Abstract
Mesenchymal stem/stromal cells (MSC) are adult stem cells which have been shown to improve survival, enhance bacterial clearance and alleviate inflammation in pre-clinical models of acute respiratory distress syndrome (ARDS) and sepsis. These diseases are characterised by uncontrolled inflammation often underpinned by bacterial infection. The mechanisms of MSC immunomodulatory effects are not fully understood yet. We sought to investigate MSC cell contact-dependent communication with alveolar macrophages (AM), professional phagocytes which play an important role in the lung inflammatory responses and anti-bacterial defence. With the use of a basic direct co-culture system, confocal microscopy and flow cytometry we visualised and effectively quantified MSC mitochondrial transfer to AM through tunnelling nanotubes (TNT). To model the human AM, primary monocytes were isolated from human donor blood and differentiated into macrophages (monocyte derived macrophages, MDM) in the presence of granulocyte macrophage colony-stimulating factor (GM-CSF), thus allowing adaptation of an AM-like phenotype ( de Almeida et al., 2000 ; Guilliams et al., 2013 ). Human bone-marrow derived MSC, were labelled with mitochondria-specific fluorescent stain, washed extensively, seeded into the tissue culture plate with MDMs at the ratio of 1:20 (MSC/MDM) and co-cultured for 24 h. TNT formation and mitochondrial transfer were visualised by confocal microscopy and semi-quantified by flow cytometry. By using the method we described here we established that MSC use TNTs as the means to transfer mitochondria to macrophages. Further studies demonstrated that mitochondrial transfer enhances macrophage oxidative phosphorylation and phagocytosis. When TNT formation was blocked by cytochalasin B, MSC effect on macrophage phagocytosis was completely abrogated. This is the first study to demonstrate TNT-mediated mitochondrial transfer from MSC to innate immune cells.
Keywords: Mesenchymal stem cells, Macrophages, Mitochondrial transfer, ARDS, Phagocytosis, Oxidative phosphorylation
Background
Data from pre-clinical studies, including studies by our group ( Xu et al., 2007 and 2008; Nemeth et al., 2009 ; Gupta et al., 2007 and 2012; Krasnodembskaya et al., 2010 and 2012; Mei et al., 2010 ; Lee et al., 2013 ; Jackson et al., 2016 ) demonstrated strong potential for MSC as a future cell-based therapy for the treatment of ARDS, an injurious hyper-inflammatory condition of the lung. In these studies MSC have displayed regenerative, immune-modulatory and anti-microbial effects which have consequently provided rationale for the design of phase I and phase II clinical trials for MSC in ARDS ( Zheng et al., 2014 ; Wilson et al., 2015 ). However, despite the rapid translation of MSC into the clinical trials, mechanisms of how MSC alleviate symptoms of ARDS still need to be fully elucidated. Recent studies have reported MSC modulate lung epithelial and endothelial cells through mitochondrial transfer via TNTs, resulting in improvement of the host cell bioenergetics ( Islam et al., 2012 ; Ahmad et al., 2014 ; Li et al., 2014 ; Liu et al., 2014 ). In ARDS, excessive pulmonary inflammation is one of the main characteristics of the disease in which alveolar macrophages (AM) are prominent cells. They orchestrate the inflammatory responses in the alveoli and play an important role in the lung bacterial clearance (Ware and Matthay, 2000; Jackson et al., 2016 ).
This protocol allowed us to study the functional effects of a TNT mediated process of an organelle transfer between MDMs both in vitro and using the same staining protocol, mouse alveolar macrophages in vivo ( Jackson et al., 2016 ). Although the major focus of our study was mitochondrial transfer, this protocol can be adapted with slight modifications for investigations of transfer of other organelles or even fluorescently labelled molecules.
