Certain visual images, even in the absence of motion or flicker, can trigger seizures in patients with photosensitive epilepsy. As of yet, there is no systematic explanation as to why some static images are likely to provoke seizures, while others pose little or no risk. Here, we examined the neurophysiology literature to assess whether the pattern of neural responses in healthy visual cortex is predictive of the pathological responses in photosensitive epilepsy. Previous studies have suggested that gamma oscillations (30-80 Hz) measured in human visual cortex may play a role in seizure generation [1, 2]. Recently, we and others have shown that increases in gamma band power can come from two very different cortical signals, one that is oscillatory (with a narrow peak between 30 Hz and 80 Hz), and another that is broadband [3]. The oscillatory signal arises from neuronal synchrony in the local population, while the broadband signal reflects the level of asynchronous neuronal activity, and is correlated with multiunit spiking [4]. These two responses have different biological origins and different selectivity for image properties. Here, we followed up on the previous proposals [1, 2] to ask whether the image features that increase seizure likelihood in photosensitive epilepsy are linked to narrowband gamma oscillations specifically, or are associated with any kind of increase in visual activity. Based on published work, we compared pairs of image classes on a number of dimensions, and show that the type of image that elicits larger narrowband gamma oscillations in healthy visual cortex is also more likely to provoke seizures or pre-seizure activity in patients with photosensitive epilepsy. In contrast, images that elicit larger broadband, multiunit, or fMRI responses are much less predictive of seizure activity. We propose that a risk factor for seizures in patients with photosensitive epilepsy is engagement of the circuitry that produces gamma oscillations.
Photosensitivity, defined as an abnormal response in the electroencephalogram (EEG) triggered by light stimulation, is common and is found in 0.3-3% of the population [5]. Photosensitive epilepsy, where light stimulation causes seizures, has a prevalence of 1 in 10,000 individuals (or 1/4,000 between ages 5-24) [5]. Highly provocative stimuli can occur in the natural environment, on TV and in computer games. In the Pokémon incident in 1997, for example, one Pokémon episode resulted in seizures and hospital visits for 685 people in Japan. In a separate incident, a video for the 2012 Olympics was removed from the website because it caused seizures. Patients with photosensitive epilepsy are advised to avoid provocative stimuli.
Neuroscientists and neurologists have started to detail the visual input that can trigger a seizure. Repetitive flashes are well known for their potential to induce seizures, but stationary patterns can also elicit seizures in about 30% of patients with photosensitive epilepsy [6]. Large, high contrast gratings of 2-4 cycles per degree are most provocative to elicit abnormal, epileptiform, responses in the EEG signal, the photoparoxysmal response (PPR, which is the clinical marker for photosensitivity); looking at these gratings for more than 500 ms can trigger a seizure. Several image features influence the degree to which a stimulus is provocative (Table 1). The likelihood that a PPR is induced by viewing a grating can be reduced by decreasing the size of the grating, by reducing the contrast, by superimposing a second grating to create a plaid or checkerboard, or by superimposing noise. Both sine and square wave gratings are provocative whereas chromatic contrast alone (isoluminant gratings) is not.
Table 1. The image features that are most provocative in photosensitive epilepsy (expressed as photo-paroxysmal responses (PPR) in the EEG), are also the most likely to induce large gamma oscillations.
When image properties vary according to the exemplars (changes in size, contrast, number of orientations, luminance/chromatic, spatial frequency, sine wave to square wave, adding noise) the number of PPR in photosensitive patients change in the same way as the amplitude of the gamma oscillations. Multiunit activity and the fMRI BOLD response change in a different manner. The direction of the arrows indicates increases or decreases, and a desaturated arrow indicates variable evidence. For gamma oscillations, spiking activity and BOLD responses, we assess an increase or decrease as the change in response of the neural population representing the stimulus center, not the signal pooled over all of visual cortex. Additional notes are provided in the Supplemental Table S1. This table includes spatial stimulus features from which the effects on provocativeness in epilepsy, induced gamma oscillations and firing rates have been described (see the Supplemental Table S1 and Table S2 for some additional stimulus features).
| Stimulus characteristic | Stimuli exemplars | I. Stimulus provocativeness in photosensitive epilepsy | II. Gamma oscillation in V1/V2 | III. Spiking activity in V1/V2 | IV. BOLD in V1/V2 |
|---|---|---|---|---|---|
| 1. Size |
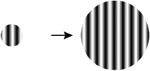
|

