Abstract
Background
Histopathological features in morphea (localized scleroderma) and their clinical correlates are poorly described.
Objective
Systematically describe histologic changes of morphea in a large, well annotated cohort and determine the association between histopathology and clinical features.
Methods
Cross-sectional study of 83 patients enrolled in the Morphea in Adults and Children (MAC) cohort. The main outcome measure was the association of microanatomical location and degree of sclerosis and inflammation seen on histologic samples with patient reported symptoms and physician-based measures of severity.
Results
Pattern of sclerosis was associated with morphea subtype, the presence of patient reported symptoms, and functional limitation. A bottom heavy pattern of sclerosis was associated with pain and tightness (P = 0.0039, 0.001 respectively). These symptoms were not associated with a top heavy pattern. Severe inflammation may be associated with pain and functional limitation (p = 0.073 for both).
Limitations
Small sample size limits ability to detect associations, particularly in subgroups.
Conclusions
Histopathological examination of morphea may assist in identifying patients who may require additional monitoring and treatment. Features such as patterns of sclerosis and severity of inflammation should be included in pathology reports to help aid in clinical management.
Keywords: Morphea, symptoms, pathology, sclerosis, severity, pain
INTRODUCTION
Morphea (localized scleroderma) is characterized by thickening of the skin resulting from inflammation and the deposition of collagen rich extracellular matrix. Although the demographic and clinical features of morphea have been the focus of many recent publications, the histological features remain poorly described.
Histopathological features of morphea may be a readily accessible aid in evaluating patients with morphea in addition to establishing the diagnosis. Existing studies are limited by reliance on very few specimens (in some cases 1–2 patients) with relatively scant clinical information, making clinicopathologic correlation difficult.1–4 Although the validation of new outcome measures in morphea are valuable, skin biopsy may provide additional information regarding depth of involvement and activity (inflammation) in cases where clinical examination is inconclusive and ultrasound or MRI are not readily available.5–8 Studies that systematically describe the histological features of morphea in a well characterized cohort of patients are needed to provide correlation between clinical and histological findings. This would allow practitioners to utilize histological in addition to clinical findings as an aid in decision making.9–11
The present study, referred to as the MAC cohort (Morphea in Adults and Children), was designed to examine demographic, clinical, and histological features in a thoroughly-phenotyped cohort of participants with morphea. By studying patients in a prospective cross sectional manner, we aimed to define the histological attributes of morphea and determine their association with clinical features.
METHODS
Patients and Procedures
The institutional review board-approved MAC Cohort contained 229 adults and children (18 years or older and 3–17 years old at enrollment, respectively) at the time of this study (details previously published).12 After consent, all data were abstracted using a case report form and medical records were reviewed.
Inclusion criteria were: enrollment in the MAC cohort, skin biopsy performed at enrollment or within 3 months of enrollment, and determination of relevant clinical variables within 3 months of the biopsy (with no intervening treatment). All patients were examined by a single physician with expertise in morphea (HJ) who assigned subtype and clinical scores. Clinical subtypes included circumscribed, linear, generalized, profunda, and morphea with lichen sclerosus features (as previously defined). 13, 14 Two generalized morphea subgroups were assigned including isomorphic (lesions occurring in areas of friction: waistline, etc; more common in older, post-menopausal women)15 and symmetrical morphea (lesions arranged symmetrically about the anatomical midline.
At the time of enrollment, 4.0 mm punch biopsies were performed at the erythematous border in inflammatory lesions, or central sclerosis of lesions without clinical inflammation (Figure 1).7 If biopsies were performed off site by the referring provider, these slides were obtained and examined in the same manner as specimens obtained by the PI. In addition, patients with biopsies done prior to enrollment were included only if their biopsy site was clearly identifiable by examination. Photos were taken to note the site of biopsy (Figure 1) for all enrolled patients. All specimens were fixed in formalin and stained with hematoxylin and eosin (H&E).
Figure 1.
