Abstract
There is at present a huge disconnect between levels of funding for basic research on fundamental mechanisms of biological aging and, given demographic projections, the anticipated enormous social and economic impacts of a litany of chronic diseases for which aging is by far the major risk factor: One valuable approach, recently instigated by Felipe Sierra & colleagues at the US National Institute on Aging, is the development of a Geroscience Interest Group among virtually all of the NIH institutes. A complementary approach would be to seek major escalations of private funding. The American Federation for Aging Research, the Paul Glenn Foundation and the Ellison Medical Foundation pioneered efforts by the private sector to provide substantial supplements to public sources of funding. It is time for our community to organize efforts towards the enhancements of such crucial contributions, especially in support of the emerging generation of young investigators, many of whom are leaving our ranks to seek alternative employment. To do so, we must provide potential donors with strong economic, humanitarian and scientific rationales. An initial approach to such efforts is briefly outlined in this manuscript as a basis for wider discussions within our community.
Keywords: Animal models of Alzheimer's disease; Geroscience; Research funding; Comparative gerontology; Healthspan/lifespan ratios; Developmental biology, intergenerational and transgenerational inheritance; Stem cell niches; Young plasma, somatic cell genetics; Induced pleuripotent stem cells; Antigeroid alleles; Epigenetic drift; Pet dogs
1. Introduction
Recent paylines for R01 project grant applications submitted to the National Institute on Aging have been as low as the 7th percentile (https://www.nia.nih.gov/research/blog/2013/12/nia-interim-payline-update). While new investigators are given additional points for the scores at these initial reviews, they have considerable difficulty in achieving fundable scores for their first renewals; many will therefore be seeking alternative careers. Fortunately, applications with a focus on Alzheimer's disease (AD) have had a significantly greater chance of being funded https://www.nia.nih.gov/research/dea/nia-funding-line-policy-fy-2016).While there are many compelling reasons for Congress to have given additional funding for AD, we have to keep making the case that, like AD (or, perhaps, several forms of AD) (Martin, 2005b), essentially all of the chronic diseases of aging are driven by fundamental pathogenetic mechanisms of aging.
Research devoted to the elucidation of these basic mechanisms should therefore also have a congressional mandate. This is the guiding principal of Jay Olshansky's “Longevity Dividend” (Olshansky, 2016, Miller, 2002, Martin et al., 2003).
The landmark creation of the NIH-wide Geroscience Interest Group by Felipe Sierra and his colleagues (Burch et al., 2014) was a major step towards getting this message across – not just for AD, but for essentially all of the many disorders of aging that limit human healthspans and are largely responsible for estimates that, by mid-century, assuming a continuation of comparable rates of growth here in the United States (rates that are greater than those of 17 other developed nations), the percent of our gross national product devoted to health care will have risen from the present level of ~17% to ~25–30% by mid-century (Martin, 2016). That rate of growth is likely to be unsustainable, a powerful argument for aggressive implementation of research programs aimed at increasing human healthspans, hopefully in the form of increased ratios of Healthspans/Lifespans. The purpose of this manuscript is to encourage members of our community to accelerate our efforts to seek additional sources of private funding for basic and translational research that will achieve that goal. In presenting our case to potential donors, we should point out areas of research that are particularly compelling and their impacts upon quality of life and the economy. Like all domains of science, such selected foci are “moving targets” that require revisions due to new theoretical insights and unanticipated discoveries. Nevertheless, the author will review his own current selections in the hope that this will motivate others to argue for alternative or additional high priority areas of research. Colleagues who attended my keynote lecture in Bregenz will observe some interesting differences in emphasis and organization, a healthy sign of flexibility as I approach my own state of advanced aging!
2. Multiple chronic diseases of aging can begin in middle age, when the force of natural selection has waned
The general public, including potential donors, often view our enterprise as being exclusively concerned with “GeriatricMedicine” – i.e., patients who are typically past the age of 65, often retired and eligible for Medicare. As noted above, this subset of human populations is undergoing unprecedented rates of growth, with vast increases in the proportion who are over 85 years of age (http://www.un.org/esa/population/publications/worldageing19502050/).
