Abstract
Increasing evidence indicates that multiple structures in the brain are associated with intelligence and cognitive function at the network level. The association between the grey matter (GM) structural network and intelligence and cognition is not well understood. We applied a multivariate approach to identify the pattern of GM and link the structural network to intelligence and cognitive functions. Structural magnetic resonance imaging was acquired from 92 healthy individuals. Source-based morphometry analysis was applied to the imaging data to extract GM structural covariance. We assessed the intelligence, verbal fluency, processing speed, and executive functioning of the participants and further investigated the correlations of the GM structural networks with intelligence and cognitive functions. Six GM structural networks were identified. The cerebello-parietal component and the frontal component were significantly associated with intelligence. The parietal and frontal regions were each distinctively associated with intelligence by maintaining structural networks with the cerebellum and the temporal region, respectively. The cerebellar component was associated with visuomotor ability. Our results support the parieto-frontal integration theory of intelligence by demonstrating how each core region for intelligence works in concert with other regions. In addition, we revealed how the cerebellum is associated with intelligence and cognitive functions.
Introduction
The biological underpinning of intelligence and cognitive ability has long been of interest to neuroscientists1. Early approaches, such as brain lesion studies, focused on specific regions of the brain and linked those regions to intelligence and cognitive function2–4. The development of neuroimaging techniques enabled neuroscientists to investigate the human brain in vivo, and evidence obtained from neuroimaging studies suggests that no single brain region has an exclusively dominant effect on intelligence5, 6. However, an extensive literature review supported the parieto-frontal integration theory, which claims that the parietal cortex and the frontal cortex are the core regions involved in intelligence7. The parieto-frontal integration theory has been substantiated by various neuroimaging studies, including focal brain lesion8, 9 and magnetic resonance spectroscopy studies10. Among structural neuroimaging studies, more than 40% have indicated that both frontal and parietal regions are correlated with intelligence7. Recent neuroimaging studies have investigated the neural basis of intelligence at the network level. Studies using diffusion tensor imaging (DTI) have revealed that the structural organization of white matter (WM) at the network level11 and its developmental trajectory12 are important in intelligence. Resting-state functional magnetic resonance imaging (MRI) studies have indicated that interactions between parietal and frontal regions are important for intellectual functioning13–15. However, to date, the network-level underpinnings of intelligence have been derived primarily from WM structural or functional MRI studies.
There is firm evidence that grey matter (GM) is associated with intelligence6, 16, 17. However, there is a limited number of studies demonstrating how the GM structural network is linked to intelligence. Previous studies have used the graph theoretical approach to assess how GM regions form structural networks18–21. However, for the graph theoretical analysis, the region of interest must be determined prior data analysis. Thus, other important regions that could potentially contribute to intelligence, such as the cerebellum, are often overlooked18. To extract structural networks without any prior assumption, we applied a recently developed approach, source-based morphometry (SBM), to structural MRI to examine the relationship of GM structural networks to intelligence and cognitive functions. SBM applies independent component analysis (ICA) to a segmented image and arranges voxels into sets that contain similar information22. Whereas the voxel-based morphometry (VBM) approach is a univariate approach that automatically segments brain images into voxelwise measures of GM23, SBM, the multivariate extension of VBM, acquires common morphological features of GM concentration among individuals at the network level and thus provides us with structural networks. We expect that SBM will enable us to uncover submerged regional networks within the brain and further provide us with a more comprehensive picture of GM structural networks. Another advantage of SBM is that this method can remove sources arising from artefacts. There is some disagreement among studies that have used VBM to demonstrate an association between intelligence and brain structure. While Gong et al. reported a correlation between intelligence and medial prefrontal GM concentration17, Colom et al. found that intelligence was associated with GM concentration in diverse brain regions, more significantly for temporal and occipital areas than for the frontal lobes24. We suggest that the brain networks subserving intelligence may be better identified with a less noisy source of interest, which can be acquired from SBM.
In addition to investigating the relationship between structural networks and intelligence, we investigated structural networks in relation to cognitive functions. Verbal fluency, processing speed, and executive functioning have been reported to be particularly relevant to brain structural networks25–30. However, this relevance has not been shown at GM structural network level. To assess the cognitive functions, we administered the Trail Making Test (TMT) and the Controlled Oral Word Association Test (COWAT) to the participants. The TMT has been reported to measure cognitive functions and consists of two parts: Part A (TMT-A), which is known to evaluate visual search and processing speed31, and Part B (TMT-B), which is known to assess set-shifting and controlled attention ability32. The COWAT, one of the most frequently applied tests used to assess verbal fluency33, is also known to measure verbal working memory34. The cognitive functions were further compared with the GM structural network.
We hypothesized that intelligence is associated with parietal and frontal GM structural network and that different structural networks interact with different domains of cognitive functions. To test this hypothesis, we first acquired GM structural networks using SBM. After acquiring structural networks, we investigated the relationship between the structural networks and intelligence. In addition, we explored how cognitive functions, assessed with the TMT and COWAT, are associated with the GM structural network.
Results
Demographic and Cognitive Characteristics
Ninety-two healthy participants aged 17 to 48 years, with intelligence quotients (IQs) ranging from 83 to 137, were included in the study. The descriptive statistics of the demographic and cognitive characteristics are presented in Table 1. There were no gender differences in demographics (e.g., age, IQ, and education year) and in neuropsychological data, except for the number of errors made in both parts A and B of TMT. Statistics on gender differences in demographic and cognitive characteristics are summarized in Supplementary Table S1.