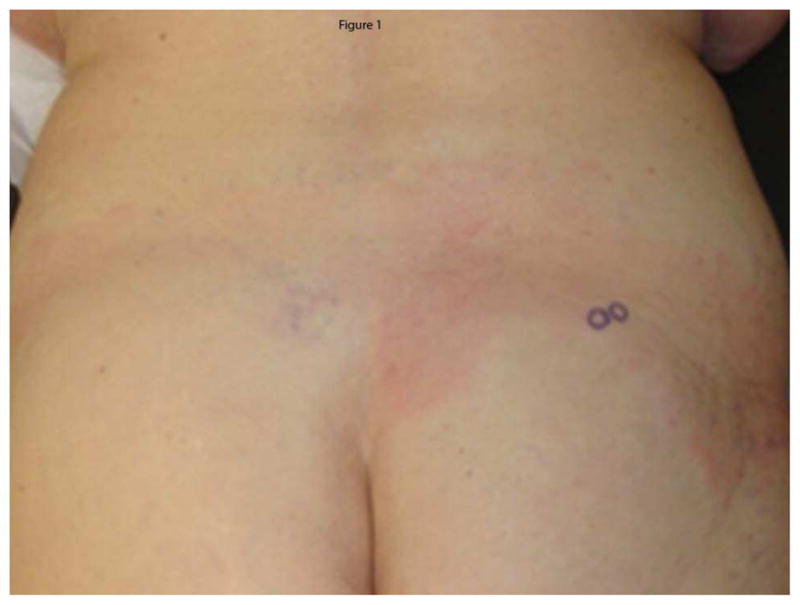
Erythematous border. Representative photograph demonstrating biopsy of erythematous border (arrow) with photograph to record biopsy site
Clinical Variables
The MAC database contains the following domains: demographic, clinical, physician based determinants of disease severity (modified Rodnan Skin Score [mRSS], functional status (presence/absence of limited range of motion, contracture, limb length discrepancy related to cutaneous morphea lesions), and patient based assessment of disease related symptoms as represented on a 10 point visual analog scale (pain, pruritus, subjective tightness of lesions). At the inception of the cohort there were no validated clinical outcome measures for morphea. Although not validated in morphea, the mRSS was selected as a measure of clinical severity because of its validation in systemic sclerosis and its use in published morphea studies at the time.16 The newly validated Localized Scleroderma Cutaneous Assessment Tool (LoSCAT) was administered to patients after 2010.7 Too few patients had LoSCAT scores for meaningful analysis in this study.
Histopathological Examination
All specimens were examined at Cockrell Dermatopathology Laboratories, Dallas, Texas, by the same blinded, board-certified dermatopathologist (JS). Twenty-five slides were randomly selected for blinded examination by a second board-certified dermatopathologist (LK) utilizing the same protocol as the primary dermatopathologist.
Histology was examined with an Olympus BX45 microscope and observations were recorded for the severity and microanatomic location of sclerosis and inflammation. Assessment of severity of sclerosis included a previously published global sclerosis score (GSS) assessing extent of histologic sclerosis (severe, moderate, or mild).10 The pattern of sclerosis was determined based on the microanatomic location of hyalinized collagen. A top-heavy pattern (Figure 2a) was defined by hyalinized collagen bundles exclusively in the papillary to superficial reticular dermis with an absence of these changes below. A bottom-heavy pattern was defined by hyalinized collagen bundles present in the deep dermis and subcutis and sparing of the papillary through mid dermis (Figure 2b), and a full-thickness pattern had thickened collagen bundles throughout the dermis. The degree of inflammation was graded as mild (mild perivascular infiltrate), moderate (dense perivascular infiltrate), or severe (dense infiltrate of inflammatory cells that may be located in a perivascular, periadnexal, and/or interstitial location or at the dermal and subcutaneous junction in which the density of the inflammatory cell infiltrate is such that it takes on the appearance of a round “nodule” of inflammatory cells. Additional assessment included documenting cell types present (plasma cells, lymphocytes, and eosinophils), considered “present” if >5 were seen at low power. Location of the inflammatory infiltrate was determined by: perivascular (> 10 cells aggregated around capillaries), periadnexal (> 10 cells aggregated around adnexal structures), interstitial, dermal subcuticular junction, septal, or lobular.