Materials and Reagents
-
Extraction of mononuclear cells from human donor Buffy Coats
50 ml Falcon tubes (SARSTEDT, catalog number: 62.554.502)
T175 culture flasks (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 178883)
Sterile Pasteur pipettes (BIOLOGIX GROUP LTD Technical, catalog number: 30-0138A1)
Cover slip
Tissue culture coated 6 well plate (Thermo Fisher Scientific, Thermo ScientificTM, catalog numbers: 140675)
Buffy coats are obtained from the Northern Ireland Blood Transfusion Service (NIBTS) following ethical approval by the School Research Ethics Committee of Queen’s University Belfast
Hank’s buffered salt solution (HBSS) No Ca2+ or Mg2+ (Thermo Fisher Scientific, catalog number: 14170138)
Ficoll-Paque (GE Healthcare, catalog number: 17-5442-03)
Complete RPMI 1640 (Thermo Fisher Scientific, GibcoTM, catalog number: 21875034)
Foetal calf serum (FCS) (heat inactivated) (Thermo Fisher Scientific, GibcoTM, catalog number: 10270106)
Granulocyte-macrophage colony stimulating factor (GM-CSF) (PeproTech, catalog number: 300-03)
Penicillin/streptomycin 10,000 U/ml (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122)
Trypan blue (Thermo Fisher Scientific, GibcoTM, catalog number: 15250061)
Complete 1% FBS RPMI (see Recipes)
Complete RPMIGM-CSF (see Recipes)
-
Culture of human bone marrow-derived mesenchymal stromal cells (MSC)
50 ml Falcon tubes (SARSTEDT, catalog number: 62.554.502)
8-well chamber slides (Sigma-Aldrich, catalog number: C7182)
Tissue culture coated 6-well and 24-well plates (Thermo Fisher Scientific, Thermo ScientificTM, catalog numbers: 140675, 142475)
Pechiney PM999 Parafilm (Bemis, catalog number: PM999)
Microscope slide
Sterile Eppendorf tubes (SARSTEDT, catalog number: 72.690.001)
Flow tubes (SARSTEDT, catalog number: 55.475)
Human bone marrow-derived MSCs are obtained from the NIH repository in Texas A&M Health Science Centre College of Medicine, Institute for Regenerative Medicine (Temple, Texas). The cells meet all the criteria for the classification as MSCs as defined by the International Society of Cellular Therapy ( Dominici et al., 2006 )
Liquid nitrogen
Dulbecco’s phosphate buffered saline (DPBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 14190094)
Complete α-MEM (Thermo Fisher Scientific, GibcoTM, catalog number: 22561021)
Penicillin/streptomycin 10,000 U/ml (Thermo Fisher Scientific, GibcoTM, catalog number: 15140122)
L-glutamine (Thermo Fisher Scientific, GibcoTM, catalog number: 25030024)
Cytochalasin B (Sigma-Aldrich, catalog number: C6762)
10x trypsin (Thermo Fisher Scientific, GibcoTM, catalog number: 15250061)
Foetal calf serum (FCS) (heat inactivated) (Thermo Fisher Scientific, GibcoTM, catalog number: 10270106)
Trypan blue (Thermo Fisher Scientific, GibcoTM, catalog number: 15250061)
MitoTracker Deep Red FM probe (APC) (Thermo Fisher Scientific, Molecular ProbesTM, catalog number: M22426)
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D8418)
Complete α-MEM (see Recipes)
Mitochondrial staining solution (1% FBS α-MEM1%MITO) (see Recipes)
Cytochalasin B solution (1%FBS α-MEM1%CYTO) (see Recipes)
-
Confocal microscopy
0.45 µm filter membrane (SARSTEDT, catalog number: 83.1826.001)
Pechiney PM999 Parafilm (Bemis, catalog number: PM999)
Paper towel
Microscope slide
Coverslip
Phosphate buffered saline (PBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10010023)
Paraformaldehyde (PFA) (Sigma-Aldrich, catalog number: 158127)
1 N NaOH (Sigma-Aldrich, catalog number: S2770)
1 N HCl (Sigma-Aldrich, catalog number: H9892)
Normal goat serum (NGS) (Thermo Fisher Scientific, Invitrogen, catalog number: 31872)
Mouse anti-human CD45 primary antibody (Abcam, catalog number: ab8216)
Mouse IgG1 isotype (Abcam, catalog number: ab81032)
Goat anti-rabbit Alexafluor 405 secondary antibody (Abcam, catalog number: ab175655)
Brightmount/Plus aqueous mounting medium (Abcam, catalog number: ab103748)
Nail varnish
1% FBS PBS (see Recipes)
4% paraformaldehyde (PFA) (see Recipes)
Strong and weak block for immunofluorescent staining (see Recipes)
-
Flow cytometry
Cell scrapers (Fisher Scientific, catalog number: 08-100-241)
Sarstedt 5 ml polystyrene round bottomed flow tubes (SARSTEDT, catalog number: 55.1578)
Phosphate buffered saline (PBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10010023)
Human FcR binding inhibitor (Thermo Fisher Scientific, eBioscienceTM, catalog number: 14-9161-73)
Anti-human CD45 (PE) antibody (Thermo Fisher Scientific, eBioscienceTM, catalog number: 12-9459-41)
Isotype control (IgG1 kappa) (Thermo Fisher Scientific, eBioscienceTM, catalog number: 12-4714)
