Abstract
Background
Cardiovascular and metabolic consequences of obesity in children, unlike adults, are still not well understood nor have they been subject to extensive research in Africa. We aimed to identify the cardio-metabolic complications associated with childhood obesity at the early phase of the management of obese children in a reference center in Cameroon.
Methods
In this cross-sectional study conducted from November 2013 to September 2014 and based on World Health Organization (WHO) classification of Obesity (BMI > 3SD under 5 years and BMI > 2SD from 5 and above), we included children aged 3 to 17 years who were being followed up for obesity at the pediatric endocrinology unit of the Mother and Child Center of the Chantal BIYA Foundation in Yaounde, Cameroon. A control group composed of children with normal BMI coming for a routine check up or vaccination was matched to the obese subjects. In both groups, we measured waist circumference (WC), blood pressure, fasting lipid profile and fasting glycaemia. We also considered the presence or absence of acanthosis nigricans. Data were analyzed using STATA software version 11.0, and presented as means, medians, compared with parametric and non-parametric statistical tests.
Results
We enrolled 38 obese children and 38 controls matched for sex and age. The majority of our participants were boys with a sex ratio of 1.24, and median age was 9.9 years. The median Z score of BMI was 3.21 in obese children. Approximately (n = 35) 90% of obese children (<6% in controls p < 0.001) presented with an abdominal obesity (WC/height ratio > 0.5) and 58% (n = 22) had acanthosis nigricans (5% (n = 2) in controls, p < 0.001). Type 2 diabetes mellitus was found in one participant, hypercholesterolemia in about 16% (n = 6) and high blood pressure in 25% (n = 8) of participants. Metabolic syndrome was present in 19% (n = 4) of obese children aged >10 years.
Conclusions
Obesity in children is associated with early onset metabolic disorders such as dyslipidemia, high blood pressure and type 2 diabetes. The screening and management of these complications is therefore recommended.
Background
In the past few decades, obesity has been on a sharp increase in children and adolescents [1]. According to WHO statistics, there were about 43 million obese children worldwide in 2011 with 35 million of them living in developing countries [2]. There is a paucity of data on childhood obesity in Sub-Saharan Africa. Notwithstanding, in Cameroon, the 2011 Health and Demographic Survey (DHS) revealed that 6% of children below 5 years were obese [3], and 10 to 15% of adolescents were affected in urban areas [4–6].
Early and late complications of childhood obesity are numerous and severe. They include metabolic diseases [7], cardiovascular diseases [8–11], bone deformities and respiratory problems [1, 12]. Moreover, childhood obesity is likely to persist throughout adulthood, thereby exposing obese subjects to cardiovascular, metabolic, respiratory, musculoskeletal and psychosocial problems. And such illnesses may lead to an increased morbidity and mortality in this population [9].
There is currently very few data on the metabolic status of obese children in sub-Saharan Africa. Therefore, the aim of this study was to identify cardio-metabolic abnormalities in obese children who attended the pediatric endocrinology outpatient clinic of the Mother and Child Centre of the Chantal BIYA Foundation in Yaounde, Cameroon, comparing them with matched children with normal Body Mass Index (BMI).
Methods
Study design, setting and participants
This cross-sectional study received ethical approval from the institutional ethical committee of “Université des Montagnes” (number 2014/047/UdM/PR/CAB/CIE). Participants were included from the pediatric endocrinology unit of the Mother and Child Centre of the Chantal BIYA Foundation in Yaoundé from November 2013 to September 2014. The Center is a multidisciplinary pediatric hospital located in the capital and second most populous city of Cameroon; it hosts the only pediatric endocrinology unit of the country. This unit allocates two separate rooms to metabolic investigations.
Selection of cases
We carried out a consecutive sampling of children and adolescents aged 2 to 17 years with obesity defined according to WHO recommendations as a body mass index (BMI) > 3 Z-score for children below 5 years and BMI > 2 Z-score for those from 5 years and above [13].
