Abstract
Full thickness rotator cuff tendon (RCT) tears have long-term effects on RC muscle atrophy and fatty infiltration, with lasting damage even after surgical tendon repair. Skeletal muscle progenitor cells (SMPs) are critical for muscle repair in response to injury, but the inability of RC muscles to recover from chronic RCT tear indicates possible deficits in repair mechanisms. Here we investigated if muscle injury state was a crucial factor during human SMP expansion and differentiation ex vivo. SMPs were isolated from muscles in patients with no, partial-thickness (PT), or full-thickness (FT) RCT tears. Despite using growth factors, physiological niche stiffness, and muscle-mimetic extracellular matrix (ECM) proteins, we found that SMPs isolated from human RC muscle with RCT tears proliferated slower but fused into myosin heavy chain (MHC)-positive myotubes at higher rates than SMPs from untorn RCTs. Proteomic analysis of RC muscle tissue revealed shifts in muscle composition with pathology, as muscle from massive RCT tears had increased ECM deposition compared with no tear RC muscle. Together these data imply that the remodeled niche in a torn RCT primes SMPs not for expansion but for differentiation, thus limiting longer-term self-renewal necessary for regeneration after surgical repair.
Keywords: proliferation, mass spectrometry, hydrogels, regenerative medicine, growth factors
Approximately 30% of the population 60+ years of age has a tear of at least one rotator cuff tendon (RCT),1 typically either the supraspinatus tendon or both supraspinatus and infraspinatus tendons. Such injuries led to nearly 300,000 surgical interventions in the United States in 2006.2 Given the often chronic presentation of RCT injuries, supraspinatus and infraspinatus muscles can degenerate, leading to fatty infiltration and muscle loss.3,4 Fatty infiltration frequently occurs in the infraspinatus muscle even when only the supraspinatus tendon is torn as a result of altered muscle loading.5 Furthermore, muscle damage, which occurs during chronic RCT injuries, does not often improve following tendon repair, and repair failure is correlated with continued progression of muscle atrophy and fatty infiltration.6 Thus, chronic RCT injury can result in permanently altered muscle, indicating possible deficits in intrinsic muscle repair mechanisms.
Skeletal muscle progenitor cells (SMPs) are responsible for muscle growth and repair in response to injury.7 While SMPs transition from a quiescent to active state in response to soluble factors released by injured muscle in vivo,8 their activation can also be modulated by insoluble factors within the niche itself,9,10 due to their location under the basement membrane surrounding muscle fibers.11 Niche characteristics typically include substrate stiffness,12 which for healthy muscle can range from 10 to 20 kiloPascals (kPa, a unit of stiffness),13,14 extracellular matrix (ECM) protein composition, including basement membrane collagens and laminins,15,16 and soluble growth and signals factors,17–20 including Notch regulation,8 HFG,18 IGF-1,19 oxytocin,20 and p38 MAP kinase (MAPK) pathway activation.21 Since SMPs are sensitive to these environmental cues, it is likely that tendon tear activates SMPs in RC muscles, as we have shown by an increase in the SMP population in muscle from partial RCT tears.22 Whereas most murine or human studies focus on substantially younger populations than ours,1 the chronicity, tear severity, and advanced age of our patient population have previously been associated with less SMP activation resulting in lower regenerative capacity.22 However, other muscle groups appear to maintain their regenerative capacity to some degree; indeed, aged murine intact myofibers contain fewer SMPs but tend to be more proliferative.23 Replating SMPs in niche with aged characteristics can even “reprogram” young cells to resemble aged SMP characteristics.23 Thus poor RCT surgical outcomes could be due to unique deficits in RC muscles created by ECM and growth factor composition of RC muscles.
To determine if the lower regenerative capacity of SMPs in muscle from torn RCTs can be “rejuvenated” by the restoration of normal niche characteristics, we extended our previous observations of SMPs that were restricted to fibronectin-coated hydrogels22 to more broadly determine whether or not SMPs isolated from supraspinatus, infraspinatus, and deltoid muscles from varying RCT tear states could be culture-expanded in muscle-mimetic niches. Using substrate stiffness,12–14 ECM protein composition,15,16 and soluble signals and growth factors,17–20 we quantified the extent to which disease state influenced expansion and subsequent differentiation, finding that tear state alone had a substantial and long-lasting effect on SMP phenotype; tear-derived SMPs fused into multinucleated myotubes at greater rates but were less proliferative than controls despite normal niche conditions. These data correlated with ECM compositional differences between tear states obtained by HPLC-MS/MS, suggesting that intrinsic niche differences may have permanently reprogrammed SMPs, thus impairing repair post-reloading of muscle.
MATERIALS AND METHODS
This study is a laboratory study.
