Abstract
Background
To test the antimicrobial activity of different extracts and fermentation broth from puffball(Bovistella radicata), the different extracts and fermentation broth of puffball were prepared, the active fraction was investigated by UPLC–UV–MS and semi-preparative chromatograph.
Results
Through zones of inhibition (ZOI) and minimum inhibitory concentrations (MIC) tests, the supernatant of fermentation possessed best antimicrobial activity in all extracts whose MIC value is 31.2 μg/ml against T. rubrum, T. mentagrophytes, S. aureus and P. aeruginosa. And ZOI value is 29.01, 21.02, 35.02, 28.01 mm against T. rubrum, T. mentagrophytes, S. aureus and P. aeruginosa. Then we compare the puffball fermentation supernatant with blank contrast by LC–MS. There are the characteristic peaks named PBR-1 and PBR-2 with the puffball fermentation supernatant, the separation of compound PBR-1 and PBR-2 was done on semi-preparative C18 column and the MIC and ZOI of compound PBR-1 and PBR-2 are 15.6 μg/ml and 34 mm with the antifungal test.
Conclusions
The fermentation supernatant and compound PBR-1 and PBR-2 have promising antifungal activity against T. rubrum and T. mentagrophytes.
Keywords: Antimicrobial activity, Tinea pedis, Puffball, Fermentation supernatant, LC–MS
Introduction
Tinea pedis is common ailment, its treatment is a serious problem due to continuous drug resistance that microorganisms soon develop against antibiotic drugs (Subissi et al. 2010), great hope rests with traditional Chinese medicine as they contain different types of primary and secondary metabolites with antimicrobial pharmacophores (Zhang et al. 2011). Trichophyton rubrum (T. rubrum) and Trichophyton mentagrophytes (T. mentagrophytes) are main tinea pedis pathogen (Koltin and Hitchcock 1997), Candida albicans (C. albicans) is opportunistic fungus pathogen (Cotter and Kavanagh 2000). S. aureus and P. aeruginosa are main tinea pedis pathogen of secondary infection (Erbagci et al. 2005), Due to the emergence of resistant pathogens and the side effects of antimicrobial agents, it becomes necessary to search for new antibiotics (Cornaglia 2009). It is important to discover novel metabolites from natural resources and it is considered as an important research field.
Traditional Chinese medicine has been practiced for many centuries (Zuo et al. 2014, 2016; Xiao et al. 2016). Over the past few decades, numerous studies have been conducted on plants to explore possible candidates for antibiotic drugs. Extracts from traditional medicinal represent a continuous effort to find new compounds with the potential to act against multi-resistant microorganisms (Zampini et al. 2009). Owing to puffball diversity in biological activities and production of novel chemical compounds, it is well known that puffball’s antibacterial activity is promising (Novakovic et al. 2015).
Puffball can be found in many provinces of china. Puffball was named as Bovistella radicata from Shuinan town, Jiangxi province in this test. The local people use it as a remedy for anti-tinea pedis and effect is significant. According to Chinese Pharmacopoeia, puffball has been reported to have a number of functions like anti-inflammatory, stanch bleeding, cough, respiratory infections etc., however, we encountered limited published information related to antimicrobial effect of puffball, especially the antifungal activity of puffball has not been considered until now. Therefore, the present study was aimed to investigate the antimicrobial activity of puffball from Jiangxi province. In order to investigate its antimicrobial activity, main kinds of tinea pedis pathogen were selected. The antimicrobial tests were done. Isolation and identification of the activity component in puffball fermentation was done based on LC–MS. Contrast tests also demonstrated that the compound PBR-1 and PBR-2 in fermentation supernatant of puffball was active particularly against T. rubrum. We can make next step to indentify the structures of the compound PBR-1 and PBR-2 to develop new antibacterial substance with stronger antimicrobial activity and more beneficial characteristics.
Materials and methods
Chemicals and instruments
Solvents used for extraction and fractionation including ethanol, chloroform, n-butanol, ethyl acetate, aether were of AR grade. All the above reagents and potato dextrose agar (PDA), glucose (Glc) and agar were purchased from Sinopharm Chemical Reagent Beijing Co., Ltd. Standard antibiotic drugs, gentamycin and terbinafine were obtained from Pharmagen, Hefei. Laminar flow cabinet was produced by Stramline Laboratory. Petri plates and glass columns were bought from Hefei Meifeng Chemical Instrument Co. Ltd. Autoclaver was produced by Shanghai ShenAn medical equipment factory.
Microorganisms
Antimicrobial microorganism (Bovistella radicata)
Sporophore and spore powder of puffball were obtained from pine forest of Shuinan town, Jishui county, Jiangxi province, China, with assistance of local traditional healers. After strain identification, it belongs to Agaricales order, Lycoperdaceae family, Bovistella genus, Bovistella radicata species, and authenticated by Professor Qingmei Zeng, College of Food Science and Engineering, Hefei University of Technology, China. Puffball(Bovistella radicata) specimen was dried and deposited at the herbarium of Department of Biology, Hefei University of Technology, China.
Test microorganisms
Test microorganisms were obtained from the Microbiology Laboratory at Department of Biology, Hefei University of Technology, Anhui. Eight strains of test microorganisms included four bacteria Staphylococcus aureus (ATCC 6538), Bacillus subtilis (ATCC 6051), Pseudomonas aeruginosa (ATCC 9027), Escherichia coli (ATCC 8739) and four fungi Trichophyton rubrum (ATCC 28188), Trichophyton mentagrophytes (ATCC 9533), Epidermophyton floccosum (ATCC 52066) and Candida albicans (ATCC 10231).