Figure 2.
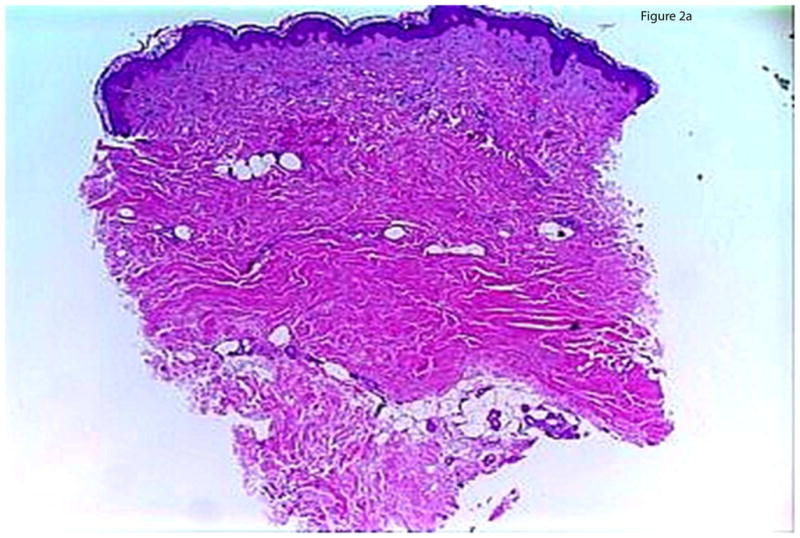
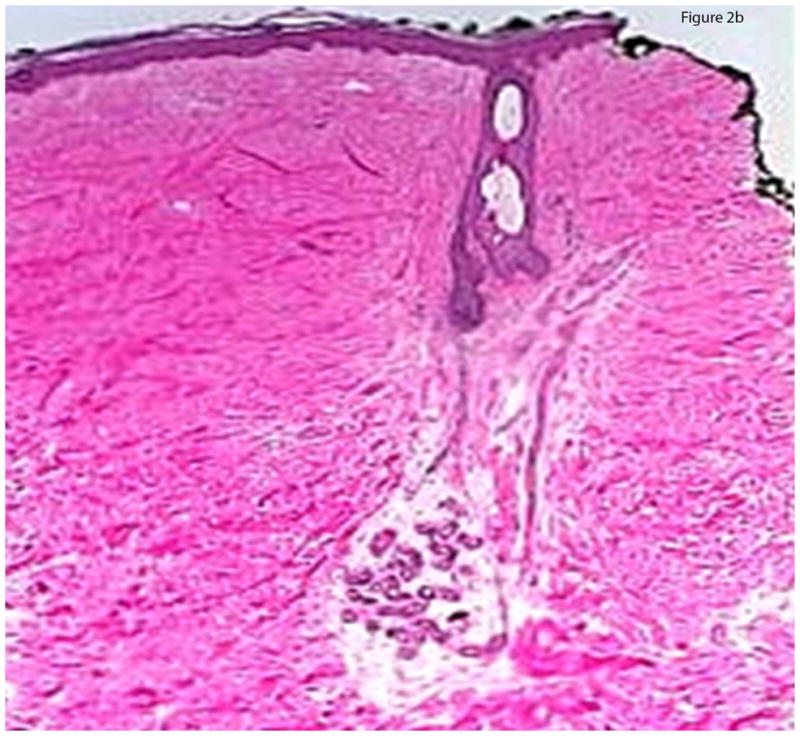
Figure 2a: Top-heavy morphea. Sclerosis is moderate to severe in the papillary and superficial reticular dermis, while the mid and deep dermis is relatively spared.
Figure 2b: Bottom-heavy morphea. Sclerosis is moderate to severe in the mid and deep dermis, while the superficial and papillary dermis is relatively spared.