Tissue Biopsies
Muscle biopsies were obtained from the distal third of the supraspinatus (SS), infraspinatus (IS), and/or deltoid (D) muscles from 15 patients of mixed gender undergoing arthroscopic or open shoulder surgery for chronic RCT tears. Patients with acute RCT tears were excluded from the study. RCTs were classified as having no tear (NT) with patients typically presenting with bursitis or instability, or tears of varying severity classified intraoperatively by the surgeon as a partial thickness tear (PT), full thickness tear (FT), or massive tear (MT). Patients classified as PT had torn one or more tendons partially but not completely through the sagittal plane of the tendon. Conversely, patients classified as FT had completely torn through the sagittal plane of the tendon. MT was categorized by FT of more than two tendons with medial retraction. Patients were classified into these groups by the operating surgeon and biopsies of approximately 10 mg of tissue were obtained using an arthroscopic rongeur. All biometric data and case notes are provided in Supplemental Table S1 with average age of 54.2 ± 15.3 years and body mass index of 26.8 ± 4.1 kg/m2. There was no significant difference in body mass index between torn and intact patients (p = 0.16), but age between torn and intact patients was significantly different (p = 0.001). This difference is consistent with the reported increasing incidence of rotator cuff tears with age.24 The institutional review board of the University of California, San Diego Human Research Protection Program approved this study (approval #090829); all participants gave written informed consent to participate.
Skeletal Muscle Progenitor (SMP) Isolation
Muscle biopsies were obtained from arthroscopic shoulder surgeries on patients with varying rotator cuff tear states. Samples from deltoid, supraspinatus, and infraspinatus muscles were procured. Samples were digested using 0.25% collagenase (Worthington Biochemical) and dispase (Stem Cell Technologies) for 30 min at 37°C, before being minced and digested for a subsequent 10 min. Cells were passed through a 70 μm filter (BD) and centrifuged at 2000 RPM for 10 min at 4°C. Cells were then resuspended in FACS buffer (2.5% normal goat serum and 1 mM EDTA in PBS) and stained using PE mouse anti human NCAM (BD 561903), eFluor450 mouse anti human CD31 (eBioscience 48-0319-42), and FITC mouse anti-human CD45 (BioLegend 304017) for 20 min on ice. Cells were centrifuged at 2000 rpm for 4 min, resuspended in FACS buffer, and sorted using a FACSAria 2 cell sorter (BD). Following sorting, SMPs were kept in 20% FBS in one well of a 24-well plate and passaged when confluent. Medium was changed every other day.
Statistical Analysis
SMP proliferation was analyzed using unsupervised hierarchical clustering in R,25 with distance metric of correlation and complete linkage calculated. Heat maps were generated using the gplots package in R. Approximately unbiased (AU) p-values for hierarchical clustering were calculated using the pvclust R package.26 HPLC-MS/MS data was graphed using a custom Matlab script. For substrate studies with C2C12s, a one-way repeated measures ANOVA was used. SMP substrate studies were analyzed using a two-way repeated measures ANOVA. Proliferation data were analyzed using a two-way repeated measures ANOVA, with factors medium and tear state. For differentiation studies, a two way ANOVA with factors medium and tear state was used. For HPLC-MS/MS data, a mixed effects model for predicting NSAFs with fixed effects tear state and GO term and random effect patient revealed significant tear state * GO term interaction (p < 10−4), indicating that the abundance of proteins with ECM or cytoskeletal GO terms varies with tear state. Data were split according to GO term association (ECM, cytoskeletal, or other), and submodels with fixed effect tear and random effect patient were calculated. Tukey’s honest significant difference post hoc testing was used to determine differences between factor levels for all ANOVAs. Statistical significance was set to p < 0.05.
Proteomic Analysis of Human Muscle Tissues
Proteomic analysis of muscle tissues was conducted using supraspinatus muscle samples from patients with either NT (n = 4) or MT (n = 3). All biopsies were flash frozen with liquid nitrogen shortly after time of biopsy. Tissue was prepared for mass spectroscopy analysis using an ECM enrichment strategy from Hill and coworkers.27 Trypsin-digested peptides were analyzed by high-pressure liquid chromatography (HPLC) coupled with tandem mass spectroscopy (LC-MS/MS) using nanospray ionization.28 The collected data were analyzed using MASCOT® (Matrix Sciences) and Protein Pilot 4.0 (ABSCIEX) for peptide identifications. Normalized spectral abundance factors (NSAFs) were calculated to correct spectral counts for proteins length and for the total peptide content of each run.29
Histological Analysis
Muscle tissue from donors described above was blocked in OCT compound (Sakura) and sectioned on a cryostat in 10 μm-thick sections. Sections were stained with picrosirus red to identify collagen content. Sections were fixed in ice cold acetone for 10 min and rehydrated in 100%–95%–70% ethanol solutions before being washed with distilled water and stained with 0.1% picrosirius red in piric acid (Electron Microscopy Sciences) for 1 h. Slides were washed with two changes of 0.5% glacial acetic acid and three changes of 100% ethanol before being mounted in Cytoseal 60 (Thermo Scientific).
