Abstract
Patients with systemic sclerosis present with varying clinical features, have different responses to therapy, and end up with different outcomes. Categorizing patients improves disease management. A new study now proposes that patients with systemic sclerosis and overlapping features of another connective tissue disease might form a distinct disease subset.
Treatment of systemic sclerosis (SSc) can be frustrating, not least because of the remarkable disease heterogeneity, with patients having variable clinical manifestations, labora tory and serological findings, complications and outcomes. Such vari ability poses a formidable challenge not only in choosing optimal therapy, but also in interpreting results from treatment studies of heterogeneous patient cohorts. Several clinical approaches to subclassify SSc have been proposed; the most widely used system divides patients, largely by the extent of cutaneous changes, into limited cutaneous (lcSSc) and diffuse cutaneous (dcSSc) subsets.1 Although it remains uncertain whether these two subsets represent truly distinct dis eases or merely different extremes of the disease spectrum, for most patients the dichotomous classification of SSc has withstood the test of time. Numerous studies have confirmed that patients with lcSSc or dcSSc have distinct autoantibody profiles, patterns of organ pathology, disease progression and outcomes;2,3 however, many patients with SSc do not fit neatly into these subclasses, suggesting the need for additional categories. In the future, classification of patients with SSc might be done according to an in-depth understanding of the genetic and molecular basis of the disease; for now, though, defining new subsets using precise analysis of clinically and laboratory-defined characteristics that are common to groups of patients with SSc seems justi fied. The ultimate goal of such subclassifica tion is, of course, to personalize disease management and improve outcomes. A study from Germany now makes a contribution to these efforts by proposing a distinct category of patients with SSc who have features that overlap with another c onnective tissue disease (CTD).4
Moinzadeh et al.4 analysed data from 3,240 patients with SSc who enrolled in the 40-centre German Network for Systemic Sclero derma, focusing on 342 patients (10% of the total cohort) with an overlap with a CTD. In the absence of established criteria, overlap was defined as having characteristic SSc features concurrently with symptoms or signs suggestive of another CTD. In con trast to patients with dcSSc, classified according to established criteria,1 patients with SSc-overlap had lower modified Rodnan skin scores, and also had less pulmonary fibrosis, less proteinuria and renal crisis, and fewer joint contractures. More over, patients with SSc-overlap were less likely to progress to pulmonary fibrosis, pulmonary hyper ten sion or cardiac dysfunction, such as pal pita tions or conduction disturbances. In sum mary, although intermediate for many cri teria, patients with SSc-overlap seem to be more similar to patients with lcSSc than those with dcSSc. Interestingly, musculo skeletal complications, including contractures, synovitis, and muscle weakness and atrophy, were more common and developed earlier in patients with SSc-overlap than in those with either dcSSc or lcSSc. Although the fre quency of overlap syndrome in patients with SSc was lower in this study (10%) than a study in the UK (20%) by Pakozdi et al.,5 over all, the findings are comparable. Both studies found that patients with SSc-overlap were more likely to have limited cutaneous involvement. The exception seems to be SSc-overlap with myositis, in which diffuse or limited cutaneous involvement is equally likely. Of note, the Pakozdi et al.5 study also showed that the most common CTD coexist ing with SSc was myositis (43%), followed by rheuma toid arthritis (32%), Sjögren's syn drome (17%) and systemic lupus erythe matosus (8%). Unfor tunately, the tem poral relation ship between the onset of SSc and of the overlapping CTD was not examined. Although SSc-specific auto antibodies and CTD-specific auto antibodies, such as anti-PM/Scl antibodies, were detected in those with overlapping myositis, or anti-Ro and anti-La anti bodies in those with Sjögren's syn drome, rheumatoid factor was detected in 50% of all patients with SSc-overlap (not only those with RA). Not surprisingly, cortico steroids and immuno suppresive drugs were used more commonly in patients with SSc-overlap than in those with dcSSc or lcSSc.
A consensus exists on the rationale and usefulness of classifying SSc into limited and diffuse cutaneous subsets. This classification helps in risk stratification, predicting the course of disease and identifying patients appropriate for treatment trials. The existence of an SSc sine subset, comprising patients with SSc-characteristic visceral organ manifestations and auto-antibodies, but lacking clinically apparent skin- thickening, is also generally accepted.6,7 The patients with SSc who fall into this category (∼10%) generally have symptoms and signs similar to patients with lcSSc.
Another group of patients with SSc whose disease might constitute a distinct subset are those who develop cancer. One study compared eight patients with SSc and anti-RNA polymerase III (RNA Pol III) auto antibodies who developed cancer within 2.5 years of SSc diagnosis with eight patients with SSc and either anti-centromere or anti- topoisomerase 1 (TOP1) auto antibodies who developed cancer a median of 14.2 years after diagnosis of SSc.8 Genetic analyses of tumour samples revealed somatic missense mutations or loss of hetero zygosity in POLR3A (which encodes RNA Pol III A subunit) in five of the eight patients who tested positive for anti-RNA Pol III autoantibodies. By contrast, no somatic mutations in the genes encoding major centromere auto antigen B (CENPB) or TOP1 were found in patients with SSc who tested positive for anti- centromere or anti-TOP1 autoanti bodies. The authors speculate that, in patients with anti-RNA Pol III auto antibodies, cancer might trigger SSc via antitumour im munity.9 Whether the presence of anti-RNA Pol III anti bodies in patients with SSc who develop cancer defines a distinct SSc subset, akin to a para-neoplastic syndrome, remains to be established. Along with similar observations from other studies, Moinzadeh et al.4 make a case that SSc-overlap is a separate disease subset. We are, therefore, now faced with five or six clinically defined and more-or-less distinct subsets of SSc (Figure 1). Whilst current clinical practice increasingly makes use of this classification scheme, further studies are needed to establish and confirm the validity of these SSc subsets, compare and contrast their genetic and pathophysio logical features and evaluate their utility in clinical decision-making.