Table 1.
The statistics for the demographic and cognitive characteristics of the study sample. IQ: Intelligence quotient, TMT: Trail Making Test, M: Male, F: Female, COWAT: Controlled Oral Word Association Test. aEstimated IQ was measured by the short form of the K-WAIS.
| Mean | SD | ||
|---|---|---|---|
| Age (years) | 26.10 | 6.87 | |
| Gender (M/F) | 54/38 | ||
| Education (years) | 14.61 | 1.77 | |
| Estimated IQa | 113.90 | 11.79 | |
| TMT | Part A (seconds) | 23.06 | 6.97 |
| (M: 50/F: 33) | Part B (seconds) | 54.83 | 17.59 |
| Part B - Part A (seconds) | 31.77 | 15.67 | |
| Part A (# of errors) | 0.14 | 0.39 | |
| Part B (# of errors) | 0.27 | 0.52 | |
| COWAT | Category (No. of responses) | 42.32 | 8.91 |
| (M: 42/F: 29) | Letter (No. of responses) | 45.15 | 10.09 |
Component Estimation and Comparison with Functional Networks
Six components were extracted from the GM images according to the minimum description length criteria (Fig. 1 and Supplementary Table S2)35. There were no significant gender differences in structural components (see Supplementary Table S1). All the components were visually inspected, and 5 out of 6 components agreed with the functional MRI-based networks. As the scale (including the sign) of the components cannot be determined by the ICA, we used an absolute value of a correlation coefficient for the comparison. Similar to the findings by Smith et al.36, the maximal absolute correlation coefficients between the functional MRI networks and SBM components in the present study were as follows: |R| = 0.55 for the “visual” network (Map 120) and precuneus component; |R| = 0.28 for the “auditory” network (Map 720) and fronto-temporal component; |R| = 0.33 for the “executive control” network (Map 820) and cerebello-parietal component; |R| = 0.34 for the “default mode network” (Map 420) and frontal component; |R| = 0.34 for the “cerebellum” network (Map 520) and cerebellar component; and |R| = 0.10 for the “sensorimotor” network (Map 620) and temporal component (see Supplementary Fig. S1). Although the Bonferroni-corrected P-values were extremely small due to the large number of voxels, one SBM component showed negligible agreement with the resting state network (RSN) (|R| = 0.10). Five other SBM components showed high agreement (between 0.28 and 0.55) with the known RSNs. This result is consistent with the previous SBM studies that have reported high correspondence between the structural and functional covariance structures37, 38.
Figure 1.
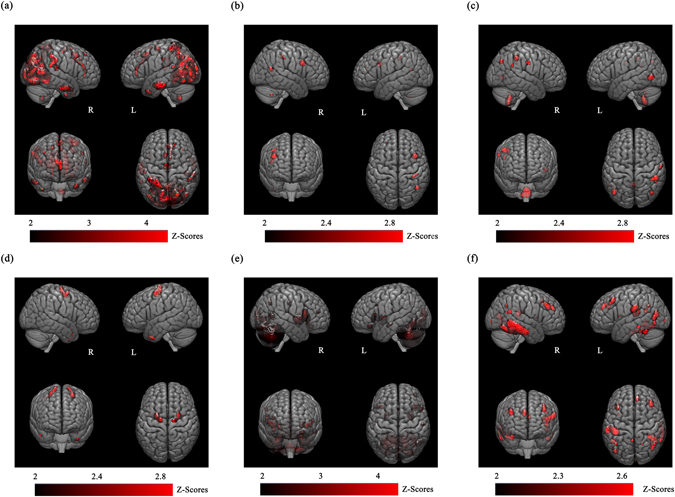
The structural components discovered by source-based morphometry. (a) Precuneus component; (b) fronto-temporal component; (c) cerebello-parietal component; (d) frontal component; (e) cerebellar component; (f) temporal component. All displayed networks had a threshold of Z > 2. The colour bar indicates the Z-score (of the contribution of each voxel to the component).
Relationship between Structural Networks and Neuropsychological Data
The correlation analysis revealed that individuals’ intelligence was associated with the cerebello-parietal component (R = 0.264, P = 0.011) (see Supplementary Fig. S1c) and the frontal component (R = 0.288, P = 0.005) (see Supplementary Fig. S1d). When the associations were separately analysed for males and females, females demonstrated a significant positive association in both the cerebello-parietal component (R = 0.330, P = 0.043) and the frontal component (R = 0.371, P = 0.022), whereas males did not show such associations (the cerebello-parietal component: R = 0.217, P = 0.116; the frontal component: R = 0.207, P = 0.134) (Fig. 2). The cerebello-parietal component and the frontal component were significantly correlated (R = 0.727, P < 0.001). When we controlled for the influence of the cerebello-parietal component on intelligence, there was no significant association between the frontal component and intelligence (R = 0.141, P = 0.182). The same was observed when the influence of the frontal component was considered for the correlation analysis between the cerebello-parietal component and intelligence (R = 0.075, P = 0.483). Multiple regression analysis predicted loading coefficients of the cerebellar component (F(1, 81) = 9.314, P = 0.003), with an R² = 0.321. The completion time (in seconds) of TMT-A (Beta = −0.321, P = 0.003) significantly contributed to the multiple regression model (Fig. 3). The completion time (in seconds) of neither TMT-B nor B-A significantly contributed to multiple regression models predicting loading coefficients of any structural component. In addition, we observed a marginally significant association between the cerebello-parietal component and the number of responses from the letter fluency of the COWAT (R = 0.235, P = 0.052).