Zombie Aqua Fixable Dye (BioLegend, catalog number: 423101)
-
Bacterial culture and phagocytosis assay
Escherichia coli K1 type strain
Phosphate buffered saline (PBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 10010023)
LB broth (Lennox) (Sigma-Aldrich, catalog number: L3022)
LB broth with agar (Lennox) (Sigma-Aldrich, catalog number: L2897)
Hank’s buffered salt solution (HBSS) No Ca2+ or Mg2+ (Thermo Fisher Scientific, catalog number: 14170138)
Complete RPMI 1640 (Thermo Fisher Scientific, GibcoTM, catalog number: 21875034)
Saponin (Sigma-Aldrich, catalog number: 47036)
Gentamycin (Sigma-Aldrich, catalog number: G1397)
Complete 1% FBS RPMI (see Recipes)
0.5% saponin (see Recipes)
Equipment
Water bath (37 °C) (Grant Instruments, model: JBN12)
Centrifuge (Eppendorf, model: 5810 R)
Laminar flow cabinet (Contained Air Solutions, model: BioMAT 2 safety cabinet class 2)
Haemocytometer and cover slips (Hawksley Medical and Laboratory Equipment, model: AC1000 Improved Neubauer, catalog number: BS 748)
Incubator (Panasonic Biomedical, model: MCO-170AIC-PE)
Dmi1 microscope (Leica Microsystems, model: DMi1)
TCS SP5 II Leica confocal microscope (Leica Microsystems, model: TCS SP5 II)
BD FACSCanto II Flow cytometer (BD, model: BD FACSCanto II)
Vortex (Cole-Palmer Instrument, catalog number: UY-86579-20)
2-20 µl pipette (Gilson, catalog number: F123600)
20-200 µl pipette (Gilson, catalog number: F144565 )
100-1,000 µl pipette (Gilson, catalog number: F144566)
Midi PlusTM pipette controller Automatic (Sartorius, catalog number: 710931)
Software
FlowJo software (FlowJo)
Prism 5 software (GraphPad Software)
LAS-AF software (Leica confocal microscopy)
FACS DIVA software (flow cytometry)
Procedure
-
Extraction of mononuclear cells from donor Buffy Coats
Single donor leukocyte buffy coats are obtained from the Blood Transfusion Centre in Belfast (NIBTS) or from volunteers following ethical approval (approx. 50 ml/blood bag/volunteer).
Pre-warm all media (HBSS, 1% FBS RPMI and 10% FBS RPMI) in a water bath 1-2 h prior to isolation and pre-warm the centrifuge to 20 °C prior to isolation.
Under a laminar flow cabinet decant the donor blood into a T75 flask and dilute (1:2) using sterile HBSS and mix by inversion.
Pipette 15 ml of Ficoll-Paque into 4 x 50 ml Falcons.
Very gently layer the diluted blood at a 45° angle onto the Ficoll, taking care not to mix the layers. Top up the remaining tube with HBSS to make a final volume of 50 ml.
Centrifuge all tubes at 480 × g, 20 °C for 20 min without brake.
After the spin, locate the pearlescent white layer (buffy layer, shown as lymphocytes, monocytes and platelets layer in Figure 1), which is sedimented through the plasma-density gradient interface. Extract into a new sterile 50 ml Falcon using a Pasteur pipette.
To wash the cells, add 40 ml of HBSS to the mononuclear cells and mix by inversion.
Centrifuge again at 290 × g, 4 °C for 5 min with brake. Aspirate off supernatant slowly (take care as the pellet may become loose and add another 40 ml HBSS). Repeat this wash step 3 times.
After the last wash, resuspend the pellet in 10 ml of complete 1% FBS RPMI and mix thoroughly by either vortexing or inversion.
Place a cover slip on a haemocytometer and load 10 μl of the cell mixture via capillary action through the loading channel.
Incubate in the 37 °C, 5% CO2 incubator for at least 5 min (see Note 1).
After this time, view the cells under a microscope at 20x magnification. Count all mononuclear cells (see Note 1).
Cells are then seeded at density of 1 x 106 cells/well of a 6 well plate using 3 ml complete 1% FBS RPMI (see Table 1 for other seeding densities). Incubate at 37 °C, 5% CO2 for at least 2 h to ensure mononuclear cells have adhered to the plastic of the well.
After this time non-adherent cells (lymphocytes) are washed off twice with HBSS (by adding 1 ml HBSS, slight agitation and then aspiration of waste).
To promote monocyte-derived macrophage (MDM) maturation, replace complete 1% FBS RPMI with 3 ml complete RPMIGM-CSF (see Recipes).
Leave cells to rest for 5-7 days at 37 °C, 5% CO2.
After 5-7 days, wash cells twice with HBSS and replace with 3 ml complete 1% FBS RPMI. Leave cells to rest for 1 h prior to stimulation experiments.
-
Human mesenchymal stromal cell culture
-
MSC expansion and storage
MSC are shipped from Texas, US in liquid nitrogen in 1 ml cryovials at passage number 1 or 2 (P1-P2) meaning they have undergone at least one or two stages of cell splitting in culture. For experimental use (see section Preparing P3 MSC for Experimental Use) cells are only used from P3-P5.