Statistical Analysis
Data were tabulated using descriptive statistics, frequency tables, and frequency distributions. Fisher’s Exact Test was used to test the association between morphea subtype and clinical characteristics with GSS, pattern of sclerosis (top heavy, bottom heavy, throughout), degree of inflammation (mild, moderate, severe), and cell type. A kappa score was calculated to assess agreement between dermatopathologists. Values greater than 0.6 were considered acceptable. A p-value <0.05 was assigned for statistical significance for all analyses. GraphPad Prism software, version 5.04. La Jolla, CA, (http://www.graphpad.com/prism/prism.htm) statistical analysis software was used.
RESULTS
Of the 229 patients in the cohort, 83 met enrollment criteria. Excluded patients were children who were not biopsied as part of enrollment (the children in the present study were biopsied by their referring dermatologist n=9) or adults who declined biopsy at enrollment (n=137). Those who declined were similar in age, race, and gender as those who were biopsied. Additionally, cosmetically sensitive sites (the face) and those prone to poor wound healing (anterior tibia and the like) were not biopsied for patient safety (N=12). Ninety-one skin biopsies were obtained from 83 patients. Of the 91 biopsies taken, 71 included the subcutis. The demographic and clinical features of the 83 patients in this study are available in Table I.
Table I.
Patient Characteristics
| Gender, n (%) | |
|
| |
| Male | 17 (20) |
| Female | 66 (80) |
|
| |
| Ethnicity, n (%) | |
| White | 63 (76) |
| Hispanic/Latino | 8 (10) |
| African American | 4 (5) |
| Other | 8 (10) |
|
| |
| Age mean, (SD) | 41 (21) |
|
| |
| Age of Onset, mean, (SD) | 37 (21) |
|
| |
| Duration of disease (years), mean (SD) | 4 (5) |
|
| |
| Morphea Clinical Subtype, n (%) * | |
| Generalized | 35 (49) |
| Linear | 26 (36) |
| Circumscribed | 11 (15) |
| Profunda | 13 (14) |
| LSA overlap | 7 (8) |
Total
HISTOLOGICAL FEATURES
Microanatomical location of sclerosis
Microanatomical locations are in Table II. Thirty-four percent of specimens (31/91) had a top-heavy sclerosis pattern, 22/91 (24%) specimens had full-thickness sclerosis, and 39/91 (43%) had a bottom heavy pattern. Microanatomical location of sclerosis was analyzed relative to clinical subtype. Specimens from patients with morphea profunda (defined as involvement of the subcutis or deeper on direct pathological examination or MRI/ultrasound imaging of the affected area) had bottom heavy or full thickness patterns of sclerosis in the dermis (13/13, p=0.012). A bottom heavy or full thickness sclerosis pattern also predominated in specimens from symmetrical generalized morphea (10/12, p=0.025). A top-heavy sclerosis pattern was more common in patients who had clinical features of lichen sclerosus accompanying morphea (6/7, p=0.0058). Specimens from linear and plaque morphea specimens exhibited all 3 patterns of sclerosis with similar frequency (there was no statistical difference in the frequency of different patterns of sclerosis).
Table II.
Distribution of pattern of sclerosis according to morphea subtype. Top heavy is defined as hyalinized collagen bundles exclusively in the papillary/reticular dermis. Bottom heavy was defined as sparing of papillary to mid dermis with hyalinized collagen bundles present in the deep dermis and subcutis. Full thickness was defined as hyalinized collagen bundles throughout the dermis and extending into subcutis. Numbers are presented as frequency and percent in parentheses. Significant associations between sclerosis pattern and subtype are indicated by *.