Selection of control subjects
The control group was made up of children with normal BMI (−2 Z-score < BMI < +1 Z-score). They were selected among healthy children who came for a routine checkup or vaccination and were matched with patients for age and sex.
Data collection and definition of terms
The waist circumference (WC) in centimeter (cm) was measured with a non-stretchable tape at a point midway between the costal margin and the anterior superior iliac spine, and the standing height (H) in cm with a wall-mounted stadiometer. The weight in kilogram (kg) was measured with a Seca® brand bathroom scale. The BMI was calculated as the weight in kg divided by the square of the height in meter. Abdominal adiposity was defined as waist-to-height ratio (WHtR = the waist circumference in cm divided by the height in cm) ≥ 0.5.
The blood pressure (BP) was measured at rest with an appropriate cuff for each child with a manual Spengler® sphygmomanometer, and the average of three measures taken at 10 min intervals was considered and then translated in percentile with the Baylor College of Medicine software [14]. Blood pressure was classified according to recommendations of the American Academy of Pediatrics as follows: hypertension for a BP ≥ 95th percentile, pre-hypertension for BP between the 90th and the 95th percentile [15]. The presence or absence of acanthosis nigricans was used as the insulin resistance indicator.
Parameters of the fasting lipid profile (total cholesterol, triglycerides, High Density Lipoprotein (HDL)-cholesterol, and Low Density Lipoprotein (LDL)-cholesterol calculated by the Friedewald formula) were measured in a 2 ml blood sample using a dry chemistry method in a Vitros® 350 automaton from Ortho Clinical Diagnosis, USA. Lipids abnormalities were defined according to age, following the recommendations of the American Academy of Pediatrics [16]. Fasting plasma glucose level was measured by the colorimetric method in the Vitros® 350 automaton. Diabetes was defined as a fasting plasma glucose ≥126 mg/dl (7 mmol/l). Impaired fasting glucose (IFG) was defined as a fasting plasma glucose level between 100 and 126 mg/dl [17]. Metabolic syndrome was defined according to International Diabetes Foundation (IDF) as an association of at least 2 metabolic disorders to abdominal obesity [18].
All control subjects underwent similar physical assessments with measurement of anthropometric parameters and blood pressure. Point-of-care devices were used to measure their biochemical parameters: cardio Check® (Polymer Technology System, USA) for capillary lipid profile analysis [19], and Accu Chek Active® (Roche Diagnostics GmbH, Germany) for capillary blood glucose [20].
Ethical approval
Before inclusion into the study, a written informed consent was signed by parents of all participants, and children above 12 years gave their assent. We also obtained ethical approval from the institutional ethical committee of “Université des Montagnes” (number 2014/047/UdM/PR/CAB/CIE).
Statistical analysis
Data were analyzed with STATA® software version 11.0 (Lakeway Drive, Texas, USA). Results were presented as mean (standard deviation: SD) for quantitative data and median (interquartile range: IQR) for qualitative data. The chi-square test was used for comparison of categorical variables. The CardioCheck® device has a lower value detection threshold for quantitative analysis; hence we considered this lower value in controls whose values were under that threshold. We used non parametric tests when variances were not homogeneous. Statistical significance was set at p value <0.05.
Results
General characteristics of participants
We included 38 out of 42 eligible participants (4 participants were excluded due to incomplete data) who were receiving care in the unit, 21 of whom were boys (55.5%). The mean age was 9.9 years (range 3–17 years). Table 1 summarizes the general characteristics of study participants and control subjects.
Table 1.