Preparation of different extracts and fermentation from puffball
After crushed and ground, the dried sporophore of puffball was mixed with spore powder. According to established protocols (Tian et al. 2011; Zhang et al. 2012; Sen et al. 2014), each 10 mg mixed puffball powder were extracted by 200 ml ethanol/aqueous/chloroform/n-butanol/Aether/Ethyl acetate for continuously 10 h. After filtration over Whatman No. 4 paper, the extracts were concentrated under a reduced pressure at 40 °C and stored at 4 °C in sterile bottles for further use.
The concentrate supernatant of puffball fermentation
The mixed powder of puffball was cultured in PDB (potato dextrose broth) at 25 °C for 2 days, the fermentation was centrifuged at 7000 rpm for 20 min to get the supernatant, the supernatant was filtered over Whatman No. 4 paper, then the filtrates were evaporated on rotary evaporator at low pressure to obtain 0.5 mg/ml concentrate supernatant, the 0.5 mg/ml concentration was prepared as stock solution for further use. From stock solution, multiple proportions dilutions were prepared in water of 500, 250, 125, 62.5, 31.2, 15.6 and 7.8 μg/ml concentrations.
Assessment of antimicrobial activity
The antimicrobial activity of puffball(Bovistella radicata) extracts was evaluated using two kinds of disk diffusion methods which were zones of inhibition (ZOI) (Geetha et al. 2015) and minimum inhibitory concentrations (MICs) (Negi et al. 2003), ZOI is qualitative analysis and MIC is quantitative analysis of antimicrobial activity (Dharajiya et al. 2015).
In this study, four bacterial strains and four fungal strains were used to determined the antimicrobial activity of puffball(Bovistella radicata). The agar cultures of S. aureus, B. subtilis, P. aeruginosa, E. coli were prepared to assess the puffball antibacterial effects. 50 ml of nutrient broth medium poured into an erlenmeyer flask, four flasks were prepared for each examined samples. The flasks including agar medium were sterilized in an autoclave at 121 °C for 15 min. For antibacterial tests, bacterial cultures were grown at 35 °C for 24 h by inoculation in Nutrient Broth (Hopper. 1992). Petri dishes with 20 ml of Nutrient Agar were prepared, previously inoculated with 100 μl of culture suspension (1%, containing 106–107 cfu/ml) and same volume of deionised water was used as a control. Three wells (5.0 mm in diameter) were cut from the agar at sterile condition. The prepared solutions of puffball were filled into the wells. The inoculated plates were incubated of 24 h at 35 °C, after which, it was observed for antimicrobial activity of the samples. At the end of the incubation period, the measurements were done basically from the edge of the zone to the edge of the well.
The same method was used for all the fungal samples. T. rubrum, T. mentagrophytes, E. floccosum and C. albicans were grown in Potato dextrose broth (PDB) at 25 °C for 48 h. 100 μl different fungal strain cultures (1%, containing 106–107 cfu/ml) were added to different flasks containing 25 ml sterile PDA at 45 °C and poured into Petri dishes. The agar was allowed to solidify at 4 °C for 1 h. The wells (5.0 mm in diameter) were cut from the agar at sterile condition. The inoculated plates were incubated of 24 h at 25 °C. PDA plates added normal saline were used as controls. The plates were then incubated at 25 °C for 2 days. The zone of inhibition (ZOI) were calculated.
For the MIC determination of a sample, agar well dilution method (Bourdeau et al. 2004) was used. Potato dextrose agar (PDA) was dissolved in distilled water with concentration 38 g/l, and autoclaved at 121 °C for 0.5 h. It was allowed to come to 50 °C before being poured into Petri plates for sample preparation. Different dilutions of each extract were prepared in this agar, with concentrations of the extract (500, 250, 125, 62.5, 31.2, 15.6 and 7.8 μg/ml). The content of each plate was mixed well and allowed to solidify. Stock solution of each extract/fraction was prepared and dilutions were made. Then, microbial cultures were transferred onto the content of each Petri plate with the help of a multipoint inoculator. Positive control and solvent control were also included. Plates were then incubated for 24 h at 37 °C, after which they were observed, the lowest concentration of antimicrobial agent at which there was no visible growth of a microorganism after incubation was taken as MIC (Petar et al. 2016) and MICs were noted.
UPLC–UV–MS analysis of puffball
The prepared solution of fermentation was also analyzed by ultra-performance liquid chromatography (UPLC) coupled with ultraviolet (UV) and Micromass-LCT Premier time of flight (TOF) mass spectrometer (Waters, MA, USA) detector. The aim was to identify the most active fractions in the compounds of puffball. A C18 reversed-phase column (Hypersil Gold 25 mm × 2.1 mm, 1.8 μm, Thermo Scientific, Massachusetts, USA) was used with the following solvent system: A = acetonitrile, B = 0.15% ammonium acetate–water. The gradient elution was 7% A in 5 min, 7–10% A in 3 min, 10% A in 2 min, 10–15% A in 5 min, 15% A in 3 min, 15–5% A in 2 min. The LC system operated at flow rate of 1.5 ml/min for 20 min. The injection volume was 5 μl. The detection was at 330 nm. The bioactive fractions were recorded on a HPLC–MS/MS system which consisted of the HPLC Premier XE (Waters, MA, USA) connected through a split to the mass spectrometer (Waters, MA, USA) detector system operating at 45 °C. The MS analyses were performed using positive ion mode electrospray ionization mass spectrometry (ESI(+)-MS). The optimized system parameters were as follows: capillary voltage 3000 V, sample cone 30 V, source temperature 120 °C, desolvation temperature of 300 °C and collision energy 4 V, cone gas flow 70 l/h and desolvation gas flow of 350 l/h. Detection was performed in positive ion mode in the m/z range 50–1000.
Testify the antifungal activity of prepared compound PBR-1 and PBR-2 from puffball fermentation
The separation was carried out on semi-preparative C18 column (Phenomenex). A stock solution of supernatant of puffball fermentation was prepared. Another stock solution of blank control (PDB medium) puffball sporophore was prepared as same steps.