| Plaque | Linear | Generalized | Profunda | LSA | Total | |
|---|---|---|---|---|---|---|
| Top heavy | 3 (27.2) | 10 (38.5) | 12 (32.4) | 0 | 6* (85.7) | 31 (33.7) |
| Bottom heavy | 4 (36.4) | 5 (19.2) | 19 (54.3) | 10 * (76.9) | 1 | 38 (42.4) |
| Full thickness | 4 (36.4) | 11 (42.3) | 4 (11.4) | 3 * (23.1) | 0 (14.3) | 22 (23.9) |
Inflammatory Cell Infiltrate
Details of inflammatory cell infiltrate are available in Table III. The majority of specimens had mild (43/91, 47%), to moderate inflammation (31/91, 34%). Degree of inflammation was equally distributed among all subtypes (no statistically significant difference in frequency). When inflammation was present, lymphocytes were the predominant cell type (83/91, 91%). Plasma cells were the second most common cell type present (68/91, 75%). Nineteen of 91 (21%) specimens had eosinophils. Inflammatory cells were present in the superficial and deep perivascular dermis (89/91, 97% 82/91, 90%). The dermal-subcutaneous interface (58/91, 64%), and periadnexal areas (59/91, 65%) were also highly enriched with inflammatory cell infiltrate. Inflammatory cells were often found along the dermal-subcutaneous interface when bottom-heavy and full-thickness sclerosis was present (41/61, 67.2%). Sclerosis and inflammation (defined as presence of mild, moderate to severe inflammation, and any sclerosis [top/bottom heavy or full thickness]) occurred simultaneously in 46.2% (42/91).
Table III.
Inflammation grade (none, mild, moderate, severe) and cell types observed, by morphea subtype. Numbers are presented as frequency and percent in parentheses. There were no statistically significant associations between degree of inflammation, cell type and morphea subtype.
| Plaque | Linear | Generalized | Profunda | LSA | Total | |
|---|---|---|---|---|---|---|
| Inflammation | ||||||
|
| ||||||
| None | 1 (9.1) | 2 (7.7) | 2 (5.9) | 3 (23.1) | 0 | 8 (8.88) |
| Mild | 3 (27.3) | 11 (42.3) | 21 (61.8) | 3 (23.1) | 5 (71.4) | 43 (47.3) |
| Moderate | 4 (36.4) | 10 (38.5) | 10 (29.4) | 5 (38.5) | 2 (28.6) | 31 (34.1) |
| Severe | 3 (27.3) | 3 (11.5) | 1 (2.9) | 2 (15.4) | 0 | 9 (9.9) |
|
| ||||||
| Cell Type Present | ||||||
|
| ||||||
| Lymphocytes | 10 (90.9) | 24 (92.3) | 32 (94.1) | 10 (76.9) | 7 (100) | 83 (91.2) |
| Plasma cells | 10 (90.9) | 22 (84.6) | 22 (64.7) | 8 (61.5) | 6 (85.7) | 68 (74.7) |
| Eosinophils | 2 (18.2) | 6 (23.1) | 5 (14.7) | 4 (30.8) | 2 (28.6) | 19 (20.9) |
CLINICOPATHOLOGIC ASSOCIATION
Table IV summarizes the results of clinicopathologic association analyses for pattern of sclerosis.
Table IV.
Clinicopathologic association for sclerosis. Association between degree of sclerosis and presence of patient reported symptoms of pain, itch/numbness, and tightness as well as physician assessed functional limitation were determined using Fisher’s Exact Test. Asterisks indicate statistically significant associations with a p value <0.05. Numbers are presented as frequency and proportion.
|
|
|||||||
|---|---|---|---|---|---|---|---|
| Pattern of Sclerosis
| |||||||
| Top Heavy | Bottom Heavy | Full Thickness | |||||
| Patient Perceived Symptoms | Frequency | Proportion | Frequency | Proportion | Frequency | Proportion | |
| Pain | Present | 6* | 0.25* | 23* | 0.62* | 8 | 0.33 |
| Absent | 18* | 0.75* | 14 | 0.38 | 16 | 0.67 | |
|
|
|||||||
| Pruritus | Present | 13 | 0.54 | *27 | *0.73 | 14 | 0.58 |
| Absent | 11 | 0.46 | 10 | 0.27 | 10 | 0.42 | |
|
|
|||||||
| Tightness | Present | 4* | 0.17* | 20* | 0.56* | 5 | 0.22 |
| Absent | 20* | 0.83* | 16 | 0.44 | 18 | 0.78 | |
|
| |||||||
| Physician Assessed | Frequency | Proportion | Frequency | Proportion | Frequency | Proportion | |
| Functional Limitation | Present | 6 | 0.25 | *14 | *0.38 | 4 | 0.17 |
| Absent | 18 | 0.75 | 23 | 0.62 | 20 | 0.83 | |
Clinicopathologic Association: Pattern of Sclerosis
Patient perceived pain and tightness were more common in specimens with bottom-heavy sclerosis (23/37, 62%, p=0.0039, 20/36, 56%, p=0.001 respectively). Patients with specimens with a top-heavy sclerosis pattern reported pain and tightness in their lesions less frequently than those with a bottom heavy pattern of sclerosis (6/24, 25%, p=0.05 and 4/24, 17%, p=0.04 respectively).