General characteristics of study participants
| Variable | Obese | Control | P value |
|---|---|---|---|
| N | 38 | 38 | |
| Boys N (%) | 21 (55.3) | 21 (55.3) | |
| Age (years)a | 9.7 ± 3.6 | 10.1 ± 3.7 | 0.729ǂ |
| Weight (Kg)a | 65.6 ± 13.7 | 35.2 ± 27.5 | <0.0001ǂ |
| Z-score-Height | 1.27 [0.29 - 1.91] | −0.15 [−0.74 - 0.60] | <0.0001¶ |
| BMI (Kg/m2)a | 29.3 ± 7.3 | 17.4 ± 2.4 | <0.0001¶ |
| Z score-BMIb | +3.3 [2–7] | 0 [−1.5 + 1] | <0.0001¶ |
| WC (cm)b | 88.5 [80.0-96.5] | 61.0 [56.0-65.0] | <0,0001¶ |
amean ± SD; b median [IQR]; ǂ t-test; ¶ Man-Whitney test
Cardiometabolic profile
Out of the 31 participants who had blood pressure values during the first consultation, 14 (45%) had high values (>90th percentile).; among these, 8 (25% of all participants) had hypertension (>95th percentile): 5 girls and 3 boys, all aged above 10 years and 6 had pre-hypertension(Fig. 1a). In the control group, 2 subjects had pre-hypertension (BP between the 90th and 95th percentile) but none had hypertension (BP > 95th percentile) (Fig. 1b). The difference in prevalence of abnormal blood pressure was significant between the two groups (p < .0001).
Fig. 1.
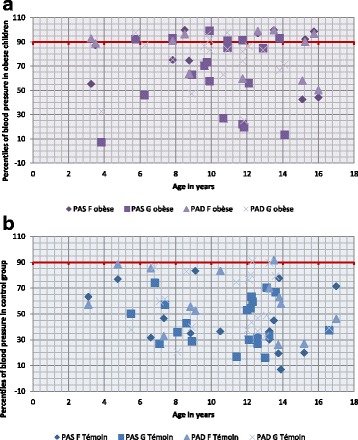
a Representation of percentiles of blood pressure in patients. b Representation of percentiles of blood pressure in controls
The median fasting blood glucose level (87 mg/dl) was similar in the two groups. However, one obese child was diagnosed of Type 2 diabetes mellitus and 4 children were diagnosed of IFG whereas there was none in the control group (p = 0.054) (Fig. 2). Compared to control subjects, obese children had higher WHtR, higher systolic and diastolic blood pressures, higher total cholesterol, LDL cholesterol, and HDL cholesterol levels (Table 2). The proportion of children with high total cholesterol, triglycerides and LDL cholesterol was higher in the obese than in the control group, whereas the proportion of children with lower HDL cholesterol level was higher in the control group (Fig. 2). Acanthosis nigricans lesions were significantly more prevalent in patients (22/38) than in control subjects (2/38) (p < 0.00001).
Fig. 2.
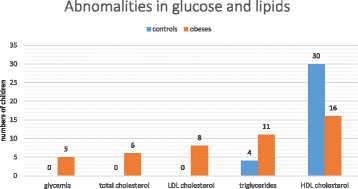
Histogram showing the numbers of children who had abnormal glucose and lipid levels
Table 2.
Cardio metabolic status in the study population
| Variable | Obese | Control | P value† |
|---|---|---|---|
| WHtRa | 0.59 [0.52–0.65] | 0.44 [0.42–0.46] | 0.0001 |
| SBP (mmHg)a | 110.0 [102.0–126.0] | 100.0 [96.0–110.0] | 0.002 |
| DBP (mmHg)a | 73.3 [68.0–79.0] | 64.1 [60.0–70.0] | 0.05 |
| Glycemia (mg/dl)a | 89 [83.7–91.0] | 85.5 [79.5–93.0] | 0.68 |
| Total cholesterola | 164.5 [142.7–187.7] | 115.0 [100.0–136.3] | 0.0001 |
| Triglycerides (mg/dl)a | 81.0 [61.0–111.0] | 61.5 [50.0–71.0] | 0.08 |
| LDL cholesterol (mg/dl)a | 102.5 [81.5–127.5] | 72.0 [64.8–85.3] | <0.0001 |
| HDL cholesterol (mg/dl)a | 42.0 [33.8–52.0] | 28.0 [24.0–36.3] | 0.04 |
amedian [IQR]; † Man-Whitney test
Excluding HDL cholesterol level, all other metabolic abnormalities were associated with the presence of abdominal obesity (Fig. 3). Out of the 21 obese children aged >10 years, 4 (19%) met the criteria of definition of metabolic syndrome while there was none in the control group.