RESULTS
Ex Vivo Human SMP Expansion Is Affected by Rotator Cuff Tear State
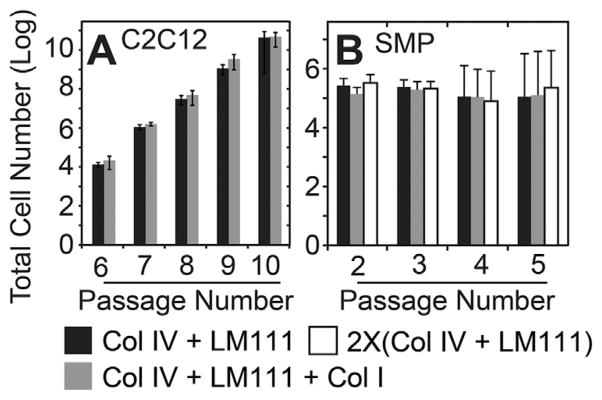
Murine SMPs have successfully been expanded on polyacrylamide (PA) hydrogels with a stiffness of ~11 kPa,13,14,30 so for human SMP expansion, we coated 11 kPa hydrogels with laminin-111 and type IV collagen15,16 and selectively with type I collagen to mirror previous descriptions of the in vivo mouse niche.30 While C2C12 mouse myoblast expansion readily occurs in both of these conditions, C2C12s are insensitive to these niche variations (Fig. 1A). Conversely, NCAM positive human SMPs (Supplementary Fig. S1) with the same niche combinations failed to proliferate over several passages (Fig. 1B); ECM protein composition of the culture substrate again made no significant difference in cell proliferation rates.
Figure 1.
ECM proteins do not significantly affect proliferation rate. (A) C2C12 mouse myoblasts were cultured on 11 kPa polyacrylamide gels, laminin-111, collagen type IV or on 11 kPa polyacrylamide gels, laminin-111, collagens type I and IV. n = 3 technical replicates with p-value = 0.32. (B) SMPs were cultured on conditions as described above. n = 3 biological replicates with p-value = 0.33. Data were analyzed using one way (A) or two way (B) repeated measures ANOVA with Tukey post-hoc. Error bars are standard error of the mean (SEM).
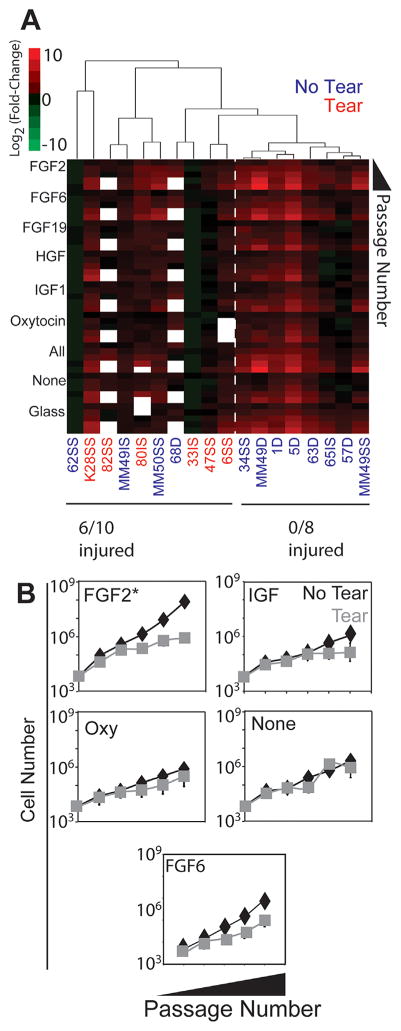
Since ECM stiffness and composition were not sufficient to induce proliferation, we next decided to also culture SMPs in the presence of growth factors to test whether a more complete niche could maintain the SMP phenotype. Based on previous literature, FGF2,17 FGF6,17 FGF19,17 HGF,18 IGF1,19 and oxytocin20 were added individually, in combination, or not at all to growth medium. Again SMPs were cultured on 11 kPa PA hydrogels with collagen IV and laminin-111 as well as on glass. The growth factor effect was significant (Fig. 2A; p < 10−4), with SMPs grown in FGF2 having the greatest average proliferation rate compared to SMPs grown without exogenously added growth factors (Fig. 2B; p < 0.05, two way repeated measures ANOVA with Tukey post-hoc testing); effects with other growth factors were not significantly better than proliferation rates without added growth factors, despite contrary observations with mouse SMPs17–20 (Fig. 2). However, tear state also had a significant effect on proliferation rates across growth factor conditions, with partial and full tear samples proliferating more slowly than no tear samples (p < 10−4). Unsupervised hierarchical clustering also revealed that SMPs from muscles with torn RCTs (either partial or full) were generally less proliferative than SMPs from cases lacking a tear (Fig. 2A). Approximately unbiased (AU) p-values calculated by multiscale nonparametric bootstrapping using the pvclust R package26 indicated high confidence for the clustering observed (Supplementary Fig. S2). In the specific case of FGF2 where the highest average expansion occurred, it is worth noting that SMPs from muscles with torn RCTs were 100-fold less proliferative than the untorn counterparts (Fig. 2B), indicating how dominant RCT tear state is as a predictor of SMP proliferation.
Figure 2.