Remarkably, centenarians are undergoing the highest rates of growth within these populations (http://www.pewresearch.org/fact-tank/2016/04/21/worlds-centenarian-population-projected-to-grow-eightfold-by-2050/). While most physicians are very aware of the increasing proportions of subjects suffering from multimorbidity in aging populations (Barnett et al., 2012), the absolute numbers of such patients have been found to be significantly greater among those younger than age 65 (Barnes, 2015). This result is to be expected, given the classical evolutionary biological theory of aging as developed by Haldane, Medawar, Hamilton, Williams and Kirkwood, among others (recently reviewed by Kowald and Kirkwood) (Kowald and Kirkwood, 2016). That theory argues that the deleterious phenotypes associated with aging emerge as the force of natural selection declines following the peak of reproduction. For our species, quantitative studies by Hamilton and Charlesworth have shown that the force of natural selection has essentially dissipated by about the age of 40 (reviewed by Martin et al., 1996). Potential private donors for our research should therefore understand that these multiple age related disorders are expressed within a very large proportion of our population and that they therefore contribute disproportionately to our rising health costs and declines in the quality and length of life. In slide presentations for potential donors, many of whom might have major fears of mainly two groups of disorders, dementias and cancers, a large litany of these disorders should be reviewed, certainly to include Dementias of the Alzheimer Type, Parkinson's Disease/Lewy Body Dementia, Frontotemporal Dementia, Multi-Infarct Dementia, Mild Cognitive Impairment, Depression, Sleep Disorders, Peripheral Neuropathies, Age-Related Macular Degeneration, Ocular Cataracts, Presbycusis, Atherosclerosis, Arteriolosclerosis, Medial Calcinosis, Ischemic & Non-Ischemic Heart Disease, Chronic Pulmonary Obstructive Disease, Chronic Renal Disease, Benign Prostatic Hyperplasia, Sarcopenia, Type 2 Diabetes Mellitus, Amyloidosis (multiple types), Osteoporosis, Osteoarthritis, Spinal Stenosis, Benign & Malignant Neoplasms, Immunosenescence (with consequences of enhanced susceptibilities to a wide range of infectious agents) and Chronic Inflammation.
3. A call to arms for more research on developmental biology
During the period of my Presidency of the Gerontological Society of America (2003), I was concerned about the lingering competition for funds by lobbying groups representing research and health care for pediatric versus geriatric patient populations. My response had always been to fully support research and public health initiatives that impact pre-natal and child care not only on the ethical grounds but also by the view that “how well one builds an organism makes a great deal of difference on how long it lasts and how well it functions!” We therefore had a focus on aspects of early developmental pathobiology, including a session on the Hutchinson-Gilford syndrome. The need for more research in this field and for more translation into preventive medicine and Public Health has been greatly strengthened by the nascent field of non-human mammalian research on transgenerational epigenetic inheritance. There is therefore the possibility that stresses associated with nutrition, environmental agents and even stressful behaviors may be passed on to subsequent generations in our species. A valuable critical review of this field has recently been published (van Otterdijk and Michels, 2016). The authors point out that truly transgenerational epigenetic inheritance (as opposed to intergenerational epigenetic inheritance) must await evidence of continued phenotypic expression in the F3 generation for a female and the F2 generation in the male. They conclude that, given the constraints on the feasibility of molecular epigenetic studies in serial generations, the existence of such transgenerational inheritance remains equivocal. Nevertheless, one can argue that the evidence of intergenerational epigenetic inheritance is already sufficiently persuasive to reinforce the importance of protecting a fetus from alterations in their epigenome with the potential to influence its healthspan and that of its own progeny.
4. Age-related increases in variegated gene expression, a potential unifying pathogenetic mechanism for the quasi-stochastic distributions of age-related disorders
As a pathologist who has performed many autopsies on both aging humans and mice, I have been impressed with what I have referred to as the “quasi-stochastic” distributions of geriatric pathologies (Martin, 2012). While it is certainly the case that many such disorders are characterized by a propensity for a particular tissue domain (e.g., the sigmoid colon for the case of adenocarcinoma of the colon or the medial temporal cortex for the case of Alzheimer's disease), within those domains, the distributions of the canonical lesions appear to be stochastic. For the case of malignant neoplasms, most of my colleagues would argue that this phenomenon can be explained by somatic mutations leading to driver mutations such as dominant oncogenes or multiple hits in recessive tumor suppressor alleles. The initial aberration, however, might be the result of age-related increases in variegated gene expression leading to the loss of proliferative homeostasis. This could occur by random fluctuations in the expressions of genes involved in the regulation of the mitotic cell cycle, with two contrasting phenotypes – multi-focal atrophy or multi-focal hyperplasia, both of which can be observed in aging humans (Martin, 1979). One could therefore posit that the first step in the genesis of age-related neoplasia is hyperplasia, as it is associated with increased DNA replication and, thus, an increased opportunity for the emergence of driver mutations. While variegated gene expression is typically associated with environmental influences, a perfectly reasonable explanation of the increasing divergence of gene expression among human identical twins (Fraga et al., 2005), I have argued that these results might also be explained by age-related stochastic epigenetic drifts in gene expression (Martin, 2005a). For the case of Alzheimer's disease, age-related drifts in gene expression involving an imbalance of gene actions for the synthesis and degradation of Abeta peptides might also explain the quasi-stochastic distribution of Abeta plaques. Although so far lacking a rigorous mathematical analysis, I have argued that variegated gene expression may have evolved as an example of an antagonistic pleiotropic type of gene action (Martin, 2009).
While age-related variegation in gene expression is usually considered to be epigenetic in origin, there may well be post-transcriptional contributions.
5. Comparative gerontology
Although there are some potential problems with the use of simple correlation coefficients in studies that compare aspects of the biology of aging in long-lived versus short-lived species (Le Bourg, 1996), this approach has proven to be very powerful and its wider use is encouraged. It has, for example, provided important support for studies ranging from tests of the classical evolutionary biological theory of aging (Austad, 1993) to support for the importance of the maintenance of proteostasis (Pride et al., 2015). Research comparing sibling species that are very closely related on a phylogenetic tree would be particularly valuable, especially if they could be combined with rigorous comparative genomics and transcriptomics (Lewis et al., 2016).