Fig. 3.
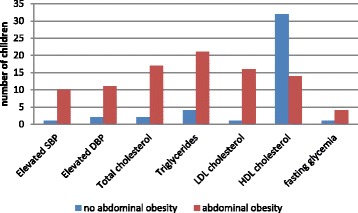
Number of children with metabolic abnormalities with respect to the abdominal obesity status
Discussion
In this study, we have found a higher prevalence of elevated blood pressure, dyslipidemia, and blood glucose abnormalities, as well as higher occurrence of acanthosis nigricans in obese children compared to their age and sex-matched lean counterparts.
Childhood obesity has been on a steep rise worldwide for the past few decades and the likelihood that this population of obese children becomes obese during adulthood is very high [9]. This phenomenon has affected developed as well as developing countries, Cameroon inclusive. Recent studies carried out in Cameroon have reported an ever-increasing prevalence of childhood obesity [3–6]. It has been observed that childhood obesity is associated with numerous and often severe short-term and long-term complications including cardiovascular, metabolic, respiratory, musculoskeletal and psychosocial problems [7–11]. However, obesity is considered as a sign of healthy living, beauty and wealth in many African cultures. As such, most parents do not consider taking their obese children to hospital for a medical check-up and follow-up [21].
The prevalence of hypertension in our study was one out of four children and about 20% had pre-hypertension. A study carried out in Bolivia found a similar prevalence, though only the systolic blood pressure was considered [22]. But another study carried out in Thailand found a lower prevalence of hypertension in obese children (20.2%) [23]. Studies have shown that the prevalence of hypertension is higher in people of African ancestry than in Asians and Europeans [24]. The difference observed is probably due to this genetic predisposition of people of African ancestry. Also, only two children in the control group had pre-hypertension (~6%). This is evidence to suggest that the high prevalence of elevated blood pressure (about 1 in 4 children) was related to obesity in these children.
Blood glucose impairment was found in 13% of obese children in our study population whereas none of the children in the control group were affected. This is further evidence to suggest that obesity in these children predisposed them to cardiometabolic abnormalities. Studies carried out elsewhere found 10.5% and 17.8% of blood glucose impairment in Thailand [23] and United States of America [25] respectively. The difference with the United States National cohort study could be due to the fact that we did not carry out an oral glucose tolerance test, thereby probably ignoring some cases of glucose intolerance.
The proportion of children with high total cholesterol, triglycerides and LDL cholesterol was higher in the obese than in the control group. This is in line with other metabolic problems which were found in this group such as glucose impairment and the presence of acanthosis nigricans (seen in about 60% of obese children compared to only about 5% in the control group). Acanthosis nigricans is a strong indicator of insulin resistance which is a very important element in the development of Type 2 diabetes mellitus. This study identified a case of Type 2 diabetes and the evidence will suggest more cases of Type 2 diabetes in this group in the near future, especially if adequate measures are not put in place to combat these complications of childhood obesity. However, we were surprised to find that the proportion of children with lower HDL cholesterol level was higher in the control group. This may suggest either the need to redefine normal standard values in children, or that the rapid test we used may not have enough accuracy and specificity to be adapted in a pediatric population.
Moreover, the cardiometabolic complications observed were mostly related to abdominal obesity. Therefore, abdominal obesity measured by the WHtR could be suggested as a quick method of identification of children with high cardiovascular risk [25].
A bigger sample is however necessary to test sensitivity and specificity. We have also studied an essentially urban sample and the results could be different with a less sedentary rural population.
We acknowledge the following potential limitations in our study: we used different laboratory analyses in testing lipid profile and blood glucose in the two groups under study but some evidence showed the accuracy of rapid photometry methods (used in control groups in our study) in the follow-up of patients affected with diabetes or dyslipidemia [19, 20].
