Abstract
Objective
Recent research has revealed a larger impairment of object perceptual discrimination than of spatial perceptual discrimination in patients with schizophrenia. It has been suggested that mental imagery may share processing systems with perception. We investigated whether patients with schizophrenia would show greater impairment regarding object imagery than spatial imagery.
Methods
Forty-four patients with schizophrenia and 20 healthy control subjects were tested on a task of object visual mental imagery and on a task of spatial visual mental imagery. Both tasks included a condition in which no imagery was needed for adequate performance, but which was in other respects identical to the imagery condition. This allowed us to adjust for nonspecific differences in individual performance.
Results
The results revealed a significant difference between patients and controls on the object imagery task (F1,63 = 11.8, p = 0.001) but not on the spatial imagery task (F1,63 = 0.14, p = 0.71). To test for a differential effect, we conducted a 2 (patients v. controls) х 2 (object task v. spatial task) analysis of variance. The interaction term was statistically significant (F1,62 = 5.2, p = 0.026).
Conclusions
Our findings suggest a differential dysfunction of systems mediating object and spatial visual mental imagery in schizophrenia.
Medical subject headings: form perception; imagery, visual; schizophrenia; space perception
Abstract
Objectif
Des recherches récentes ont révélé une déficience plus importante de la discrimination perceptuelle des objets que de la discrimination perceptuelle de l'espace chez des patients atteints de schizophrénie. On a laissé entendre que l'imagerie mentale peut avoir des systèmes de traitement en commun avec la perception. Nous avons cherché à déterminer si des patients atteints de schizophrénie montreraient une plus grande déficience au niveau de l'imagerie des objets que de l'imagerie de l'espace.
Méthodes
Nous avons soumis 44 patients atteints de schizophrénie et 20 sujets témoins en bonne santé à une tâche d'imagerie mentale d'un objet visuel et d'un espace visuel. Les deux tâches comportaient une condition selon laquelle aucune imagerie n'était nécessaire pour produire un résultat adéquat, mais qui était à d'autres égards identique à la condition d'imagerie, ce qui nous a permis d'effectuer un rajustement en fonction de différences non spécifiques au niveau du rendement individuel.
Résultats
Les résultats ont révélé une différence importante entre les patients et les témoins au niveau de l'imagerie de l'objet (F1,63 = 11,8, p = 0,001), mais non au niveau de l'imagerie de l'espace (F1,63 = 0,14, p = 0,71). Pour déterminer s'il y a un effet différentiel, nous avons procédé à une analyse d'écart 2 (patients c. témoins) !#!khcy; 2 (tâche objet c. tâche espace). La condition d'interaction était statistiquement significative (F1,62 = 5,2, p = 0,026).
Conclusions
Nos résultats indiquent une dysfonction différentielle des systèmes médiateurs de l'imagerie mentale visuelle de l'espace et des objets dans les cas de schizophrénie.
Introduction
A recent study1 reported a larger impairment of object perceptual discrimination than of spatial perceptual discrimination in patients with schizophrenia. The authors related this functional dissociation to neuroanatomical pathways involved in object and spatial processing, respectively. As it has been suggested that perception and mental imagery may share information-processing systems in the brain,2 we hypothesized that a similar dissociation between object and spatial processing might occur with regard to mental imagery in patients with schizophrenia.
Subjectively, most people report being able to conjure up from memory the recollection of visual events that seem to have at least some of the characteristics of the initial sensory experience. Interestingly, when mentally scanning a map (with the “mind's eye”) containing various objects, reaction times have been shown to be directly proportional to the distance between 2 points, as would be the case when scanning a physical map with the real eye.2 Functional neuroimaging studies have also provided evidence of a sharing of processing systems in perception and imagery.3,4,5 Indeed, the main difference between visual perception and visual imagery is that the former relies on stimulus input via visual stimulation, thus involving bottom-up mechanisms (from the retina through the thalamus to cortical areas), whereas mental imagery is totally dependent on top-down mechanisms.
It is well established that visual information processing involves 2 functional and anatomically segregated systems in the brain for the processing of spatial (“where”) and object (“what”) information.6 Spatial processing occurs mainly in the occipitoparietal lobe (or “dorsal stream”), whereas object processing is confined to the occipitotemporal lobe (“ventral stream”). A further equivalence of imagery and perceptual processing has been suggested with regard to these processing pathways by Luzzatti et al,7 who reported the case of a neurologic patient with deficient spatial but not object imagery. Evidence from functional neuroimaging has also suggested that the “what” and “where” pathways are also involved in mental imagery.8 We investigated whether the findings of Tek et al1 concerning a differential deficit of object versus spatial perception in schizophrenia would extend to mental imagery, namely, whether patients with schizophrenia would show greater impairment regarding object imagery than spatial imagery.