6. Genetic approaches to the discovery of intraspecific “antigeroid” alleles, including major suppressor alleles
Our understanding of basic mechanisms of aging has been enriched by research that takes advantage of experiments of nature that, via constitutional mutations, have led to segmental progeroid syndromes in human subjects (Martin, 1978). A cogent example is the identification of a variety of such progeroid mutations that are based upon genomic instability. A recent example involves mutations in POLD1 (Lessel et al., 2015). Given the quite reasonable preoccupation of medical geneticists with allelic variants that result in diminished structure and function, it is not surprising that there are few studies that have investigated allelic variants that lead to unusual enhancements of structure and function resulting in what might be referred to as antigeroid syndromes (Hisama et al., 2016). One such neglected approach could involve large scale genetic studies of middle aged subjects that employ highly sensitive and highly specific assays of a range of physiological functions (Martin, 2002). Another neglected approach would be to engage the power of somatic cell genetics. Another example would be searches for suppressor alleles of age-related phenotypes expressed in relevant types of cultured cells. The exciting advances in induced pluripotent stem cell biology (Barral and Kurian, 2016) and in trans-differentiations of somatic cells (Prasad et al., 2016) could provide a wide range of relevant phenotypes for suppressor hunts.
7. Can we “wake up” aging stem cells?
The now classic paper on the regeneration of myogenic stem cells of the skeletal muscle of aging mice via heterochronic parabioses (Conboy et al., 2005) has led to a focus upon basic and translational research that addresses the microenvironment or “niche” of aging stem cells and on the potential to “wake up” aging stem cells via small molecular weight compounds that by-pass or abrogate the apparent defect(s) in the microenvironments of these and other types of stem cells (Villeda et al., 2011). Such research has been partly responsible for the recent surge of interest in the therapeutic properties of “young plasma” (Kaiser, 2016). The situation is far more complicated, however, as suggested by the thoughtful review of Helen Blau and colleagues (Blau et al., 2015).
8. Arguments pointing to the pathogenetic heterogeneity of Alzheimer's disease, the role of basic mechanisms of aging and the need for a systematic search for spontaneous animal models of AD and related dementias
The vast majority of research on Alzheimer's disease continues to deal with a pathogenetic sequence that addresses the genesis and neuropathological consequences of abnormal amounts and types of beta amyloid peptides and associated abnormal forms of tau, gliosis and inflammatory responses. The fact that all three autosomal dominant constitutional gene mutations responsible for early onset familial AD were shown to involve the synthesis of beta amyloid peptides provided a strong rationale for that focus (Sandbrink et al., 1996) and the conclusion that “… Alzheimer's disease (AD) is a single disease with a common metabolic APP- beta A4-amyloid pathway” (Sandbrink et al., 1996). A more heuristic hypothesis, however, would embrace the hypothesis that there may be several distinct upstream pathogenetic pathways that are driven by one or more basic mechanisms of biological aging, all of which lead to a common downstream pathway of beta amyloidosis, tauopathies and inflammation; if so, a nomenclature of “Dementias of the Alzheimer Type” (DAT) would be preferable (Martin, 2005b). Early precursors of DAT might conceivably range from nature-nature interactions and somatic mutations/epimutations that impact age-related aberrations in proteostasis (Yerbury et al., 2016) to the emergence of viral agents as a result of either immunosenescence (Shaw et al., 2010) or age-related alterations in the stability of chromatin leading to the expression of an endogenous retroviral agent (Christensen, 2016). Another argument in favor of pathogenetic heterogeneity is the common finding of mixtures of what have been considered to be distinct types of neuropathology, including frontotemporal dementia, Lewy Body dementia and multi- infarct dementia, especially for the case of older subjects (Rahimi and Kovacs, 2014).
While the several genetically engineered mouse models of AD based upon the dominance of the beta amyloid hypothesis have been helpful, controversial aspects persist http://www.alzforum.org/news/research-news/do-app-knock-ins-call-overexpression-models-ad-question. The use of a mouse model expressing the human APOE4 allele has also provided useful information (Wang et al., 2005). Such mice lack potentially important human cis and trans regulatory domains, however.
All of the above leads me to conclude that a more systematic search for animal models of naturally occurring age-related dementias is a high priority for our community. We would require sufficient funds for ensuring end of life autopsies of the great majority of a wide range of species, with special attention to non-human primates. A research partnership with organizations such as The World Association of Zoos and Aquariums would be desirable (http://www.waza.org/en/site/home).
9. A call for more research on genetic and environmental influences upon the ratios of healthspan/lifespan in model organisms and in human subjects
Until quite recently, the field of biogerontology has been almost entirely dominated by research on mean, median and maximum lifespans. With the emergence of the broader conceptual framework of Geroscience (Kennedy et al., 2014), our community has begun to consider parameters of healthspan as well as lifespan in their experimental designs. Particularly urgent, in my view, would be the development and application of highly specific, sensitive and relatively non-invasive physiological and morphological assays for the determination of rates of declines in a range of parameters that could lead to quantifications of “good health” versus “bad health”. This would have the potential to more precisely define various summarizations of healthspan, the ratios of healthspans/lifespans, and reliable tests of the extent to which these ratios might be modulated by constitutional genetics, a wide range of environmental variables and stochastic events.
