Abstract
Background
Parenteral fat emulsions are important components of parenteral nutrition (PN). For patients who develop PN-associated liver disease (PNALD), use of fish oil (FO) fat emulsions reverses cholestasis. The European Pharmacopeia contains two FO monographs. One is “fish oil; rich in omega-3 fatty acids,” (NFO). The other is “omega-3 acids,” (PFO) derived from NFO but enriched in omega-3 fatty acids, particularly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). The purpose of this study is to compare the effects of 20% NFO and PFO emulsions produced in the laboratory and tested in a murine model.
Methods
Lipid emulsions (20% oil) were compounded containing different oils: United States Pharmacopoeia (USP)-grade soybean oil (SO), NFO, PFO with 66% of the purified fatty acids in triglyceride form (PFO66), and PFO with 90% of the purified fatty acids in triglyceride form (PFO90). Chow-fed C57BL/6 mice received saline, one of the above emulsions, or a commercial FO (OM) by tail vein injection (2.4g/kg/day) for 19 days. Effects after each dose were recorded. On day 19, animals were euthanized and livers, spleens, and lungs were procured for histologic analysis.
Results
Animals administered OM, SO, NFO, and PFO90 tolerated injections well clinically, while those administered PFO66 developed tachypnea and lethargy for ~1 minute following injections. At euthanasia, PFO66- and PFO90-treated animals had organomegaly compared to the other groups. On histologic analysis, PFO66 and PFO90 groups had splenic fat-laden macrophages and hepatic sinusoidal lipid-laden Kupffer cells with no inflammation or necrosis. Lungs in these groups had scattered fat deposits. All other groups had normal-appearing livers, spleens, and lungs.
Conclusions
Use of PFO lipid emulsions is an attractive possibility for improving systemic inflammation in PN-dependent patients and optimizing management of PNALD by concentrating anti-inflammatory EPA and DHA. However, when compounded as a 20% emulsion using similar methods used to formulate currently available commercial parenteral lipid emulsions, PFO monotherapy was poorly tolerated and resulted in adverse end organ sequelae. These results suggest that PFO may not be safe as a 20% emulsion, or that different manufacturing methods may need to be developed to formulate a safe 20% PFO emulsion. Although PFO may meet pharmacopeial standards, and may appear to be tolerated clinically, as was the case with the PFO90 emulsion, it may not be optimal for use in high amounts in parenteral lipid emulsions.
Keywords: Parenteral lipid emulsions, Fish oil, Parenteral nutrition-associated liver disease, Intestinal failure, USP 729
Introduction
Fat is an important component of parenteral nutrition (PN) that serves several purposes. Parenteral fat provides essential fatty acids (EFA) that cannot be synthesized de novo including the long chain polyunsaturated omega-3 and omega-6 fatty acids. Omega-3 fatty acids include α-linolenic acid (ALA), which can be converted through a series of elongation and desaturation steps to eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Omega-6 fatty acids include the parent compound linoleic acid (LA), which can be enzymatically converted to arachidonic acid (ARA) by the same elongases and desaturases that act on omega-3 fatty acids. Fat is also a dense source of calories that prevents the need for excess carbohydrates to meet caloric demands. It has been demonstrated that fat-free, high carbohydrate PN can result in upregulation of de novo lipogenesis and hepatic fat accumulation1–3.
Intravenous fat is compounded as an oil-in-water emulsion. Dispersions used to create stable fat emulsions are comprised of an emulsifier (i.e. egg phospholipid), glycerin, a stabilizer such as sodium oleate, and water. The oil contains fatty acids that confer nutritional value, fulfill the EFA requirement, and are precursors of lipid-derived second messengers and bioactive mediators. For example, ARA is a precursor of thromboxane and prostacyclin, which are important in platelet aggregation and coagulation. ARA is also metabolized to produce pro-inflammatory leukotrienes and prostaglandins. EPA and DHA are precursors of series 5 leukotrienes and series 3 prostaglandins4, resolvins, defensins, and maresins, which are anti-inflammatory mediators5.