Shortly after arrival, defrost 1 vial and culture in pre-warmed 15 ml complete 16% FBS α-MEM in a T175 flask for less than 24 h.
Retain the passage number by splitting cells less than 24 h later and expanding them using a density of 21,000 cells per flask. A P2 vial can undergo an additional expansion to be stored at P3. Store cells (1 million cells/vial) at either P2 or P3 in liquid nitrogen storage.
-
Preparing P3 MSC for experimental use
Once ready to use, defrost 1x P3 cryovial of MSC in the water bath (37 °C) and place entire 1 ml into 15-20 ml of complete 16% FBS α-MEM, making sure to feed every other day. Cells are maintained in culture until P5-P6.
-
-
Direct co-culture of Human Mesenchymal Stromal Cells (MSC and Monocyte-derived Macrophages (MDM)
-
Pre-warm DPBS, HBSS, complete 10% FBS RPMI, 1% FBS RPMI, 16.5% FBS α-MEM and 1% FBS α-MEM in the water bath at 37 °C for at least half an hour before the start of the experiment.
Following work has to be completed in a biosafety cabinet with cabinet light switched off.
At 70-80% confluency, to pre-stain MSC mitochondria, aspirate older 16% FBS α-MEM from the T175 flask and add 20 ml of staining solution (1% FBS α-MEMMITO [see Recipes and Note 2]), incubate for approximately 30 min at 37 °C, 5% CO2.
For MSC being pre-treated with cytochalasin B, 20 ml of 1% FBS α-MEMCYTO is added to cells for 1-2 h at least prior to staining solution (1% FBS α-MEMMITO). As Cyto B is soluble in DMSO, make sure to have a control flask of cells for DMSO vehicle control.
Aspirate 1% FBS α-MEMCYTO from the flask and replace with 1% FBS α-MEMMITO, and incubate for 30 min at 37 °C, 5% CO2.
After this time, the staining solution is aspirated from MSC and the cells are washed at least three times in 5 ml DPBS.
Aspirate waste DPBS and add 7 ml 1x trypsin (diluted in DPBS) and leave in the incubator for approximately 2-5 min.
Once the cells are suitably detached, add 7 ml of complete α-MEM to counteract trypsin action and pipette the cells into a 50 ml Falcon.
Centrifuge the cells at 290 × g for 5 min.
During this time, prepare the MDMs by aspirating older culture medium and washing twice with 1-2 ml of HBSS.
Aspirate the remaining HBSS and leave the MDMs to rest in the incubator in 200 μl (chamber slide), 1 ml (24 well plate) or 3 ml (6 well plate) of 1% FBS RPMI.
After MSC centrifugation, aspirate the supernatant and resuspend the pellet in 1 ml 1% FBS RPMI (see Note 3).
Count cells using a haemocytometer, using trypan blue exclusion and depending on well size needed for experiment, seed corresponding cell densities and co-culture with MDMs for 24 h (see Table 1).
Proceed to either confocal microscopy or flow cytometry procedures.
-
-
Confocal microscopy staining procedure
After co-culture on a chamber slide, carefully aspirate off culture medium, and wash cells twice using 200 μl of ice cold PBS.
Aspirate the PBS and fix the cells using 100 μl of 4% PFA solution.
Incubate at room temperature with gentle agitation for approximately 15 min.
After washing twice again with PBS, proceed to blocking the cells with 100 μl strong block for at least 1 h.
Wash twice with PBS and add 50 μl of primary antibody mouse anti-human CD45 primary antibody or isotype control Mouse IgG1 (diluted 1:200) in weak block. Seal slide with Parafilm and incubate slide at 4 °C overnight with agitation.
The next day, repeat the PBS wash step as before and then add 50 μl of secondary antibody goat anti-rabbit Alexafluor 405 secondary antibody (diluted 1:1,000 in weak block) for one hour. Incubate in the dark at room temperature for one hour.
Immediately after, repeat the PBS wash step, taking care not to expose slide to light for longer than 10 min.
Gently tap the chamber slide onto a paper towel to remove any excess PBS wash and then carefully dislodge the plastic chamber portion to separate the underlying microscope slide.
Add approximately 2-3 drops of mounting medium and place a coverslip over the area of stained cells ensuring all air bubbles are removed. Allow to air dry for 30-45 min in the dark.
When dry, use commercial nail varnish around the edges to act as glue and fix the coverslip in place.
Leave nail varnish to dry in the dark at room temperature. The slide may also be stored at 4 °C long-term sealed with Parafilm in the dark for future analysis.
Proceed to viewing using a confocal microscope (Figure 2).