We compared the antifungal activity of the compound PBR-1 and PBR-2 from puffball fermentation with blank control (retention time from 8 to 12.5 min) by ZOI test, T. rubrum and T. mentagrophytes are main tinea pedis pathogens (Djeridane et al. 2006), so they were choosed as test microorganisms. T. rubrum and T. mentagrophytes were inoculated in a preliminarily melted and tempered to 45–48 °C PDA medium. The inoculated PDA media were transferred in quantity of 20 ml in sterilized Petri dishes (d = 9 cm) and allowed to solidify. After this, three wells (d = 6 mm) per dish were cut. Then the separation compound PBR-1 and PBR-2 from puffball fermentation was added into three wells (100 μl/well).
Results
In vitro antimicrobial assay
MIC values of prepared solution from puffball against fungi and bacteria are reported in Table 1. The most polar extracts of puffball showed antibacterial and antifungal activity against most tested microorganism. Fermentation supernatant from puffball showed good activity against the tested microorganism and the strongest activity was seen against T. rubrum, T. mentagrophytes, S. aureus and P. aeruginosa (MIC = 31.2 μg/ml), In addition, B. subtilis resisted all prepared solution of puffball (MIC ≥500 μg/ml). Chloroform, aether and ethyl acetate extracts of puffball demonstrated very close activities against all microorganism, all microorganism showed resistance for three extracts(MIC ≥500 μg/ml), while T. rubrum and T. mentagrophytes were also sensitive fungus to the n-butanol extract (MIC = 62.5 and 31.2 μg/ml) and aqueous extract (MIC = 31.2 μg/ml). E. floccosum and C. albicans were inhibited by high concentrations of aqueous and ethanol extracts (MIC = 125 and 62.5 μg/ml).
Table 1.
MIC values of fermentation supernatant and extracts of puffball
| Extracts/fractions of puffball | MIC (μg/ml) | |||
|---|---|---|---|---|
| Fungus | ||||
| T. rubrum | T. mentagrophytes | E. floccosum | C. albicans | |
| Fermentation supernatant | 31.2 | 31.2 | 62.5 | 125 |
| Ethanol extract | 62.5 | 62.5 | 125 | 125 |
| Chloroform extract | >500 | >500 | >500 | >500 |
| Aqueous extract | 31.2 | 31.2 | 62.5 | 62.5 |
| Ethyl acetate extract | >500 | >500 | >500 | >500 |
| n-butanol extract | 62.5 | 31.2 | 62.5 | 62.5 |
| Aether | >500 | >500 | >500 | >500 |
| Terbinafine | 31.2 | 15.6 | 62.5 | 7.8 |
| Extracts/fractions of puffball | MIC (μg/ml) | |||
|---|---|---|---|---|
| Bacteria | ||||
| B. subtilis | S. aureus | E. coli | P. aeruginosa | |
| Fermentation supernatant | 500 | 31.2 | 62.5 | 31.2 |
| Ethanol extract | 500 | 62.5 | 125 | 31.2 |
| Chloroform extract | >500 | >500 | >500 | >500 |
| Aqueous extract | 500 | 62.5 | 62.5 | 15.6 |
| Ethyl acetate extract | >500 | >500 | >500 | >500 |
| n-Butanol extract | 500 | 31.2 | 62.5 | 31.2 |
| Aether | >500 | >500 | >500 | >500 |
| Gentamicin sulfate | 15.6 | 15.6 | 31.2 | 31.2 |
The ZOI of the fermentation supernatant from puffball exhibited strong activity against test microorganisms in Table 2. Regarding antifungal activity, the strongest antifungal activity was observed using the fermentation supernatant from puffball with the ZOI value of 29.01 ± 2.17 mm, while the strongest antibacteria activity was the ZOI value of 35.02 ± 2.03 mm. The standard drug terbinafine achieved the antifungal activity against T. rubrum (MIC = 31.2 μg/ml, ZOI = 26.09 ± 3.06 mm), and gentamicin sulfate achieved the highest antibacteria activity against B. subtilis (MIC = 15.6 μg/ml, ZOI = 43.19 ± 0.98 mm).
Table 2.
ZOI values of fermentation supernatant and extracts of puffball
| Extracts/fractions of puffball | Zone of inhibition (mm) | |||
|---|---|---|---|---|
| Fungus | ||||
| T. rubrum | T. mentagrophytes | E. floccosum | C. albicans | |
| Fermentation supernatant | 29.01 ± 2.17 | 21.02 ± 2.05 | 12.03 ± 1.14 | 8.03 ± 1.02 |
| Ethanol extract | 10.03 ± 2.02 | 8.02 ± 1.12 | 0.00 ± 0.00 | 5.81 ± 0.35 |
| Chloroform extract | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Aqueous extract | 15.08 ± 1.05 | 17.28 ± 1.67 | 10.02 ± 1.21 | 11.36 ± 1.33 |
| Ethyl acetate extract | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| n-Butanol extract | 18.22 ± 3.06 | 15.65 ± 2.16 | 17.12 ± 2.14 | 12.36 ± 1.89 |
| Aether | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Terbinafine | 26.09 ± 3.06 | 25.04 ± 2.13 | 34.12 ± 3.02 | 38.41 ± 2.25 |
| Extracts/fractions of puffball | Zone of inhibition (mm) | |||
|---|---|---|---|---|
| Bacteria | ||||
| B. subtilis | S. aureus | E. coli | P. aeruginosa | |
| Fermentation supernatant | 8.01 ± 1.12 | 35.02 ± 2.03 | 15.03 ± 1.35 | 28.01 ± 1.59 |
| Ethanol extract | 0.00 ± 0.00 | 12.02 ± 1.12 | 6.03 ± 1.32 | 6.69 ± 1.79 |
| Chloroform extract | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Aqueous extract | 12.08 ± 1.38 | 22.28 ± 1.12 | 3.02 ± 0.92 | 15.22 ± 1.13 |
| Ethyl acetate extract | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| n-Butanol extract | 0.00 ± 0.00 | 18.11 ± 1.02 | 16.32 ± 0.97 | 17.35 ± 1.33 |
| Aether | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Gentamicin sulfate | 43.19 ± 0.98 | 31.03 ± 1.76 | 28.16 ± 2.06 | 32.12 ± 2.03 |
The date are mean ± SD of three repeats
Due to the resistance of pathogens soon developing against current drugs, it is important to questing for more effective and safer antimicrobial therapies. Natural product being reservoirs of various types of bioactive molecules are target of extensive research worldwide. In the present work, many extracts and fermentation of puffball were subjected to antimicrobial study against a number of pathogenic microorganism.