The oil in lipid emulsions also contains excipients including phytosterols and α-tocopherol. Phytosterols are structurally similar to cholesterol, and abundant in plant-based oils. They are poorly metabolized by mammals when administered intravenously, and can inhibit the hepatic Farsenoid-X receptor to impair bile flow6. α-tocopherol (Vitamin E) is an antioxidant with anti-inflammatory properties7 that is added to fish oil (FO) to prevent it from becoming rancid. Plant-based oils contain very little α-tocopherol.
The oils in emulsions can have different physiologic effects by virtue of their differing fatty acid composition and additive content8–10. SO is rich in omega-6 fatty acids, with LA comprising ~50% of its fatty acid content. It is rich in phytosterols and contains little α-tocopherol. FO contains an abundance of omega-3 fatty acids, fewer omega-6 fatty acids, high levels of α-tocopherol, and only trace amounts of phytosterols. Parenteral nutrition-associated liver disease (PNALD), characterized by hepatic inflammation and cholestasis that can progress to cirrhosis and end-stage liver disease if untreated, is associated with use of SO parenteral fat sources11. FO as the parenteral fat source can reverse cholestasis.12,13. While some studies have concluded that a high phytosterol level renders SO hepatotoxic14, others have suggested that the abundance of α-tocopherol and omega-3 fatty acid precursors of anti-inflammatory mediators renders FO hepato-protective15. Olive oil is rich in nonessential omega-9 monounsaturated fatty acids and coconut oil is rich in medium-chain triglycerides. Both oils contain very little EFAs. While the effect of olive oil as a sole parenteral fat source has not been reported, use of coconut oil as the sole fat source can result in essential fatty acid deficiency (EFAD)16.
Parenteral lipid emulsions and their oil components are required to meet specific pharmacopeial criteria meant to ensure the integrity and safety of emulsions for parenteral use. In the United States, Chapter <729> of the United States Pharmacopoeia (USP) requires that parenteral fat emulsions have a mean fat globule size < 500nm and a percent of fat globules > 5μm (PFAT5) ≤ 0.05%17. These criteria are in place to avoid coalescence of emulsions and maintain stability during transport and storage prior to use18–20.
Oil sources for parenteral lipid emulsions also have pharmacopeial monographs that define criteria for safe use in pharmaceutical applications. FO has 2 distinct monographs in the European Pharmacopeia. Monograph number 1912 [“fish oil, rich in omega-3 acids,” or natural fish oil (NFO)] contains a minimum of 28% omega-3 fatty acids (at least 22% EPA and DHA), and reflects the fatty acid content of the oil extracted from fish21. Monograph number 1352 [“omega-3 acids,” or purified fish oil (PFO)] originates from NFO, but is further enriched in EPA, DHA, and total omega-3 fatty acids (at least 45% EPA and DHA, and a least 60% omega-3 fatty acids)22. Given the anti-inflammatory properties of the omega-3 fatty acids, use of PFO in parenteral emulsions to provide more anti-inflammatory mediators per unit volume is an attractive possibility. However, comparisons of the effects of NFO and PFO have not been reported in models of PN dependence or in human populations. The purpose of this study is to compare the effects of PFO and NFO as oil sources in parenteral lipid emulsions in a murine model.
Materials and Methods
Lipid Emulsion Production
Lipid emulsions were generated via high-pressure homogenization. Figure 1 demonstrates a schematic of the emulsion formulation process. To formulate the lipid dispersion, Lipoid E80 egg phospholipid (Lipoid LLC, Newark, NJ) was added to heated, (75–90°C), USP-grade sterile water for injection (SWFI) (Hospira, Lake Forest, IL), under high-speed shear mixing conditions. Temperature was allowed to decrease to 40–45°C. Sodium oleate (Lipoid LLC, Newark, NJ) was then added and shear mixing continued at 3900–4000 RPM for 40 minutes. Heated SWFI was serially added to maintain the temperature at 40–45°C. Glycerin (Sigma-Aldrich, St. Louis, MO) was added under continuous shear mixing. This resulted in a dispersion comprised of 12% egg phospholipid, 25% glycerin, and 0.3% sodium oleate. The crude dispersion was transferred to a Panda Plus homogenizer (GEA Niro Saovi, Columbia, MD) and homogenized at 9000psi at 40–45°C for 20 cycles, filtered through a 0.45μm membrane, and pH was adjusted to 10.4 with 0.5N sodium hydroxide (NaOH). All steps were performed under a nitrogen atmosphere.