Growth factor and tear state affect long term SMP proliferation rates. (A) SMPs were grown on matrices of collagen IV and laminin-111 with the addition of growth factors. Unsupervised hierarchical clustering was used to order patient data at the third passage. Growth factor effect p < 0.001, tear effect p < 0.001. (B) FGF2 significantly enhanced SMP proliferation over adding no growth factors (p < 0.01). Data were analyzed using two way repeated measures ANOVA with Tukey post-hoc. Error bars are SEM.
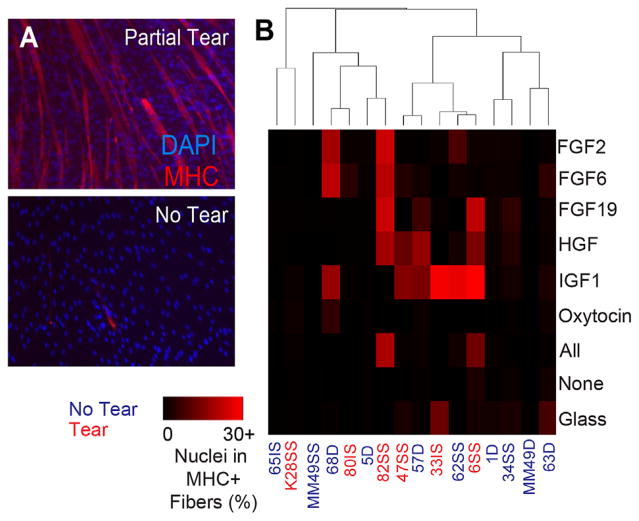
Myotube Fusion Rate Trends With Rotator Cuff Tear State
While the RCT injury niche may reprogram SMPs to limit their expansion, poor clinical outcomes5 might further suggest limits on the ability of existing SMPs to fuse into and repair mature muscle. Thus, after expansion in a muscle mimetic niche, SMP differentiation potential was assessed for each growth factor. We found that differentiation rates, calculated as the percentage of nuclei within myosin heavy chain (MHC) positive myotubes (Fig. 3A) after 5 days in differentiation media, were significantly affected by tear state of the RCT and specific growth factors used during cell expansion. When unsupervised hierarchical clustering was performed and cluster confidence was evaluated using the Pvclust R package,26 AU p-values from multiscale nonparametric bootstrapping indicated high confidence (>95%) for each cluster generated (Supplementary Fig. S3). Differentiation rates were significantly greater for SMPs from torn cuffs than for SMPs from intact cuffs (Fig. 3B; p < 10−4, two-way ANOVA). Growth factor effects were also significant (p < 0.01), as was the medium and tear state interaction (p = 0.005). Thus, the effects of expansion in IGF1, which produced the highest average rates of differentiation, were not uniform across tear state. The dependence of SMP expansion and differentiation on tear state and growth factors, as well as prior observations that injury can affect SMP expansion in vivo,22 suggests that expansion may differently affect SMPs’ underlying self-renewal status, i.e., the difference between proliferating and differentiating SMP.8,31 These data suggest that specific niche conditions could prime cells for differentiation after expansion, which would impact the ability of differentiated progeny of SMPs in RC muscles to repair post injury.
Figure 3.
SMP differentiation capacity varies by tear state and proliferation medium. (A) Cells were seeded at high confluency at the end of the proliferation experiment (P7-8) and allowed to differentiate for 5 days in differentiation medium (5% horse serum and 10 μg/ml insulin). Representative images of cells are shown from partial tear and no tear. (B) Differentiation was quantified as the number of nuclei that were in myosin heavy chain (MHC)-positive myotubes. Growth factor effect p = 0.00698, tear effect p < 10−4, growth factor*tear interaction p = 0.00547. Data were analyzed using a two way ANOVA with Tukey post-hoc analysis. (C) RNA expression of myogenic markers MyoD and Pax7 shown as a ratio of MyoD/Pax7 expression after cell expansion. Error bars are SEM.
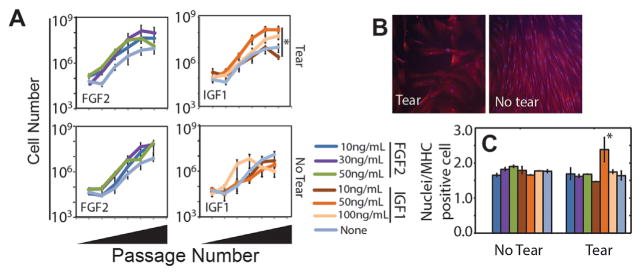
Since FGF2 and IGF1 produced the most robust expansion and MHC positive fibers, respectively, we next determined the concentrations that optimized SMP proliferation and subsequent differentiation to ascertain if specific niche conditions could improve both. Using FGF2 and IGF1 concentrations above and below those reported to affect murine SMPs,17,19 we found a significant growth factor effect in the no tear sample (p < 10−4, one way ANOVA) and the highest average SMP proliferation for FGF2, consistent with Figure 2. While there were few significant effects within FGF2 or IGF1 concentrations, maximal expansion for SMPs from muscle from torn RCTs occurred at 30 ng/ml IGF1 (p = 0.037, Tukey post-hoc following one way ANOVA, Fig. 4A). To determine if there was a concentration dependence on subsequent differentiation, we assessed myotube fusion based on the number of nuclei per MHC positive cell. SMPs from intact RCT muscles were not impacted by growth factor condition versus media without exogenous growth factors, consistent with Figure 2. However for SMPs from torn RC muscles, we again found that IGF1 produced a more robust response than FGF2, but specifically with an optimum at 50 ng/ml (Fig. 4B and C). Thus, 50 ng/ml IGF1 appears to prime SMPs from torn RC muscles, while not affecting SMPs from untorn RCT muscles. Given prior observations that injury can affect SMP expansion in vivo22 and our current observation that niche conditions affect expansion, these data implicate specific growth factor dosing in combination could better prime or encourage post-injury SMP expansion and differentiation.
