Abstract
Netrin-1 is an evolutionarily conserved secreted extracellular matrix protein discovered using genetic and biochemical screens for its role in axon guidance at the central nervous system (CNS) midline1,2. Netrin-1 is expressed by cells localized at CNS midline, such as the floor plate in vertebrate embryos1,3. Growth cone turning assays and 3D gel diffusion assays showed that netrin-1 can attract commissural axons2,4–6. Loss-of-function experiments further demonstrated that commissural axon extension to the midline is severely impaired in absence of netrin-13,7–9. Together these data support a model in which commissural axons are attracted by a netrin-1 gradient diffusing from the midline. Here, we selectively ablated netrin-1 expression in floor plate cells using a Netrin-1 conditional mouse line. We found that hindbrain and spinal cord commissural axons develop normally in absence of floor plate-derived netrin-1. Furthermore, we show that netrin-1 is highly expressed by cells in the ventricular zone with the potential to release it at the pial surface where it binds to commissural axons. Importantly, netrin-1 deletion from the ventricular zone phenocopies commissural axon guidance defects previously described in Netrin-1 knockout mice. These results show that the classical textbook view that attraction of commissural axons is mediated by a gradient of floor plate-derived netrin-1 is inaccurate and that netrin-1 primarily acts locally by promoting growth cone adhesion.
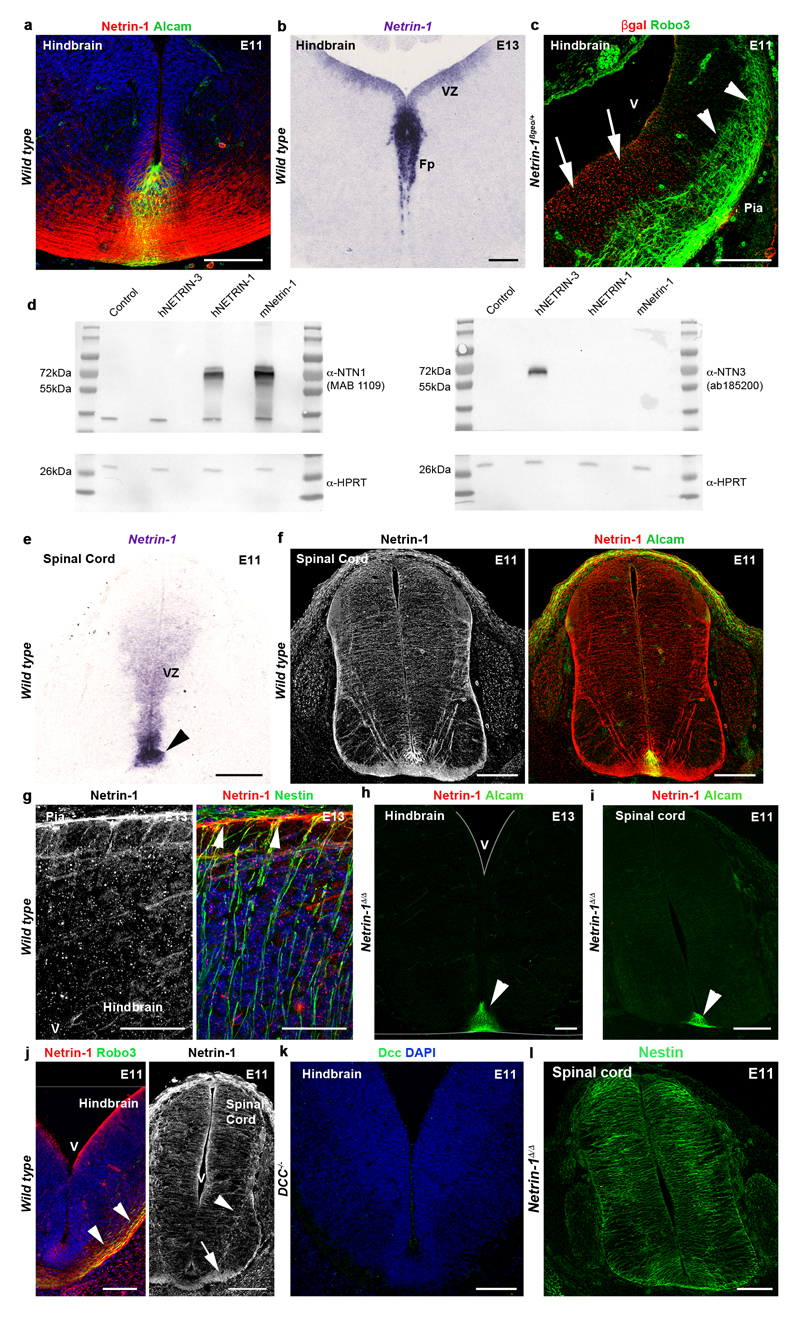
Mouse commissural neurons are diverse and comprise many subtypes10. In the midbrain, hindbrain and spinal cord, commissural neurons transiently express the Robo3 receptor 11,12 (Fig. 1a). At embryonic day 9.5 (E9.5), the first commissural neurons cross the floor plate13 where netrin-1 expression is the highest 4,7,14. As previously shown, netrin-1 mRNA is also expressed in neural progenitors of the basal plate ventricular zone (VZ) (Fig. 1b, Fig. 2, Extended Data Figs 1 and 2). Netrin-1 protein is in the floor plate (labeled with Alcam/BEN a floor plate marker12) and along commissural axons (Fig. 1c, Extended Data Figs 1 and 2). The netrin-1 antibody, did not cross-react with netrin-3 (Extended Data Fig. 1d).
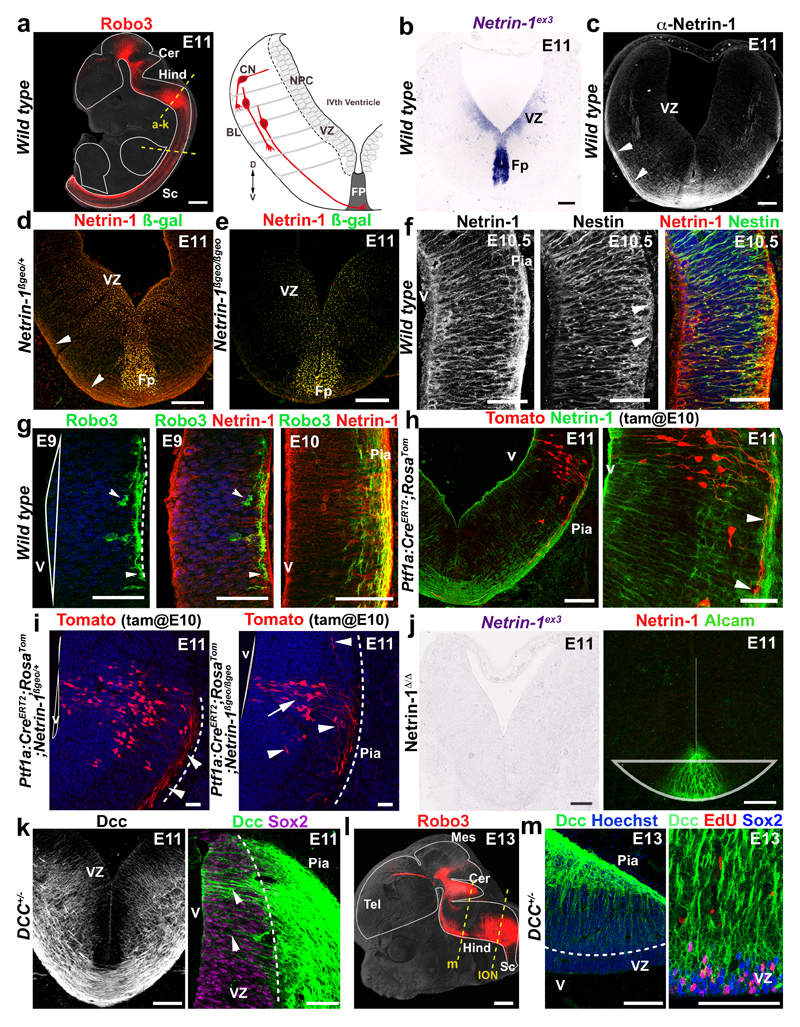
Figure 1. Netrin-1 is expressed by ventricular zone neural progenitors.