Figure 1. Schematic of the lipid emulsion formulation process by high-pressure homogenization.
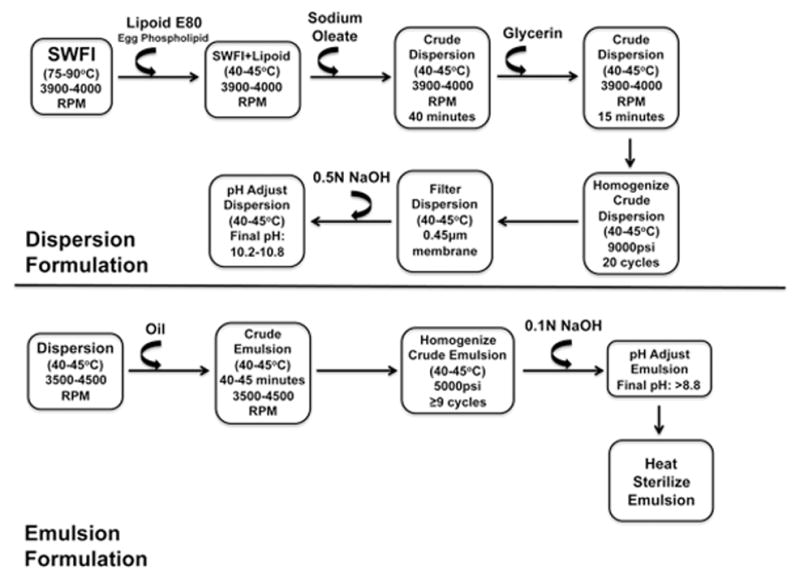
The two major steps of lipid emulsion formulation are dispersion formulation (top half of the page) and emulsion formulation (bottom half of the page). Curved arrows indicate addition of the substance noted above the arrow. SWFI = sterile water for injection; RPM = revolutions per minute in the high-speed shear mixer; cycles refer to the number of times the total volume of dispersion/emulsion passes through the homogenizer at the pressure indicated. All steps are performed under a nitrogen gas atmosphere.
Emulsions were made with the following oils: USP grade SO (Spectrum Chemicals, New Brunswick, NJ), NFO, PFO with 66% of the fatty acids as triglycerides (PFO66), and PFO with 90% of the fatty acids as triglycerides (PFO90), (NFO and both PFO varieties are from Pronova Biopharma, Oslo, Norway). NFO contained 41% of fatty acids as EPA and DHA and ≥94% of total fatty acids were in triglyceride form. PFO66 contained 91.6% omega-3 fatty acids (77.5% EPA and DHA) purified and re-esterified such that 66% of the fatty acids were in triglyceride form (PFO66) and the remaining 34% were present as diglycerides, monoglycerides, ethylesters, and free fatty acids. PFO90 contained 66.2% of fatty acids as EPA and DHA and 90.5% of all fatty acids in triglyceride form. Table 1 demonstrates the properties of NFO according to its certificate of analysis. NFO met the majority of the European Pharmacopeia Monograph Number 1912 specifications. Table 2 demonstrates the properties of the PFO66 and PFO90 oils, which met the majority of the European Pharmacopeia Number 1352 specifications. Analyses were completed in adherence to USP <401> specifications. Table 2 outlines the emulsions and their abbreviations. The final composition of each emulsion was 20% oil, 1.2% egg phospholipid, 2.5% glycerin, and 0.03% sodium oleate. This composition is similar to currently available commercial products.
Table 1.