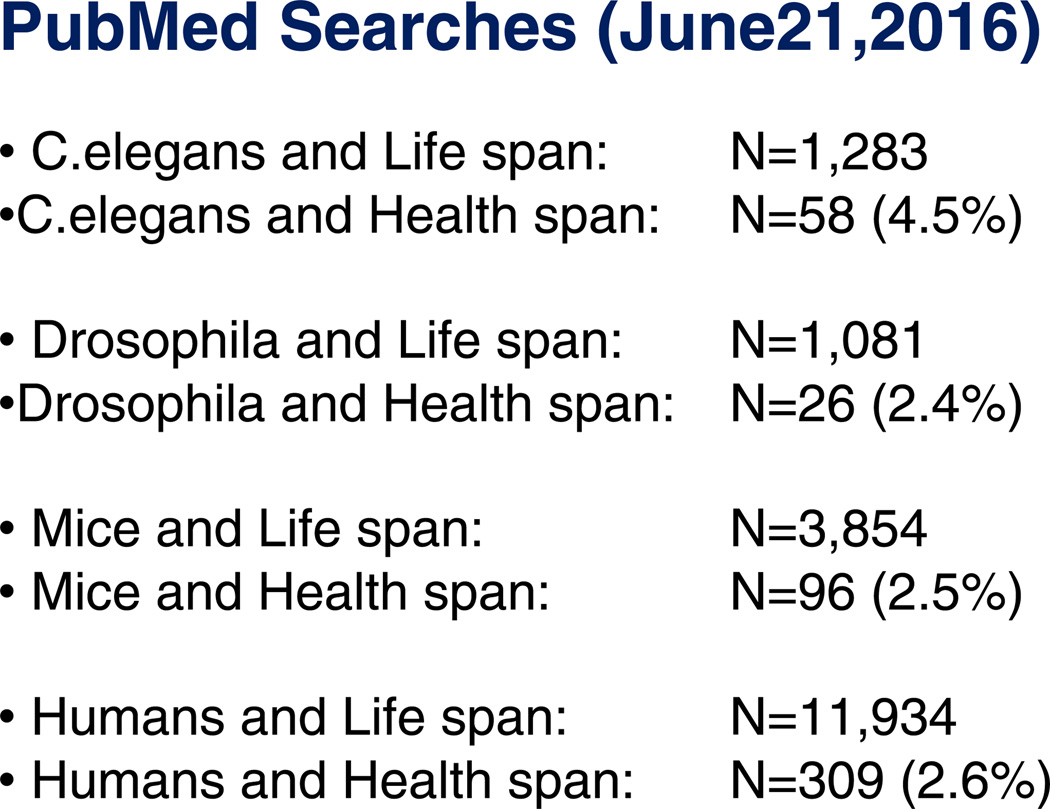
While probably biased to some extent by the difficulty in searching for a range of suitable synonyms of “healthspan” in the scientific literature, Fig. 1 supports the assertion that the types of research advocated above have barely begun. Kudos can be given to such scientists as Monica Driscoll (Onken and Driscoll, 2010), Matt Kaeberlein (Sutphin et al., 2012), Barry Halliwell (Ng et al., 2014), Heidi Tissenbaum (Bansal et al., 2015), Coleen Murphy (Hahm et al., 2015), Malene Hansen (Gelino et al., 2016) and others for having pioneered healthspan research in C. elegans. Let us hope we can see a similar expansion of such research in other model organisms, certainly to include mammalian models (Richardson et al., 2016).
Fig. 1.
The results of PubMed searches on June 21, 2016 for papers on healthspan and lifespan conducted with model organisms. The results show that the vast majority of such publications have been concerned with lifespan, not healthspan
Jim Fries pioneered studies on the compression of morbidity in human subjects, the key concept related to the above discussion about the ratio of healthspan/lifespan (Fries, 1988). More recent studies of exceptionally long lived individuals by Tom Perls and colleagues have supported the concept that there is in fact a compression of morbidity in such human subjects (Andersen et al., 2012). It will be very important to seek confirmation of this very encouraging conclusion, particularly to minimize the possibility of any bias in ascertainment. As regards the general population, the most likely scenario is that there is considerable variation in such rations. A recent publication has in fact demonstrated considerable heterogeneity (Lowsky et al., 2014).
