unknown size, PWS-27. Test probes specific to D15S541, NIPA1, chr15: 21,263,422, chr15:21,402,665, D15S63, AS-SRO, IC1, IC2, SNRPN-exon1, OCA2, and PWS26.04Mb as well as the control ACTB probe were used in the QMH reaction for each subject. QMH results showed that PWS-26 was duplicated for all test probes, while PWS-27 was duplicated only for NIPA1, chr15: 21,263,422, chr15:21,402,665, and D15S63 with all other loci intact (Table II). A second QMH reaction using the D15S128 probe was performed for PWS-27, and the MFI ratio indicated that this test probe was also duplicated (Table II). This defined a duplicated region of approximately 2.045 Mb in PWS-27.
Statistical Analysis of QMH Results for All Tested Loci
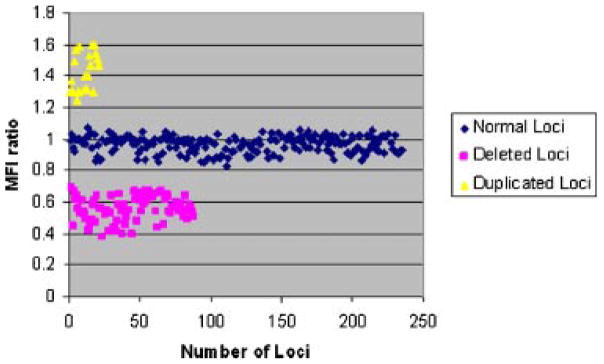
Analysis of the MFI values for all normal loci tested by QMH (n = 191) showed a mean of 0.98 ± 0.075 with a 95% confidence level of 0.011 (Fig. 2) and the MFI values for all deleted loci (n = 80) have a mean of 0.58 ± 0.072 with a 95% confidence level of 0.016 (Fig. 2). The MFI values for all duplicated loci (n = 22) have a mean of 1.44 ± 0.1 with a 95% confidence level of 0.082. No overlap between the MFI values for normal and deleted loci (n = 271) was observed and genotypes were easily distinguishable for all subjects which passed the initial PCR quality control step (Fig. 2).
Fig. 2.
Summary statistics for the PWS QMH assay. The MFI ratios for all tested loci are plotted to illustrate that MFI ratios for various genotypes do not overlap. The mean MFI ratio for deleted loci (pink) was 0.56 ± 0.09 (n = 88) as compared to the MFI ratios for normal loci (blue), 0.96 ± 0.06 (n = 236), and duplicated loci (yellow), 1.44 ± 0.1 (n = 22).
DISCUSSION
This study applies the previously developed QMH assay [Newkirk, patent pending, 2008] for the detection of copy number changes in the PWS region on chromosome 15q11–q13. The use of distinct spectrally encoded microspheres enabled accurate analysis of test and reference probes in a single tube in this study. Using QMH, type I and type II deletions were readily distinguished in a single assay and refined more precisely in a second QMH assay. Smaller deletions within the IC region were also defined. A few subjects evaluated in this study revealed atypical type I and type II deletion intervals, which could represent new classes or sub-classes of deletions, evidence for which has been documented by others [Bittel et al., 2005; Butler et al., 2008]. The QMH assay was able to detect deletions in the IC region in six PWS subjects, one of which was previously classified as having a non-deletion status [Ohta et al., 1999]. This illustrates the utility of QMH in identifying IC defects and limitations in current testing technologies to properly identify IC defects. The identification of an IC deletion is critical from a genetic counseling standpoint with a 50% recurrence risk if the father contains the same IC defect on his mother’s chromosome 15. Hence, proper genetic diagnosis can be useful for quality of life issues, education, clinical management, and for genetic counseling.
This study of the PWS region is the first presentation of a clinical application of the QMH assay. We present this as a suitable diagnostic alternative to other copy number determination methods not requiring context-dependent sequence identification. Other techniques to assess copy number in the same region have been reported and include FISH, Southern hybridization, microarray, aCGH, MLPA, and MAPH. We found that QMH reliably detected copy number differences at a genomic resolution of ≥60 bp in this study, which exceeds the resolution achievable by FISH, Southern hybridization, and aCGH (which is limited by the size and density of cloned probes, [Bejjani and Shaffer, 2004]), and is similar to MAPH and MLPA in probe density [Schouten et al., 2002]. An extension of the QMH method which detects methylation abnormalities is underway (data unpublished), which would enable this technique to detect both types of deletions (type I and type II), IC defects as well as UPD, which none of the aforementioned techniques can accomplish. QMH using unique sequence probes does not require either target DNA amplification or the addition of competitor DNA (i.e., C0t-1 DNA) for the suppression of repetitive sequences. The addition of competitor DNA has been shown to compromise the accuracy and reproducibility of genomic hybridization assays [Newkirk et al., 2005], which would not be ideal for a clinical diagnostic test.