Properties of Natural Fish Oil Used in Emulsion Formulation
| Oil Component | NFO | Reference Range |
|---|---|---|
|
| ||
| Triglycerides (%) | ≥ 94 | None provided |
| Free Fatty Acids (% Oleic) | 0.3 | None provided |
| Omega-3 Fatty Acids (%) | ≥ 28 | ≥ 28 |
| EPA+DHA (%) | 41 | ≥ 22 |
| Acid Value (mg KOH/g oil) | 0.6 | ≤ 0.5 |
| Anisidine Value | 8.8 | ≤ 30 |
| Peroxide Value (MEq/kg) | 1.8 | ≤ 10 |
| Absorbance at 233nm (AU) | Not Reported | ≤ 0.7 |
| Oligomers (area %) | Not Reported | ≤ 1.5 |
Table 2.
Properties of Purified Fish Oil Used in Emulsion Formulation
| Oil Component | PFO66 | PFO90 | Reference Range |
|---|---|---|---|
|
| |||
| Triglycerides (%) | 66 | 90.5 | None provided |
| Diglycerides (%) | 28.1 | 6.8 | * |
| Monoglycerides (%) | 1.9 | 0 | * |
| Ethylesters (%) | 3.8 | 2.7 | None provided |
| Free Fatty Acids (% oleic) | 0 | 0 | None provided |
| Omega-3 Fatty Acids (%) | 91.6 | 80 | ≥ 60 |
| EPA+DHA (%) | 77.5 | 66.5 | ≥ 45 |
| Acid Value (mg KOH/g oil) | 0 | 0.005 | ≤ 3 |
| Anisidine Value | 5.3 | 6.4 | ≤ 30 |
| Peroxide Value (MEq/kg) | 0.1 | 2.7 | ≤ 10 |
| Absorbance at 233nm (AU) | 0.31 | 0.35 | ≤ 0.73 |
| Oligomers (area %) | < 0.03 | 0 | ≤ 3 |
Reference range for total partial glycerides ≤ 50%
To compound the emulsions, oil was added to the dispersion agent in a thin stream under continuous shear mixing conditions at 3500–4500 RPM for 40–45 minutes, maintaining a 40–45°C temperature. The resulting crude emulsions were transferred to the Panda Plus homogenizer and homogenized at 5000psi and 40–45°C for no less than 9 cycles of the emulsion. The pH of the emulsions was buffered to > 8.8 using 0.1N NaOH. All steps of the compounding process were performed under a nitrogen atmosphere. The finished emulsions were aliquoted into 20mL glass serum vials and headspaces were flooded with nitrogen gas before sealing. All vials were heat sterilized. To determine whether emulsions met USP <729> specifications, each product underwent independent mean globule size and PFAT5 testing (Micro Measurements, Deerfield, IL). Table 3 specifies the 4 emulsions formulated in the laboratory that were used in this study.
Table 3.
Emulsions Formulated
| Abbreviation Used | Oil | Oil Fraction (%) |
|---|---|---|
|
| ||
| SO | Soybean Oil | 20 |
| PFO66 | Purified Fish Oil | 20 |
| PFO90 | Purified Fish Oil | 20 |
| NFO | Natural Fish oil | 20 |
Murine Tolerance Experiments
All experiments were approved by the Boston Children’s Hospital Institutional Animal Care and Use Committee. As the purpose of this study was to assess solely safety and tolerance of the emulsions and was not meant to provide a source of calories or essential fatty acids, animals were fed a standard chow diet throughout the experiments. Six to eight week-old C57BL/6 male mice (Jackson Labs, Bar Harbor, ME) maintained on an ad lib chow diet were injected with 2.4g/kg/day of one of the laboratory compounded emulsions (20% SO, 20% NFO, 20% PFO66, or 20% PFO90), the commercially available 10% FO emulsion Omegaven® (Fresenius Kabi, Bad Homburg, Germany) (OM), or saline every other day for 19 days. The commercial fish oil emulsion and saline control were included as the commercial fish oil emulsion is well tolerated and has been safe to use in this mouse model. Thus, the use of the commercial fish oil emulsion afforded the ability to assess whether the process used to compound the test emulsions yielded similar histological findings and to ascertain whether the compounding technique may be responsible for observed findings in the groups receiving the test emulsions. Body mass was measured prior to each tail vein injection, and observed responses to each injection were recorded. After 19 days, animals were euthanized. Livers, spleens, and kidneys were weighed and masses recorded. Livers, spleens, and lungs were fixed in 10% formalin for hematoxylin and eosin histologic analysis. A board-certified pathologist blinded to the experimental groups performed the histologic analysis.