Clinicopathologic Association: Degree of Inflammation
Of the specimens with severe inflammation on biopsy, there was a trend toward increased presence of pain and functional limitation versus those with little or no inflammation (6/8 75%, p=0.073 had painand5/8, 63% ,p=0.073 had functional impairment).
Clinicopathologic Association: Global Sclerosis Score
There were no significant associations between global sclerosis scores and any clinical variables. Functional limitation and deep involvement were more frequent in grade 3 sclerosis but failed to reach significance.
MEASURES OF AGREEMENT
Interobserver correlation studies of global sclerosis scores yielded an interuser agreement ratio of 87.5%, k=0.755; degree of inflammation interuser agreement ratio=80.0%, k=0.706, sclerosis pattern interuser agreement ratio=75%, k=0.602.
DISCUSSION
To date, few studies have systematically examined the histopathological findings in morphea using predetermined clinical and histological criteria. This cross sectional study was designed to address this deficit. We found that specific histological characteristics were associated with morphea subtype and patient reported symptoms.
Results of the present study are similar to prior reports describing the presence of sclerosis at any level of the dermis including the superficial papillary dermis extending into the panniculus producing septal sclerosis in morphea.1–3 We confirmed the findings of McNiff, et al that morphea may be limited to the superficial reticular dermis.4 In fact, this occurred in 34% of our specimens, which we termed a top heavy sclerosis pattern. Unique to this study, we found that all patterns of sclerosis occur with nearly equal frequency in morphea overall indicating that the term “morphea profunda” may not be indicative of a distinct morphea subtype, but rather reflect a finding that may occur in linear, plaque, and generalized subtypes.
We also examined the relationship between the pattern of sclerosis and morphea subtypes and symptoms. The three microanatomic locations of sclerosis (top heavy, bottom heavy, and throughout) were present in all morphea subtypes, but in different frequencies. In the case of linear and circumscribed subtypes, all 3 patterns were present with equal frequency. Not unexpectedly, morphea profunda predominated with deep or throughout patterns while patients with lichen sclerosus overlap displayed top heavy sclerosis. We found two patterns of sclerosis dominated in patients who met criteria for generalized morphea. In one subset, generalized symmetrical morphea, bottom heavy sclerosis predominated. In patients with isomorphic morphea (which our group described previously) top heavy patterns of sclerosis predominated.15 Isomorphic lesions occur in sights of chronic friction (waistband area). The superficial distribution of histological changes in isomorphic morphea further supports chronic superficial trauma in the pathogenesis of these lesions. Moreover, the presence of a bottom heavy, or deeper, pattern of sclerosis was associated with the presence of symptoms like pain, tightness, and functional impairment in patients with all subtypes, while patients with top heavy sclerosis patterns had less frequent pain, tightness, and functional impairment. Prior reports indicate perineural inflammation is present in morphea.2, 3 The presence of deep sclerosis, in some cases perineurally, may account for the presence of pain. Taken together, these results indicate that the pattern of sclerosis, not clinical subtype alone, may predict the clinical severity of morphea. This may account for the clinical heterogeneity of morphea which ranges from mild cosmetic impairment to extreme pain and disability even within the same subtype.17–19 This supports the utilization of the pattern of sclerosis as a clinical indicator in addition to subtype and distribution of lesions in evaluation.