Figure 2.
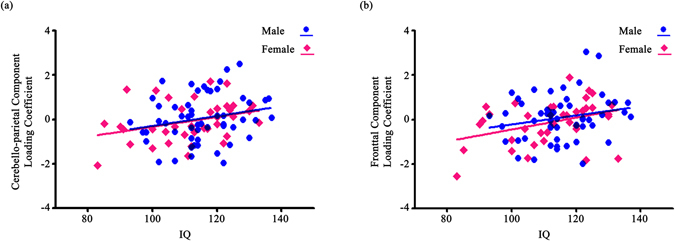
The association between structural networks and intelligence quotient (IQ). (a) Correlation between intelligence and the cerebello-parietal component; (b) correlation between intelligence and the frontal component.
Figure 3.
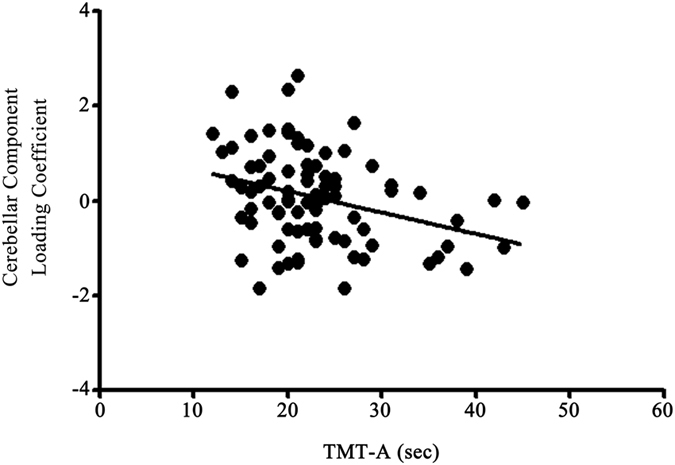
The association between the cerebellar component and results from the Trail Making Test, Part A (TMT-A).
Discussion
Our study examined GM structural networks by applying a multivariate morphometric procedure and further revealed the networks’ relationships with intelligence and visuomotor ability (TMT-A). Our results showed that the cerebello-parietal component and the frontal component were associated with intelligence. To our knowledge, this is the first study to link GM anatomical connectivity between the parietal cortex and cerebellum in humans in vivo and to further associate this structural connectivity with intelligence. In addition, the cerebellar component was associated with TMT-A, which measures visual search ability and processing speed. By determining the morphometric variation patterns of the brain through the use of SBM, we demonstrated how the core regions are associated with other brain regions and contribute to intelligence.
Five of the 6 structural networks were spatially similar to previously reported functional networks. In multimodal studies in which ICA was applied across different modalities, the structural networks were comparable to the networks extracted from DTI and functional MRI studies38, 39. Similarly, in our structural components, we observed characteristics that were assumed to be unique to the functional networks. Our cerebello-parietal component and the frontal component demonstrated patterns similar to the executive control network and the default-mode network, respectively. Both functional networks, the executive control network and the default-mode network, were reported to be associated with intelligence14, 40. Functional MRI indirectly measures neuronal cell activity, which mainly occurs in GM41. However, only a few studies have established the association between the GM structural networks and functional networks38, 42, 43. By linking the GM structural networks to the functional networks and further to intelligence and cognitive functions, our results shed light on the importance of GM structural investigation at the network level.
Cerebellum and parietal regions form the cerebello-parietal component, which was associated with intelligence. In primates, an anatomical connection between the parietal and pontine regions was revealed44, and the pons was further shown to be extensively connected with cerebellar cortical areas via the middle cerebellar peduncle45. This WM parieto-ponto-cerebellar tract was also revealed in human studies using DTI46. During phonological storage processing, co-activation of the cerebellar and parietal regions was reported47. In addition, during verbal encoding, transcranial direct current stimulation over the cerebellum affected the interaction between the cerebellum and the parietal cortex48. Both studies emphasize the importance of the cerebellar-parietal connection on the processes supporting verbal intelligence. In accordance with previous reports linking the cerebello-parietal network and verbal intelligence, we observed a marginally significant association between the cerebello-parietal component and verbal fluency. In addition, it is interesting to note that our structural analysis is consistent with other functional studies with regards to the more detailed anatomical characteristics of the cerebellum. In a resting-state functional MRI study, Buckner et al. investigated associations between the cerebellar and cerebral regions49. The study revealed that the default-mode network, lateral temporal cortex, and inferior parietal lobule were functionally connected with Crus I and II in the cerebellar cortex. Previous studies indicated the potential influence of this network on higher-level cognition49–51, and our cerebello-parietal component, composed of Crus II and the inferior parietal lobule, was associated with both intelligence and verbal fluency. Although many studies have reported that frontal and parietal regions play a critical role in intelligence, some studies have emphasized the importance of the cerebellum52–55. As noted by Jung and Haier7, relatively overlooked brain regions, such as the cerebellum, are also important for brain function, and the importance of the cerebellum in intelligence was revealed in our results.