a Robo3+ commissural axons (left) and hindbrain schematic (right). Dashed lines show section levels. b, In wild type, netrin-1 mRNA is in VZ and Fp (n=6). c, Netrin-1 protein is in Fp and commissural axons (arrowheads; n=6). d,e, In Netrin-1ßgeo/+ (d) and Netrin-1ßgeo/ßgeo (e), the netrin-1-ß-gal protein is in the VZ and Fp (n=6 and 6). Commissural axons (arrowheads) are netrin-1+ but ßgal-. f, netrin-1 is detected in Nestin+ radial processes extending to the pia (arrowheads; n=6). g, the first Robo3+ commissural growth cones (arrowheads; n=6) extend under the pia (dotted line) in a netrin-1-rich domain. h,i, Tomato+ inferior olivary growth cones (arrowheads) extend ventrally in a netrin-1-rich domain in Ptf1a:CreERT2;RosaTom and Netrin-1ßgeo/+ (n=6). In Netrin-1ßgeo/ßgeo (n=6), axons (arrowheads) stall at the pial surface and some enter the VZ (arrow). j, Absence of netrin-1 mRNA and protein (n=6 each) in Netrin-1Δ/Δ hindbrain. k, DCC labels hindbrain commissural axons and radial processes (arrowheads) extending from the Sox2+ VZ (n=6). l, Robo3+ commissural axons at E13. m, Sox2+/EdU+ cerebellar VZ progenitors express DCC (n=6). Abbreviations: Cerebellum (Cer); Hindbrain (Hind), spinal cord (Sc); Neural precursors (NPC), ventricular zone (VZ), basal lamina (BL); commissural neurons (CN), floor plate (Fp). Scale bars, 500 µm (a, l); 50 µm (g, h right panel, m); 100 µm (all other panels).
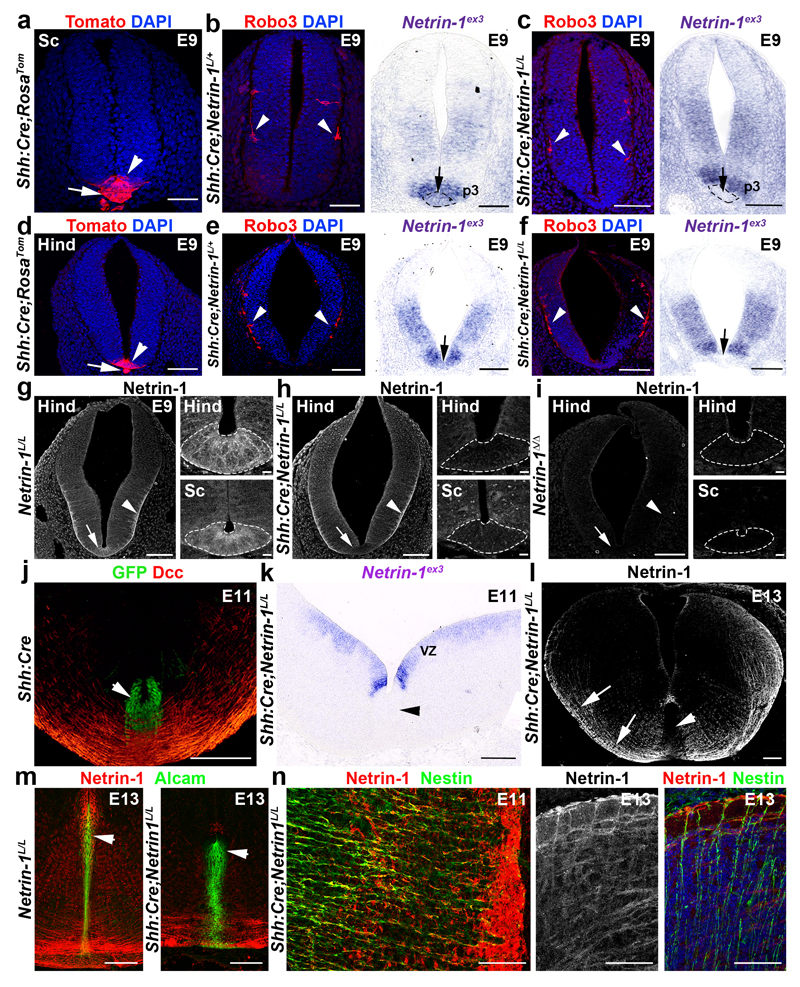
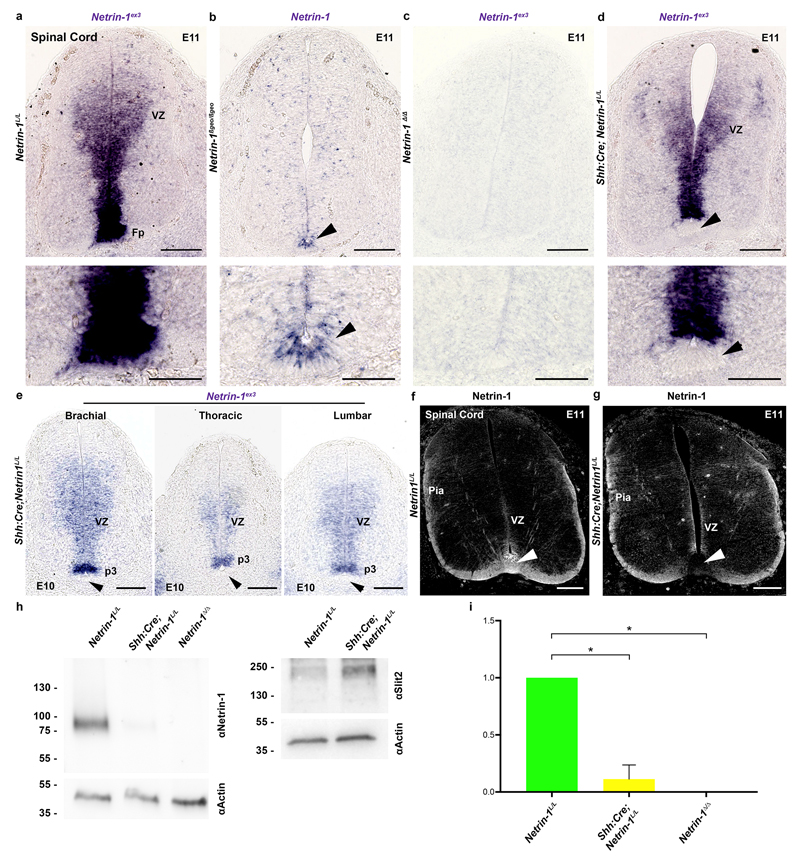
Figure 2. Floor plate-specific deletion of netrin-1 in Shh:Cre;Netrin-1lox embryos.
a-f, Tomato is expressed in the floor plate (arrowhead) and notochord (arrow) in Shh:Cre;RosaTom spinal cord (a) and hindbrain (d) (n=6). Robo3+ commissural axons (arrowheads in b, c, e and f) have not reached the midline. Netrin-1 mRNA is in the floor plate (short arrows) in Shh:Cre;NetrinL/+ but not in Shh:Cre;NetrinL/L. g-i, Netrin-1 is in floor plate (arrow) and pial surface (arrowhead) in the wild type (g; n=6). In Shh:Cre;Netrin-1L/L (h; n=6), netrin-1 is absent from floor plate (arrow and dotted lines) but present at the pial surface (arrowhead). Netrin1 is completely absent in Netrin-1Δ/Δ (i; n=6). j, In Shh:Cre, GFP is in floor plate (arrowhead; n=6). Commissural axons express Dcc. k, Netrin-1 is absent from floor plate but maintained in VZ (n=6). l-n, In Shh:Cre;Netrin-1L/L, floor plate (arrowheads in l) lacks Netrin-1, but not commissural axons (short arrows in l) and Nestin+ neural progenitors (n; n=6). Alcam expression is unchanged in floor plate (arrowheads in m; n=6). In Shh:Cre;Netrin-1L/L (n) Nestin+ processes still express netrin-1 (n=6).