Results
USP <729> Standards
The PFO66 emulsion met both mean globule size and PFAT5 pharmacopeial standards (210nm and 0.03% respectively). The SO emulsion met pharmacopeial standards, with a mean globule size of 279.8nm and PFAT5 of 0.019%. The NFO emulsion met the mean globule size pharmacopeia standard at 249nm but exceeded the 0.05% PFAT5 threshold at 0.06%. The PFO90 emulsion also met mean globule size specifications at 215 nm but exceeded the PFAT5 threshold at 0.059%.
Clinical Tolerance
Animals in all treatment groups demonstrated similar weight gain over the study period (data not shown). Mice receiving the NFO, SO, OM, and saline intravenously exhibited no symptoms following each injection. Animals receiving the PFO66 emulsion became transiently tachypneic and lethargic for ~1 minute following each injection before returning to normal respiratory and activity status.
Given that the SO, NFO, and PFO66 emulsions were made with the same dispersion using the same compounding methods and the SO and NFO were well tolerated, we hypothesized that a property of the oil rather than the dispersion or the compounding process resulted in the poor tolerance of the PFO66 emulsion. We next hypothesized that improving the re-esterification efficiency of PFO may result in improved tolerance of a 20% PFO parenteral emulsion. When the tolerance experiment was repeated using PFO90, animals tolerated each injection with no symptoms, similar to the effects of SO, NFO, OM, and saline.
Gross Organ Assessment and Histology
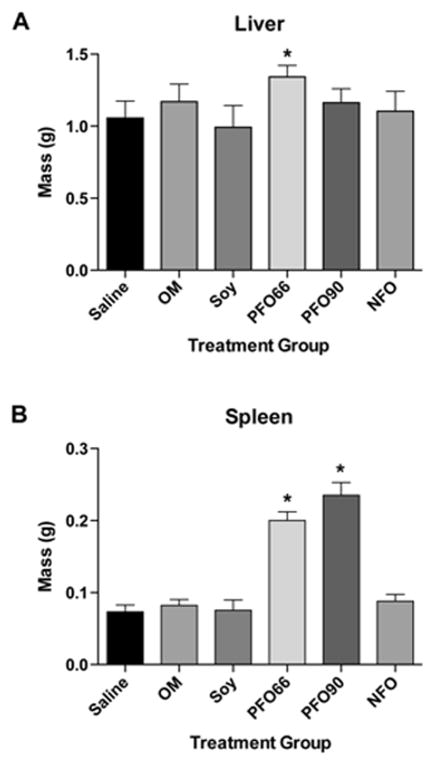
At organ procurement, animals administered the PFO66 had marked hepatosplenomegaly compared to the SO, NFO, OM, and saline groups. Despite being asymptomatic with intravenous administration of the PFO90 emulsion, animals in this group had splenomegaly at time of organ procurement (Figure 2).
Figure 2. PFO66 and PFO90 result in organomegaly, while NFO preserves normal organ size.
Liver (A) and spleen (B) masses after 19 days of 2.4mg/kg/day intravenous lipid emulsion administration. (*) indicated p<0.05 compared to the saline treated group. In A, PFO66 liver mass was significantly different than all groups except the OM group by single-factor ANOVA with Tukey multiple comparison test. Saline, SO, OM, PFO90, and NFO liver masses were not significantly different. In B, PFO66 and PFO90 groups were significantly different than saline, OM, SO, and NFO groups by single-factor ANOVA with Tukey multiple comparison test. Saline, OM, SO, and NFO groups were not significantly different from each other.
On histologic analysis, the PFO66 and PFO90 groups had hepatic and splenic lipid-laden macrophages with no necrosis or evidence of inflammation; as well as scattered pulmonary fat deposits. The SO, NFO, OM, and saline groups had histologically normal livers, spleens, and lungs (Figure 3).