|

|

|

|
| 2. Contrast |
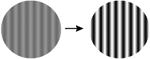
|

|

|

|

|
| 3. Number of orientations |
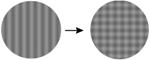
|

|

|

|

|
| 4. Luminance→ chromatic |
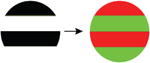
|

|

|
-- |

|
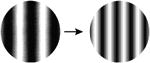
| 5. Spatial frequency low to middle (1-4cpd) |
|

|

|

|

|
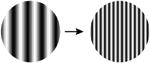
| 6. Spatial frequency middle (l-4cpd) to high |
|

|

|

|

|
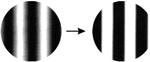
| 7. Sinusoidal → square wave |
|
-- | -- |

|
-- |
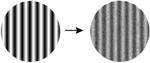
| 8. Increasing noise |
|

|

|

|

|
A comprehensive overview of the neurophysiology literature indicates that the same image properties that can trigger seizures also elicit gamma oscillations in the local field potential in visual cortex. Gamma oscillations are most strongly driven by large, high contrast gratings and can be reduced in amplitude by decreasing the size of the grating, by reducing the contrast, by superimposing a second grating to create a plaid or checkerboard, or by superimposing noise [7]. In addition, gamma oscillations in visual cortex peak at a spatial frequency around 2-4 cycles per degree, sine and square wave gratings both induce gamma oscillations, and isoluminant gratings induce little or no gamma oscillations [8]. These stimulus features are highly similar to those that are provocative to induce seizures in photosensitive epilepsy (Table 1; additional references from the original reports are in Supplemental Table S1).
Importantly, the stimulus manipulations that increase both gamma oscillations and PPRs differ from those that increase multiunit firing rates [7] and the fMRI Blood Oxygen Level Dependent (‘BOLD’) response (Table 1). For example, gamma oscillations increase with larger stimuli, while the level of local neuronal firing and BOLD amplitude decrease (due to surround suppression). When a grating is converted to a plaid by overlaying additional orientations, gamma oscillations decrease, while increasing neuronal population firing rates and the BOLD signal. Superimposing white noise on a grating decreases gamma oscillations while not influencing firing rates. Chromatic contrast (e.g., an isoluminant grating) elicits high firing rates and a large BOLD response, but does not elicit a large gamma oscillation [8]. The stimuli that are most provocative in photosensitive epilepsy therefore match the stimuli that strongly drive gamma oscillations, and do not match the stimuli that strongly drive the overall level of neuronal firing or the metabolic demand as measured by BOLD fMRI.
This review focuses on the link between photosensitive epilepsy and gamma oscillations induced by spatial features of images. Narrowband gamma oscillations are highly dependent on the visual stimulus [3, 7] and these oscillations are associated with engagement of specific types of cells and circuitry in visual cortex (e.g., [12, 13]). We hypothesize that engagement of this circuitry is a factor that increases the likelihood of seizure activity, perhaps because this circuit does not self-stabilize in some patients with photosensitive epilepsy. Stroboscopic (mean field) flicker across many frequencies (3 Hz to 60 Hz) can also be highly provocative in photosensitive epilepsy (Supplemental Table S2). Periodic stimuli elicit periodic responses; however, it is unclear whether these stimuli also engage the same specialized circuitry that produces induced (non-stimulus-locked) gamma oscillations. Hence the mechanism by which flicker induces seizure activity remains an important topic for further exploration.