Extracts and fermentation of puffball showed antimicrobial activity in a dose-dependent manner against the test microorganisms. T. rubrum, T. mentagrophytes, P. aeruginosa, and S. aureus were the most sensitive microorganisms to the fermentation of puffball (MIC = 31.2), while the strongest activity was demonstrated against P. aeruginosa (MIC = 15.6 μg/ml) using the aqueous extract. Besides, fermentation and extracts of puffball showed better toxicity against T. rubrum, ZOI of fermentation supernatant, ethanol, aqueous and n-butanol were about 29.01, 10.03, 15.08 and 18.22 mm respectively, better antibacterial activity against S. aureus with ZOI of fermentation supernatant, aqueous, n-butanol and ethanol being about 35.02, 22.28, 18.11 and 12.02 mm respectively in Table 2. Notably, fermentation of puffball showed effective against bacteria and fungus. Anti-fungal and anti-bacteria compounds of puffball appeared in polar solvents and are of hydrophilic nature. This may provide a lead for an antibiotic for pathogen. In general, the fermentation supernatant and more polar extracts were better antimicrobial agents than less polar counterparts. The fermentation displayed especially notable antimicrobial efficacy, this may be due to the spore germination and the active substances are liberated by rupture of spore or the major secondary metabolites were released. T. rubrum and S. aureus were the most susceptible. Further investigation into this fraction has great prospects to yield exploitable natural products for future drugs.
Identification of active principle using liquid chromatography–mass spectrometry (LC–MS) analysis
As stated above, based on Chinese Pharmacopoeia puffball has not been reported to have the antifungal activity. In a preliminary set of MIC and ZOI experiments, fermentation of puffball show best antifungal activity, so it is important to separate antifungal activity substance from puffball fermentation supernatant. However, in most cases, the base peak was broad with a low peak purity and the separation of these compounds with satisfactory resolution was not achieved. Besides that, anti-tinea activity maybe due to synergistic effects of different substance. The separation of purified substance is a very difficult task. Thus, we decided to validate an HPLC/UV method to determine the total content of antifungal activity and LC/MS spectra method to determine characteristic [M + H] ion m/z.
Puffball was collected in the same place in Jishui county, Jiangxi province and showed similar antifungal activities. Through contrast test, antifungal activity of puffball fermentation supernatant was done. Standard PDB medium (without puffball) was set as blank control. These supernatants were analyzed by LC–MS. Puffball and blank control presented different chromatographic profile. The chromatogram of puffball revealed the presence of two main peaks F1 (9.69 min) and F2 (10.34 min) while blank control without these peaks. The whole process is represented in the chromatograms shown in Fig. 1.
Fig. 1.
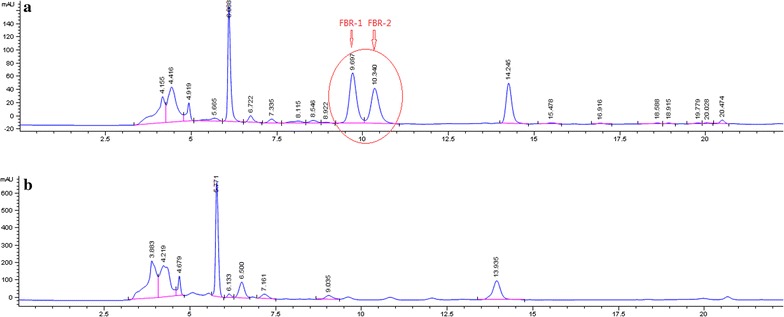
HPLC chromatograms of a the fermentation supernatant of puffball and b the blank control (PDB)
The characteristic peaks of fermentation were collected by preparation chromatographic for further studies. This compound was identified by comparison of HPLC retention time with blank control (standard PDB medium).
The difference is the characteristic peaks P1 and P2 inside the red circle between HPLC chromatograms of A and B, the component in P1 and P2 was named as PBR-1 and PBR-2, then we collected the compound PBR-1 and PBR-2, the same was for standard PDB medium (retention time from 8 to 12.5 min), antifungal activity test of the compound PBR-1 and PBR-2 was done. Test microorganisms were T. rubrum and T. mentagrophytes. Test method was ZOI (zone of inhibition). The test results are as follows.
Antifungal activity test of characteristic peak of puffball
Through ZOI test, we verified the antifungal activity of the compound PBR-1 and PBR-2 and PDB medium (retention time from 8 min to 12.5 min), the activity of antifungal was showed in Fig. 2. The red circle indicates the size of ZOI. The results showed the inhibition concentration is 50 μg/ml and the ZOI of T. rubrum and T. mentagrophytes are 34 and 27 mm respectively. The results showed in Table 3.
Fig. 2.