-
Flow cytometry staining procedure
Immediately after co-culture place the 6 well plate on ice and aspirate off culture medium.
Add 1 ml of ice cold 1% FBS PBS to the cell co-culture and lightly scrape using a cell scraper all over the well, so as to gently detach cells from the plastic surface.
Once detached, place the cells into 5 ml polystyrene round bottomed flow tubes and centrifuge the cells at 800 × g for 5 min.
In order to test viability of the cells after culture and detachment, it is necessary to include a live/dead control. This requires a separate tube of unstained cells and the addition of a live/dead marker stain Zombie Aqua (use according to manufacturer’s protocol).
Decant off the supernatant, leaving the pellet submerged in approximately 100 µl of 1% FBS PBS.
Vortex vigorously and add 2.5 μg/1 x 106 cells of FcR binding inhibitor to the cells and vortex this again.
Leave in the dark and on ice for 30 min.
After this time directly add 5 μl/1 x 106 cells of anti-human CD45 (PE) or Isotype control (IgG1 kappa) to the cell suspension and vortex again to mix thoroughly.
Leave in the dark and on ice for 20 min.
Following this, add an additional 1 ml of 1% FBS PBS to wash the cells and dispose of any unbound antibody. Vortex thoroughly.
Centrifuge the suspension as before at 800 × g for 5 min.
Decant the supernatant and repeat this wash step a final time. Resuspend the pellet in 200 μl of 1% FBS PBS and place the cells on ice in preparation for flow cytometry.
Use FACS DIVA software to perform analysis. To rule out MSC-macrophage aggregates, single cells were gated as W-FSC vs. A-FSC and from this we defined CD45-PE+MitoRed-APC- population as MDM and also MitoRed-APC+CD45-PE- population as MSC (Figure 3).
-
Bacterial culture and phagocytosis assay
Inoculate 15 ml of LB broth with a stored aliquot of E.coli K1 type strain (sterile LB broth/15% glycerol) and incubate at 37 °C to grow an overnight culture.
Use a 24-well plate to set up a direct co-culture with MDM and MSC (see Table 1 for seeding densities).
For E. coli, a total viable count of 1 x 108 colony forming units (CFU/ml) equates to an optical density (OD) of approximately 0.25-0.3 at 600 nm.
Spin bacteria at 15,000 × g, for 5 min and resuspend the pellet using sterile 1 ml HBSS.
After 24 h of MDM and MSC co-culture infect cells using a Multiplicity of Infection of 10 (MOI 10, see Note 4 for MOI calculation), using HBSS as a vehicle control.
Incubate for 4 h at 37 °C, 5% CO2.
After this time, retrieve the supernatant from the cells for quantification of extracellular bacteria and make a dilution series of 10-1 to 10-6 in sterile PBS.
Pipette 3 x 20 μl spots of each dilution out onto LB agar and incubate overnight at 37 °C.
For intracellular bacteria wash the cells in 1 ml sterile PBS 3 times, aspirate wash and then add 300 μg/ml of gentamycin (diluted in 1% FBS RPMI), 100 μl/well and incubate at 37 °C for 1 h to kill adherent bacteria.
After this time wash the cells in 1 ml sterile PBS 3 times, aspirate wash and add 100 μl of 0.5% saponin to lyse the cells and leave the bacteria intact.
After 5 min, add an additional 900 μl of sterile PBS to each well to dilute saponin, and repeat the dilution series again, plating out each dilution. Incubate plates at 37 °C.
Count CFU/ml within 24 h (Figures 4D-4G).
Figure 1. Schematic diagram to illustrate monocyte isolation from peripheral blood.

After blood has been layered onto the Ficoll and centrifuged as described above without brake, blood components are separated into layers. A Pasteur pipette is used to carefully remove the lymphocyte, monocyte and platelet layer to use in downstream processing.
Table 1. MSC and MDM (1:20 ratio) seeding densities.
| No. of MSC/well |
No. of MDM/well |
Type of plate | Final volume (ml) of 1% FBS RPMI | Experiment analysis technique |
|---|---|---|---|---|
| 5 x 104 | 1 x 106 | 6 well | 3 | Flow cytometry/Western |
| 1.5 x 104 | 3 x 105 | 24 well | 1 | ELISA/bioplex/phagocytosis assays |
| 2.5 x 103 | 5 x 104 | 8 well chamber slide | 0.2 | Confocal microscopy |
Figure 2. Visualization of transfer of MSC mitochondria to MDM by confocal microscopy.