Scale bars are 100 µm except on the higher magnifications of g, h, I where they are 10µm.
Is dorsal netrin-1 produced locally in the VZ or does it diffuse from the FP? We detected netrin-1 on the radial processes and basal endfeet of VZ neural progenitors which are bipolar cells extending from the ventricular surface to the pia (Fig. 1, Extended Data Fig. 1). We confirmed the presence of netrin-1 in VZ precursors using Netrin-1 hypomorphs (Netrin-1ßgeo) in which netrin-1 is fused to ß-galactosidase (ß-gal) and trapped into endosomes (Fig. 1d)7. In Netrin-1ßgeo/+ embryos, netrin-1+/ß-gal+ puncta are in the floor plate and VZ but not in commissural neurons. However, commissural axons were netrin-1-immunoreactive (due to the wild type allele; Fig. 1d and Extended Data Fig. 1c). The specificity of the netrin-1 axonal-immunolabeling is supported by its absence in Netrin-1ßgeo/ßgeo embryos (Fig. 1e). A ventricular source of netrin-1 was confirmed with nestin, a marker of neural progenitors. At E10.5-E13, neural progenitor processes extending to the pial surface coexpressed netrin-1 and nestin (Fig. 1f and Extended Data Fig. 1g and Fig. 4h). This suggests that in the dorsal hindbrain and spinal cord, netrin-1 originates from VZ precursors rather than floor plate.
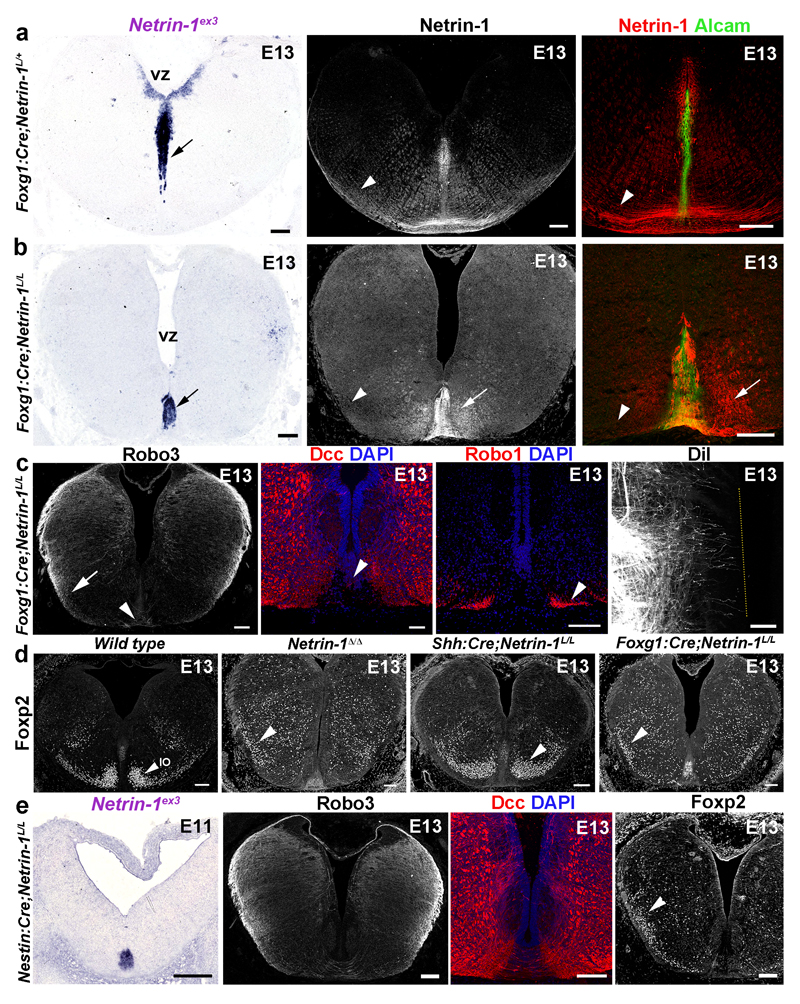
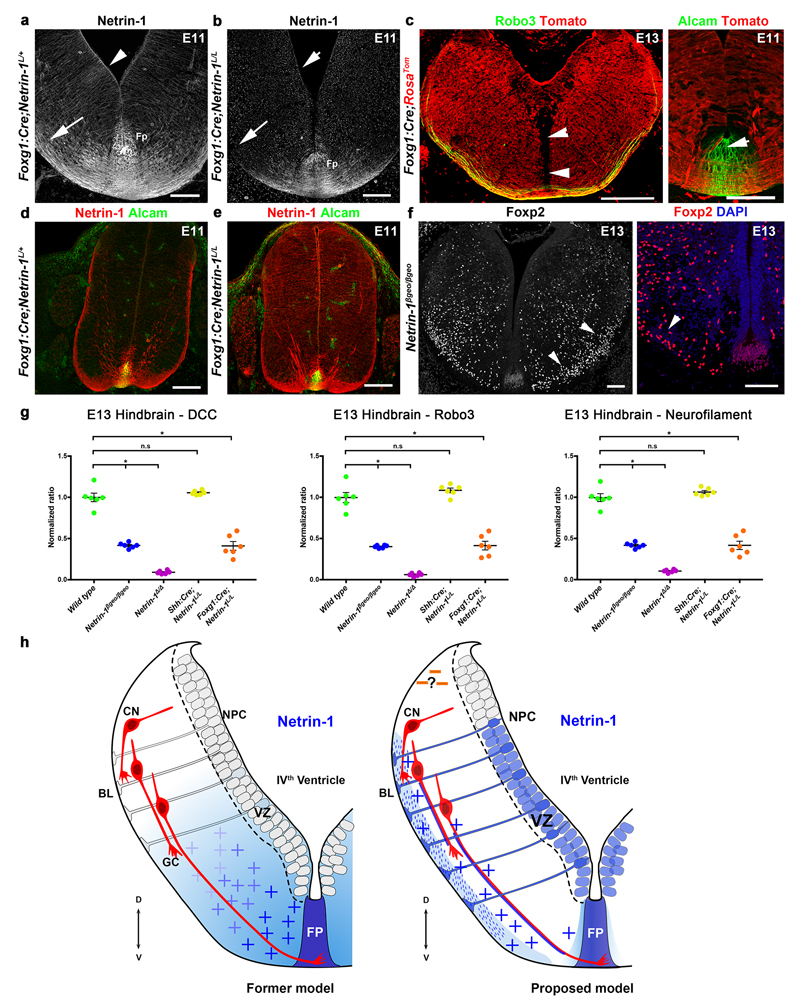
Figure 4. Ventricular zone-derived netrin-1 controls commissural axon guidance.
a, b, VZ-Netrin-1 is absent in Foxg1:Cre;Netrin-1L/L and reduced on commissural axons (arrowheads). Netrin-1 is still found in floor plate (short arrows; 5/5). c, A few commissural axons (arrowheads; n=6) cross the midline (arrow) and DiI-labeled axons fail to cross (dotted line; n=6). d, in wild type Foxp2+ olivary neurons (IO) have started to reach the floor plate (arrowhead; n=7). Most IO neurons fail to migrate ventrally (arrowheads; n=6) in Netrin-1Δ/Δ and Foxg1:Cre;Netrin-1L/L, unlike in Shh:Cre;Netrin-1L/L (n=7). e, Netrin-1 expression (n=6). Midline crossing and IO neuron migration are impaired (n=6).
Scale bars,100 µm.