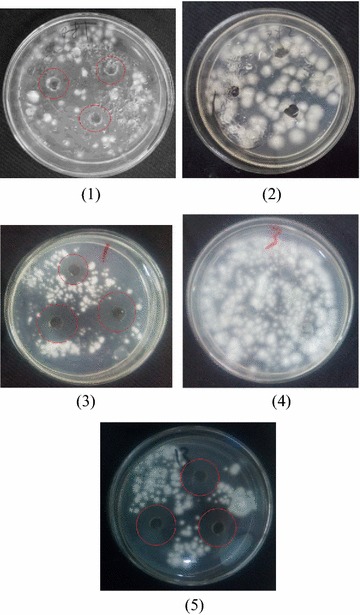
Antifungal activity of fermentation and aqueous extracts. (1) Contrast 1 is antifungal test of terbinafine, (2) contrast 2 is antifungal test of distilled water, (3) antifungal test of fermentation of puffball, (4) antifungal test of PDB medium, (retention time from 8 to 12.5 min), (5) antifungal activity of compound FBR-1 and FBR-2
Table 3.
MIC/ZOI values of compound PBR-1 and PBR-2 from puffball against pathogen
| Test items | T. rubrum | T. mentagrophytes | |
|---|---|---|---|
| PBR-1 and PBR-2 | MIC (μg/ml) | 15.6 | 15.6 |
| ZOI (mm) | 34 | 27 | |
| Terbinafine hydrochloride | MIC (μg/ml) | 31.2 | 15.6 |
| ZOI (mm) | 26 | 25 |
Minimum inhibitory concentration (MIC) was carried out to determine the active concentration of the compound PBR-1 and PBR-2 that inhibited the growth of tested microorganism. MIC of the compound PBR-1 and PBR-2 against pathogenic microbes T. Rubrum and T. mentagrophytes was 15.6 μg/ml (Table 3). Notably, the compound PBR-1 and PBR-2 showed better antifungal activity than positive control (terbinafine hydrochloride) and fermentation of puffball. The compound PBR-1 and PBR-2 provide a lead result for antifungal activity.
The compound PBR-1 and PBR-2 of puffball were the most abundant antifungal activity bioactive fractions. The following molecular weight were identified based on the UV spectrum, mass spectra and fragmentation patterns.
Purification and identification of the bioactive metabolite
The compound PBR-1 and PBR-2 were subjected to LCQTOF-MS/MS analysis in positive mode ionizations. The MS of bioactive F1 in positive mode revealed a peak of m/z 353 (M + H), while F2 in positive mode revealed a peak of m/z 353 (M + H) and a peak of m/z 413 (M + H) (Fig. 3). The MS/MS of 353 resulted in formation of fragments with m/z 191, 217, 279, 299. The MS/MS of 413 resulted in formation of fragments with m/z 375, 381, 399, 411, (Fig. 3). According to mass spectra and its fragmentation pattern corresponded to bioactive fractions which have molecular mass of 353 and 413 Da (Klyba et al. 2014; Vukics et al. 2008). Machining with molecular bank, molecular formula of 353 and 413 Da are C21H36O4 (352.49) and C24H44O5 (412.58) respectively.
Fig. 3.
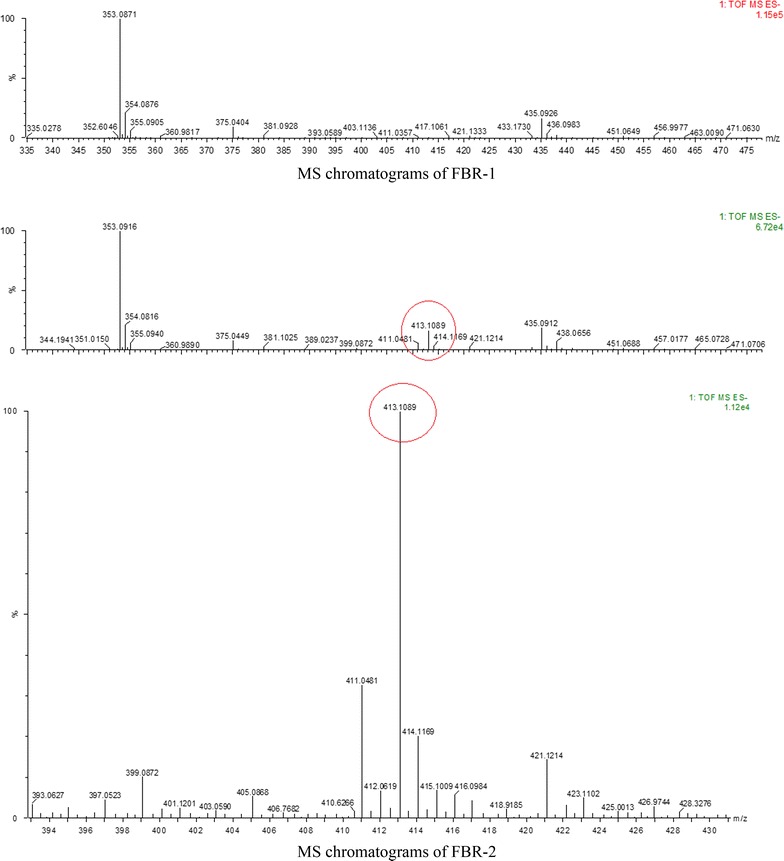
MS chromatograms of compound PBR-1 and PBR-2, the red circle in PBR-2 show m/z 413 (M + H) and detailed chromatograms
Fermentation supernatant of puffball has high activity against fungus, we conclude that water soluble antibacterial substances contain a lot of hydroxyl groups, based on Chinese pharmacopoeia, biological activity include steroidal, sesquiterpenes, flavonoids, alkaloids, puffball polysaccharide, fatty acid, proteins, amino acid and so on in puffball. It need to further study to identify molecular structure of antifungal properties from puffball and modify it to improve it’s anti-microbiological activity.