The frontal component partially includes middle and superior temporal gyri of temporal region. For decades, many studies have reported that the frontal lobe is related to intelligence56–58, and to a lesser degree, it is also reported that the temporal lobe is involved in intelligence59. In the functional MRI and DTI studies that assessed brain regional network aspects, it is reported that the connectivity between frontal and temporal is related to cognitive function60–62, and that linkage abnormality between the two regions may be related to the pathophysiology of the mental illness63, 64. A previous study reported the influence of the language network, composed of frontal and temporal regions, on not only semantic but also syntactic processing65. We suggest that the intricate interaction between semantic and syntactic processing affects intelligence, and the interaction was reflected in the frontal component at GM structural level in our study.
Both the cerebello-parietal component and the frontal component are associated with intelligence. However, when the cerebello-parietal component was controlled for, the association between the frontal component and intelligence became non-significant. The same was observed in the relationship between the cerebello-parietal component and intelligence when the frontal component was controlled for. Hence, we suggest that two networks are intimately related in contributing to processes involved in intelligence. The parieto-frontal integration theory proposed two important brain regions associated with intelligence, and our results further suggest that both regions work in concert with different regions in the brain to support intelligence. It is interesting to note that the negative weighting of the cerebello-parietal component included the frontal regions, while the negative weighting of the frontal component included the parietal regions (see Supplementary Table S2). The negative weighting region of the SBM component means that the region has negative contributions to the structural network, indicating that the region demonstrates an opposite trend of GM concentration to the positively weighted regions39. By demonstrating an antithetical pattern of parietal and frontal interaction in both intelligence-related structural networks, we speculate that there may be a more complex mechanism underlying intelligence that cannot be monolithically explained by increased or decreased GM regional density. We suggest that greater GM density does not always indicate a higher intelligence level. How effectively neural cells are organized66, 67 and how efficiently brain regions interact each other may influence intelligence, but this possibility must be further explored.
We found that TMT-A performance was associated with the cerebellar component. The cerebellar component comprised of mainly cerebellar sensorimotor areas (lobules IV, V, and VI) along with occipital visual areas. Hence, it is not surprising to find an association between the cerebellar component and performance on the TMT-A that measures visual search ability and processing speed31. Strong evidence has recently emerged of a much more fundamental role for the cerebellum in higher cognitive processing than was previously considered53, 68. Accordingly, cerebellar volume changes were observed in patients with various mental disorders69–71, and that those with cerebellar damage had cognitive abnormalities in various domains72, 73. In this light, one might expect the cerebellar component to be associated with TMT-B (or TMT-B minus TMT-A), which measures the ability to control attention and set-shifting32. However, we did not find such an association. Thus, our results highlight the specificity of the cerebellar component for sensorimotor function measured with the TMT-A.
There are some limitations of our study. First, although some existing studies have applied SBM to explore structural networks by estimating structural covariance74, 75, more studies are needed to further validate this relatively new approach. Because our results were consistent with previous studies regarding the relationship between brain regions and cognition, the present study provides additional face validity for the SBM approach. Second, neuropsychological assessment tests were not applied to all participants. The performance of SBM analysis is expected to be better with more data22. For instance, the number of components that could be extracted is proportional to the number of participants35. Therefore, we tried to include more participants in our study, but missing data due to administration issues hindered the inclusion of more participants (e.g., the TMT outcome variables for 9 out of 92 participants and the COWAT outcome variables for 21 out of 92 participants were not available). We anticipate that a large amount of data will help us to extract more biologically interpretable sources that are beneficial in revealing structural networks related to the complex mechanisms of intelligence and cognition.
In summary, our multivariate morphometric analysis revealed structural networks consistent with previously reported functional networks. Among the extracted structural networks, the cerebello-parietal component and the frontal component were associated with intelligence. The multivariate approach enabled us to emphasize the role of a previously overlooked region, the cerebellum, in intelligence in conjunction with the parietal lobe. To our knowledge, this study is the first to link GM structural connectivity between parietal and cerebellum regions and to further link this connectivity with intelligence. In addition, the cerebellum was involved in verbal fluency within the cerebello-parietal component and visuomotor processing speed in conjunction with visual areas. By emphasizing the role of the frontal and parietal lobes in intelligence, our results provide additional information on how the cerebellum is associated with intelligence and visuomotor ability at the structural network level. Based on our study’s findings, future studies should investigate the association of the brain’s structural networks with intelligence and cognitive function using various approaches.
Methods
Participants
Ninety-two participants were selected from the subject pool of prospective cohort study projects at the Seoul National University Hospital and the Department of Brain and Cognitive Sciences at Seoul National University. The participants were recruited from the community via internet advertisement and screened with the Structured Clinical Interview of the DSM-IV, Non-Patient Version (SCID-NP)76. To control for any possible confounding variables and to only include mentally healthy individuals in the present study, exclusion criteria consisted of the following: (i) head injury, medical or neurological disorders, or any substance abuse; (ii) IQ below 80; (iii) less than 17 years of age; (iv) less than 10 years of education; or (v) any first- or second-degree relatives with a history of any psychiatric illness. During recruitment, only participants with the appropriate quality of MRI data were included in our cohort after the MRI data quality check, which was conducted by 2 independent researchers. Specifically, participants with MRI signal loss due to orthodontic appliances (n = 2) or dental implants (n = 1), were not included in our current dataset. Written informed consent was obtained from all participants after they had been completely informed of the study protocols. This study was approved by the Institutional Review Board of Seoul National University Hospital and all methods were performed in accordance with the relevant guidelines and regulations.