Which source of netrin-1 do pioneer commissural axons encounter at the onset of their extension? At E9-E9.5, the first Robo3+ commissural growth cones appear in the marginal layer of the hindbrain containing high-levels of netrin-1 (Fig. 1g). This was confirmed using a Ptf1a:CreERT2;RosaTom reporter line15 (Fig. 1h). Ptfa1 is expressed by diverse hindbrain commissural neuron progenitors, including those of the inferior olivary nucleus16 (ION; see below). Importantly, commissural axon guidance errors were observed in Netrin-1ßgeo/ßgeo hypomorphs embryos as soon as growth cones appear (Fig. 1i). These results suggest that netrin-1 is released or transported locally by neural progenitors to the pial surface and guides pioneer commissural axons by promoting their initial extension at the CNS periphery in the first leg of their trek. Netrin-1 accumulation on commissural axons might create a permissive pathway for follower axons.
The role of floor-plate-derived netrin-1 in mouse commissural axon guidance in vivo is supported by the phenotypic analysis of Netrin-1 hypomorphs7 and Netrin-1 null embryos8,9. To identify the critical source of netrin-1 for mediating commissural axon guidance, we crossed a novel netrin-1 conditional mouse line (Netrin-1L/L) to three mouse lines expressing Cre recombinase: (1) ubiquitously, (2) only in the floor plate, or (3) in all the neural tube except the floor plate. In homozygous (Netrin-1Δ/Δ) null embryos, in which netrin-1 was ubiquitously deleted, no netrin-1 mRNA is detectable in the hindbrain and spinal cord (Fig. 1j and not shown), in contrast to a residual expression in Netrin-1ßgeo/ßgeo hypomorphs (Extended Data Fig. 2a-c). Likewise, netrin-1 immunolabeling is abrogated in E11-E13 Netrin-1Δ/Δ embryos (Fig. 1j, Extended Data Fig. 1h,i). Netrin-1 immunoreactivity persisted on commissural axons in absence of permeabilization suggesting that some netrin-1 is at their extracellular surface (Extended Data Fig. 1j). This suggests that commissural axons and precursors could accumulate or internalize netrin-1, possibly in a Dcc-dependent manner as shown in Drosophila 17. Accordingly, Dcc is not only expressed by E11 and E13 commissural axons, but also by neural progenitors in the hindbrain (Fig. 1k). The specificity of the Dcc antibody was demonstrated by the lack of staining in Dcc knockout embryos18 (Extended Data Fig. 1k). In the E13 cerebellar plate (Fig. 1l), 3-hour pulse labeling with EdU (5-ethynyl-2’-deoxyuridine) and immunostaining for the stem cell marker Sox2 confirmed that Dcc expression by neural progenitors (Fig. 1m). Radial progenitor processes extended normally in Netrin-1Δ/Δ embryos (Extended Data Fig. 1l).
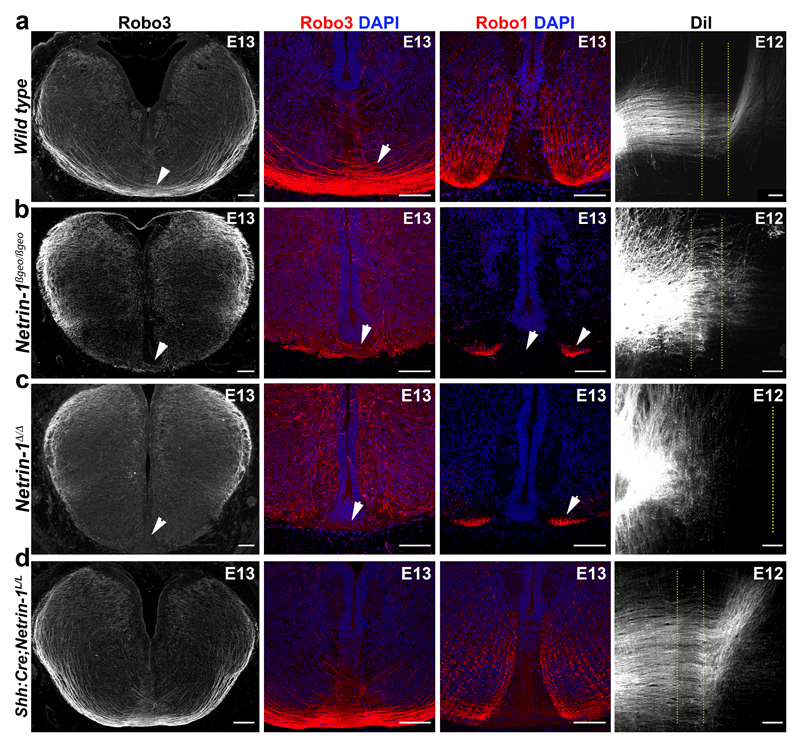
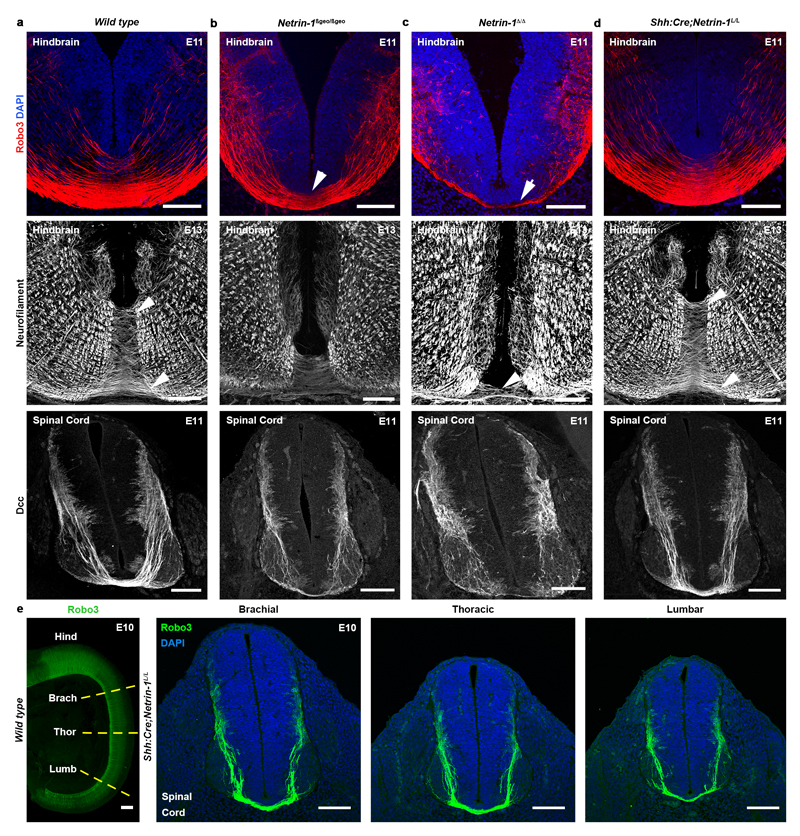
Next, we analyzed the consequences from floor plate-specific deletion of netrin-1 on commissural axon development (Figs 2 and 3). As performed previously19, we used a line expressing Cre recombinase fused to green fluorescent protein (GFP) under the control of the sonic hedgehog (Shh) promoter. Crossing Shh:Cre with RosaTom line showed that Cre was active in E9 floor plate, before commissural axons reach it (Fig. 2a, d). Neither Tomato nor GFP were expressed in the VZ (Fig. 2j). In Shh:Cre:Netrin-1L/L embryos, Netrin-1 mRNA was ablated from the floor plate all along the spinal cord and hindbrain at E9, E10, E11 and E13 (Fig. 2b, c, e, f and k). Netrin-1 protein was also eliminated from the floor plate at E9 but maintained in the VZ (Fig. 2g,h). Netrin-1 was not detected in E9 Netrin-1Δ/Δ embryos (Fig. 2i). Therefore, in Shh:Cre;Netrin-1L/L embryos, netrin-1 ablation well precedes midline crossing. The absence of netrin-1 from Shh:Cre;Netrin-1L/L floor plate was confirmed at E10-E13 (Fig. 2k, l, m and Extended Data Fig. 2a-d). Western blot analysis of floor plate extracts confirmed that netrin-1 is undetectable in Netrin-1Δ/Δ embryos and almost completely absent in Shh:Cre:Netrin-1L/L embryos compared to controls (Extended Data Fig. 2h, i. For gel source data see Supplementary figure 1). However, netrin-1 was still present in commissural axons, VZ and neural progenitor processes (Fig. 2l-n and Extended Data Fig. 2f, g). To determine if the absence of floor-plate netrin-1 perturbed midline crossing, we visualized commissural axons in Shh:Cre:Netrin-1L/L embryos with immunostaining for Neurofilament, Robo3, Dcc and Robo1 receptors. Robo3 is expressed by precrossing axons11, Dcc before and after crossing11, and Robo1 after crossing11. As previously described, the number of axons crossing the midline was severely reduced in the hindbrain and spinal cord in E11 and E13 Netrin-1ßgeo/ßgeo embryos compared to controls (Shh:Cre;Netrin-1L/+; Netrin-1ßgeo/+ or wild type; Fig. 3a, b and Extended Data Fig. 3a, b). This was confirmed by the parallel reduction in Robo1 staining. As described 8,9 , this phenotype was exacerbated in Netrin-1Δ/Δ embryos in which midline crossing was almost completely abolished (Fig. 3c and Extended Data Fig. 3c). Remarkably, midline crossing was not perturbed in E10-E13 Shh:Cre;Netrin-1L/L embryos, neither in the hindbrain, nor at any levels of the spinal cord (Fig. 3d and Extended Data Fig. 3d, e and data not shown). At E12, 1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine (DiI) injection in open-book hindbrains and spinal cords showed that commissural axons cross the midline and turn longitudinally in wild type and Shh:Cre;Netrin-1L/L; embryos, whereas crossing is severely perturbed in Netrin-1ßgeo/ßgeo and Netrin-1Δ/Δ embryos (Fig. 3 and not shown). Therefore, floor plate-derived netrin-1 is not required for commissural axon guidance to the floor plate.