Discussion
Tinea pedis fungal infection pathogens mainly include T. rubrum, T. mentagrophytes and C. albicans, about 70% of clinical fungal infection pathogens is T. rubrum, 15% of clinical fungal infection pathogens is T. mentagrophytes, C. albicans and other bacterials share 15%. Puffball are widespread in natural environments and puffball species have been and continue to be extensively screened for their potential for producing useful natural products (Nedelcheva et al. 2007). Previously many researchers have reported that puffball produced antibacteria activity (Canli et al. 2016; Ng et al. 2003). The present study showed that fermentation supernatant of puffball(Bovistella radicata) had potent antifungal and antibacterial activity with MIC value of 31.2 μg/ml, Maximum zone of inhibition (ZOI) was observed against T. rubrum (29.01 mm), T. mentagrophytes (21.02 mm), E. floccosum (12.03 mm), C. albicans (8.03 mm), B. subtilis (8.01 mm), S. aureus (35.02 mm), E. coli (15.03 mm), P. aeruginosa (28.01 mm). The antifungal activity of puffball may be attributed to an array of secondary metabolites by its spore germination, then the active substances are liberated by rupture of spore or the major secondary metabolites were produced just like steroid saponins, sesquiterpenes, flavonoids, alkaloids, puffball polysaccharide, fatty acids, amino acid, proteins and peptides and so on (Cantrell et al. 1999; Lam et al. 2001; Kamo et al. 2006). The present study revealed that puffball isolated from JiangXi province showed good antimicrobial activity against tested microbes in preliminary screening.
Steroid saponins have been reported to possess a wide range of biological activities including antibacterial (Gan et al. 2014), antifungal (Wang et al. 2015), immuno inhibitory (Nunez 1988). The bioactivity of steroidal is due to the presence of a steroid nucleus in its structure, which can be damage to the membrane and leakage of cellular materials, ultimately leading to bacteria and fungus death (Moulin-Traffort et al. 1998). So steroidal saponins maybe also have a significant role against the growth of T. rubrum and T. mentagrophytes. The role of the isolated steroidal compounds on persons’ health should be further investigated to understand its action in vivo. Sesquiterpene lactones possessed very high antifungal activity (Vajs et al. 1999). The biological activity of sesquiterpene lactones is generally attributed to the alkylating property of the α-methylene-γ-lactone moiety, and the presence of other alkylating sites (epoxides and conjugated carbonyl groups) may enhance their biological activities. Moreover, their lipophilicity seems to play an important role in antifungal activity. Since the chemical composition of the fungal cells walls is highly lipophilic, they generally represent strong barriers for the penetration of hydrophilic compounds, and the transport of polar compounds through the outer lipid layer of fungi is retarded (Skaltsa et al. 2000). Flavonoids can complex with extracellular soluble proteins and bacterial cell walls, maybe it is the reason which Flavonoids possess antimicrobial activity (Hossain et al. 2013), more lipophilic flavonoids may also disrupt microbial membranes (Ghanbari et al. 2012).
The main mechanism of the anti-microorganism of polysaccharide is to suppress signal recognition mechanism of the microorganism, which may be related with microorganism growth and mutual recognition (Schmidtchen and Malmsten 2015). Alkaloids isolated from plant are commonly found to have antimicrobial properties (Jiang et al. 2016). Berberine and hormone are important representatives of the alkaloid group. The mechanism of action of highly aromatic planar quaternary alkaloids such as berberine and harmane is attributed to their ability to intercalate with DNA (Jiang et al. 2011).
The chemical profile was analyzed in fermentation supernatant of puffball; it revealed the diversity of secondary metabolites. In future we may isolate the active molecules from the fermentation and use it as drugs for the control of microbes causing infectious diseases.
Puffball(Bovistella radicata) from Jiangxi province showed antibacterial and antifungal activity in contrast to the rest of puffball which had no detectable antifungal activity. Based on the Pharmacopeia, the main anti-microorganism function of puffball is anti-S. aureus and P. aeruginosa, the different results concerning the anti-microorganism activity of puffball might be due to different geographic sources of the material used, different types of strains used, and different assay methods (Awadh et al. 2003).
Since puffball demonstrated activity against the most prevalent microorganism, the use of puffball as anti-tinea pedis is validated, scientifically supported by the results obtained in this work.
Authors’ contributions
YY collected the data, created figures, and drafted the manuscript with the guidance of corresponding author (QZ). Co-authors helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The author wish to thank Dr. Kun liu and Qinghua Zeng (from School of Food Science and Engineering, Hefei University of Technology , Anhui, China) for help in experiment.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data are fully available without restriction.
Consent for publication
This manuscript does not contain any individual person’s data.
Ethics approval and consent to participate
No animal or human subjects were used in this work.
Funding
This study was funded by the National Natural Science Foundation of China (Grant Nos. 31371844; 31071556) and Science and Technology Department of Anhui province, PR of China (Grant No. 1301032155).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- UPLC
ultra-performance liquid chromatography
- UV
ultraviolet
- MS
mass
- TOF
time of flight
- ZOI
zones of inhibition
- MIC
minimum inhibitory concentrations
- PBR
peak of Bovistella radicata
- PDA
potato dextrose agar
- PDB
potato dextrose broth
Contributor Information
Yong Ye, Email: yeyong_wen@126.com.
Kun Liu, Email: 729462498@qq.com.
Qinghua Zeng, Email: 121148625@qq.com.
Qingmei Zeng, Email: zengqingmei-1@163.com.