Neuropsychological Data
Intelligence was assessed with the short form of the Korean version of the Wechsler Adult Intelligence Scale (WAIS), which consists of Vocabulary, Arithmetic, Block Design, and Picture Arrangement subtests77. In addition, the TMT was administered to 83 participants. The TMT is composed of two parts: (i) TMT-A involves sequentially connecting numbers with a continuous line; (ii) TMT-B involves alternately connecting numbers and letters. The total number of errors and the response time (in seconds) were recorded. The COWAT78 was administered to 71 participants to measure their ability to generate words within a specific category and to name words beginning with a certain letter. The number of responses during the time limit (within 1 minute) was recorded. TMT (n = 9) or COWAT (n = 21) data were not available for 21 participants, as they were from our different neuroimaging studies. Nevertheless, these individuals were included because they followed identical MRI and IQ assessment procedures as with the remainder of participants.
Image Acquisition
Structural images were obtained with a Siemens 3 T Trio MRI scanner (Siemens Magnetom Trio, Erlangen, Germany) using a 12-channel head coil. The T1-weighted anatomical image was acquired using magnetization-prepared rapid gradient echo imaging (echo time [TE]/repetition time [TR] = 1.89/1670 ms, field of view [FOV] = 250 mm, flip angle = 9°, matrix = 256 × 256, voxel size = 1.0 × 1.0 × 1.0 mm3, 208 slices). The time required to collect the structural scan was 3 minutes and 54 seconds.
Image Preprocessing
Structural images were preprocessed according to the VBM pipeline79 in the Computational Anatomy Toolbox (CAT; http://dbm.neuro.uni-jena.de/cat/), which runs in the Statistical Parametric Mapping toolbox version 12 (SPM12; http://www.fil.ion.ucl.ac.uk/spm/). The toolbox segments a native structural image into GM, WM, and cerebrospinal fluid (CSF), based on the maximum a posteriori approach, which does not require information about the tissue probability prior to segmentation80. Then, affine registration to MNI space was applied, and the non-linear deformation parameter was subsequently calculated using the high-dimensional Dartel algorithm81. To remove noise, a multithreaded denoising filter was applied82. As suggested in previous studies22, 83, the GM images were smoothed with a full width at half maximum (FWHM) Gaussian kernel of 12 mm.
SBM Analysis
SBM applies ICA to segmented and smoothed structural images from the preprocessing step22. ICA was calculated using the Infomax algorithm implemented in the GIFT Toolbox (http://mialab.mrn.org/software/gift/). The number of estimated components was determined according to the minimum description length criteria35. We used ICASSO84 and repeated the estimation 20 times with bootstrapping to increase the stability of the estimated components. Each of the preprocessed GM volumes was separately converted into a one-dimensional vector. Ninety-two preprocessed images were arrayed into a GM image matrix in the dimension of the number of subjects by the number of GM voxels. Then, the image matrix was decomposed into a mixing matrix and a source matrix. As it was estimated according to the minimum description length criteria35, six components were extracted. Thus, the 92 subject-by-the number of GM voxels matrix was decomposed to a 92 subject-by-6 components matrix (mixing matrix) and a 6 component-by-GM voxels matrix (source matrix). The mixing matrix involves loading coefficients that demonstrate how each structural component contributes to the 92 subjects and thus contains information about the relationship between each subject and each component. The (Z-transformed) loading coefficients were further used to investigate the association between structural components and neuropsychological variables. The source matrix demonstrates how each component contributes to different GM voxels and thus involves spatial information about the structural components. The (Z-transformed) source matrix was used for visualization and labelling of structural components. Geographical information of each component identified in the source matrix was used in the visual inspection step to exclude artefact components in further analyses.
Comparison with Functional Networks
It has been demonstrated that the GM-based components are spatially well matched to the resting-state fMRI-based components35, 36. To validate that the source separation in our data was also biologically meaningful, each component extracted from SBM was compared with previously reported functional networks36. The functional networks were composed of 10 well-matched maps that were independently derived from both the activation meta-analysis and resting-state data36. The component maps were downloaded from (http://fsl.fmrib.ox.ac.uk/analysis/brainmap+rsns/), and a detailed description of how the functional connectivity maps were obtained can be found in Smith et al.36. The components extracted from the SBM and functional network components were compared based on spatial cross-correlation (i.e., Pearson’s correlation between vectorized images).
Statistical Analysis
The associations between the GM structural components and neuropsychological data were estimated. As the measure of intelligence was already adjusted for the effect of age and gender, Pearson’s correlation analysis was applied to find the association between intelligence and structural connectivity loading coefficient values. In addition, for the structural networks that were significantly associated with intelligence, we conducted the Pearson’s correlation analysis separately for males and females. To control for the effect of age and gender, partial correlation analysis was applied to find the association between the COWAT outcome values and the loading coefficient values of each structural component. Because the TMT-A reflects visual processing abilities and the TMT-B reflects working memory and set-shifting abilities, the response time of TMT-B was subtracted by TMT-A (B-A) to remove the effect of processing speed ability and to observe executive control abilities85. Multiple regression analyses were conducted to examine the relationship between structural networks and TMT outcomes, including completion time (in seconds) of TMT and number of errors made during TMT. Each multiple regression analysis was performed with the loading coefficient of each structural component as the dependent variable. Independent variables consisted of TMT outcomes, including completion time of TMT and number of errors made during TMT, as well as other factors that could affect the TMT outcome, such as age and gender. Each of the TMT outcomes, TMT-A, TMT-B, and B-A, was analysed separately using different multiple regression analyses. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY).
Electronic supplementary material
Acknowledgements
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Science, ICT and Future Planning (Grant no. 2016R1E1A1A02921618).