10. Pre-clinical research: from mice to humans via pet dogs
Most of the above discussions have dealt with Basic Geroscience. This is not to minimize the importance of Translational Geroscience. There is a great need, however, to develop animal models within phylogenetic mammalian niches that are closer to humans than the traditional rodent models, yet can be cost effective and have the potential to be closer to human environments. My close colleagues Matt Kaeberlein and Daniel Promislow have concluded that pet dogs could admirably fill that need and have so far received enthusiastic support from pet dog owners and veterinary physicians, all of whom are keen to enhance both the healthspans and lifespans of these greatly loved human companions. As of this writing a Phase 1 study of the effects of rapamycin has been completed and a Phase 2 study is being developed http://dogagingproject.com/project-details/. Meanwhile, there is an ongoing Phase 1 clinical trial of rapamycin in combination with lonafarnib (a farnesyl transferase inhibitor) to ameliorate the progression of the Hutchinson-Gilford Syndrome, a prototypic type of segmental progeroid syndrome http://progeriaresearch.org/assets/files/pdf/Statement-on-PRFs-Triple-Drug-Trial-July-11-2016.pdf. My own laboratory has also published results that envisage the potential use of suitable doses of rapamycin or its analogs for the treatment of the classical adult onset segmental progeroid syndrome, the Werner syndrome (Saha et al., 2014).
11. A tribute to the pioneers of private support for basic and translational geroscience research and the need to develop a consortium of partner organizations
Three non-profit organizations have provided magnificent models of how private funds can advance Geroscience research. The American Federation for Aging Research (AFAR), http://www.afar.org/ features a large number of expert committees for the peer review of applications in support of research grant applications that deal with basic aspects of the biology of aging. It was founded in 1981 by the late Irving S. Wright, MD, a New York cardiologist.
AFAR has since awarded research grants, totaling approximately $172 million, to some 3,300 investigators at more than 500 leading research institutions. Its emphasis has been on helping to develop the research careers of young investigators, but it also has a large portfolio of grant programs suitable for junior, mid-career and senior investigators. This includes a successful program to support medical students who wish to participate in research within Geroscience laboratories. While research on specific age-related disorders is supported, particularly those that deal with Alzheimer's disease and related disorders (which has been generously supported by funds from the Rosalinde and Arthur Gilbert Foundation), AFAR strongly encourages approaches that examine the underlying pathogenetic mechanisms of biological aging that “set the stage” for diseases of aging. AFAR seeks support from a wide range of private sources, most recently from the Arthritis National Research Foundation, including generous support from the Glenn Foundation for Medical Research and the Ellison Medical Foundation, both of which are discussed below.
The Glenn Foundation for Medical Research is the most venerable of the three organizations I am honoring in this review, having been founded by Paul F. Glenn in 1965. Its mission is “ to extend the healthy years of life through research on mechanisms of biology that govern normal human aging and its related physiological decline, with the objective of translating research into interventions that will extend healthspan with lifespan” (http://glennfoundation.org/). In addition to funding a range of programs implemented by AFAR, including post-doctoral fellowships and “BIG” Awards (Breakthroughs in Gerontology), it currently supports eleven specialized Glenn Centers for Research In Aging at distinguished institutions around the country and continues to provide a substantial number of smaller awards to particularly promising investigators in our field of research.
The Ellison Medical Foundation (EMF) http://www.ellisonfoundation.org/) developed as a result of a close relationship between the late Joshua Lederberg, a world famous geneticist and educator, and Lawrence J. Ellison, the CEO and founder of Oracle, a world-leading software company. In their discussions on areas of science particularly ready to profit from an infusion of private funding, they concluded that a program of support for basic research on the biology of aging would be a sound investment: “…they talked about different fields for the foundation and aging was one that stuck," Dr. Lederberg said. "It was clear it was an area that was not quite integrated into what most molecular biologists were doing." (http://www.ellisonfoundation.org/content/history) and began awarding grants to both New Scholars and Senior Scholars in 1998 after peer review by a scientific advisory committee that had wide interests and long experience in the evaluation of science. Interested readers are invited to examine the awardees, perhaps beginning with the first group of Ellison Senior Scholars (http://www.ellisonfoundation.org/awardlist/aging-senior-scholar/1998). The extent of support by the Ellison Medical Foundation grew to approximately $50 million per year and was an enormous contribution to the development of the Geroscience that exists today. Unfortunately, the last set of awards (to Ellison New Scholars) was made in 2013 and after these periods of funding cease, no further awards will be made. The EMF has now become the Lawrence Ellison Foundation and will hopefully continue to support worthy biomedical research initiatives.
One unusual Ellison award is worth pointing out because of its potentially important impact upon future opportunities for the private funding of research on basic mechanisms of aging. In 2011, following discussions by two members of the American Aging Association (Elliot Bergman and myself), the EMF co-hosted, together with the National Institute on Aging, a meeting of distinguished health economists and leaders in gerontological research at the Bethesda offices of the EMF. We asked our guests to discuss approaches to the calculations of the economic impact of research that led to some degree of slowing of the rate of aging in human subjects. The conclusion was that there are indeed useful methodologies for addressing this question. The EMF, together with AFAR, the Gerontological Society of America, the American Aging Association and the Alliance for Aging Research concluded that such a study was worthy of support. The results of the study were published two years later in a high profile journal (Goldman et al., 2013). Although the authors were careful to point out limitations of their study, they concluded that “Overall, greater investment in research to delay aging appears to be a highly efficient way to forestall disease, extend healthy life, and improve public health” (Goldman et al., 2013).