Figure 4.
Growth factor dose effects. (A) SMPs were expanded on 11 kPa polyacrylamide gels, laminin-111, collagens type I and IV in the presence of the indicated growth factors and their concentrations. Data are plotted as total cell number versus passage number. n = 3 technical replicates with one biological replicate per tear state. (B) Immunofluorescent staining for MHC from SMPs with the indicated tear state. (C) Number of nuclei per MHC positive cell plotted for the growth factors and concentrations indicated in panel A. *p < 0.05 for comparisons to all other conditions using a post-hoc Tukey test.
Analysis of Protein Composition Changes in Muscle With Rotator Cuff Tear State
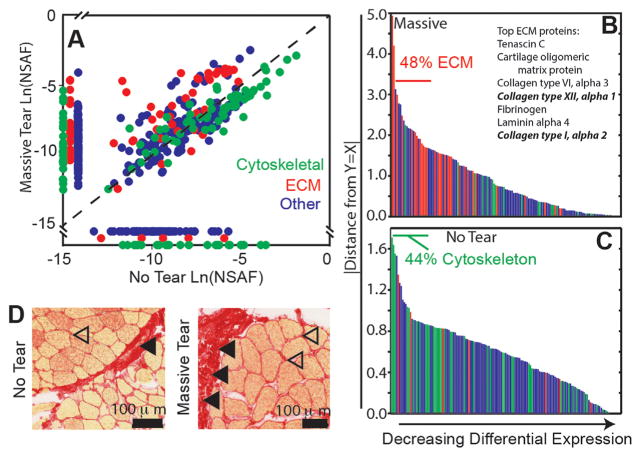
Given that the niche significantly affected SMP behavior, we next characterized differences in the niche in vivo to correlate it with matrix and growth factor combinations used in vitro. We used high-pressure liquid chromatography coupled with tandem mass spectroscopy (HPLC-MS/MS) and an ECM enrichment strategy for sample preparation27 to evaluate bulk protein changes in supraspinatus muscle from massive tear (n = 3) and untorn (n = 4) cases. A total of 10,252 unique tryptic peptides were detected (Supplementary Table S2) accounting for a total of 447 (massive tear) and 337 (no tear) non-redundant proteins (Supplemental Table S3); of these proteins, 277 were common between tear states. ECM and cytoskeletal proteins were the most abundant gene ontology (GO) terms based on BioMart annotations32 and in accordance with the ECM enrichment strategy.27 Comparisons of normalized spectral abundance factors (NSAFs)29 for each protein common between tear states showed ECM protein enrichment in biopsies of massive tears, that is, a shift away from equal expression between cuff states (Fig. 5A, red; upward and leftward shift).
Figure 5.
Mass spectroscopy reveals that muscle composition varies with disease state. (A) Natural log of mean normalized spectral abundance factors (NSAFs) plotted for massive tear (n = 3) versus no tear (n = 4). Dashed line is y = x reference line. Proteins are colored by gene ontology (GO) terms for cytoskeletal (green), ECM (red), and other (blue). Proteins expressed in either massive tear or no tear samples but not both are located along each respective axis. (B, C) The absolute value of the distance from each point to the y = x reference line was calculated to indicate differential protein expression. Panel B indicates those proteins expressed at higher levels in massive tear samples (i.e., to the left of the y = x line), while panel C indicates those expressed at higher levels in no tear samples. Panel B also annotates those ECM proteins that had the highest overall expression, with fibrillar collagens noted in bold. (D) Representative images of picrosirius red staining of no tear and massive tear samples show increased ECM content and collagen deposition in massive tear samples, with some collagen deposition in no tears likely due to bursitis.