Author Contributions
Y.B.Y. analysed the data and wrote the manuscript. T.Y.L. was responsible for recruitment and study procedures. Y.S.S. and J.W.H. were involved in the neuropsychological evaluation. K.K.C. and W.S.S. acquired the MRI data. K.H.L. and S.G.K. participated in theoretical development. J.S.K. was responsible for all aspects of the study. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-02304-z
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gall, F. J. Sur les fonctions du cerveau et sur celles de chacune de ses parties (J.-B. Baillière, 1825).
- 2.Takayama Y, Sugishita M, Akiguchi I, Kimura J. Isolated acalculia due to left parietal lesion. Arch. Neurol. 1994;51:286–291. doi: 10.1001/archneur.1994.00540150084021. [DOI] [PubMed] [Google Scholar]
- 3.Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J. Neurosci. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duncan J, Burgess P, Emslie H. Fluid intelligence after frontal lobe lesions. Neuropsychologia. 1995;33:261–268. doi: 10.1016/0028-3932(94)00124-8. [DOI] [PubMed] [Google Scholar]
- 5.Duncan J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn. Sci. 2010;14:172–179. doi: 10.1016/j.tics.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Basten U, Hilger K, Fiebach CJ. Where smart brains are different: a quantitative meta-analysis of functional and structural brain imaging studies on intelligence. Intelligence. 2015;51:10–27. doi: 10.1016/j.intell.2015.04.009. [DOI] [Google Scholar]
- 7.Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav. Brain Sci. 2007;30:135–154. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- 8.Woolgar A, et al. Fluid intelligence loss linked to restricted regions of damage within frontal and parietal cortex. Proc. Natl. Acad. Sci. USA. 2010;107:14899–14902. doi: 10.1073/pnas.1007928107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbey AK, et al. An integrative architecture for general intelligence and executive function revealed by lesion mapping. Brain. 2012;135:1154–1164. doi: 10.1093/brain/aws021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel T, Blyth JC, Griffiths G, Kelly D, Talcott JB. Moderate relationships between NAA and cognitive ability in healthy adults: implications for cognitive spectroscopy. Front. Hum. Neurosci. 2014;8:39. doi: 10.3389/fnhum.2014.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, et al. Brain anatomical network and intelligence. PLoS Comput. Biol. 2009;5:e1000395. doi: 10.1371/journal.pcbi.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamnes CK, et al. Intellectual abilities and white matter microstructure in development: a diffusion tensor imaging study. Hum. Brain Mapp. 2010;31:1609–1625. doi: 10.1002/hbm.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song M, et al. Brain spontaneous functional connectivity and intelligence. Neuroimage. 2008;41:1168–1176. doi: 10.1016/j.neuroimage.2008.02.036. [DOI] [PubMed] [Google Scholar]
- 14.van den Heuvel MP, Stam CJ, Kahn RS, Hulshoff Pol HE. Efficiency of functional brain networks and intellectual performance. J. Neurosci. 2009;29:7619–7624. doi: 10.1523/JNEUROSCI.1443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langeslag SJ, et al. Functional connectivity between parietal and frontal brain regions and intelligence in young children: the Generation R study. Hum. Brain Mapp. 2013;34:3299–3307. doi: 10.1002/hbm.22143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narr KL, et al. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cereb. Cortex. 2007;17:2163–2171. doi: 10.1093/cercor/bhl125. [DOI] [PubMed] [Google Scholar]
- 17.Gong QY, et al. Voxel-based morphometry and stereology provide convergent evidence of the importance of medial prefrontal cortex for fluid intelligence in healthy adults. Neuroimage. 2005;25:1175–1186. doi: 10.1016/j.neuroimage.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 18.Khundrakpam BS, et al. Imaging structural covariance in the development of intelligence. Neuroimage. 2016;16:30426–8. doi: 10.1016/j.neuroimage.2016.08.041. [DOI] [PubMed] [Google Scholar]
- 19.Alexander-Bloch A, Giedd JN, Bullmore E. Imaging structural co-variance between human brain regions. Nat. Rev. Neurosci. 2013;14:322–336. doi: 10.1038/nrn3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassett DS, et al. Hierarchical organization of human cortical networks in health and schizophrenia. J. Neurosci. 2008;28:9239–9248. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He Y, Chen ZJ, Evans AC. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb. Cortex. 2007;17:2407–2419. doi: 10.1093/cercor/bhl149. [DOI] [PubMed] [Google Scholar]
- 22.Xu L, Groth KM, Pearlson G, Schretlen DJ, Calhoun VD. Source-based morphometry: the use of independent component analysis to identify gray matter differences with application to schizophrenia. Hum. Brain Mapp. 2009;30:711–724. doi: 10.1002/hbm.20540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 24.Colom R, Jung RE, Haier RJ. Finding the g-factor in brain structure using the method of correlated vectors. Intelligence. 2006;34:561–570. doi: 10.1016/j.intell.2006.03.006. [DOI] [Google Scholar]
- 25.Reijmer YD, et al. Structural network alterations and neurological dysfunction in cerebral amyloid angiopathy. Brain. 2015;138:179–188. doi: 10.1093/brain/awu316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gläscher J, et al. Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proc. Natl. Acad. Sci. USA. 2012;109:14681–14686. doi: 10.1073/pnas.1206608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Sullivan M, et al. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/WNL.57.4.632. [DOI] [PubMed] [Google Scholar]
- 28.Turken U, et al. Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. Neuroimage. 2008;42:1032–1044. doi: 10.1016/j.neuroimage.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duering M, et al. Identification of a strategic brain network underlying processing speed deficits in vascular cognitive impairment. Neuroimage. 2013;66:177–183. doi: 10.1016/j.neuroimage.2012.10.084. [DOI] [PubMed] [Google Scholar]
- 30.Caeyenberghs K, et al. Altered structural networks and executive deficits in traumatic brain injury patients. Brain Struct. Funct. 2014;219:193–209. doi: 10.1007/s00429-012-0494-2. [DOI] [PubMed] [Google Scholar]
- 31.Reitan, R. M. & Wolfson, D. The Halstead-Reitan neuropsychological test battery: theory and clinical interpretation (Neuropsychology Press, 1986).