Figure 3. Commissural axons develop normally without floor plate-derived netrin-1.
Coronal sections and flat-mounts of E12 and E13 hindbrains. a, In wild type, Robo3+ commissural axons cross the floor plate (arrowheads) and Robo1 is on post-crossing axons (n=7). DiI-labeled commissural axons cross the midline (dotted lines) and turn longitudinally (n=5). b, In Netrin-1ßgeo/ßgeo, midline crossing is reduced (arrowheads) and most DiI-labeled axons fail to cross (n=3). c, Crossing is strongly reduced in Netrin-1Δ/Δ (n=6). Robo1 is still expressed on the few crossing axons (arrowheads in b and c; n=6). d, Commissures look similar to control in Shh:Cre;Netrin-1L/L (n=6).
Scale bars, 100 µm.
To confirm this result, we crossed Netrin-1L/L mice to lines expressing Cre in the hindbrain. In Foxg1:Cre;Netrin-1L/L embryos, netrin-1 mRNA is undetectable in the hindbrain VZ, but highly expressed in the floor plate (Fig. 4a, b). Accordingly, netrin-1 protein is almost absent from commissural axons and pial surface of E11 and E13 Foxg1:Cre;Netrin-1L/L hindbrains (Fig. 4a, b and Extended Data Fig. 4a, b). Netrin-1 immunoreactivity in floor plate and its vicinity, suggests that netrin does not diffuse far from it. A RosaTom reporter line confirmed that Foxg1:Cre drives Cre expression in most of the hindbrain but only a few floor plate cells (Extended Data Fig. 4c). At E13, hindbrain commissures are strongly reduced in Foxg1:Cre;Netrin-1L/L embryos, phenocopying Netrin-1 null embryos (Fig. 4c and Extended Data Fig. 4g and data not shown). DiI tracing of hindbrain commissural axons confirms the absence of crossing at E13 (Fig. 4c). Since VZ-netrin-1 persisted in Foxg1:Cre;Netrin-1L/L spinal cord, we could not determine if the VZ is also the netrin-1 source for spinal cord commissural axons (Extended Data Fig. 4d, e). We next studied the development of inferior olivary nucleus (ION) neurons, whose migration from the dorsal hindbrain to the floor plate is perturbed in Netrin-1ßgeo/ßgeo embryos20 (see also Fig. 1i). At E13, Foxp2+ migrating ION neurons have started to reach the floor plate in wild type mice21 (Fig. 4d). By contrast, in Netrin-1ßgeo/ßgeo (Extended Data Fig. 4f) and Netrin-1Δ/Δ embryos (Fig. 4d) most ION neurons either fail to migrate ventrally or migrate inside the hindbrain (Figs 4d and 1i). Similar defects occurred in Foxg1:Cre;Netrin-1L/L embryos but the ION migrated normally in Shh:Cre;Netrin-1L/L embryos (Fig. 4d). The role of VZ-derived netrin-1 was further investigated in E11 Nestin:Cre;Netrin-1L/L embryos22, which lack netrin-1 mRNA in VZ but not floor plate (Fig. 4e). In this mutant, a severe reduction of midline crossing was observed (Fig. 4e) an IO neuron migration was abnormal (Fig. 4e).
Netrin-1 was initially proposed as a diffusible cue, but sequence similarities with laminins and X-ray structural analyses have strengthened the view that netrin-1 is an extracellular matrix protein impacting cell adhesion4,23. This questioned the ability of netrin-1 to simply act as a soluble cue. Accordingly, studies in Drosophila netrin-1 mutants showed that the expression of a membrane-tethered netrin-1 in midline glia rescues midline crossing24. Likewise, in the Drosophila visual system, Netrin-1/Dcc interaction promotes attachment to target cells rather than chemoattraction25. In the mouse, netrin-1 attachment to a substrate is required for commissural axon extension26. We show here that during their ipsilateral extension, commissural axons respond to netrin-1 produced dorsally by neural progenitors in the hindbrain and spinal cord and that floor-plate netrin-1 is not essential. The presence of netrin-1 in dorsal spinal cord extracts14 most likely reflects its local production by neural progenitors rather than its diffusion from the floor plate. We propose that netrin-1 promotes growth cone attachment and haptotaxis, anchoring pioneer commissural axons close to the pial surface (Extended Data Fig. 4h).
Our results suggest that long-range attraction by gradient of chemoattractants is not a major guidance mechanism for commissural axons, as previously proposed 27. The role of floor plate netrin-1 is still unclear as Shh:Cre;Netrin1L/L mice are viable without any obvious behavioral defects. By contrast, Foxg1:Cre;Netrin1L/L and Nestin:Cre;Netrin-1L/L mutants die at birth. Yet, in the spinal cord, floor plate- and VZ-derived netrin-1 might act redundantly. In the light of our results it will be important to consider floor-plate independent cellular mechanisms for the ipsilateral guidance of commissural axons.
Methods
Mouse strains and genotyping
Netrin-1ßgeo 7 and DCC 18 knockout lines were previously described and genotyped by PCR. The Netrin-1 conditional knockout was created (Genoway, Lyon, France) by inserting two Loxp sites flanking the coding sequences containing both the principal ATG (Based on netrin-1 cDNA sequence NM_008744) and the cryptic ATG (Based on netrin-1 cDNA: BC141294) and the alternative promoter described in intron 328.