Methods
Forty-four patients with a diagnosis of schizophrenia according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV),9(confirmed by a standardized interview, the Comprehensive Assessment of Symptoms and History10) and 20 healthy comparison subjects participated in the present study. The patients' symptoms and their severity were rated with the Positive and Negative Syndrome Scale (PANSS).11 Patients were recruited from the Department of Psychiatry, University Medical Center, Utrecht, and were also participants in a larger, ongoing study on the neurocognitive basis of hallucinations. Control subjects were recruited by advertisement in a local newspaper. The protocol was approved by the Ethics Committee of the University Medical Center. All patients and controls gave their written informed consent. All patients were treated with atypical antipsychotics at the moment of testing.
Two visual mental imagery measures that have been described previously were used:12 one of object mental imagery and one of spatial mental imagery. Both tasks included a condition in which no imagery was needed for adequate performance, but which was in other respects identical to the imagery condition. This allowed us to adjust for nonspecific individual performance differences, by subtracting accuracy scores (total number correct) of the imagery condition from the scores in the no-imagery condition.
The object imagery task concerned a quantitative comparison between an imagery and a no-imagery condition of visual form characteristics of common objects (the task was adapted from Mehta et al13). The task involved 22 names of objects printed on cards and 22 triads of line drawings of common objects.14 From the triads of line drawings, subjects had to indicate which item was the odd one out in terms of visual form, namely, “which one is different from the other two objects in terms of the visual form of the outline.” In the no- imagery condition the line drawings were actually presented, whereas in the imagery condition the object names were read from cards. For example, in the perceptual (no-imagery) condition, pictures of the following 3 objects were presented: “pumpkin,” “lettuce” and “tomato,” whereas in the imagery condition only the names of these 3 objects were presented to the subject. Thus, the imagery condition required the participants to form mental images in order to be able to make a correct judgement (which in the example given would be “lettuce”). The odd one out could not be determined correctly on the basis of only semantic information, and all subjects reported the use of imagery to perform the task. Performance was scored as the percentage of correct responses for the perception and imagery conditions separately.
For the spatial imagery, we adapted the letter imagery task used by Kosslyn et al.15 The subject was asked whether an X, presented in a 4 х 5 grid, fell on a capital letter. In the imagery condition, the letter was not actually presented in the grid but had to be imagined by the subject in a predefined way, as shown by the experimenter during the instruction. For example, a fixation point was presented for 1 second in the middle of the screen to draw the subject's attention, after which a lowercase letter “f” was presented, followed by an empty grid with the X at the lower right corner. The subject had to decide whether the target would fall on an uppercase letter “F” or not. In the no-imagery condition, the letter actually appeared in the grid. Eight letters were randomly presented during the task: “c,” “f,” “h,” “j,” “l,” “p,” “s,” “u.” Each condition of the task consisted of 32 trials, 4 trials for each of the letters (2 “on” trials, where the X fell on the imagined letter, and 2 “off” trials, where the X did not fall on the imagined letter). We modified the task slightly, in that we allowed the X to appear only in cells in which the chance that the X would cover a letter was equal (thus, no X appeared in the column that was furthest to the left, because most capital letters would cover these cells). The percentage of correct responses was recorded for the imagery and no-imagery conditions, and subtraction of the latter from the former yielded the difference score.
Repeated-measures analysis of variance (ANOVA) was carried out on the difference scores for the object and spatial imagery tasks. The results were considered statistically significant at p < 0.05 (2-tailed).