A. MDM express surface CD45 marker (blue); B. MSC mitochondria are stained with MitoTracker probe (red); C. After 24 h of co-culture mitochondrial positive TNT are observed, protruding from the MSC (arrows) ranging up to 200 µm in length. Mitochondrial transfer from MSC to MDM is evidenced by co-localisation of blue and red staining (pink). (Images were taken at magnification 10 x 63, scale bar = 75 µm). (Adapted from Jackson et al., Stem Cells. 2016 Aug; 34(8): 2210-23)
Figure 3. Quantification of mitochondrial transfer from MSC to MDM by flow cytometry.
A-B. Live single cells were gated as W-FSC vs. A-FSC and from this we defined C. C. CD45-PE+ MDM population without co-culture with MSC is MitoRed-APC negative. D. APC+ MSC population alone is positive for mitochondria but negative for macrophage marker, CD45-PE-. E. After 4 h in co-culture MDM had acquired 92% of MSC mitochondria shown by the MitoRed fluorescence marker APC. F. Histogram illustrates a reduction in MitoRed fluorescence in the MSC population indicating robust donation of mitochondria to the MDM population. (Adapted from Jackson et al., Stem Cells. 2016 Aug; 34(8): 2210-23)
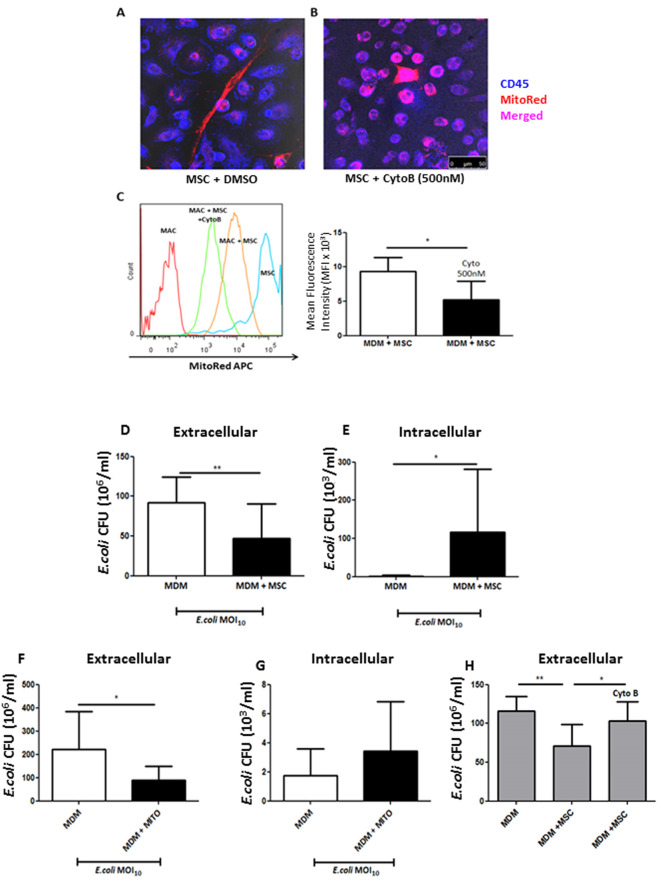
Figure 4. Cytochalasin B blocks TNT-mediated mitochondrial transfer thereby preventing MSC functional effects on MDM.
A. Confocal microscopy shows distinctive spindle MSC shape (red) with MDM (blue) in co-culture. TNT is shown to be present with robust mitochondrial transfer to MDM. B. Cytochalasin B pre-treatment of MSC inhibited actin filament elongation, causing them to appear rounded with loss of TNT structures. Mitochondrial transfer is still however evident due to probable microvesicle secretion from MSC to MDM (magnification 10 x 63, scale bar = 50 µm). C. Cytochalasin B treatment of MSC showed that MitoRed mean fluorescence intensity (MFI) decreased by 50% in MDMs in co-culture (*P < 0.05 Mann Whitney U test). D. After 4 h of live E.coli infection of the co-culture untreated MSC significantly reduced extracellular CFU, whilst simultaneously elevated intracellular CFU in macrophages, an effect recapitulated by stimulation with isolated MSC mitochondria (F + G). H. This effect was completely abrogated with cytochalasin B-treated MSC as even in their presence MDM phagocytosis remained unaffected. (Adapted from Jackson et al., Stem Cells. 2016 Aug; 34(8): 2210-23)
Data analysis
In vitro experiments using 96-well and 24-well plates were performed in triplicate, of which means ± SD were calculated for at least 3 independent experiments. For flow cytometry median fluorescence intensity was calculated using FlowJo software version 7. Data were tested for normality by plotting histograms of frequency distribution and using the D’Agostino and Pearson Omnibus normality test in GraphPad Prism 5. Comparisons of parametric data were analysed by Student’s t-test, one-way or two-way ANOVA for multiple groups. Post hoc analysis using the Bonferroni method was then used to test where significance lay. For non-parametric data the Mann Whitney U and Kruskal Wallis test were used with the Dunn’s method as a post test. Statistical significance was considered when P < 0.05 and all data are displayed as mean ± SD. All Statistical analysis was performed using GraphPad Prism version 5. All of the above statistical information and supporting documentation can be found in Jackson et al., Stem Cells. 2016 Aug; 34(8):2210-23 with URL: http://onlinelibrary.wiley.com/doi/10.1002/stem.2372/full.