The targeting vector was constructed as follows: three fragments of 2.1kb, 3.4kb and 4.6kb (respectively, the 5’, floxed and 3’ arms) were amplified by PCR using 129Sv/Pas ES DNA as a template and sequentially subcloned into the pCR4-TOPO vector (Invitrogen). These fragments were used for the construction of the targeting vector in which a FRT-flanked Neomycin cassette was inserted in 5’ of the Loxp-flanked region. The linearized construct was electroporated into 129Sv/Pas mouse embryonic stem (ES) cells. After selection, targeted clones were identified by PCR using external primers and further confirmed by Southern blot analysis both with a neo and a 5' external probe. The positive ES cell clones were injected into C57BL/6J blastocysts and gave rise to male chimeras with a significant ES cell contribution. Breeding was established with C57BL/6 mice expressing the Flp- recombinase, to produce the heterozygous Netrin-1 conditional knockout line devoid of the Neomycin cassette. To generate a null-allele of netrin-1, Netrin-1L/L mice were crossed to Krox20:Cre mice which express Cre recombinase in the male and female germline after sexual maturity29. To ablate netrin-1 expression in the floor plate we used the Shh:Cre line30 (Jackson laboratories). In this line, the eGFP reporter was also inserted in the Shh locus. Last, we crossed Netrin-1L/L mice to Foxg1:Cre mice31 and Nestin:Cre mice22 (Jackson laboratories). The Ai9 RosatdTomato reporter line (RosaTom; Jackson Laboratories) was used to monitor Cre expression. Developing inferior olivary neurons were visualized by crossing the RosaTom line with the Ptf1aCreERT2 line15. They were also further crossed to Netrin-1ßgeo mice. All mice are kept in C57BL/6 background. The day of the vaginal plug was counted as embryonic day 0.5 (E0.5). Mice were anesthetized with Ketamine (100mg/ml) and Xylazine (10mg/ml). All animal procedures were carried out in accordance to institutional guidelines and approved by the UPMC University ethic committee (Charles Darwin). Embryos of either sex were used.
Tamoxifen injection
Ptf1aCreERT2;RosaTom pregnant mice were intraperitoneally injected at E10 with 1mg of tamoxifen (Sigma, T-5648) dissolved in corn oil (Sigma, C-8267). The embryos were collected at E11.
EdU labeling
Pregnant females were injected i.p. with EdU(1mg/10g) and sacrificed three hours later.
The proliferating cells were visualized after immunohistochemistry using the Alexa Fluor 647 Click-iT EdU Imaging Kit (Invitrogen).
In situ hybridization
Antisense riboprobes were labeled with digoxigenin-11-d-UTP (Roche Diagnostics, Indianapolis, IN) as described elsewhere12, by in vitro transcription of cDNA encoding mouse netrin-1 7 or mouse netrin-1 exon 3.
Dil tracing
4%PFA-fixed E12-E13 hindbrains in an open book configuration were injected using a glass micropipette with DiI crystals or small drops of DiI (Invitrogen) diluted in Dymethyl sulfoxyde (DMSO, Sigma). Samples were kept for 24hours at 37°C in 4%PFA.
Immunohistochemistry
Embryos were fixed by immersion in 4% paraformaldehyde in 0.12 M phosphate buffer, pH 7.4 (PFA) over night at 4°C. Samples were cryoprotected in a solution of 10% sucrose in 0.12M phosphate buffer (pH7.2), frozen in isopentane at 50°C and then cut at 20µm with a cryostat (Leica). Immunohistochemistry was performed on cryostat sections after blocking in 0.2% gelatin in PBS containing 0.5% Triton-X100 (Sigma). Sections were then incubated O/N with the following primary antibodies: goat anti-human Robo3 (1:250, R&D Systems AF3076), goat anti-Dcc (1:500, Santa Cruz sc-6535), goat anti-Robo1 (1:500, R&D Systems AF1749), rat anti-mouse Netrin-1 (1:500, R&D Systems MAB1109), mouse anti-Nestin-Alexa488 (1:1000, Abcam ab197495), mouse anti Neurofilament (1:300, DSHB 2H3), goat anti-Foxp2 (1:1000, Santa Cruz sc-21069), rabbit anti-Foxp2 (1:1000, Abcam AB16046), rabbit anti-Sox2 (1:500, Abcam ab97959), rabbit anti-βgal (1:500, Cappel 55976), goat anti-human Alcam (1:500, R&D Systems AF656), rabbit anti-GFP (1:800, Life Technologies A11122), rabbit anti-Dsred (1:500, Clontech 632496) followed by 2 hours incubation in species-specific secondary antibodies directly conjugated to fluorophores (Cy-5, Cy-3, Alexa-Fluor 647 from Jackson ImmunoResearch, or from Invitrogen). For netrin-1 immunostaining, an antibody retrieval treatment was performed on the sections before to process them for immunochemistry. The sections were boiled in citrate buffer (pH 6) during 9 minutes. Sections were counterstained with DAPI (1:1000,Sigma). In the case of netrin-1 immunostaining on non-permeabilized tissue, the Triton was removed from all the steps. Slides were scanned with a Nanozoomer (Hamamatsu) and laser scanning confocal microscope (FV1000, Olympus). Brightness and contrast were adjusted using Adobe Photoshop.
Whole-mount labeling and 3DISCO clearing
Whole-mount immunostaining and 3DISCO optical clearing procedure has been described previously32. 3D imaging was performed with an ultramicroscope using Inspector Pro software (LaVision BioTec).
Western blotting
HEK-293T cells (from ATCC, not authenticated, tested for mycoplasma contamination with a negative result) were transfected with pCDNA3, pCDNA3-human NTN3, pCDNA3-human NTN1 or pCDNA3-mouse ntn1 plasmids using Fugene HD transfection reagent (Promega) according to the manufacturer’s instructions. Cells were harvested and lysed 36 hr after transfection. Cells were lysed using RIPA buffer (150 mM NaCl, 50 mM Tris pH 8.0, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, complete protease inhibitor cocktail (Roche)) and incubated 1 hr at 4°C. Floor plates were micro-dissected from hindbrains and spinal cords from Netrin-1L/L, Shh:Cre;Netrin-1L/L and Netrin-1Δ/Δ E11 embryos. Floor plates were lysed in RIPA buffer and incubated 20 min at 4°C. Protein content was determined by a BCA assay. 25 µg of total proteins were loaded on a 10% Mini Protean TGX precast gel (Biorad) and blotted onto a nitrocellulose membrane (Biorad). Membranes were blocked with 5% dried milk and 3% of BSA in TBS-0.1% Tween (TBS-T) for 1 hr at room temperature and incubated for 1 hr and 30 min at room temperature with primary antibodies: anti-Actin (Sigma, A5060, rabbit polyclonal, 1:1500), anti-HPRT (Abcam, ab109021, rabbit monoclonal EPR5299, 1:10000), anti-Netrin-1 (R&D Systems, MAB1109, rat monoclonal, 1:500), anti-Netrin-3 (Abcam, ab185200, rabbit polyclonal, 1:1000) and anti-Slit2 (Abcam, ab134166, rabbit monoclonal, 1:400). After three washes in TBS-T, membranes were incubated with the appropriate HRP-conjugated secondary antibody (1:5000, Jackson ImmunoResearch, Suffolk, UK). Detection was performed using Pierce ECL Western Blotting Substrate (ThermoScientific).
Data quantification and statistics
All data quantification was done by an observer blinded to the experimental conditions. We did not perform randomization into groups. Data were presented as mean values ± standard error of the mean. Statistical significance was calculated using one-sided unpaired tests for non-parametric tendencies (Kruskal-Wallis and Mann-Whitney). For western blot, at least 3 independent cases were quantified from independent experiments using densitometric analysis (Image J) by normalizing phospho-signals to total protein levels. The control cases were normalized to 1 and for the mutants, data were presented as mean values ± standard error of the mean (0.1133 ± 0.05 for Shh:Cre; 1 for Netrin-1L/L and 0 for the Netrin-1Δ/Δ). Differences were considered significant (*) when P<0.05. Statistical test data were as follows; Extended Data 2i, Netrin-1L/L to Shh:Cre; Netrin-1L/L or Netrin-1Δ/Δ Mann-Whitney test, P<0.05 (P=0.0022 for both). The thickness of hindbrain commissural bundles was quantified for each embryo on 9 coronal sections. The sections were representative of 3 different hindbrain antero-posterior levels (3 sections for each level). To minimize the developmental variations, mutant embryos and littermate controls were compared (except for Netrin-1Δ/Δ embryos which were compared with wild type embryos). The ratio of the commissural axon bundle size was normalized to controls. Six embryos of each genotype were quantified, from at least two different litters. Data were presented as mean values ± standard error of the mean. For the Wt (Dcc 0.994 ± 0.052; Robo3 1 ± 0.061; Neurofilament 1 ± 0.048), Netrin-1ßgeo/ßgeo (Dcc 0.416 ± 0.013; Robo3 0.402 ± 0.007; Neurofilament 0.416 ± 0.01), Netrin-1Δ/Δ (Dcc 0.091 ± 0.008; Robo3 0.061 ± 0.008; Neurofilament 0.104 ± 0.007) Shh:Cre;Netrin-1L/L (Dcc 1.051 ± 0.011; Robo3 1.084 ± 0.028; Neurofilament 1.063 ± 0.019) and Foxg1:Cre;Netrin-1L/L (Dcc 0.411 ± 0.053; Robo3 0.369 ± 0.056; Neurofilament 0.416 ± 0.050).