Conclusion
Metabolic disturbances are prevalent in obese children in Yaounde, as compared to those with normal BMI. One quarter of obese children have high blood pressure, 21% of dyslipidemia and 13% of glucose intolerance with a case of type 2 diabetes. The metabolic syndrome affects 19% of our adolescents and girls seem to be more at risk. The various abnormalities are associated with the presence of abdominal obesity. Prevention should be emphasized not only on the occurrence of complications, but better still on the occurrence of obesity in children, given the cardiovascular and metabolic risk and the heavy socio-economic repercussion in developing countries such as Cameroon.
Acknowledgements
We thank the personnel of the endocrinology and diabetes unit of the Mother and Child Center of the Chantal Biya Foundation, especially Mr. Dang and Mr. Bayiha.
Funding
None.
Availability of data and materials
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.
Authors’ contributions
Study concept and design: ECN, SS, SPC, DN, OKN. Data collection and analysis: ECN, SS, INT. Drafting: ECN, SS. Manuscript critical revision: ECN, SS, SPC, INT, DN, OKN. All the authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study received ethical approval from the Institutional Ethics Committee of “Université des Montagnes” (number 2014/047/UdM/PR/CAB/CIE). We obtained written informed consent from parents for the participation of their children as controls, and assent of all children above 12 years; for patients, we used data from their medical records.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- BMI
Body Mass Index
- BP
Blood Pressure
- HDL
High Density Lipoprotein
- IDF
International Diabetes Foundation
- IFG
Impaired Fasting Glucose
- LDL
Low Density Lipoprotein
- WC
Waist Circumference
- WHO
World Health Organization
- WHtR
Waist to Height Ratio
Contributor Information
Eunice Chedjou-Nono, Phone: +237 696 784 681, Email: eunicevannono@gmail.com.
Suzanne Sap, Email: suzysap@gmail.com.
Simeon-Pierre Choukem, Email: schoukem@gmail.com.
Issa Ngosso Tetanye, Email: icetetanye@yahoo.com.
Daniel Nebongo, Email: dnebongo@gmail.com.
Olivier Koki Ndombo, Email: koki_paul@hotmail.com.
References
- 1.Han JC, Lawlor DA, Kimm SYS. Childhood obesity. Lancet. 2010:1737–48. [DOI] [PMC free article] [PubMed]
- 2.World Health Organization. World health statistics. World Health Stat [Internet]. 2011; Available from: http://www.who.int/whosis/whostat/FR_WHS2011_Full.pdf. Accessed 12 Dec 2013.
- 3.National institute of Statistics and ORC Macro. Demographic Health Survey and MICS [Internet]. 2011. Available from: http://www.dhsprogram.com/pubs/pdf/FR260/FR260.pdf. Accessed 8 Dec 2013.
- 4.Wamba PC, Enyong J, Cianflone K. Prevalence of overweight, obesity, and thinness in Cameroon urban children and Adolescents. J Obes. 2013:1–9. [DOI] [PMC free article] [PubMed]
- 5.Mekone KI. Risk factors of obesity in children aged 10 to 15 years in secondary schools in Yaounde [dissertation of pediatrics] Cameroon: University of Yaounde I; 2014. [Google Scholar]
- 6.Kamdeu J. Prevalence and determining factors of childhood overweight and obesity in primary schools in Douala. MD Thesis. Faculty of Medicine and Pharmaceutical Sciences, University of Douala, Cameroon. 2013.
- 7.Mancini MC. Metabolic syndrome in children and adolescents - criteria for diagnosis. Diabetol Metab Syndr. 2009;1(1):20. doi: 10.1186/1758-5996-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oduwole AA, Ladapo TA, Fajolu IB, Ekure EN, Adeniyi OF Obesity and elevated blood pressure among adolescents in Lagos, Nigeria: a cross-sectional study. BMC Public Health. 2012;12:616. doi: 10.1186/1471-2458-12-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365(20):1876–1885. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]
- 10.De Ferranti S, Osganian S. Epidemiology of paediatric metabolic syndrome and type 2 diabetes mellitus. Diab Vasc Res. 2007;4(4):285–296. doi: 10.3132/dvdr.2007.055. [DOI] [PubMed] [Google Scholar]
- 11.Forga L, Petrina E, Barbería JJ. Complications of obesity. An Sist Sanit Navar. 2002;25(Suppl 1):117–126. doi: 10.23938/ASSN.0820. [DOI] [PubMed] [Google Scholar]
- 12.Reilly JJ, Methven E, McDowell ZC, Hacking B, Alexander D, Stewart L, et al. Health consequences of obesity. Arch Dis Child. 2003;88(9):748–752. doi: 10.1136/adc.88.9.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO child growth standard 2006 [Internet]. World Health Organization; Available from: http://www.who.int/childgrowth/standards/fr/. Accessed 8 Dec 2013.