12. A tentative plan towards the creation of an organized effort, by and for Geroscientists, to enhance private sources of funding for both basic and translational research
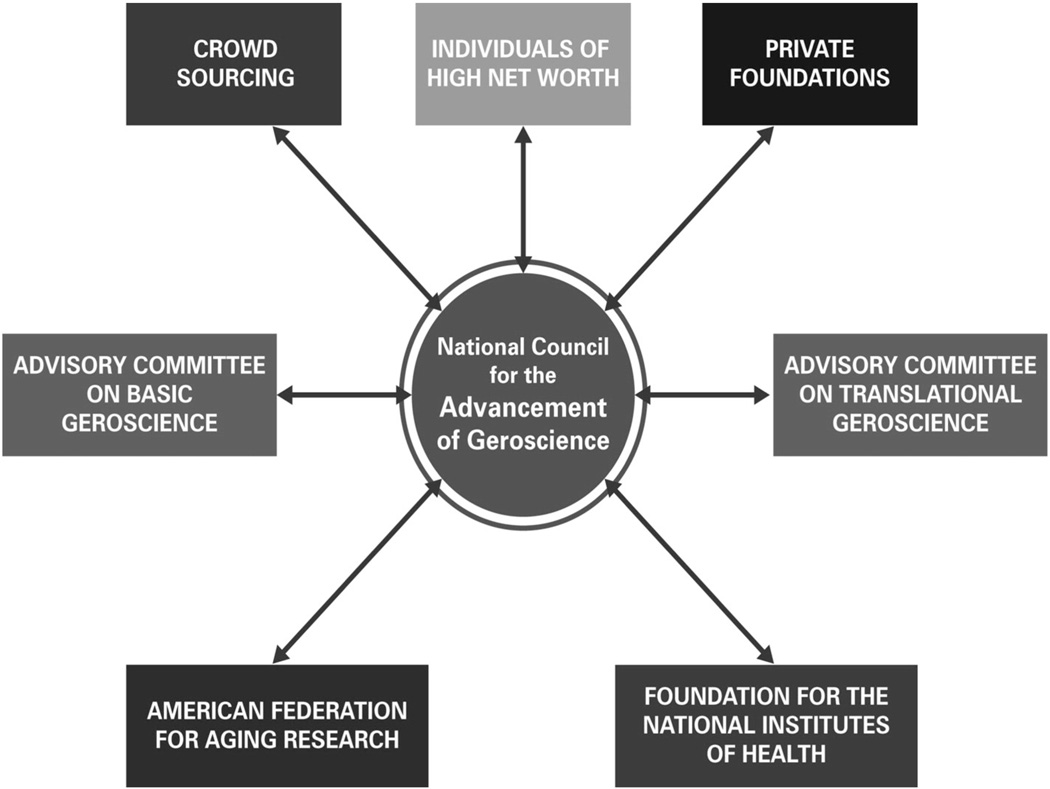
A tentative graphic summarization of one such plan is summarized in Fig. 2. The plan envisages a coordinating group (National Council for the Advancement of Geroscience, NCAG) consisting of representatives of the major funding and granting agencies as well as a range of other non-profit professional organizations concerned with Geroscience research, education and practice. This body would seek advice from committees of experts with major interests in either basic or translational aspects of Geroscience in order to develop research priorities in both domains. The NCAG would initiate contacts with a wide range of potential private sources of funding in order to substantially supplement the budgets of AFAR and the Foundation for the National Institutes of Health (http://www.fnih.org/), an organization devoted to raising private funds to enhance the budgets of the National Institutes of Health, including what we would hope would include a special program to enhance research portfolios developed by the NIH Geroscience Interest Group. Discussions on alternative plans by my colleagues would be most welcome.
Fig. 2.
An initial draft of an administrative structure designed to provide substantial increments of private sources of funding in order to accelerate progress on both basic and translational research in Geroscience via both federal and non-federal granting agencies. The focus of such research would be comparable to that of the NIH Geroscience Interest Group – i.e., to support research that addresses both fundamental and applied Geroscience research with the potential to slow the rate of biological aging and thus to delay, ameliorate or prevent multiple disorders of aging. The goal of such research would be to enhance the healthspans as well as the lifespans of human populations and of pet animals. Such research must also address the important issue of how such interventions may impact the ratios of Healthspan/Lifespan (see text).
Acknowledgments
The author is grateful for early discussions with several University of Washington colleagues concerning various approaches to the development of the plan of Fig. 2, notably Peter S. Rabinovitch, Matthew Kaeberlein, Daniel Promislow and Paula Ladd. He takes full responsibility, however, for any perceived deficiencies with the plan envisioned in Fig. 2.
Footnotes
Disclosures
The author is a past President and Scientific Director Emeritus of the American Federation for Aging Research and the former Chairman of the Scientific Advisory Board of the Ellison Medical Foundation. He currently serves as a member of the Scientific Advisory Board of the Glenn Foundation for Medical Research and is a member of the President's Cabinet of the Gerontological Society of America.