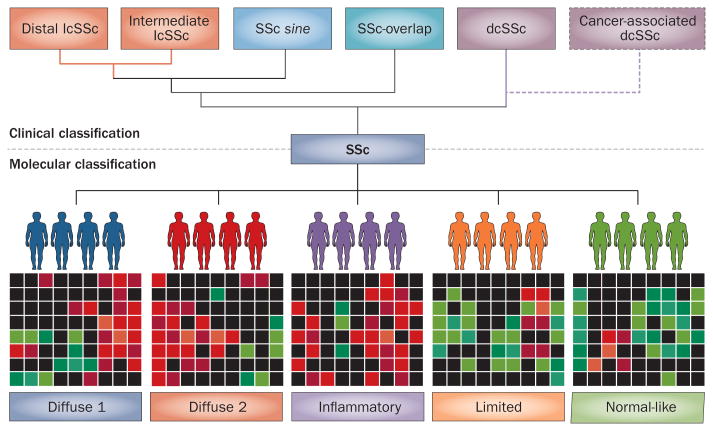
Figure 1.
Alternative systems of SSc classification. Currently, clinical classification separates patients on the basis of limited versus diffuse skin involvement. Moinzadeh et al.4 suggest a new SSc subset, intermediate to lcSSc and dcSSc, comprising patients with SSc who have features of an overlapping connective tissue disease. In the future, patients might be classified by gene expression patterns or molecular ‘skin signatures’. For example, using microarrays, Milano et al.9 classified biopsy-obtained SSc skin samples into five molecular subsets distinct from clinically classified subsets. A combination approach to classification might provide an enhanced personalized medicine approach to treatment. Abbreviations: dcSSc, diffuse cutaneous SSc; lcSSc, limited cutaneous SSc; SSc, systemic sclerosis.
We anticipate that, in the future, precise classi fication of patients with SSc will be based on an integrated ‘systems’ strategy. A molecular classification of SSc that incor porates traditional clinical variables com bined with information from serum auto antibody testing and high-throughput analytical approaches, such as functional gen omics, proteomics or metabolo mics, might pro vide substantial added insight into dis ease hetero geneity, and have implica tions for a personalized medicine approach to patient management. Already, genome-wide expres sion profiling of biopsy-obtained skin sam ples from patients with SSc has revealed intriguing molecular hetero geneity that is uncoupled from clinically-defined subclasses (Figure 1). For instance, Milano et al.9 identified five gene signatures in SSc skin samples that define distinct molecular sub sets. Pilot studies have provided ‘proof-of-concept’ that molecular sub classification of patients with SSc might facilitate the selec tion of targeted therapies and improve out comes.10 Using advanced classification approaches in clinical trials will be necessary for validating preliminary observations and defining their predictive value and clinical utility.
Moinzadeh et al.4 propose that we should con sider SSc-overlap as a distinct disease subset. Recognizing the 10–20% of patients with SSc who fall within this subset should help in choosing targeted therapeutic strategies. Further research to define the dis tinct genetic and pathophysiological characteristic s of SSc subsets is urgently needed.
Acknowledgments
The authors' work is supported by grants from the NIH (AR42309 and HD55884).
Footnotes
Competing interests: The authors declare no competing interests.
References
- 1.LeRoy EC, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–205. [PubMed] [Google Scholar]
- 2.Gliddon AE, et al. Antinuclear antibodies and clinical associations in a British cohort with limited cutaneous systemic sclerosis. J Rheumatol. 2011;38:702–705. doi: 10.3899/jrheum.100754. [DOI] [PubMed] [Google Scholar]
- 3.Meyer OC, Fertig N, Lucas M, Somogyi N, Medsger TA., Jr Disease subsets, antinuclear antibody profile, and clinical features in 127 French and 247 US adult patients with systemic sclerosis. J Rheumatol. 2007;34:104–109. [PubMed] [Google Scholar]
- 4.Moinzadeh P, et al. Disease progression in systemic sclerosis-overlap syndrome is significantly different from limited and diffuse cutaneous systemic sclerosis. Ann Rheum Dis. doi: 10.1136/annrheumdis-2013-204487. http://dx.doi.org/10.1136/annrheumdis-2013-204487. [DOI] [PMC free article] [PubMed]
- 5.Pakozdi A, et al. Clinical and serological hallmarks of systemic sclerosis overlap syndromes. J Rheumatol. 2011;38:2406–2409. doi: 10.3899/jrheum.101248. [DOI] [PubMed] [Google Scholar]
- 6.Poormoghim H, Lucas M, Fertig N, Medsger TA., Jr Systemic sclerosis sine scleroderma: demographic, clinical, and serologic features and survival in forty-eight patients. Arthritis Rheum. 2000;43:444–451. doi: 10.1002/1529-0131(200002)43:2<444::AID-ANR27>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 7.Marangoni RG, et al. Systemic sclerosis sine scleroderma: distinct features in a large Brazilian cohort. Rheumatology (Oxford) 2013;52:1520–1524. doi: 10.1093/rheumatology/ket163. [DOI] [PubMed] [Google Scholar]
- 8.Joseph CG, et al. Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science. 2014;343:152–157. doi: 10.1126/science.1246886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milano A, et al. Molecular subsets in the gene expression signatures of scleroderma skin. PLoS ONE. 2008;3:e2696. doi: 10.1371/journal.pone.0002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinchcliff M, et al. Molecular signatures in skin associated with clinical improvement during mycophenolate treatment in systemic sclerosis. J Invest Dermatol. 2013;133:1979–1989. doi: 10.1038/jid.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]