A mixed effects model for predicting NSAFs with fixed effects tear state and GO term and random effect patient revealed significant tear state * GO term interaction (p < .0001), indicating that the abundance of proteins with ECM or cytoskeletal GO terms varies with tear state. In a subsequent model with the cytoskeletal fraction of the data with fixed effect tear state and random effect patient, cytoskeletal proteins were significantly increased in no tear muscle (p = 0.046). To quantitatively illustrate this, differential expression was computed as distance of each protein’s NSAF from the y = x line, plotted in the order of decreasing distance. For proteins with greater expression in massive tear samples (Fig. 5B; Supplemental Table S4), ECM GO terms comprised 48% of the 25 most differentially expressed proteins. The most abundant matrix proteins are also listed, with fibrillar collagens highlighted in bold. Given their relatively high abundance, the presence of several fibrillar collagens indicates possible niche remodeling. Conversely for no tear samples (Fig. 5C; Supplemental Table S5), cytoskeletal GO terms comprised 44% of the 25 most differentially expressed proteins whereas only 4 of the top 100 terms were ECM-related. Furthermore, the few ECM proteins enriched in no tear RC muscle were laminins, fibronectin, and nidogen, all of which are associated with the SMP niche. These data indicate that fibrosis may substantially change the composition of the SMP niche in vivo in muscle with RCT tears, and to confirm this, picrosirius red staining of the same samples was performed. Staining indicated that while muscle from untorn RCTs had some degree of fibrosis possibly associated with bursitis, there was substantial collagen deposition within muscle from torn RCTs (Fig. 5D), consistent again with an altered SMP niche in vivo. These data further suggest that changes within the niche during the chronic phase of remodeling could negatively impact SMP repair ability post-surgery.
DISCUSSION
While most murine and human studies with SMPs report age effects to varying degrees,21,33,34 our results highlight the importance of disease state and cell culture conditions in the ability of the SMP to proliferate and differentiate. While the former was assessed in vitro, it is possible that the significant differences we observed within the niche itself could have longer-term implications for repairing chronically torn RCTs.
Human Versus Murine Differences in SMP Behavior
Previous studies have implicated numerous growth factors, including FGF2,17 FGF19,17 FGF6,17 HGF,18 IGF1,19 and oxytocin,20 in maintaining the proliferative state of murine SMPs in vitro. Using human SMPs, however, we found only FGF2 significantly improved cell expansion and IGF1 significantly improved cell fusion. Additionally, the effects of muscle-mimetic substrate stiffness and ECM protein composition were not sufficient to prolong human SMP proliferation, in contrast to murine models.30 However, such comparisons may be problematic due to population differences resulting from SMP isolation.31,35–37 While populations can be evaluated for Pax7 expression, isolation differences could result in different Pax7+ subsets and thus different outcomes as we observed, for example, growth factor concentrations reported in mouse literature resulted in different outcomes here; thus, additional combinations of niche conditions could further improve human SMPs expansion and their regenerative capacity.8,31
RCT Tear State Affects SMP Phenotype Maintenance Ex Vivo
Here we showed that SMPs from muscle with torn RCTs proliferated at slower rates over several passages in culture but differentiated significantly better than SMPs from untorn RCTs. Our study has several limitations, including the difficultly of decoupling tear effects from any possible muscle group effects. As a RCT tear generally involves the supraspinatus or both the supraspinatus and infraspinatus tendons, these muscles will be directly unloaded following injury, while the deltoid remains mechanically unaffected. The data presented here include some deltoid samples as intact control muscle, so it is possible that some of the differential clustering observed is due to differences between muscle groups. However, previous studies have investigated human SMPs from different muscle groups and pooled results with success,38 indicating that SMPs from diverse muscle groups may have similar properties. However as that study had a patient cohort with substantially different demographics from this RCT repair cohort, exact comparisons to their expansion and engraftment may be difficult. Furthermore, while the mean age of the tear group was significantly greater than that of the no tear group (p = 0.001, 65 and 45 years, respectively), it is not known to what degree such an age difference affects SMP qualities when both ages are neither juvenile nor geriatric. Regardless, our finding that tear state influenced the growth factor response, specifically with FGF2 and IGF1, indicates that subsequent analysis requires more careful tissue analysis and consideration of injury status. Moreover, it suggests that possible clinical intervention with these specific factors in acute tears could be beneficial by maximizing expansion and repair, though more direct in vivo evidence is required.
RCT Tear State Affects SMP Niche Components
As the SMP niche has a demonstrated importance in maintaining the SMP phenotype, it is likely that the altered niche SMPs encounter in an injury or pathological state affects SMP quality. Indeed, mouse models bear this out; knock out of collagen IV impaired regeneration and reduced SMP self-renewal after injury.10 Conversely, excessive fibrosis also limits SMP renewal through chronic inflammatory responses that block entry into muscle fibers.39 Our examination of proteomic changes in muscles from torn versus untorn RCTs illustrates the increased ECM deposition and loss of cytoskeletal proteins seen in RCT tear muscles, which suggests that fibrotic responses can alter the SMP niche. Severe, chronic RCT injuries could then impair SMP self-renewal within the niche22 as well as in culture, as we observed. Despite all of these significant remodeling events, especially those associated with the basement membrane that surrounds muscle fibers in vivo, it is important to note that HPLC-MS/MS evaluates bulk level protein expression in the sample. Thus the changes we observed were likely due to global differences in the connective tissue of the muscle belly rather than specific differences within the SMP niche. This limitation of HPLC-MS/MS accordingly leads to the loss of protein localization data. Myofibroblast-associated matrix could make SMP niche changes in response to injury difficult to detect. Although histological analyses are an alternative to HPLC-MS/MS, the changes we observed in vivo and their ability to be modulated in vitro with growth factors that inhibit fibrosis lead us to conclude that HPLC-MS/MS provided a reasonable snapshot of the RC muscles.