Results
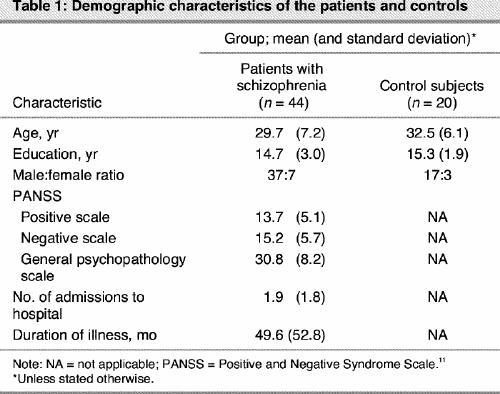
The demographic characteristics of the groups can be found in Table 1. The groups did not differ in age and level of education. The percentage of correct responses was examined in the control group in order to confirm that the tasks were not too difficult and that there were no ceiling effects. Their performance was 81% (standard deviation [SD] 8.6%) correct for the imagery condition and 90% (SD 7.3%) correct for the no-imagery condition of the object task, whereas their performance was 86% (SD 14.2%) correct for the imagery condition and 95% (SD 4.4%) correct for the no-imagery condition of the spatial task. The statistical analysis (ANOVA) with the difference scores as a dependent variable, revealed a significant difference between patients and controls on the object task (F1,63 = 11.8, p = 0.001) but not on the spatial task (F1,63 = 0.14, p = 0.71). To test whether the group differences were larger for the object imagery test than for the spatial imagery test, we conducted a 2 (patients v. controls) х 2 (object task v. spatial task) ANOVA. The interaction term was statistically significant (F1,62 = 5.2, p = 0.026). Patients made significantly more errors on the object imagery task (relative to the no-imagery condition) than on the spatial imagery task (relative to the no- imagery condition), compared with control subjects. Mean percentage values (and SDs) for the difference in correct scores (imagery v. no-imagery) for these tasks were 11.8% (9.6%) for patients and 2.4% (11.2%) for controls on the object task, and 10.0% (9.9%) and 8.9% (12.6%) for patients and controls, respectively, on the spatial task. Patients did not differ from controls on the no-imagery conditions of the 2 tasks.
Table 1
Discussion
Our findings show a differential impairment of object imagery relative to spatial imagery in patients with schizophrenia. The differential performance on the object and spatial imagery task is not trivial, in that we have reported in a previous study that object and spatial imagery are affected to the same extent in congenitally blind people.16 It has been argued that persuasive evidence for a differential deficit on behavioural tasks requires that the tasks be matched on psychometric properties, specifically difficulty level and reliability or true score variance.17 In contrast, others have maintained that this solution neglects important issues of process specification. To test specific hypotheses about underlying cognitive mechanisms, it can be necessary to change stimulus and task characteristics that may be intricately associated with task difficulty. Matching on psychometric characteristics in such paradigms causes unmatching regarding process and inevitably confounds the processes being measured, resulting in theoretically ambiguous findings.18 Notably, in the present study, there were no ceiling effects in the performance of healthy controls, and they performed in the same range on both tasks (> 80% to < 95% correct). Furthermore, as every subject in the present study was his own “control” in a way (by subtracting scores on the no-imagery condition from the imagery condition), the role of difficulty is minimized in the group comparison.
In conclusion, our results reveal differential dysfunction of systems mediating object and spatial visual mental imagery in schizophrenia, thereby extending the findings reported by Tek et al1 regarding a dissociation between object and spatial visual perception in schizophrenia. Gabrovska et al19 reported specific impairment of visual object processing in schizophrenia and interpreted their findings as evidence for an associative agnosic deficit. The findings are also consistent with considerable evidence of temporal lobe dysfunction in schizophrenia. Indeed, structural volume reductions have been shown to be more prominent for the temporal lobes than for the parietal lobes in schizophrenia.20 Our finding of intact spatial imagery is consistent with a previous study in which patients with schizophrenia performed identically to comparison subjects on a task requiring visual imagery to compare spatial dimensions.21 It is important to note, however, that several previous studies have reported dysfunction of the dorsal route in schizophrenia.22,23 Indeed, Coleman et al24 recently reported evidence of both spatial and object working memory impairments in patients with schizophrenia. However, none of these studies concerned mental imagery.
A limitation of the present study concerns the difference in stimuli between the object and spatial task. More specifically, the object task allowed for a more idiosyncratic interpretation and generation of mental images than the spatial task, which might have contributed to differences between patients and control subjects. Future studies should control for the nature of the stimuli.
In sum, our finding of differential object versus spatial imagery impairment in schizophrenia warrants a more detailed functional neuroimaging investigation of the “what” and “where” pathways in schizophrenia.
Footnotes
Competing interests: None declared.
Correspondence to: Dr. André Aleman, Division of Neuroscience, Department of Psychiatry, A01.126, University Medical Center, Heidelberglaan 100, 3584 CX, Utrecht, The Netherlands; fax 31 30 2505443; a.aleman@azu.nl
Submitted Apr. 2, 2003; Revised Aug. 21, 2003; Nov. 13, 2003; Jan. 7, 2004; Accepted Jan. 22, 2004
References
- 1.Tek C, Gold J, Blaxton T, Wilk C, McMahon RP, Buchanan RW. Visual perceptual and working memory impairments in schizophrenia. Arch Gen Psychiatry 2002;59:146-53. [DOI] [PubMed]
- 2.Kosslyn SM. Image and brain. Cambridge: The MIT Press; 1994.