We found QMH easily adaptable to the PWS region, despite the high density of surrounding repetitive sequences and limited number of unique sequence regions available for probe design. Assay optimization was minimal (see Materials and Methods Section) and results were consistently reproducible between samples with similar genotypes with negligible differences between MFI ratios (Fig. 2; 0.96 ± 0.06 for intact loci; 0.56 ± 0.09 for deleted loci, and 1.44 ± 0.1 for duplicated loci). No false positive or false negative results were obtained for samples analyzed in this study as determined by QPCR and the genotypes of all known samples matched those found by QMH. We present this QMH assay as an initial diagnostic test for the high-throughput screening of subjects with PWS. This test allows for the refinement of PWS deletion intervals using subsequent QMH assays, and FISH could be used to confirm the chromosomal context of deletions detected by QMH. Deletions smaller than the probes used in conventional FISH (~100 kb) would need to be confirmed using unique sequence probe cocktails [4–6 kb; Newkirk and Bi, patent pending, 2008] since conventional probes would provide a false negative result for deletion due to their large size. Identification of the genetic subtype in PWS may impact on clinical management and genetic counseling for family members.
Acknowledgments
We kindly thank Danielle Savastano and Nataliya Kibiryeva for their time and involvement in this study.
References
- Bejjani BA, Shaffer LG. A cytogeneticist’s perspective on genomic microarrays. Hum Reprod Update. 2004;10:221–226. doi: 10.1093/humupd/dmh022. [DOI] [PubMed] [Google Scholar]
- Bettio D, Rizzi N, Giardino D, Grugni G, Briscioli V, Selicorni A, Carnevale F, Larizza L. FISH analysis in Prader-Willi and Angelman syndrome patients. Am J Med Genet. 1995;56:224–228. doi: 10.1002/ajmg.1320560222. [DOI] [PubMed] [Google Scholar]
- Bittel DC, Butler MG. Prader-Willi syndrome: Clinical genetics, cytogenetics and molecular biology. Expert Rev Mol Med. 2005;7:1–20. doi: 10.1017/S1462399405009531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittel DC, Kibiryeva N, Talebizadeh Z, Butler MG. Microarray analysis of gene/transcript expression in Prader-Willi syndrome: Deletion versus UPD. J Med Genet. 2003;40:568–574. doi: 10.1136/jmg.40.8.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittel DC, Kibiryeva N, Talebizadeh Z, Driscoll DJ, Butler MG. Microarray analysis of gene/transcript expression in Angelman syndrome: Deletion versus UPD. Genomics. 2005;85:85–91. doi: 10.1016/j.ygeno.2004.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittel DC, Kibiryeva N, Butler MG. Expression of 4 genes between chromosome 15 breakpoints 1 and 2 and behavioral outcomes in Prader-Willi syndrome. Pediatrics. 2006;118:1276–1283. doi: 10.1542/peds.2006-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittel DC, Kibiryeva N, McNulty SG, Driscoll DJ, Butler MG, White RA. Whole genome microarray analysis of gene expression in an imprinting center deletion mouse model of Prader-Willi syndrome. Am J Med Genet Part A. 2007;143A:422–429. doi: 10.1002/ajmg.a.31504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buiting K, Saitoh S, Gross S, Dittrich B, Schwartz S, Nicholls RD, Horsthemke B. Inherited microdeletions in the Angelman and Prader-Willi syndromes define an imprinting centre on human chromosome 15. Nat Genet. 1995;9:395–400. doi: 10.1038/ng0495-395. [DOI] [PubMed] [Google Scholar]
- Butler MG, Bittel DC, Kibiryeva N, Talebizadeh Z, Thompson T. Behavioral differences among subjects with Prader-Willi syndrome and type I or type II deletion and maternal disomy. Pediatrics. 2004;113:565–573. doi: 10.1542/peds.113.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Fischer W, Kibiryeva N, Bittel DC. Array comparative genomic hybridization (aCGH) analysis in Prader-Willi syndrome. Am J Med Genet Part A. 2008;146A:854–860. doi: 10.1002/ajmg.a.32249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coder DM, Redelman D, Vogt RF. Computing the central location of immunofluorescence distributions: Logarithmic data transformations are not always appropriate. Cytometry. 1994;18:75–78. doi: 10.1002/cyto.990180204. [DOI] [PubMed] [Google Scholar]