CONCLUSIONS
This study demonstrates the importance of the SMP niche in maintaining proliferation and differentiation capacity in vitro. We show the difficulties of translating findings in soluble factors for murine SMPs to human SMPs, as only FGF2 substantially improves long-term expansion ex vivo. Furthermore, we establish a relationship between the injury state of the muscle used for SMP isolation and SMP phenotype maintenance ex vivo. Our data indicate that SMPs from muscles with a RCT tear proliferate more slowly but differentiate at greater rates after several passages than SMPs from muscles without RCTs. HPLC-MS/MS analysis of proteomic changes in response to RCT tear shows an accumulation of ECM proteins and a decrease in cytoskeletal proteins in massive RCT tear muscle. These shifts in protein expression could alter the in vivo niche for SMPs that affect their ability to expand in vitro, irrespective of culture conditions. These data suggest the importance of context and injury-specific considerations in treating RCTs with exogenous factors to expand and prime SMPs in vivo.
Supplementary Material
Acknowledgments
Grant sponsor: National Institutes of Health; Grant numbers: R01HD073180, DP2OD006460; Grant sponsor: Muscular Dystrophy Association; Grant number: 241665; Grant sponsor: California Institute for Regenerative Medicine; Grant sponsor: National Institutes of Health; Grant number: T32AR060712.
The authors would like to thank Drs. Eugene Sato and Gretchen Meyer for assistance with sample acquisition and Dr. Majid Ghassemian and the UCSD Proteomics core facility for their expertise in mass spectroscopy. The authors acknowledge research grant support from the National Institutes of Health (R01HD073180 to S.R.W. and DP2OD006460 to A.J.E.) and Muscular Dystrophy Association (241665 to A.J.E.) as well as training grant support from the California Institute for Regenerative Medicine (to K.A.T.) and National Institutes of Health (T32AR060712 to M.C.G.).
Footnotes
AUTHORS’ CONTRIBUTIONS
Experiments were designed by K.A.T., S.R.W., and A. J.E., experiments were performed by K.A.T and M.C. G., samples were collected in surgeries by J.G.L. and A.S., and all contributed to writing and editing the manuscript. All authors have read and approved the final manuscript.
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- 1.Tempelhof S, Rupp S, Seil R. Age-related prevalence of rotator cuff tears in asymptomatic shoulders. J Shoulder Elb Surg. 1999;8:296–299. doi: 10.1016/s1058-2746(99)90148-9. [DOI] [PubMed] [Google Scholar]
- 2.Colvin AC, Egorova N, Harrison AK, et al. National trends in rotator cuff repair. J Bone Jt Surg. 2012;94:227–233. doi: 10.2106/JBJS.J.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuchs B, Weishaupt D, Zanetti M, et al. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elb Surg. 1999;8:599–605. doi: 10.1016/s1058-2746(99)90097-6. [DOI] [PubMed] [Google Scholar]
- 4.Gerber C, Schneeberger AG, Hoppeler H, et al. Correlation of atrophy and fatty infiltration on strength and integrity of rotator cuff repairs: a study in thirteen patients. J Shoulder Elb Surg. 2007;16:691–696. doi: 10.1016/j.jse.2007.02.122. [DOI] [PubMed] [Google Scholar]
- 5.Cheung S, Dillon E, Tham SC, et al. The presence of fatty infiltration in the infraspinatus: its relation with the condition of the supraspinatus tendon. Arthrosc – J Arthrosc Relat Surg. 2011;27:463–470. doi: 10.1016/j.arthro.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Gladstone JN, Bishop JY, Lo IKY, et al. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35:719–728. doi: 10.1177/0363546506297539. [DOI] [PubMed] [Google Scholar]
- 7.Shi X, Garry DJ. Muscle stem cells in development, regeneration, and disease. Genes Dev. 2006;20:1692–1708. doi: 10.1101/gad.1419406. [DOI] [PubMed] [Google Scholar]
- 8.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lepper C, Conway SJ, Fan C-M. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature. 2009;460:627–631. doi: 10.1038/nature08209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urciuolo A, Quarta M, Morbidoni V, et al. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat Commun. 2013;4:1964. doi: 10.1038/ncomms2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boonen KJM, Baaijens FPT, Van Der Schaft DWJ, et al. Essential environmental cues from the satellite cell niche: optimizing proliferation and differentiation. Am J Cell Physiol. 2009;2:1338–1345. doi: 10.1152/ajpcell.00015.2009. [DOI] [PubMed] [Google Scholar]
- 13.Collinsworth AM, Zhang S, Kraus WE, et al. Apparent elastic modulus and hysteresis of skeletal muscle cells throughout differentiation. Am J Physiol Cell Physiol. 2002;283:C1219–C1227. doi: 10.1152/ajpcell.00502.2001. [DOI] [PubMed] [Google Scholar]