References
- Awadh Ali NA, Mothana RA, Lesnau A, Pilgrim H, Lindequist U. Antiviral activity of Inonotus hispidus. Fitoterapia. 2003;74:483–486. doi: 10.1016/S0367-326X(03)00119-9. [DOI] [PubMed] [Google Scholar]
- Bourdeau P, Marchand A, Etoré F. P-17 In vitro activity of posaconazole and other antifungals against Malassezia pachydermatis isolated from dogs. Vet Dermatol. 2004;15:45–46. [Google Scholar]
- Canli K, Altuner EM, Akata I, Turkmen Y, Uzek U. In vitro antimicrobial screening of Lycoperdon lividium and determination of the ethanol extract composition by gas chromatography/mass spectrometry. Bangl. J. Pharmacol. 2016;2:391–394. [Google Scholar]
- Cantrell CL, Rajab MS, Franzblau SG, Fronczek FR, Fischer NH. Antimycobacterial ergosterol-5, 8-endoperoxide from Ajuga remota. Planta Med. 1999;65:732–734. doi: 10.1055/s-1999-14053. [DOI] [PubMed] [Google Scholar]
- Cornaglia G. Fighting infections due to multidrug-resistant Gram-positive pathogens. Clin Microbiol Infect. 2009;15:209–211. doi: 10.1111/j.1469-0691.2009.02737.x. [DOI] [PubMed] [Google Scholar]
- Cotter G, Kavanagh K. Adherence mechanisms of Candida albicans. Br J Biomed Sci. 2000;57:241–249. [PubMed] [Google Scholar]
- Dharajiya D, Patel P, Moitra N. Antibacterial activity of Emblica officinalis (Gaertn.) Fruits and Vitex negundo (L.) Leaves. Curr Trends Biotechnol Pharm. 2015;9:357–368. [Google Scholar]
- Djeridane A, Djeridane Y, Ammar-Khodja A. Epidemiological and aetiological study on tinea pedis and onychomycosis in Algeria. Mycoses. 2006;49:190–196. doi: 10.1111/j.1439-0507.2006.01230.x. [DOI] [PubMed] [Google Scholar]
- Erbagci Z, Tuncel A, Zer Y, Balci I. A prospective epidemiologic survey on the prevalence of onychomycosis and dermatophytosis in male boarding school residents. Mycopathologia. 2005;159:347–352. doi: 10.1007/s11046-004-5493-2. [DOI] [PubMed] [Google Scholar]
- Gan C, Cui J, Su S, Lin Q, Jia L, Fan L, Huang Y. Synthesis and antiproliferative activity of some steroidal thiosemicarbazones, semicarbazones and hydrozones. Steroids. 2014;87:99–107. doi: 10.1016/j.steroids.2014.05.026. [DOI] [PubMed] [Google Scholar]
- Geetha R, Sathian CT, Prasad V, Gleeja VL. Efficacy of purified antimicrobial peptides from lactic acid bacteria against bovine mastitis pathogen. Asian J Dairy Food Res. 2015;34:259–264. doi: 10.18805/ajdfr.v34i4.6874. [DOI] [Google Scholar]
- Ghanbari R, Anwar F, Alkharfy KM, Gilani AH, Saari N. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.)—a review. Int J Mol Sci. 2012;13:3291–3340. doi: 10.3390/ijms13033291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper S (1992) Standard methods for the examination of dairy products, 11th ed. American Public Health Association, Washington. 11:940–941
- Hossain MA, AL-Raqmi KA, AL-Mijizy ZH, Weli AM, Al-Riyami Q, Mohammad AH, Khulood AS. Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown Thymus vulgaris. Asian Pacific J Trop Biomed. 2013;3:705–710. doi: 10.1016/S2221-1691(13)60142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Eliaz I, Sliva D. Suppression of growth and invasive behavior of human prostate cancer cells by ProstaCaid: mechanism of activity. Int J Oncol. 2011;38:1675–1682. doi: 10.3892/ijo.2011.996. [DOI] [PubMed] [Google Scholar]
- Jiang QW, Chen MW, Cheng KJ, Yu PZ, Wei X, Shi Z. Therapeutic potential of steroidal alkaloids in cancer and other diseases. Med Res Rev. 2016;36:119–143. doi: 10.1002/med.21346. [DOI] [PubMed] [Google Scholar]
- Kamo T, Kashiwabara M, Tanaka K, Tanaka K, Ando S, Shibata H, Hirota M. Plant growth inhibitory activity of azo-and azoxyformamides from Calvatia craniiformis and Lycoperdon hiemale. Nat Prod Res. 2006;20:507–510. doi: 10.1080/14786410600649596. [DOI] [PubMed] [Google Scholar]
- Klyba L, Nedolya N, Tarasova O, Sanzheeva E. Mass spectra of new heterocycles Main fragmentation pathways of the molecular ions of 5-methylsulfanyl-1-vinyl-1 H-pyrrol-2-amines under electron impact and chemical ionization. Russ J Org Chem. 2014;50:35–44. doi: 10.1134/S1070428014010072. [DOI] [Google Scholar]
- Koltin Y, Hitchcock CA. The search for new triazole antifungal agents. Curr Opin Chem Biol. 1997;1:176–182. doi: 10.1016/S1367-5931(97)80007-5. [DOI] [PubMed] [Google Scholar]
- Lam YW, Ng TB, Wang HX. Antiproliferative and antimitogenic activities in a peptide from puffball mushroom Calvatia caelata. Biochem Biophys Res Commun. 2001;289:744–749. doi: 10.1006/bbrc.2001.6036. [DOI] [PubMed] [Google Scholar]
- Moulin-Traffort J, Favel A, Elias R, Regli P. Study of the action of alpha-hederin on the ultrastructure of Candida albicans. Mycoses. 1998;41:411–416. doi: 10.1111/j.1439-0507.1998.tb00362.x. [DOI] [PubMed] [Google Scholar]
- Nedelcheva D, Antonova D, Tsvetkova S, Marekov I, Momchilova S, Nikolova-Damyanova B, Gyosheva M. TLC and GC-MS probes into the fatty acid composition of some Lycoperdaceae mushrooms. J Liq Chromatogr Relat T. 2007;30:2717–2727. doi: 10.1080/10826070701560629. [DOI] [Google Scholar]