References
- Andersen SL, Sebastiani P, Dworkis DA, Feldman L, Perls TT. Health span approximates life span among many supercentenarians: compression of morbidity at the approximate limit of life span. J. Gerontol. A Biol. Sci. Med. Sci. 2012;67:395–405. doi: 10.1093/gerona/glr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austad SN. Retarded senescence in an insular population of Virginia opossums (Didelphis-Virginiana) J. Zool. 1993;229:695–708. [Google Scholar]
- Bansal A, Zhu LJ, Yen K, Tissenbaum HA. Uncoupling lifespan and healthspan in Caenorhabditis elegans longevity mutants. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E277–E286. doi: 10.1073/pnas.1412192112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. Mechanisms of development of multimorbidity in the elderly. Eur Respir J. 2015;45:790–806. doi: 10.1183/09031936.00229714. [DOI] [PubMed] [Google Scholar]
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- Barral S, Kurian MA. Utility of induced pluripotent stem cells for the study and treatment of genetic diseases: focus on childhood neurological disorders. Front. Mol. Neurosci. 2016;9:78. doi: 10.3389/fnmol.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau HM, Cosgrove BD, Ho AT. The central role of muscle stem cells in regenerative failure with aging. Nat. Med. 2015;21:854–862. doi: 10.1038/nm.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch JB, Augustine AD, Frieden LA, Hadley E, Howcroft TK, Johnson R, Khalsa PS, Kohanski RA, Li XL, Macchiarini F, et al. Advances in geroscience: impact on healthspan and chronic disease. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69(Suppl. 1):S1–S3. doi: 10.1093/gerona/glu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen T. Human endogenous retroviruses in neurologic disease. APMIS. 2016;124:116–126. doi: 10.1111/apm.12486. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suner D, Cigudosa JC, Urioste M, Benitez J, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl. Acad. Sci. U. S. A. 2005;102:10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries JF. Aging, illness, and health policy: implications of the compression of morbidity. Perspect. Biol. Med. 1988;31:407–428. doi: 10.1353/pbm.1988.0024. [DOI] [PubMed] [Google Scholar]
- Gelino S, Chang JT, Kumsta C, She X, Davis A, Nguyen C, Panowski S, Hansen M. Intestinal Autophagy Improves Healthspan and Longevity in C. elegans during Dietary Restriction. PLoS Genet. 2016;12:e1006135. doi: 10.1371/journal.pgen.1006135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman DP, Cutler D, Rowe JW, Michaud PC, Sullivan J, Peneva D, Olshansky SJ. Substantial health and economic returns from delayed aging may warrant a new focus for medical research. Health Aff (Millwood) 2013;32:1698–1705. doi: 10.1377/hlthaff.2013.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm JH, Kim S, DiLoreto R, Shi C, Lee SJ, Murphy CT, Nam HG. C. elegans maximum velocity correlates with healthspan and is maintained in worms with an insulin receptor mutation. Nat Commun. 2015;6:8919. doi: 10.1038/ncomms9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisama FM, Oshima J, Martin GM. How research on human progeroid and antigeroid syndromes can contribute to the longevity dividend initiative. Cold Spring Harb Perspect Med. 2016;6:a025882. doi: 10.1101/cshperspect.a025882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J. BIOMEDICINE. Antiaging trial using young blood stirs concerns. Science. 2016;353:527–528. doi: 10.1126/science.353.6299.527. [DOI] [PubMed] [Google Scholar]
- Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowald AaK, Kirkwood TBL. Can aging be programmed? A critical review. Aging Cell. 2016;15:986–998. doi: 10.1111/acel.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourg E. Correlational analysis in comparative gerontology: an examination of some problems. Exp. Gerontol. 1996;31:645–653. doi: 10.1016/s0531-5565(96)00098-8. [DOI] [PubMed] [Google Scholar]
- Lessel D, Hisama FM, Szakszon K, Saha B, Sanjuanelo AB, Salbert BA, Steele PD, Baldwin J, Brown WT, Piussan C, et al. POLD1 germline mutations in patients initially diagnosed with Werner syndrome. Hum. Mutat. 2015;36:1070–1079. doi: 10.1002/humu.22833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KN, Soifer I, Melamud E, Roy M, McIsaac RS, Hibbs M, Buffenstein R. Unraveling the message: insights into comparative genomics of the naked mole-rat. Mamm. Genome. 2016;27:259–278. doi: 10.1007/s00335-016-9648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowsky DJ, Olshansky SJ, Bhattacharya J, Goldman DP. Heterogeneity in healthy aging. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69:640–649. doi: 10.1093/gerona/glt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GM. Genetic syndromes in man with potential relevance to the pathobiology of aging. Birth Defects Orig Artic Ser. 1978;14:5–39. [PubMed] [Google Scholar]
- Martin GM. Proliferative homeostasis and its age-related aberrations. Mech. Ageing Dev. 1979;9:385–391. doi: 10.1016/0047-6374(79)90080-0. [DOI] [PubMed] [Google Scholar]