Differences were considered significant (*) when P<0.05. Statistical test data were as follows; Extended Data Fig. 4g, Dcc, Robo3 and Neurofilament Kruskal-Wallis, P<0.05. Extended Data Fig. 4g, comparison between wild type and the different conditions for Dcc, Robo3 and Neurofilament, Mann-Whitney, P<0.05. Except the comparison between wild type and Shh:Cre;Netrin-1L/L where, Dcc Mann-Whitney, P=0.0649, Robo3 Mann-whitney, P=0.1797, Neurofilament Mann-whitney, P=0.0649. Statistical analyses of the mean and variance were performed with Prism 7 (GraphPad Software).
Extended Data
Extended Data Figure 1. Netrin-1 distribution in Hindbrain and Spinal Cord.
Coronal cryostat sections of the hindbrain and spinal cord (brachial level) of E11 and E13 embryos.
a, At E11, the floor plate (Alcam+ positive cells in green) but also commissural axons are immunoreactive for netrin-1 (n=6). b, At E13 netrin-1 mRNA is still expressed in the floor plate (Fp) and VZ of the basal plate (n=6). c, In a E11 Netrin1ßgeo/+, Robo3+ commissural neurons in the dorsal hindbrain (arrowheads) are not immunoreactive for ßgal unlike the basal plate neuroepithelium (arrows; n=6). d, Western blot analysis of HEK-293T cells overexpressing hNETRIN-3, hNETRIN-1 or mNetrin-1 proteins (n=3). Left, the monoclonal anti-NTN1 antibody (MAB1109) specifically recognizes Netrin-1 proteins (human and mouse) and not Netrin-3. Right, Netrin-3 is specifically recognized by the polyclonal anti-NTN3 antibody (ab185200) unlike Netrin-1. e, At E11, netrin-1 is expressed in the spinal cord by floor plate (arrowhead) and VZ progenitors (n=6). f, The floor plate (Alcam+), commissural axons, radial processes of neural progenitors and basal lamina are immunoreactive for netrin-1 (n=6). g, At E13 (n=7), netrin-1 is still highly distributed in Nestin+ radial processes of neural progenitors and at the pial surface. h,i Netrin-1 protein is absent from the hindbrain of Netrin-1Δ/Δ at E13 (h) and the spinal cord at E11 (i) (n=6 for each). Floor plate cells (arrowhead) still express Alcam (green). j, Netrin-1 immunostaining without permeabilization at E11. Commissural axons are still labeled (arrowheads) including post-crossing ones (arrow). Commissural axons are also stained with anti-Robo3 on the left panel. Ventricle (V). k, Shows the absence of Dcc-immunoreactivity on a hindbrain section from a DCC-/- E11 embryo (DAPI counterstaining, n=6). l, Neural progenitors radial processes are present in Netrin-1Δ/Δ embryos (n=6). Scale bars, 100 µm except in g, 50 µm.
Extended Data Figure 2. Floor plate-specific deletion of netrin-1 in Shh:Cre;Netrin1lox mutants.
Coronal cryostat sections of the hindbrain and spinal cord of E10 and E11 embryos.
a-d, In situ hybridization for netrin-1 on E11 spinal cord (brachial level). In Netrin-1L/L embryo (a) netrin-1 mRNA is highly expressed in floor plate (Fp) and ventricular zone (VZ) (n=6). A weak netrin-1 expression is still detected in the floor plate (arrowhead) of a Netrin1ßgeo/ßgeo hypomorph (b) (n=5), whereas no signal is seen in a Netrin-1Δ/Δ (c) embryo (n=6). In Shh:Cre;Netrin1L/L embryo (d), netrin-1 mRNA is not expressed in the floor plate (arrowhead) but still present in the VZ (n=6). e, E10 Shh:Cre;NetrinL/L spinal cord sections at brachial, thoracic and lumbar levels. At all levels, Netrin-1 is found in the VZ, with the highest levels in the p3 progenitor domain, but is absent from the floor plate (arrowheads, n=6). f,g, In Netrin-1L/L (f) commissural axons, basal lamina (Pia) and floor plate (arrowhead in f) are immunoreactive for netrin-1. By contrast, the floor plate is not labeled in Shh:Cre;Netrin1L/L embryos (arrowhead in g) whereas netrin-1 remains expressed along neural progenitor processes and basal lamina (Pia; n=6/6). h, Western blot with Netrin-1 antibody on floor plate extracts from Netrin-1L/L, Shh:Cre;NetrinL/L and Netrin-1Δ/Δ E11 embryo hindbrain and spinal cord (at least 3 cases for each from 3 independent experiments). Netrin-1 is undetectable in Netrin-1Δ/Δ and reduced of 90% in Shh:Cre;NetrinL/L. i, Western blot quantification. Wild type values were normalized to 1 and mutant values were compared to it using a non-parametric Mann-Whitney test. Mutant values are represented as the mean ± SEM (*P<0.05). Scale bars, 100 µm, except on a, b, c and d higher magnifications, 50 µm.
Extended Data Figure 3. Floor plate-derived netrin-1 is not necessary for the midline crossing in hindbrain and spinal cord.
Coronal cryostat sections of the hindbrain and spinal cord (brachial level) of E10, E11 and E13 embryos . a-d, E11 and E13 hindbrain sections (upper and middle panels) and E11 spinal cord sections (lower panels). In wild type (a) Robo3+ and Dcc+ commissural axons cross the floor plate (n=6). Midline crossing is reduced in Netrin1ßgeo/ßgeo hypomorphs (b; arrowheads; n=3) and almost absent in Netrin-1Δ/Δ embryos (c; n=6). By contrast, no midline crossing defects are present in Shh:Cre;Netrin1L/L embryos (d) (n=9). e, Coronal sections at 3 rostro-caudal levels of the spinal cord of an E10 Shh:Cre;Netrin-1L/L embryo labeled with anti-Robo3. Commissural axons cross the floor plate at all levels. The dashed lines on the left panel shows the level of the sections. Abbreviations: Brach: brachial; Hind, hindbrain; Thor, thoracic; Lumb, lumbar. Scale bars, 100 µm, except on e left panel, 400 µm.
Extended Data Figure 4. Analysis of the Foxg1:Cre ;Netrin-1lox mice.
Coronal cryostat sections of the hindbrain of E11 and E13 embryos and spinal cord (brachial level) of E11 embryos . a-b, In Foxg1:Cre;Netrin-1L/+ embryos as in wild type, netrin-1 is in the hindbrain VZ (arrowhead) and commissural axons (arrow). This is not the case in Foxg1:Cre;Netrin-1L/L mutant. The floor plate is still labelled (Fp). Note that netrin-1 is present in the vicinity of the Fp (n=6). c, Foxg1:Cre drives Cre expression in E13 (left) and E11 (right) hindbrain cells (Tomato+ cells in red) but not in the floor plate (arrowheads; n=3/3). A few Alcam+ floor plate cells are Tomato+. d, e, Netrin-1 distribution is similar in the spinal cord of Foxg1:Cre;Netrin-1L/+ (d) and Foxg1:Cre;Netrin-1L/L (e) embryos (n=5). f, In Netrin1ßgeo/ßgeo, Foxp2+ olivary neurons fail to migrate (arrowheads) ventrally and only few of them are able to reach to the floor plate (arrowheads; n=6). g, Quantification of the size of hindbrain commissures in the different mutants compared to controls. Six embryos of each genotype and 9 sections from each were quantified. Data are normalized with the wt and are represented as the mean ± SEM (One-way Kruskal-Wallis with Mann-Whitney post-test, *P<0.05, ns, not significant). h, Netrin-1 guidance mechanisms of hindbrain commissural axons: past and current models. In the initial model, soluble netrin-1 secreted by floor plate (FP) forms a ventro-dorsal gradient, which attracts ventrally commissural axon (CN) growth cones (GC). In the revised model, pioneer CN axons form in a superficial region containing high levels of netrin-1 produced by neural progenitor cells (NPCs) extending from the ventricular zone (VZ) to the basal lamina (BL) at the surface of the hindbrain. CN axons might also capture Netrin-1 and establish a netrin-1-rich pathway guiding follower axons. Their ventral extension might be facilitated by chemorepellents (question mark) produced in the dorsal hindbrain. Scale bars, 100µm.