- 14.USDA/ARS Children’s Nutrition Research Center. Age-based pediatric blood pressure reference charts [Internet]. Body composition Laboratory-Baylor College of medicine; Available from: https://www.bcm.edu/bodycomplab/Flashapps/BPVAgeChartpage.html. Accessed 13 May 2014.
- 15.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The Fourth Report on the Diagnosis, Evaluation and Treatment of High Blood Pressure in Children and Adolescents [Internet]. 2004 p. 555. Report No.: 114. Available from: http://pediatrics.aappublications.org/content/114/Supplement_2/555. Accessed 15 May 2014.
- 16.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk. 2011 p. 213. Report No.: 128. Available from: http://pediatrics.aappublications.org/content/128/Supplement_5/S213. Accessed 8 June 2014. [DOI] [PMC free article] [PubMed]
- 17.American Diabetes Association. Standards of medical care in diabetes. 2014 p. 14–80. Report No.: 37.
- 18.Zimmet P, Alberti K, George MM, Kaufman F, Tajima N, Silink M. The metabolic syndrome in children and adolescents – an IDF consensus report. Pediatr Diabetes. 2007;8:299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 19.Plüddemann A, Thompson M, Price CP, Wolstenholme J, Heneghan C. Point-of-care testing for the analysis of lipid panels: primary care diagnostic technology update. Br J Gen Pract. 2012;62(596):224–226. doi: 10.3399/bjgp12X630241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freckmann G, Baumstark A, Jendrike N, Zschornack E, Kocher S, Tshiananga J, et al. System accuracy evaluation of 27 blood glucose monitoring systems according to DIN EN ISO 15197. Diabetes Technol Ther. 2010;12(3):221–231. doi: 10.1089/dia.2009.0128. [DOI] [PubMed] [Google Scholar]
- 21.Correia J, Pataky Z, Golay A. Understanding obesity in Africa: the effect of [economic] development and [mental] concepts. Rev Médicale Suisse. 2014;10(423):712–716. [PubMed] [Google Scholar]
- 22.Caceres M, Teran CG, Rodriguez S, Medina M. Prevalence of insulin resistance and its association with metabolic syndrome criteria among Bolivian children and adolescents with obesity. BMC Pediatr. 2008;8(1):31. doi: 10.1186/1471-2431-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rerksuppaphol S. Prevalence of Metabolic Syndrome in Thai Children: A Cross- sectional Study. J Clin Diagn Res [Internet]. 2014 [cited 2015 Oct 15]; Available from: http://www.jcdr.net/article_fulltext.asp?issn=0973-709x&year=2014&volume=8&issue=4&page=PC04&issn=0973-709x&id=4287. Accessed 8 Dec 2013. [DOI] [PMC free article] [PubMed]
- 24.Blascovich SJ, Spencer S, Quinn D, Steele C. African Americans and high blood pressure: the role of stereotypes threat. Psychol Sci. 2001;12(3):225–229. doi: 10.1111/1467-9280.00340. [DOI] [PubMed] [Google Scholar]
- 25.Martínez-Gómez D, Eisenmann JC, Gómez-Martínez S, Veses A, Marcos A, Veiga OL. Sedentary behavior, adiposity, and cardiovascular risk factors in Adolescents. The AFINOS study. Rev Esp Cardiol Engl Ed. 2010;63(3):277–285. doi: 10.1016/S0300-8932(10)70086-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.