- 14.Engler AJ, Griffin MA, Sen S, et al. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nandan D, Clarke EP, Ball EH, et al. Ethyl-3,4-dihydroxybenzoate inhibits myoblast differentiation: evidence for an essential role of collagen. J Cell Biol. 1990;110:1673–1679. doi: 10.1083/jcb.110.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ocalan M, Goodman SL, Kühl U, et al. Laminin alters cell shape and stimulates motility and proliferation of murine skeletal myoblasts. Dev Biol. 1988;125:158–167. doi: 10.1016/0012-1606(88)90068-1. [DOI] [PubMed] [Google Scholar]
- 17.Yousef H, Conboy MJ, Mamiya H, et al. Mechanisms of action of hESC-secreted proteins that enhance human and mouse myogenesis. Aging. 2014;6:1–19. doi: 10.18632/aging.100659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada M, Tatsumi R, Yamanouchi K, et al. High concentrations of HGF inhibit skeletal muscle satellite cell proliferation in vitro by inducing expression of myostatin: a possible mechanism for reestablishing satellite cell quiescence in vivo. Am J Physiol Cell Physiol. 2010;298:C465–C476. doi: 10.1152/ajpcell.00449.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacquemin V, Furling D, Bigot A, et al. IGF-1 induces human myotube hypertrophy by increasing cell recruitment. Exp Cell Res. 2004;299:148–158. doi: 10.1016/j.yexcr.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 20.Elabd C, Cousin W, Upadhyayula P, et al. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat Commun. 2014;5:4082. doi: 10.1038/ncomms5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosgrove BD, Gilbert PM, Porpiglia E, et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med. 2014;20:255–264. doi: 10.1038/nm.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer GA, Farris AL, Sato E, et al. Muscle progenitor cell regenerative capacity in the torn rotator cuff. J Orthop Res. 2015;33:421–429. doi: 10.1002/jor.22786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lacraz G, Rouleau A-J, Couture V, et al. Increased stiffness in aged skeletal muscle impairs muscle progenitor cell proliferative activity. PLoS ONE. 2015;10:e0136217. doi: 10.1371/journal.pone.0136217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sher JS, Uribe JW, Posada A, et al. Abnormal findings on magnetic resonance images of asymptomatic shoulders. J Bone Jt Surg Am. 1995;77:10–15. doi: 10.2106/00004623-199501000-00002. [DOI] [PubMed] [Google Scholar]
- 25.R Core Team. R: A language and environment for statistical computing. 2016. [Google Scholar]
- 26.Suzuki R, Shimodaira H. Pvclust: an R package for assessing the uncertainty in hierarchical clustering. Bioinformatics. 2006;22:1540–1542. doi: 10.1093/bioinformatics/btl117. [DOI] [PubMed] [Google Scholar]
- 27.Hill RC, Calle EA, Dzieciatkowska M, et al. Quantification of extracellular matrix proteins from a rat lung scaffold to provide a molecular readout for tissue engineering. Mol Cell Proteomics. 2015;14:961–973. doi: 10.1074/mcp.M114.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mccormack AL, Schieltz DM, Goode B, et al. Direct analysis and identification of proteins in mixtures by LC/MS/MS and database searching at the low-femtomole level. Anal Chem. 1997;69:767–776. doi: 10.1021/ac960799q. [DOI] [PubMed] [Google Scholar]
- 29.Paoletti AC, Parmely TJ, Tomomori-Sato C, et al. Quantitative proteomic analysis of distinct mammalian mediator complexes using normalized spectral abundance factors. Proc Natl Acad Sci USA. 2006;103:18928–18933. doi: 10.1073/pnas.0606379103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilbert PM, Havenstrite KL, Magnusson KEG, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boldrin L, Muntoni F, Morgan JE. Are human and mouse satellite cells really the same? J Histochem Cytochem. 2010;58:941–955. doi: 10.1369/jhc.2010.956201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smedley D, Haider S, Durinck S, et al. The BioMart community portal: an innovative alternative to large, centralized data repositories. Nucleic Acids Res. 2015;43:W589–W598. doi: 10.1093/nar/gkv350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cerletti M, Jurga S, Witczak CA, et al. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conboy IM, Conboy MJ, Wagers AJ, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 35.Dellavalle A, Sampaolesi M, Tonlorenzi R, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 36.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Zheng B, Cao B, Crisan M, et al. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotechnol. 2007;25:1025–1034. doi: 10.1038/nbt1334. [DOI] [PubMed] [Google Scholar]
- 38.Charville GW, Cheung TH, Yoo B, et al. Ex vivo expansion and in vivo self-renewal of human muscle stem cells. Stem Cell Rep. 2015;5:1–12. doi: 10.1016/j.stemcr.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mann CJ, Perdiguero E, Kharraz Y, et al. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle. 2011;1:21. doi: 10.1186/2044-5040-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.