- 32.Lezak, M. D. Neuropsychological assessment (Oxford University Press, 2004).
- 33.Loonstra AS, Tarlow AR, Sellers AH. COWAT metanorms across age, education, and gender. Appl. Neuropsychol. 2001;8:161–166. doi: 10.1207/S15324826AN0803_5. [DOI] [PubMed] [Google Scholar]
- 34.Duff K, Schoenberg MR, Scott JG, Adams RL. The relationship between executive functioning and verbal and visual learning and memory. Arch. Clin. Neuropsych. 2005;20:111–122. doi: 10.1016/j.acn.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Li YO, Adali T, Calhoun VD. Estimating the number of independent components for functional magnetic resonance imaging data. Hum. Brain Mapp. 2007;28:1251–1266. doi: 10.1002/hbm.20359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith SM, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo L, et al. Constrained source-based morphometry identifies structural networks associated with default mode network. Brain Connect. 2012;2:33–43. doi: 10.1089/brain.2011.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Segall JM, et al. Correspondence between structure and function in the human brain at rest. Front. Neuroinform. 2012;6:10. doi: 10.3389/fninf.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim SG, Jung WH, Kim SN, Jang JH, Kwon JS. Alterations of gray and white matter networks in patients with obsessive-compulsive disorder: a multimodal fusion analysis of structural MRI and DTI using mCCA + jICA. PLoS One. 2015;10:e0127118. doi: 10.1371/journal.pone.0127118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cole MW, Yarkoni T, Repovs G, Anticevic A, Braver TS. Global connectivity of prefrontal cortex predicts cognitive control and intelligence. J. Neurosci. 2012;32:8988–8999. doi: 10.1523/JNEUROSCI.0536-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Logothetis NK. The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2002;357:1003–1037. doi: 10.1098/rstb.2002.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calhoun VD, et al. Method for multimodal analysis of independent source differences in schizophrenia: combining gray matter structural and auditory oddball functional data. Hum. Brain Mapp. 2006;27:47–62. doi: 10.1002/hbm.20166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brodal P. Principles of organization of the monkey corticopontine projection. Brain Res. 1978;148:214–218. doi: 10.1016/0006-8993(78)90392-X. [DOI] [PubMed] [Google Scholar]
- 45.Brodal P. The pontocerebellar projection in the rhesus monkey: an experimental study with retrograde axonal transport of horseradish peroxidase. Neuroscience. 1979;4:193–208. doi: 10.1016/0306-4522(79)90082-4. [DOI] [PubMed] [Google Scholar]
- 46.Kamali A, Kramer LA, Frye RE, Butler IJ, Hasan KM. Diffusion tensor tractography of the human brain cortico-ponto-cerebellar pathways: a quantitative preliminary study. J. Magn. Reson. Imaging. 2010;32:809–817. doi: 10.1002/jmri.22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen SA, Desmond JE. Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. Neuroimage. 2005;24:332–338. doi: 10.1016/j.neuroimage.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 48.Macher K, Bohringer A, Villringer A, Pleger B. Cerebellar-parietal connections underpin phonological storage. J. Neurosci. 2014;34:5029–5037. doi: 10.1523/JNEUROSCI.0106-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barton RA, Venditti C. Rapid evolution of the cerebellum in humans and other great apes. Curr. Biol. 2014;24:2440–2444. doi: 10.1016/j.cub.2014.08.056. [DOI] [PubMed] [Google Scholar]
- 51.Londei A, et al. Sensory-motor brain network connectivity for speech comprehension. Hum. Brain Mapp. 2010;31:567–580. doi: 10.1002/hbm.20888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee JY, et al. Intellect declines in healthy elderly subjects and cerebellum. Psychiatry Clin. Neurosci. 2005;59:45–51. doi: 10.1111/j.1440-1819.2005.01330.x. [DOI] [PubMed] [Google Scholar]
- 53.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 54.Bischoff-Grethe A, Ivry RB, Grafton ST. Cerebellar involvement in response reassignment rather than attention. J. Neurosci. 2002;22:546–553. doi: 10.1523/JNEUROSCI.22-02-00546.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hogan MJ, et al. Cerebellar brain volume accounts for variance in cognitive performance in older adults. Cortex. 2011;47:441–450. doi: 10.1016/j.cortex.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Halstead, W. C. Brain and intelligence; a quantitative study of the frontal lobes (Univ. of Chicago Press, 1947).