Notes
The 5 min incubation step allows mononuclear cells to adhere to the haemocytometer and are easily distinguished next to lymphocytes. Monocytes appear ‘blurry’, ‘splodgy’ or ‘ghost-like’ whereas lymphocytes appear much clearer and sharper. In the case where the original suspension is too dense with cells, a 1:10 or 1:20 dilution is required to properly visualise mononuclear cells.
As the MitoTracker probe is supplied in a reduced form, it may be susceptible to oxidases in serum, and therefore the staining solution consists of up to 1% FCS content to allow for maximum staining potential (https://www.thermofisher.com/order/catalog/product/M22426)
All stimulation experiments with co-culture of MSC and MDMs are performed in 1% FBS RPMI media.
-
Calculation of multiplicity of infection MOI
For E. coli K1, an optical density of 0.25-0.3 at 600 nm equates to approximately 1 x 108 CFU/ml of bacteria. This varies for each strain of E.coli and indeed for different types of bacteria. Therefore beginning bacterial work, a growth curve (CFU/ml vs. time) and also absorbance (OD) vs. CFU/ml must be performed to accurately estimate bacterial rates of growth and also how OD varies with bacterial concentration. Once this is established, MOI can then be estimated. This is the number of bacterial cells added per MDM in each infection. See example below.
Initial MDM seeding density = 3 x 105 cells (24 well plate)
Starting OD600 nm (0.25-0.3) = 1 x 108 CFU/ml of E. coli
Therefore MOI 1 = 1 x 105 CFU/ml of E. coli (1 bacterial cell:1 MDM cell)
MOI 10 = 1 x 106 CFU/ml of E. coli (10:1)
MOI 100 = 1 x 107 CFU/ml of E. coli (100:1)
Recipes
-
Complete 1% FBS RPMI
500 ml RPMI 1640
5 ml of FCS (1%) or 50 ml FCS (10%)
5 ml Pen-Strep (1%)
-
Complete RPMIGM-CSF
500 ml RPMI 1640
50 ml FCS (10%)
5 ml Pen-Strep (1%)
10 ng/ml GM-CSF
-
Complete α-MEM
500 ml α-MEM
5 ml of FCS (1%) or 80 ml FCS (16.5%)
5 ml Pen-Strep (1%)
5 ml L-glutamine (1%)
-
Mitochondrial staining solution (1% FBS α-MEMMITO)
500 ml α-MEM
5 ml of FCS (1%)
5 ml Pen-Strep (1%)
5 ml L-glutamine (1%)
200 nM MitoTracker Deep Red FM probe
-
Cytochalasin B solution (1% FBS α-MEMCYTO)
500 ml α-MEM
5 ml of FCS (1%)
5 ml Pen-Strep (1%)
5 ml L-glutamine (1%)
500 nM cytochalasin B or same volume for DMSO (vehicle control)
-
1% FBS PBS
50 ml sterile PBS
0.5 ml of FCS (1%)
-
4% paraformaldehyde (PFA)
Add 4 g of paraformaldehyde to 50 ml of double distilled H2O (ddH2O)
Add 1 ml 1 N NaOH to the solution and heat on the heating block at 60 °C until PFA is dissolved
Add 10 ml of 10x PBS to the mixture and allow to cool to room temperature
The pH is then adjusted to 7.4 with 1 N HCl
Adjust the final volume of the mixture to 100 ml using ddH2O
Use a 0.45 µM membrane to filter the solution and remove debris. Aliquots may be stored up to 3-6 months at -20 °C
-
Strong and weak block for immunofluorescent staining
Strong block: 45 ml PBS + 5 ml of NGS (10%)
Weak block: 49 ml PBS + 1 ml of NGS (2%)
-
0.5% saponin
50 mg of saponin powder
10 ml sterile PBS
Acknowledgments
This work was funded by Medical Research Council of the UK MR/L017229/1 (ADK), NHLBI HL51854 (MAM). Some of the materials employed in this work were provided by the Texas A&M Health Science Center College of Medicine Institute for Regenerative Medicine at Scott & White through a grant from NCRR of the NIH, Grant # P40RR017447. This protocol was adapted from our publication ( Jackson et al., 2016 ).