Figure 3. PFO66 and PFO90 result in histologic abnormalities in liver, spleen, and lungs, while NFO preserves normal organ architecture.
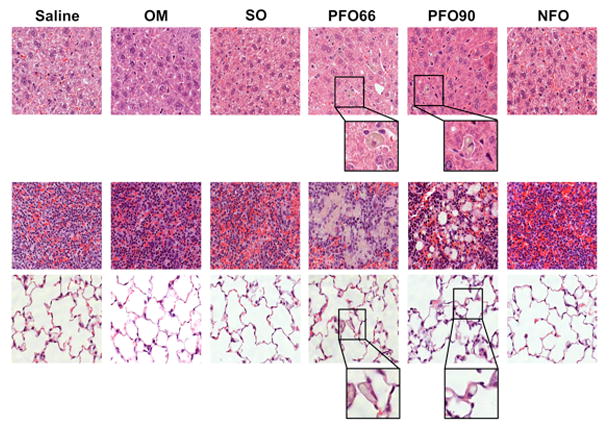
Saline, OM, SO, and NFO groups demonstrated histologically normal livers (top row), spleens (middle row), and lungs (bottom row) after 19 days of treatment. PFO66 and PFO90 groups developed hepatic and splenic lipid-laden macrophages without evidence of inflammation or necrosis; as well as pulmonary fat deposits. All panels are stained with hematoxylin and eosin. Magnification is 400X for all groups. For PFO66 and PFO90 groups, a portion of hepatic and pulmonary slides further magnified to emphasize lipid-laden macrophages and pulmonary fat.
Discussion
PFO emulsions are modified to contain a higher proportion of the anti-inflammatory fatty acids EPA and DHA than NFO. Thus, PFO represents an attractive potential parenteral fat source for improving inflammatory states in chronically PN-dependent patients.23 Use of PFO as a parenteral fat source also offers the possibility of meeting EFA requirements in smaller volumes given the greater EPA and DHA concentrations. This could be of potential benefit in PN-dependent neonates with respiratory distress syndrome, in whom minimizing intravenous fluid volume may be important to optimize respiratory function. However, to date, no study has addressed the safety of PFO compared to NFO as parenteral fat sources.
Here, we demonstrate that the 20% PFO emulsions were poorly tolerated and resulted in adverse end organ sequelae while 20% NFO emulsions were well tolerated when administered as monotherapy. Improving the re-esterification efficiency of the PFO improved clinical tolerance, but still resulted in histologic abnormalities. These data suggest that PFO oils meeting the European Pharmacopeia monograph 1352 (“omega-3 acids”) may result in adverse end organ effects even if they appear to be well tolerated clinically.
It remains uncertain which part of the process of generating PFO from NFO confers physiologically incompatible properties to PFO for intravenous administration. One possibility is that while the unprocessed fatty acids in NFO are virtually all in triglyceride form, generation of PFO requires hydrolysis of triglycerides to purify and enrich EPA and DHA fatty acids, followed by re-esterification. If the re-esterification process is not 100% efficient, the resulting oil contains fatty acids in diglyceride, monoglyceride, and free fatty acid form, in addition to triglycerides. This study demonstrates that while increasing the re-esterification efficiency from 66% to 90% improved clinical tolerance of the PFO emulsions, both emulsions resulted in adverse end organ sequelae. To further investigate whether fatty acids in non-triglyceride form contribute to the poor tolerance of PFO emulsions, studies using emulsions containing PFO re-esterified to triglycerides with near 100% efficiency, or complete removal of non-triglyceride fats from PFO would need to be performed. However, such oils are not currently available. It is also possible that an alternative emulsion compounding method could yield a safe, well-tolerated 20% PFO emulsion, however, a new technique for compounding emulsions would have to be developed.