We still know little about the underlying mechanisms of photosensitive epilepsy, in part because there is no animal model that translates well to humans1 and because there is no experimental paradigm for studying risk factors for photosensitive epilepsy in healthy human subjects. In contrast, neither of these limitations applies to gamma oscillations. The circuitry involved in gamma oscillations in visual cortex is extensively studied at the cellular level in animal models, at the systems level in healthy human subjects, and at the level of computational modeling. In particular, studies at all of these levels propose that the interaction between excitatory neurons and inhibitory interneurons is important for the generation of gamma oscillations [9] and fast spiking basket interneurons have resonant properties in the gamma frequencies and are hypothesized to play a role in gamma oscillations. Moreover, animal recordings have shown that large stimuli compared to small stimuli increase the power of gamma oscillations, and drive fast spiking interneurons more strongly [10]. Competing computational models (reviewed in [9]) have different implications for the type of stimuli and neuronal states that are likely to modulate the level of gamma oscillations in healthy visual cortex. If our conjecture is correct, then a critical question to address is why engagement of this circuitry leads to seizures or atypical responses in the EEG in some people, but not in others. Therefore, the tools used to study gamma oscillations – at the computational, cellular, circuit, and systems level - might be marshaled to explain not only why gamma synchrony occurs, but also how excessive synchrony can occur in epilepsy.
Supplementary Material
Acknowledgments
This work was supported by the Netherlands Organization for Scientific Research grant 016_VENI_178_048 (D.H.), the National Institutes of Health grants R00-EY022116 (J.W.) and R01-MH111417 (J.W.) and the EU program Marie Curie MEXCT-CT-2005-024224 “Visual Sensitivity” (DKNT).
Footnotes
There are photosensitive baboons, but the seizure focus is fronto-centrally located, whereas the focus in humans is in visual cortex.
Author contribution: All authors contributed to writing and editing the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parra J, Kalitzin SN, Iriarte J, Blanes W, Velis DN, Lopes da Silva FH. Gamma-band phase clustering and photosensitivity: is there an underlying mechanism common to photosensitive epilepsy and visual perception? Brain. 2003;126:1164–1172. doi: 10.1093/brain/awg109. [DOI] [PubMed] [Google Scholar]
- 2.Perry G, Brindley LM, Muthukumaraswamy SD, Singh KD, Hamandi K. Evidence for increased visual gamma responses in photosensitive epilepsy. Epilepsy Res. 2014;108:1076–1086. doi: 10.1016/j.eplepsyres.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Hermes D, Miller KJ, Wandell BA, Winawer J. Stimulus Dependence of Gamma Oscillations in Human Visual Cortex. Cereb Cortex. 2015;25:2951–2959. doi: 10.1093/cercor/bhu091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ray S, Maunsell JH. Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Biol. 2011;9:e1000610. doi: 10.1371/journal.pbio.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher RS, Harding G, Erba G, Barkley GL, Wilkins A. Photic- and pattern-induced seizures: a review for the Epilepsy Foundation of America Working Group. Epilepsia. 2005;46:1426–1441. doi: 10.1111/j.1528-1167.2005.31405.x. [DOI] [PubMed] [Google Scholar]
- 6.Kasteleijn-Nolst Trenite DG. Photosensitivity in epilepsy. Electrophysiological and clinical correlates. Acta Neurol Scand Suppl. 1989;125:3–149. [PubMed] [Google Scholar]
- 7.Jia X, Xing D, Kohn A. No consistent relationship between gamma power and peak frequency in macaque primary visual cortex. J Neurosci. 2013;33:17–25. doi: 10.1523/JNEUROSCI.1687-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swettenham JB, Muthukumaraswamy SD, Singh KD. BOLD Responses in Human Primary Visual Cortex are Insensitive to Substantial Changes in Neural Activity. Front Hum Neurosci. 2013;7:76. doi: 10.3389/fnhum.2013.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buzsaki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haider B, Krause MR, Duque A, Yu Y, Touryan J, Mazer JA, McCormick DA. Synaptic and network mechanisms of sparse and reliable visual cortical activity during nonclassical receptive field stimulation. Neuron. 2010;65:107–121. doi: 10.1016/j.neuron.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