- Delach JA, Rosengren SS, Kaplan L, Greenstein RM, Cassidy SB, Benn PA. Comparison of high resolution chromosome banding and fluorescence in situ hybridization (FISH) for the laboratory evolution of Prader-Willi syndrome and Angelman syndrome. Am J Med Genet. 1994;52:85–91. doi: 10.1002/ajmg.1320520117. [DOI] [PubMed] [Google Scholar]
- Dunbar S, Godbout R, Newkirk H, Hetzel J. Microsphere suspension array technology for SNP detection in cattle. IEEE Eng Med Biol Mag, July/August 2003. 2003;22:158–162. doi: 10.1109/memb.2003.1237526. [DOI] [PubMed] [Google Scholar]
- Kirkwood TBL. Geometric means and measures of dispersion [Letter] Biometrics. 1979;35:908–909. [Google Scholar]
- Kuwano A, Mutirangura A, Dittrich B, Buiting K, Horsthemke B, Saitoh S, Niikawa N, Ledbetter SA, Greenberg F, Chinault AC. Molecular dissection of the Prader-Willi/Angelman syndrome region (15q11-13) by YAC cloning and FISH analysis. Hum Mol Genet. 1992;1:417–425. doi: 10.1093/hmg/1.6.417. [DOI] [PubMed] [Google Scholar]
- Locke DP, Segraves R, Nicholls RD, Schwartz S, Pinkel D, Albertson DG, Eichler EE. BAC microarray analysis of 15q11-q13 rearrangements and the impact of segmental duplications. J Med Genet. 2004;41:175–182. doi: 10.1136/jmg.2003.013813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapendano CK, Kishino T, Miyazaki K, Kondo S, Yoshiura KI, Hishikawa Y, Koji T, Niikawa N, Ohta T. Expression of the Snurf-Snrpn IC transcript in the oocyte and its putative role in the imprinting establishment of the mouse 7C imprinting domain. J Hum Genet. 2006;51:236–243. doi: 10.1007/s10038-005-0351-8. [DOI] [PubMed] [Google Scholar]
- Newkirk H. Genomic Copy Number Determination Using Microsphere-based Suspension Hybridization. Children’s Mercy Hospitals and Clinics; 2008. USPTO Serial no. 60/708,734. [Google Scholar]
- Newkirk H, Bi C. Novel process for identification of unique sequence regions in genomic DNA: Unique Genomic Sequence Hunter. Children’s Mercy Hospital and Clinics; 2008. USPTO Serial no. 12/058,659. [Google Scholar]
- Newkirk H, Knoll JHM, Rogan PK. Distortion of quantitative genomic and expression hybridization by Cot-1 DNA: Mitigation of this effect. Nucleic Acid Res. 2005;33:191. doi: 10.1093/nar/gni190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newkirk H, Miralles M, Rogan PK, Knoll JHM. Determination of genomic copy number with quantitative microsphere hybridization. Hum Mut. 2006;27:376–386. doi: 10.1002/humu.20312. [DOI] [PubMed] [Google Scholar]
- Nicholls RD, Saitoh S, Horsthemke B. Imprinting in Prader-Willi and Angelman syndromes. Trends Genet. 1998;14:194–200. doi: 10.1016/s0168-9525(98)01432-2. [DOI] [PubMed] [Google Scholar]
- Nygren AOH, Ameziane N, Duarte HMB, Vijzelaar RNCP, Waisfisz Q, Hess CJ, Schouten JP, Errami A. Methylation-specific MLPA (MS-MLPA): Simultaneous detection of CpG methylation and copy number changes of up to 40 sequences. Nucleic Acid Res. 2005;33:128. doi: 10.1093/nar/gni127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T, Gray TA, Rogan PK, Buiting K, Gabriel JM, Saitoh S, Muralidhar B, Bilienska B, Krajewska-Walasek M, Driscoll DJ, Horsthemke B, Butler MG, Nicholls RD. Imprinting-mutation mechanisms in Prader-Willi syndrome. Am J Hum Genet. 1999;64:397–413. doi: 10.1086/302233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp AK. Advances in fluorescence in situ hybridization. Mutat Res. 1998;400:287–298. doi: 10.1016/s0027-5107(98)00029-3. [DOI] [PubMed] [Google Scholar]
- Sahoo T, Shaw CA, Young AS, Whitehouse NL, Schroer RJ, Stevenson RE, Beaudet AL. Array-based comparative genomic hybridization analysis of recurrent chromosome 15q rearrangements. Am J Med Genet Part A. 2005;139A:106–113. doi: 10.1002/ajmg.a.31000. [DOI] [PubMed] [Google Scholar]
- Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acid Res. 2002;30:57. doi: 10.1093/nar/gnf056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankiewicz P, Beaudet A. Use of array CGH in the evaluation of dysmorphology, malformations, developmental delay, and idiopathic mental retardation. Curr Opin Genet Dev. 2007;17:182–192. doi: 10.1016/j.gde.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Zarcone J, Napolitano D, Peterson C, Breidbord J, Ferraioli S, Caruso-Anderson M, Holden L, Butler MG, Thompson T. The relationship between compulsive behaviour and academic achievement across the three genetic subtypes of Prader-Willi syndrome. J Intellect Disabil Res. 2007;51:478–487. doi: 10.1111/j.1365-2788.2006.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]