This study provides preclinical evidence suggesting caution in the use of PFO sources meeting the European Pharmacopoeia monograph 1352 “omega-3 acids” as the sole source in 20% parenteral lipid emulsions. PFO with re-esterification efficiency up to 90% resulted in adverse effects on livers, spleens, and lungs. It is notable that despite clinically appearing to tolerate the PFO90 emulsion, adverse end-organ effects still occurred. These data suggest caution in using even highly re-esterified PFO in parenteral emulsions at lower concentrations, especially in patients with long-term PN-dependence in whom adverse end organ effects may be cumulative. Furthermore, 20% NFO emulsions were well tolerated and preserved organ integrity similarly to the commercially available 10% NFO emulsion. Use of 20% NFO emulsions could provide a means of reducing the volume necessary to administer to meet EFA and fat calorie requirements for PN-dependent patients with intravenous volume restrictions. Additionally, these results indicate that 20% NFO and SO emulsions compounded in the laboratory, which were safe and well tolerated in our mouse model, may be used as research tools to better understand the role of parenteral fat in modulating systemic inflammation and PN-related hepatic toxicity.
Clinical Relevancy Statement.
This study compares the effects of 2 FO sources that comply with different pharmacopeial monographs, and which may be used in parenteral fat emulsions for PN-dependent patients. The results presented suggest that one oil source may be preferable and better tolerated than the other for parenteral use.
Acknowledgments
We acknowledge Bernard Mikrut for providing expertise and consultation in formulating the lipid emulsions. This research was funded by the Boston Children’s Hospital Surgical Foundation, BASF, NIH grant 1F32DK104525-01 (GLF), the Joshua Ryan Rappaport Fellowship (PN), NIH grant 5T32HL007734-22 (MAB), and NIH grant T35HL110843 (BSC).
Footnotes
Disclosures
A license agreement for the use of Omegaven® has been signed by Boston Children’s Hospital and Fresenius Kabi, and a patent has been submitted by Boston Children’s Hospital on behalf of M. Puder and K.M. Gura. M. Puder and K.M. Gura serve on the Board of Advisors for BASF. The NFO, PFO66, and PFO90 oils used in this study were generously donated by Pronova Biopharma.
References
- 1.Guenst JM, Nelson LD. Predictors of total parenteral nutrition-induced lipogenesis. Chest. 1994;105(2):553–9. doi: 10.1378/chest.105.2.553. [DOI] [PubMed] [Google Scholar]
- 2.Ling P-R, Andersson C, Strijbosch R, et al. Effects of glucose or fat calories in total parenteral nutrition on fat metabolism and systemic inflammation in rats. Metabolism. 2011;60(2):195–205. doi: 10.1016/j.metabol.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Ito K, Hao L, Wray AE, Ross AC. Lipid emulsion administered intravenously or orally attenuates triglyceride accumulation and expression of inflammatory markers in the liver of nonobese mice fed parenteral nutrition formula. J Nutr. 2013;143(3):253–9. doi: 10.3945/jn.112.169797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wada M, DeLong CJ, Hong YH, et al. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J Biol Chem. 2007;282(31):22254–66. doi: 10.1074/jbc.M703169200. [DOI] [PubMed] [Google Scholar]
- 5.Serhan CN, Dalli J, Colas RA, Winkler JW, Chiang N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim Biophys Acta. 2015;1851(4):397–413. doi: 10.1016/j.bbalip.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter BA, Taylor OA, Prendergast DR, et al. Stigmasterol, a soy lipid-derived phytosterol, is an antagonist of the bile acid nuclear receptor FXR. Pediatr Res. 2007;62(3):301–6. doi: 10.1203/PDR.0b013e3181256492. [DOI] [PubMed] [Google Scholar]