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Ahmad T., Mukherjee S., Pattnaik B., Kumar M., Singh S., Kumar M., Rehman R., Tiwari B. K., Jha K. A., Barhanpurkar A. P., Wani M. R., Roy S. S., Mabalirajan U., Ghosh B. and Agrawal A.(2014). Miro1 regulates intercellular mitochondrial transport& enhances mesenchymal stem cell rescue efficacy. EMBO J 33(9): 994-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Almeida M. C., Silva A. C., Barral A. and Barral Netto M.(2000). A simple method for human peripheral blood monocyte isolation. Mem Inst Oswaldo Cruz 95(2): 221-223. [DOI] [PubMed] [Google Scholar]
- 3.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D. and Horwitz E.(2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 8(4): 315-317. [DOI] [PubMed] [Google Scholar]
- 4.Guilliams M., De Kleer I., Henri S., Post S., Vanhoutte L., De Prijck S., Deswarte K., Malissen B., Hammad H. and Lambrecht B. N.(2013). Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med 210(10): 1977-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta N., Krasnodembskaya A., Kapetanaki M., Mouded M., Tan X., Serikov V. and Matthay M. A.(2012). Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia . Thorax 67(6): 533-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta N., Su X., Popov B., Lee J. W., Serikov V. and Matthay M. A.(2007). Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol 179(3): 1855-1863. [DOI] [PubMed] [Google Scholar]
- 7.Islam M. N., Das S. R., Emin M. T., Wei M., Sun L., Westphalen K., Rowlands D. J., Quadri S. K., Bhattacharya S. and Bhattacharya J.(2012). Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med 18(5): 759-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson M. V., Morrison T. J., Doherty D. F., McAuley D. F., Matthay M. A., Kissenpfennig A., O'Kane C. M. and Krasnodembskaya A. D.(2016). Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS . Stem Cells 34(8): 2210-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krasnodembskaya A., Samarani G., Song Y., Zhuo H., Su X., Lee J. W., Gupta N., Petrini M. and Matthay M. A.(2012). Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol 302(10): L1003-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krasnodembskaya A., Song Y., Fang X., Gupta N., Serikov V., Lee J. W. and Matthay M. A.(2010). Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells 28(12): 2229-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J. W., Krasnodembskaya A., McKenna D. H., Song Y., Abbott J. and Matthay M. A.(2013). Therapeutic effects of human mesenchymal stem cells in exvivo human lungs injured with live bacteria . Am J Respir Crit Care Med 187(7): 751-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X., Zhang Y., Yeung S. C., Liang Y., Liang X., Ding Y., Ip M. S., Tse H. F., Mak J. C. and Lian Q.(2014). Mitochondrial transfer of induced pluripotent stem cell-derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke-induced damage. Am J Respir Cell Mol Biol 51(3): 455-465. [DOI] [PubMed] [Google Scholar]
- 13.Liu K., Ji K., Guo L., Wu W., Lu H., Shan P. and Yan C.(2014). Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia-reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer . Microvasc Res 92: 10-18. [DOI] [PubMed] [Google Scholar]
- 14.Mei S. H., Haitsma J. J., Dos Santos C. C., Deng Y., Lai P. F., Slutsky A. S., Liles W. C. and Stewart D. J.(2010). Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med 182(8): 1047-1057. [DOI] [PubMed] [Google Scholar]
- 15.Nemeth K., Leelahavanichkul A., Yuen P. S., Mayer B., Parmelee A., Doi K., Robey P. G., Leelahavanichkul K., Koller B. H., Brown J. M., Hu X., Jelinek I., Star R. A. and Mezey E.(2009). Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 15(1): 42-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ware L. B. and Matthay M. A.(2000). The acute respiratory distress syndrome. N Engl J Med 342(18): 1334-1349. [DOI] [PubMed] [Google Scholar]
- 17.Wilson J. G., Liu K. D., Zhuo H., Caballero L., McMillan M., Fang X., Cosgrove K., Vojnik R., Calfee C. S., Lee J. W., Rogers A. J., Levitt J., Wiener-Kronish J., Bajwa E. K., Leavitt A., McKenna D., Thompson B. T. and Matthay M. A.(2015). Mesenchymal stem(stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med 3(1): 24-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu J., Qu J., Cao L., Sai Y., Chen C., He L. and Yu L.(2008). Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. J Pathol 214(4): 472-481. [DOI] [PubMed] [Google Scholar]
- 19.Xu J., Woods C. R., Mora A. L., Joodi R., Brigham K. L., Iyer S. and Rojas M.(2007). Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol 293(1): L131-141. [DOI] [PubMed] [Google Scholar]
- 20.Zheng G., Huang L., Tong H., Shu Q., Hu Y., Ge M., Deng K., Zhang L., Zou B., Cheng B. and Xu J.(2014). Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res 15: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]