- Negi PS, Anandharamakrishnan C, Jayaprakasha GK. Antibacterial activity of Aristolochia bracteata root extracts. J Med Food. 2003;6:401–403. doi: 10.1089/109662003772519994. [DOI] [PubMed] [Google Scholar]
- Ng TB, Ying WL, Wang H. Calcaelin, a new protein with translation-inhibiting, antiproliferative and antimitogenic activities from the mosaic puffball mushroom Calvatia caelata. Planta Med. 2003;69:212–217. doi: 10.1055/s-2003-38492. [DOI] [PubMed] [Google Scholar]
- Novaković AR, Karaman MA, Matavulj MN, Pejin BM, Belović MM, Radusin TI, Ilić NM. An insight into in vitro bioactivity of wild-growing puffball species Lycoperdon perlatum (Pers) 1796. Food Feed Res. 2015;42:51–58. doi: 10.5937/FFR1501051N. [DOI] [Google Scholar]
- Nunez EA. Modulation of cell-mediated immune response by steroids and free fatty acids in AIDS patients: a critical survey. Tumour Biol. 1988;9:225–232. doi: 10.1159/000217566. [DOI] [PubMed] [Google Scholar]
- Petar R, Ivica D, Jelena T, Tanja B, Irena V, Dušanka M, Slaviša S. Antimicrobial activity of serbian propolis evaluated by means of MIC, HPTLC. Bioautography Chemom Plos One. 2016;11:1–15. doi: 10.1371/journal.pone.0157097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtchen A, Malmsten M. (Lipo) polysaccharide interactions of antimicrobial peptides. J Colloid Interface Sci. 2015;449:136–142. doi: 10.1016/j.jcis.2014.11.024. [DOI] [PubMed] [Google Scholar]
- Sen A, Gurbuz B, Gurer U, Bulut G, Bitis L. Flavonoids and biological activities of Centaurea stenolepis. Chem Nat Compd. 2014;50:128–129. doi: 10.1007/s10600-014-0886-z. [DOI] [Google Scholar]
- Skaltsa H, Lazari D, Panagouleas C, Georgiadou E, Garcia B, Sokovic M. Sesquiterpene lactones from Centaurea thessala and Centaurea attica. Antifung Act Phytochem. 2000;55:903–908. doi: 10.1016/S0031-9422(00)00254-5. [DOI] [PubMed] [Google Scholar]
- Subissi A, Monti D, Togni G, Mailland F. Ciclopirox recent nonclinical and clinical data relevant to its use as a topical antimycotic agent. Drugs. 2010;70:2133–2152. doi: 10.2165/11538110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Tian S, Shi Y, Zhou X, Ge L, Upur H. Total polyphenolic (flavonoids) content and antioxidant capacity of different Ziziphora clinopodioides Lam. Extr Pharmacogn Mag. 2011;7:65–68. doi: 10.4103/0973-1296.75904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajs V, Todorović N, Ristić M, Tesević V, Todorović B, Janaćković P, Marin P, Milosavljević S. Guaianolides from Centaurea nicolai: antifungal activity. Phytochemistry. 1999;52:383–386. doi: 10.1016/S0031-9422(99)00207-1. [DOI] [PubMed] [Google Scholar]
- Vukics V, Kery A, Bonn GK, Guttman A. Major flavonoid components of heartsease (Viola tricolor L.) and their antioxidant activities. Anal Bioanal Chem. 2008;390:1917–1925. doi: 10.1007/s00216-008-1885-3. [DOI] [PubMed] [Google Scholar]
- Wang AR, Song HC, An HM, Huang Q, Luo X, Dong JY. Secondary metabolites of plants from the genus chloranthus: chemistry and biological activities. Chem Biodivers. 2015;12:451–473. doi: 10.1002/cbdv.201300376. [DOI] [PubMed] [Google Scholar]
- Xiao JC, Chen HM, Dian K. Qualitatively and quantitatively investigating the regulation of intestinal microbiota on the metabolism of panax notoginseng saponins. J Ethnopharmacol. 2016;194:324–336. doi: 10.1016/j.jep.2016.09.027. [DOI] [PubMed] [Google Scholar]
- Zampini IC, Cuello S, Alberto MR, Ordoñez RM, D’Almeida Solorzano ER, Isla MI. Antimicrobial activity of selected plant species from “the Argentine Puna” against sensitive and multi-resistant bacteria. J Ethnopharmacol. 2009;124:499–505. doi: 10.1016/j.jep.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Zhang BL, Fan CQ, Dong L, Wang FD, Yue JM. Structural modification of a specific antimicrobial lead against Helicobacter pylori discovered from traditional Chinese medicine and a structure-activity relationship study. Eur J Med Chem. 2011;45:5258–5264. doi: 10.1016/j.ejmech.2010.08.045. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhu X, Luo F, Sun C, Huang J, Li X, Chen K. Separation and purification of neohesperidin from the albedo of Citrus reticulata cv. Suavissima by combination of macroporous resin and high-speed counter-current chromatography. J Sep Sci. 2012;35:128–136. doi: 10.1002/jssc.201100695. [DOI] [PubMed] [Google Scholar]
- Zuo GY, Han ZQ, Hao XY. Synergy of aminoglycoside antibiotics by 3-Benzylchroman derivatives from the Chinese drug Caesalpinia sappan against clinical methicillin-resistant Staphylococcus aureus (MRSA) Phytomedicine. 2014;21(7):936–941. doi: 10.1016/j.phymed.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Zuo GY, Wang CJ, Han J, Li YQ, Wang GC. Synergism of coumarins from the Chinese drug Zanthoxylum nitidum with antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA) Phytomedicine. 2016;23(14):1814–1820. doi: 10.1016/j.phymed.2016.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are fully available without restriction.