- 7.Brigelius-Flohé R. Bioactivity of vitamin E. Nutr Res Rev. 2006;19(2):174–86. doi: 10.1017/S0954422407202938. [DOI] [PubMed] [Google Scholar]
- 8.Vanek VW, Seidner DL, Allen P, et al. A.S.P.E.N. Position Paper: Clinical Role for Alternative Intravenous Fat Emulsions. Nutr Clin Pract. 2012;27(2):150–192. doi: 10.1177/0884533612439896. [DOI] [PubMed] [Google Scholar]
- 9.Xu Z, Harvey KA, Pavlina TM, Zaloga GP, Siddiqui RA. Tocopherol and tocotrienol homologs in parenteral lipid emulsions. Eur J Lipid Sci Technol. 2015;117(1):15–22. doi: 10.1002/ejlt.201400182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Z, Harvey KA, Pavlina T, et al. Steroidal compounds in commercial parenteral lipid emulsions. Nutrients. 2012;4(8):904–21. doi: 10.3390/nu4080904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teng J, Arnell H, Bohlin K, Nemeth A, Fischler B. Impact of parenteral fat composition on cholestasis in preterm infants. J Pediatr Gastroenterol Nutr. 2015;60(6):702–7. doi: 10.1097/MPG.0000000000000739. [DOI] [PubMed] [Google Scholar]
- 12.de Meijer VE, Gura KM, Le HD, Meisel Ja, Puder M. Fish oil-based lipid emulsions prevent and reverse parenteral nutrition-associated liver disease: the Boston experience. JPEN J Parenter Enteral Nutr. 2009;33(5):541–7. doi: 10.1177/0148607109332773. [DOI] [PubMed] [Google Scholar]
- 13.Puder M, Valim C, Meisel Ja, et al. Parenteral fish oil improves outcomes in patients with parenteral nutrition-associated liver injury. Ann Surg. 2009;250(3):395–402. doi: 10.1097/SLA.0b013e3181b36657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Kasmi KC, Anderson AL, Devereaux MW, et al. Phytosterols promote liver injury and Kupffer cell activation in parenteral nutrition-associated liver disease. Sci Transl Med. 2013;5(206):206ra137. doi: 10.1126/scitranslmed.3006898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng K, Stoll B, Chacko S, et al. Vitamin E in New-Generation Lipid Emulsions Protects Against Parenteral Nutrition-Associated Liver Disease in Parenteral Nutrition-Fed Preterm Pigs. JPEN J Parenter Enteral Nutr. 2015 Jan; doi: 10.1177/0148607114567900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ling P-R, De Leon CE, Le H, Puder M, Bistrian BR. Early development of essential fatty acid deficiency in rats: fat-free vs. hydrogenated coconut oil diet. Prostaglandins Leukot Essent Fatty Acids. 83(4–6):229–37. doi: 10.1016/j.plefa.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pharmacopoeia TUS. United States Pharmacopeia and National Formulary (USP 36 - NF 31) 2012. Chapter 729: Globule Size Distribution in Lipid Injectable Emulsions; pp. 321–323. [Google Scholar]
- 18.Driscoll DF. Lipid injectable emulsions: Pharmacopeial and safety issues. Pharm Res. 2006;23(9):1959–69. doi: 10.1007/s11095-006-9092-4. [DOI] [PubMed] [Google Scholar]
- 19.Driscoll DF, Silvestri AP, Bistrian BR, Mikrut BA. Stability of total nutrient admixtures with lipid injectable emulsions in glass versus plastic packaging. Am J Health Syst Pharm. 2007;64(4):396–403. doi: 10.2146/ajhp060062. [DOI] [PubMed] [Google Scholar]
- 20.Driscoll DF, Ling P-R, Bistrian BR. Physical stability of 20% lipid injectable emulsions via simulated syringe infusion: effects of glass vs plastic product packaging. JPEN J Parenter Enteral Nutr. 31(2):148–53. doi: 10.1177/0148607107031002148. [DOI] [PubMed] [Google Scholar]
- 21.Pharmacopoeia E. FISH OIL, RICH IN OMEGA-3-ACIDS. European Pharmacopoeia 8.0. 2014:3941–3943. [Google Scholar]
- 22.Pharmacopoeia E. OMEGA-3-ACID TRIGLYCERIDES. European Pharmacopoeia 7.5. 2012:4677–4678. [Google Scholar]
- 23.Compher C, Pazianas M, Benedict S, Brown JC, Kinosian BP, Hise M. Systemic inflammatory mediators and bone homeostasis in intestinal failure. JPEN J Parenter Enteral Nutr. 31(2):142–7. doi: 10.1177/0148607107031002142. [DOI] [PubMed] [Google Scholar]