We also examined the characteristics of the inflammatory cell infiltrate in morphea. Our results confirm those of Fleischmajer, et al in which 84% of 43 patients with morphea had lymphoplasmacytic infiltrates present both in the dermis and subcutis.1, 2 We found lymphocytes in 91.2% of our specimens and plasma cells in 74.7% of specimens. The presence of plasma cells in addition to lymphocytes in 75% of specimens is congruent with current theories of the mechanism of fibrosis implicating a Th2 dominated immune response.20, 21 Skewed activation of the adaptive immune response producing a Th2 dominant pattern of cytokine release is thought to be a key mediator of fibrosis and is associated with antibody production by plasma cells. Although prior studies2 implicate a stepwise progression from inflammatory to sclerotic phases in morphea, inflammation and sclerosis were simultaneously present in this series. This implies that some patients with morphea have ongoing inflammation driving prolonged periods of sclerosis.20, 21
Limitations of the present study include low number of specimens especially when examining association between specific clinical and histological features and lack of subcutaneous tissue in 20 of the specimens. Also, some biopsies were performed prior to clinical assessment by the morphea expert who evaluated each patient in the cohort. Every attempt was made to enhance the validity of these findings by only including patients who had biopsies done within 3 months of clinical assessment, who did not have intervening treatment, and where the biopsy site was identifiable and was taken from the inflammatory border of lesions. Lesion duration was not ascertained as it is prone to recall bias. Additionally, disease duration has not been found to correlate with severity of individual lesions in morphea patients.19 Additional limitations include lack of biopsies from pediatric patients and those with lesions in cosmetically sensitive sites or areas prone to poor wound healing (as histological features may differ in these sites). This also underscores that use of skin biopsy for evaluation may be limited in these clinical scenarios.
This study has several implications for practice. First, pathological findings, particularly pattern of sclerosis and degree of inflammation, have a role in patient evaluation and may function as an adjunct to subtype determination and sites of involvement for determining the probability of significant symptoms or functional impairment. Thus, biopsy in morphea may not just be indicated for diagnosis, but also assessment. Consequently, pathology reports should include descriptions of these findings. In turn, clinicians should be aware that the presence of a bottom heavy pattern of sclerosis and severe inflammation may indicate the patient is at increased risk for morphea related symptoms and functional impairment. In the context of other clinical findings, this should prompt frequent monitoring and initiation of aggressive treatment (systemic immunosuppressives), particularly in those with active generalized or linear morphea.
Acknowledgments
Rose Ann Cannon, Travis Vandergriff, MD
Funding/Support: This work was conducted with support from UT-STAR, NIH/NCRR/NCATS Grant Number UL1RR024982. The content is solely the responsibility of the authors and does not necessarily represent the official views of UT-STAR, The University of Texas Southwestern Medical Center at Dallas and its affiliated academic and health care centers, the National Center for Research Resources, or the National Institutes of Health.
Research for this manuscript was supported in part by NIH Grant No. K23AR056303-4.
ABBREVIATIONS & ACRONYMS
- MAC
Morphea in Adults and Children
- LoSCAT
Localized Scleroderma Cutaneous Assessment Tool
- GSS
Global Sclerosis Score
Footnotes
Conflicts of Interest: None
IRB Approval: UT Southwestern Medical Center
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Torres JE, Sanchez JL. Histopathologic differentiation between localized and systemic scleroderma. Am J Dermatopathol. 1998;20:242–5. doi: 10.1097/00000372-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Fleischmajer R, Nedwich A. Generalized morphea. I. Histology of the dermis and subcutaneous tissue. Arch Dermatol. 1972;106:509–14. doi: 10.1001/archderm.106.4.509. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery H, O'Leary PA, Ragsdale WE., Jr Dermatohistopathology of various types of scleroderma. AMA Arch Derm. 1957;75:78–87. doi: 10.1001/archderm.1957.01550130080008. [DOI] [PubMed] [Google Scholar]