- Martin GM. Help wanted: physiologists for research on aging. Sci. Aging Knowl. Environ. 2002:vp2. doi: 10.1126/sageke.2002.9.vp2. [DOI] [PubMed] [Google Scholar]
- Martin GM. Epigenetic drift in aging identical twins. Proc. Natl. Acad. Sci. U. S. A. 2005a;102:10413–10414. doi: 10.1073/pnas.0504743102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GM. Genetic modulation of senescent phenotypes in Homo sapiens. Cell. 2005b;120:523–532. doi: 10.1016/j.cell.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Martin GM. Epigenetic gambling and epigenetic drift as an antagonistic pleiotropic mechanism of aging. Aging Cell. 2009;8:761–764. doi: 10.1111/j.1474-9726.2009.00515.x. [DOI] [PubMed] [Google Scholar]
- Martin GM. Stochastic modulations of the pace and patterns of ageing: impacts on quasi-stochastic distributions of multiple geriatric pathologies. Mech. Ageing Dev. 2012;133:107–111. doi: 10.1016/j.mad.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GM. Views on the ethical struggle for universal, high quality, affordable health care and its relevance for gerontology. Exp. Gerontol. 2016 doi: 10.1016/j.exger.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GM, Austad SN, Johnson TE. Genetic analysis of ageing: role of oxidative damage and environmental stresses. Nat. Genet. 1996;13:25–34. doi: 10.1038/ng0596-25. [DOI] [PubMed] [Google Scholar]
- Martin GM, LaMarco K, Strauss E, K LK. Research on aging: the end of the beginning. Science. 2003;299:1339–1341. doi: 10.1126/science.299.5611.1339. [DOI] [PubMed] [Google Scholar]
- Miller RA. Extending life: scientific prospects and political obstacles. Milbank Q. 2002;80:155–174. doi: 10.1111/1468-0009.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng LF, Gruber J, Cheah IK, Goo CK, Cheong WF, Shui G, Sit KP, Wenk MR, Halliwell B. The mitochondria-targeted antioxidant MitoQ extends lifespan and improves healthspan of a transgenic Caenorhabditis elegans model of Alzheimer disease. Free Radic. Biol. Med. 2014;71:390–401. doi: 10.1016/j.freeradbiomed.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Olshansky SJ. Articulating the Case for the Longevity Dividend. Cold Spring Harb Perspect Med. 2016;6:a025940. doi: 10.1101/cshperspect.a025940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onken B, Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One. 2010;5:e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A, Manivannan J, Loong DT, Chua SM, Gharibani PM, All AH. A review of induced pluripotent stem cell, direct conversion by trans-differentiation, direct reprogramming and oligodendrocyte differentiation. Regen. Med. 2016;11:181–191. doi: 10.2217/rme.16.5. [DOI] [PubMed] [Google Scholar]
- Pride H, Yu Z, Sunchu B, Mochnick J, Coles A, Zhang Y, Buffenstein R, Hornsby PJ, Austad SN, Perez VI. Long-lived species have improved proteostasis compared to phylogenetically- related shorter-lived species. Biochem. Biophys. Res. Commun. 2015;457:669–675. doi: 10.1016/j.bbrc.2015.01.046. [DOI] [PubMed] [Google Scholar]
- Rahimi J, Kovacs GG. Prevalence of mixed pathologies in the aging brain. Alzheimers Res. Ther. 2014;6:82. doi: 10.1186/s13195-014-0082-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A, Fischer KE, Speakman JR, de Cabo R, Mitchell SJ, Peterson CA, Rabinovitch P, Chiao YA, Taffet G, Miller RA, et al. Measures of healthspan as indices of aging in mice-arecommendation. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71:427–430. doi: 10.1093/gerona/glv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha B, Cypro A, Martin GM, Oshima J. Rapamycin decreases DNA damage accumulation and enhances cell growth of WRN-deficient human fibroblasts. Aging Cell. 2014;13:573–575. doi: 10.1111/acel.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandbrink R, Hartmann T, Masters CL, Beyreuther K. Genes contributing to Alzheimer's disease. Mol. Psychiatry. 1996;1:27–40. [PubMed] [Google Scholar]
- Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Curr. Opin. Immunol. 2010;22:507–513. doi: 10.1016/j.coi.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutphin GL, Bishop E, Yanos ME, Moller RM, Kaeberlein M. Caffeine extends life span, improves healthspan, and delays age-associated pathology in Caenorhabditis elegans. Longev Healthspan. 2012;1:9. doi: 10.1186/2046-2395-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Otterdijk SD, Michels KB. Transgenerational epigenetic inheritance in mammals: how good is the evidence? FASEB J. 2016;30:2457–2465. doi: 10.1096/fj.201500083. [DOI] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Wilson WA, Moore SD, Mace BE, Maeda N, Schmechel DE, Sullivan PM. Human apoE4-targeted replacement mice display synaptic deficits in the absence of neuropathology. Neurobiol. Dis. 2005;18:390–398. doi: 10.1016/j.nbd.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Yerbury JJ, Ooi L, Dillin A, Saunders DN, Hatters DM, Beart PM, Cashman NR, Wilson MR, Ecroyd H. Walking the tightrope: proteostasis and neurodegenerative disease. J. Neurochem. 2016;137:489–505. doi: 10.1111/jnc.13575. [DOI] [PubMed] [Google Scholar]