Supplementary Material
Acknowledgements
We thank Dr Marc Tessier-Lavigne for providing the DCC and Netrin-1 knockout Dr Christopher Wright for the Ptf1a:CreERT2 line and Dr Patrick Charnay for the Krox20:Cre line. We also thank Alex Kolodkin and Robin Vigouroux for critical reading of the manuscript and Stéphane Fouquet of the Vision Institute imaging facility for its technical support. This work was supported by grants from the Agence Nationale de la Recherche (ANR-14-CE13-0004-01) (AC). It was performed in the frame of the LABEX LIFESENSES (reference ANR-10-LABX-65) supported by French state funds managed by the ANR within the Investissements d'Avenir programme under reference ANR-11-IDEX-0004-02 (AC). This work was also supported by grants from INCA, ERC, ANR and Fondation Bettencourt (PM). C.D was recipient of a PhD fellowship from the Fondation pour la recherche médicale.
Footnotes
Authors contributions
AC, AB and PM, designed the experiments. CD, JAMB, NR, PV, QR and SRP performed the experiments. AC, CD and JAMB prepared the figures. AC and PM supervised the project and wrote the manuscript.
Authors information
Reprints and permissions information is available at www.nature.com/reprints.
The authors declare no competing interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Serafini T, et al. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 2.Ishii N, Wadsworth WG, Stern BD, Culotti JG, Hedgecock EM. UNC-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron. 1992;9:873–881. doi: 10.1016/0896-6273(92)90240-e. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell KJ, et al. Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons. Neuron. 1996;17:203–215. doi: 10.1016/s0896-6273(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy TE, Serafini T, de la Torre J, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- 5.Ming GL, et al. cAMP-dependent growth cone guidance by netrin-1. Neuron. 1997;19:1225–1235. doi: 10.1016/s0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- 6.de la Torre JR, et al. Turning of retinal growth cones in a netrin-1 gradient mediated by the netrin receptor DCC. Neuron. 1997;19:1211–1224. doi: 10.1016/s0896-6273(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 7.Serafini T, et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–14. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 8.Yung aR, Nishitani aM, Goodrich LV. Phenotypic analysis of mice completely lacking Netrin-1. Development. 2015;142:3686–3691. doi: 10.1242/dev.128942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bin JMM, et al. Complete Loss of Netrin-1 Results in Embryonic Lethality and Severe Axon Guidance Defects without Increased Neural Cell Death. Cell Rep. 2015;12:1099–106. doi: 10.1016/j.celrep.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 10.Chédotal A. Development and plasticity of commissural circuits: from locomotion to brain repair. Trends Neurosci. 2014;37:551–62. doi: 10.1016/j.tins.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Sabatier C, et al. The divergent Robo family protein rig-1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell. 2004;117:157–69. doi: 10.1016/s0092-8674(04)00303-4. [DOI] [PubMed] [Google Scholar]
- 12.Marillat V, et al. The slit receptor Rig-1/Robo3 controls midline crossing by hindbrain precerebellar neurons and axons. Neuron. 2004;43:69–79. doi: 10.1016/j.neuron.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Wentworth LE. The development of the cervical spinal cord of the mouse embryo. II. A Golgi analysis of sensory, commissural, and association cell differentiation. J Comp Neurol. 1984;222:96–115. doi: 10.1002/cne.902220109. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy TE, Wang H, Marshall W, Tessier-Lavigne M. Axon guidance by diffusible chemoattractants: a gradient of netrin protein in the developing spinal cord. J Neurosci. 2006;26:8866–8874. doi: 10.1523/JNEUROSCI.5191-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleming JT, et al. The Purkinje neuron acts as a central regulator of spatially and functionally distinct cerebellar precursors. Dev Cell. 2013;27:278–92. doi: 10.1016/j.devcel.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renier N, et al. Genetic Dissection of the Function of Hindbrain Axonal Commissures. PLoS Biol. 2010;8:e1000325. doi: 10.1371/journal.pbio.1000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiramoto M, Hiromi Y, Giniger E, Hotta Y. The Drosophila Netrin receptor Frazzled guides axons by controlling Netrin distribution. Nature. 2000;406:886–889. doi: 10.1038/35022571. [DOI] [PubMed] [Google Scholar]
- 18.Fazeli A, et al. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature. 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- 19.Joksimovic M, et al. Wnt antagonism of Shh facilitates midbrain floor plate neurogenesis. Nat Neurosci. 2009;12:125–31. doi: 10.1038/nn.2243. [DOI] [PubMed] [Google Scholar]
- 20.Bloch-Gallego E, Ezan F, Tessier-Lavigne M, Sotelo C. Floor plate and netrin-1 are involved in the migration and survival of inferior olivary neurons. J Neurosci. 1999;19:4407–20. doi: 10.1523/JNEUROSCI.19-11-04407.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujita H, Sugihara I. FoxP2 expression in the cerebellum and inferior olive: development of the transverse stripe-shaped expression pattern in the mouse cerebellar cortex. J Comp Neurol. 2012;520:656–77. doi: 10.1002/cne.22760. [DOI] [PubMed] [Google Scholar]
- 22.Tronche F, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 23.Grandin M, et al. Structural Decoding of the Netrin-1/UNC5 Interaction and its Therapeutical Implications in Cancers. Cancer Cell. 2016;29:173–185. doi: 10.1016/j.ccell.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Brankatschk M, Dickson BJ. Netrins guide Drosophila commissural axons at short range. Nat Neurosci. 2006;9:188–94. doi: 10.1038/nn1625. [DOI] [PubMed] [Google Scholar]
- 25.Akin O, Zipursky SL. Frazzled promotes growth cone attachment at the source of a Netrin gradient in the Drosophila visual system. Elife. 2016;5 doi: 10.7554/eLife.20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore SW, Biais N, Sheetz MP. Traction on immobilized netrin-1 is sufficient to reorient axons. Science. 2009;325:166. doi: 10.1126/science.1173851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matise M, Lustig M, Sakurai T, Grumet M, Joyner A. Ventral midline cells are required for the local control of commissural axon guidance in the mouse spinal cord. Development. 1999;126:3649–3659. doi: 10.1242/dev.126.16.3649. [DOI] [PubMed] [Google Scholar]
- 28.Delloye-Bourgeois C, et al. Nucleolar Localization of a Netrin-1 Isoform Enhances Tumor Cell Proliferation. Sci Signal. 2012;5:ra57–ra57. doi: 10.1126/scisignal.2002456. [DOI] [PubMed] [Google Scholar]
- 29.Voiculescu O, Charnay P, Schneider-Maunoury S. Expression pattern of a Krox-20/Cre knock-in allele in the developing hindbrain, bones, and peripheral nervous system. genesis. 2000;26:123–6. doi: 10.1002/(sici)1526-968x(200002)26:2<123::aid-gene7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 30.Harfe BD, et al. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 31.Hébert JM, Mcconnell SK. Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev Biol. 2000;222:296–306. doi: 10.1006/dbio.2000.9732. [DOI] [PubMed] [Google Scholar]
- 32.Belle M, et al. A Simple Method for 3D Analysis of Immunolabeled Axonal Tracts in a Transparent Nervous System. Cell Rep. 2014;9:1191–1201. doi: 10.1016/j.celrep.2014.10.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.