- 57.Roca M, et al. Executive function and fluid intelligence after frontal lobe lesions. Brain. 2010;133:234–247. doi: 10.1093/brain/awp269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duncan J, Emslie H, Williams P, Johnson R, Freer C. Intelligence and the frontal lobe: the organization of goal-directed behavior. Cognitive Psychol. 1996;30:257–303. doi: 10.1006/cogp.1996.0008. [DOI] [PubMed] [Google Scholar]
- 59.Meyer V. Cognitive changes following temporal lobectomy for relief of temporal lobe epilepsy. AMA Arch. NeurPsych. 1959;81:299–309. doi: 10.1001/archneurpsyc.1959.02340150031004. [DOI] [PubMed] [Google Scholar]
- 60.Crossley NA, et al. Superior temporal lobe dysfunction and frontotemporal dysconnectivity in subjects at risk of psychosis and in first-episode psychosis. Hum. Brain Mapp. 2009;30:4129–4137. doi: 10.1002/hbm.20834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex. 2008;44:953–961. doi: 10.1016/j.cortex.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Colom R, Karama S, Jung RE, Haier RJ. Human intelligence and brain networks. Dialogues Clin. Neurosci. 2010;12:489–501. doi: 10.31887/DCNS.2010.12.4/rcolom. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoon YB, et al. Altered Fronto-temporal functional connectivity in individuals at ultra-high-risk of developing psychosis. PloS one. 2015;10:e0135347. doi: 10.1371/journal.pone.0135347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benetti S, et al. Auditory verbal hallucinations and brain dysconnectivity in the perisylvian language network: a multimodal investigation. Schizophr. Bull. 2015;41:192–200. doi: 10.1093/schbul/sbt172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Friederici AD, Ruschemeyer SA, Hahne A, Fiebach CJ. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb. Cortex. 2003;13:170–177. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- 66.Shaw P, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 67.Schnack HG, et al. Changes in thickness and surface area of the human cortex and their relationship with intelligence. Cereb. Cortex. 2015;25:1608–1617. doi: 10.1093/cercor/bht357. [DOI] [PubMed] [Google Scholar]
- 68.Leiner HC, Leiner AL, Dow RS. Cognitive and language functions of the human cerebellum. Trends Neurosci. 1993;16:444–447. doi: 10.1016/0166-2236(93)90072-T. [DOI] [PubMed] [Google Scholar]
- 69.Rasser PE, et al. Cerebellar grey matter deficits in first-episode schizophrenia mapped using cortical pattern matching. Neuroimage. 2010;53:1175–1180. doi: 10.1016/j.neuroimage.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 70.Roman-Urrestarazu A, et al. Brain structure in different psychosis risk groups in the Northern Finland 1986 birth cohort. Schizophr. Res. 2014;153:143–149. doi: 10.1016/j.schres.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 71.Henze R, et al. Gray matter alterations in first-admission adolescents with schizophrenia. J. Neuroimaging. 2011;21:241–246. doi: 10.1111/j.1552-6569.2010.00504.x. [DOI] [PubMed] [Google Scholar]
- 72.Cooper FE, et al. The contribution of the cerebellum to cognition in Spinocerebellar Ataxia Type 6. Behav. Neurol. 2010;23:3–15. doi: 10.1155/2010/724861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gottwald B, Wilde B, Mihajlovic Z, Mehdorn HM. Evidence for distinct cognitive deficits after focal cerebellar lesions. J. Neurol. Neurosurg. Psychiatry. 2004;75:1524–1531. doi: 10.1136/jnnp.2003.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palaniyappan L, et al. Structural correlates of formal thought disorder in schizophrenia: an ultra-high field multivariate morphometry study. Schizophr. Res. 2015;168:305–312. doi: 10.1016/j.schres.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Depping MS, et al. Common and distinct structural network abnormalities in major depressive disorder and borderline personality disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2015;65:127–133. doi: 10.1016/j.pnpbp.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 76.First, M. B., Spitzer, R. L., Gibbon, M. & Williams, J. B. Structured Clinical Interview for DSM-IV Axis I Disorders, Non-Patient Edition (SCID-NP) (Biometrics Research Department, New York State Psychiatric Institute, 1995).
- 77.Kim J, Lee Y. Validity of short forms of the Korean-Wechsler adult intelligence scale. Korean J. Clin. Psychol. 1995;14:111–116. [Google Scholar]
- 78.Benton A. Development of a multilingual aphasia battery: progress and problems. J. Neurol. Sci. 1969;9:39–48. doi: 10.1016/0022-510X(69)90057-4. [DOI] [PubMed] [Google Scholar]
- 79.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 80.Rajapakse JC, Giedd JN, Rapoport JL. Statistical approach to segmentation of single-channel cerebral MR images. IEEE Trans. Med. Imaging. 1997;16:176–186. doi: 10.1109/42.563663. [DOI] [PubMed] [Google Scholar]
- 81.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 82.Manjon JV, Coupe P, Marti-Bonmati L, Collins DL, Robles M. Adaptive non-local means denoising of MR images with spatially varying noise levels. J. Magn. Reson. Imaging. 2010;31:192–203. doi: 10.1002/jmri.22003. [DOI] [PubMed] [Google Scholar]
- 83.Kasparek T, et al. Source-based morphometry of gray matter volume in men with first-episode schizophrenia. Hum. Brain Mapp. 2010;31:300–310. doi: 10.1002/hbm.20865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Himberg J, Hyvarinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage. 2004;22:1214–1222. doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 85.Sanchez-Cubillo I, et al. Construct validity of the Trail Making Test: role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J. Int. Neuropsychol. Soc. 2009;15:438–50. doi: 10.1017/S1355617709090626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