- 4.McNiff JM, Glusac EJ, Lazova RZ, et al. Morphea limited to the superficial reticular dermis: an underrecognized histologic phenomenon. Am J Dermatopathol. 1999;21:315–9. doi: 10.1097/00000372-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Li SC, Liebling MS, Haines KA, et al. Initial evaluation of an ultrasound measure for assessing the activity of skin lesions in juvenile localized scleroderma. Arthritis care & research. 2011;63:735–42. doi: 10.1002/acr.20407. [DOI] [PubMed] [Google Scholar]
- 6.Nezafati KA, Cayce RL, Susa JS, et al. 14-MHz ultrasonography as an outcome measure in morphea (localized scleroderma) Arch Dermatol. 2011;147:1112–5. doi: 10.1001/archdermatol.2011.243. [DOI] [PubMed] [Google Scholar]
- 7.Arkachaisri T, Vilaiyuk S, Torok KS, et al. Development and initial validation of the localized scleroderma skin damage index and physician global assessment of disease damage: a proof-of-concept study. Rheumatology (Oxford) 2010;49:373–81. doi: 10.1093/rheumatology/kep361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horger M, Fierlbeck G, Kuemmerle-Deschner J, et al. MRI findings in deep and generalized morphea (localized scleroderma) AJR Am J Roentgenol. 2008;190:32–9. doi: 10.2214/AJR.07.2163. [DOI] [PubMed] [Google Scholar]
- 9.Kissin EY, Merkel PA, Lafyatis R. Myofibroblasts and hyalinized collagen as markers of skin disease in systemic sclerosis. Arthritis Rheum. 2006;54:3655–60. doi: 10.1002/art.22186. [DOI] [PubMed] [Google Scholar]
- 10.Verrecchia F, Laboureau J, Verola O, et al. Skin involvement in scleroderma--where histological and clinical scores meet. Rheumatology (Oxford) 2007;46:833–41. doi: 10.1093/rheumatology/kel451. [DOI] [PubMed] [Google Scholar]
- 11.Furst DE, Clements PJ, Steen VD, et al. The modified Rodnan skin score is an accurate reflection of skin biopsy thickness in systemic sclerosis. J Rheumatol. 1998;25:84–8. [PubMed] [Google Scholar]
- 12.Johnson W, Jacobe H. Morphea in adults and children cohort II: Patients with morphea experience delay in diagnosis and large variation in treatment. J Am Acad Dermatol. 2012;67:881–9. doi: 10.1016/j.jaad.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Laxer RM, Zulian F. Localized scleroderma. Curr Opin Rheumatol. 2006;18:606–13. doi: 10.1097/01.bor.0000245727.40630.c3. [DOI] [PubMed] [Google Scholar]
- 14.Rahbari H. Histochemical differentiation of localized morphea-scleroderma and lichen sclerosus et atrophicus. J Cutan Pathol. 1989;16:342–7. doi: 10.1111/j.1600-0560.1989.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 15.Grabell D, Hsieh C, Andrew R, et al. The role of skin trauma in the distribution of morphea lesions: A cross-sectional survey of the Morphea in Adults and Children cohort IV. J Am Acad Dermatol. 2014 doi: 10.1016/j.jaad.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clements PJ, Lachenbruch PA, Seibold JR, et al. Skin thickness score in systemic sclerosis: an assessment of interobserver variability in 3 independent studies. J Rheumatol. 1993;20:1892–6. [PubMed] [Google Scholar]
- 17.Zulian F, Athreya BH, Laxer R, et al. Juvenile localized scleroderma: clinical and epidemiological features in 750 children. An international study Rheumatology (Oxford) 2006;45:614–20. doi: 10.1093/rheumatology/kei251. [DOI] [PubMed] [Google Scholar]
- 18.Fett N, Werth VP. Update on morphea: part II. Outcome measures and treatment. J Am Acad Dermatol. 2011;64:231–42. doi: 10.1016/j.jaad.2010.05.046. quiz 43–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saxton-Daniels S, Jacobe HT. An evaluation of long-term outcomes in adults with pediatric-onset morphea. Arch Dermatol. 2010;146:1044–5. doi: 10.1001/archdermatol.2010.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wynn TAR, TR Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nature Medicine. 2012;18:1028–40. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurzinski K, Torok KS. Cytokine profiles in localized scleroderma and relationship to clinical features. Cytokine. 2011;55:157–64. doi: 10.1016/j.cyto.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