- 3.Kosslyn SM, Pascual-Leone A, Felician O, Camposano S, Keenan JP, Thompson WL, et al. The role of area 17 in visual imagery: convergent evidence from PET and rTMS. Science 1999;284:167-70. [DOI] [PubMed]
- 4.Aleman A, Schutter DJLG, Ramsey NF, van Honk J, Kessels RPC, Hoogduin JH, et al. Functional neuroanatomy of top-down visuospatial processing in the human brain: evidence from rTMS. Cogn Brain Res 2002;14:300-2. [DOI] [PubMed]
- 5.Formisano E, Linden DE, Di Salle F, Trojano L, Esposito F, Sack AT, et al. Tracking the mind's image in the brain I: time-resolved fMRI during visuospatial mental imagery. Neuron 2002;35:185-94. [DOI] [PubMed]
- 6.Mishkin M, Ungerleider LG, Macko KA. Object vision and spatial vision: two cortical pathways. Trends Neurosci 1983;6:414-7.
- 7.Luzzatti C, Vecchi T, Agazzi D, Cesa-Bianchi M, Vergani C. A neurological dissociation between preserved visual and impaired spatial processing in mental imagery. Cortex 1998;34:461-9. [DOI] [PubMed]
- 8.Mellet E, Petit L, Mazoyer B, Denis M, Tzourio N. Reopening the mental imagery debate: lessons from functional anatomy. Neuroimage 1998;2:129-39. [DOI] [PubMed]
- 9.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 1994.
- 10.Andreasen NC. Comprehensive assessment of symptoms and history. Iowa City: University of Iowa; 1987.
- 11.Kay SR, Opler LA, Fizbein A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987;13:261-76. [DOI] [PubMed]
- 12.Aleman A, Nieuwenstein M, Böcker KBE, De Haan EHF. Mental imagery and perception in hallucination-prone individuals. J Nerv Ment Dis 2000; 188: 830-6. [DOI] [PubMed]
- 13.Mehta Z, Newcombe F, De Haan EHF. Selective loss of imagery in a case of visual agnosia. Neuropsychologia 1992;30: 645-55. [DOI] [PubMed]
- 14.Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol [Hum Learn] 1980;6:174-215. [DOI] [PubMed]
- 15.Kosslyn SM, Cave CB, Provost D, Von Gierke S. Sequential processes in image generation. Cogn Psychol 1988;20:319-43. [DOI] [PubMed]
- 16.Aleman A, Van Lee L, Mantione M, Verkoijen I, De Haan EHF. Visual imagery without visual experience: evidence from congenitally totally blind people. Neuroreport 2001;12:2601-4. [DOI] [PubMed]
- 17.Chapman LJ, Chapman JP. Commentary on two articles concerning generalized and specific cognitive deficits. J Abnorm Psychol 2001; 110:31-9. [DOI] [PubMed]
- 18.Knight RA, Silverstein SM. A process-oriented approach for averting confounds resulting from general performance deficiencies in schizophrenia. J Abnorm Psychol 2001;110:15-30. [DOI] [PubMed]
- 19.Gabrovska VS, Laws KR, Sinclair J, McKenna PJ. Visual object processing in schizophrenia: evidence for an associative agnosic deficit. Schizophr Res 2003; 59:277-86. [DOI] [PubMed]
- 20.Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry 2000; 157:16-25. [DOI] [PubMed]
- 21.David AS, Cutting JC. Visual imagery and visual semantics in the cerebral hemispheres in schizophrenia. Schizophr Res 1993;8:263-71. [DOI] [PubMed]
- 22.O'Donnell BF, Swearer JM, Smith LT, Nestor PG, Shenton ME, McCarley RW. Selective deficits in visual perception and recognition in schizophrenia. Am J Psychiatry 1996;153:687-92. [DOI] [PubMed]
- 23.Braus DF, Weber-Fahr W, Tost H, Ruf M, Henn FA. Sensory information processing in neuroleptic-naive first-episode schizophrenic patients: a functional magnetic resonance imaging study. Arch Gen Psychiatry 2002;59:696-701. [DOI] [PubMed]
- 24.Coleman MJ, Cook S, Matthysse S, Barnard J, Lo Y, Levy DL, et al. Spatial and object working memory impairments in schizophrenia patients: a Bayesian item-response theory analysis. J Abnorm Psychol 2002;111:425-35. [DOI] [PubMed]


