Abstract
Numerous researches supported that microbiota can influence behavior and modulate cognitive function through “microbiota-gut-brain” axis. Our previous study has demonstrated that ZiBuPiYin recipe (ZBPYR) possesses excellent pharmacological effects against diabetes-associated cognitive decline. To elucidate the role of ZBPYR in regulating the balance of gut microbiota to improve psychological-stress-induced diabetes-associated cognitive decline (PSDACD), we compared blood glucose, behavioral and cognitive functions and diversity of the bacterial community among experimental groups. The Zucker diabetic fatty (ZDF) rats with PSDACD exhibited behavioral and cognitive anomalies showing as increased anxiety- and depression-like behaviors and decreased learning and memory abilities. High-throughput sequencing of the bacterial 16S rRNA gene revealed that Roseburia and Coprococcus were decreased in ZDF rats with PSDACD compared with control group. Notably, these changes were reversed by ZBPYR treatment. Our findings indicate that ZBPYR might prevent PSDACD by maintaining the compositions of gut microbiota, which could be developed as a new therapy for T2D with PSDACD.
Keywords: ZiBuPiYin recipe, diabetes, psychological-stress, cognitive decline, gut microbiota, Pathology Section
INTRODUCTION
Type 2 diabetes (T2D) is characterized by chronic hyperglycemia with progressive failure of pancreatic insulin secretion and increased peripheral insulin resistance [1]. More recent investigations show that cognitive impairment is found in T2D patients prior to the onset of these conditions [2–4]. Diabetes-associated cognitive decline (DACD), as slight cognitive decrements, is generally thought across species to a central nervous systems (CNS) complication of diabetes [5–7]. The development of DACD is a complex process involving genetic susceptibility and environmental factors [8, 9], yet the mechanisms have not been fully unmasked.
The immediate psychosocial stress (PS) is not thought to be the problem affecting health, however, chronic activation of PS is considered to be the key [10]. Chronic psychosocial stress increased the incidence of approaching impaired glucose tolerance to diabetes [11]. Previous reports deem the mechanism as that insulin sensitivity is declined and insulin resistance is enhanced under the control of the hypothalamus, the limbic system of the emotional loop [12, 13]. However, the exact mechanism of insulin signal transduction disorders inducing cognitive dysfunction is not well established. Since psychological-stress-induced diabetes-associated cognitive decline (PSDACD) adversely influences quality of life in T2D patients [13], developing a prospective strategy for PSDACD is of great importance.
ZiBuPiYin recipe (ZBPYR), a traditional formula of Chinese medicine documented in the book of Bujuji written by Wu Cheng in the Qing dynasty, is originated from Zicheng Decoction for clinical therapy of cognitive impairment [14]. Our previous work has demonstrated that ZBPYR improved DACD in db/db mice effectively [15], which examined the beneficial effects of ZBPYR on cognitive impairment and suggested that ZBPYR could increase brain leptin, improve insulin resistance and ameliorate peripheral high glucose environment. We also found that ZBPYR protected hippocampal neurons against Aβ amyloid- and glutamate-induced neurotoxicity [16, 17]. A recent study performed by our group revealed the characterization of gastrointestinal microbes (GM) in ZDF rat GI tract, suggesting that the microbiota might serve as a biomarker of impending or fully manifest T2D and altering the GI microbial communities might be a prospective strategy for treating obesity and T2D [18].
Hyperactive hypothalamus-pituitary-adrenal (HPA) axis leads to changes of the gut microbial interacting with the brain via the gut-brain axis to modulate brain development, stress responses, the overall glucose and lipid metabolism [19–22]. To elucidate the role of ZBPYR in regulating the balance of gut microbiota affecting psychological-stress-induced diabetes-associated cognitive decline (PSDACD), we used male Zuker diabetes fatty (ZDF) rat as T2D model, which presents developing hyperinsulinemia and hyperglycaemia [23] and is initiated with a mutation in the leptin receptor gene [24, 25].
RESULTS
ZBPYR improves glucose metabolism in ZDF rats exposed to PS
Three chronic PS (restriction, rotation, and congest) were imposed on ZDF rat to establish a model of PSDACD, while ZDF rat without PS was as control. As shown in Figure 1A, compared with controls, fasting blood glucose levels were remarkably elevated in PSD (ZDF rats with PSDACD) group at 30, 60, 90, and 120minutes (P < 0.01). The glucose total AUC of PSD group was extremely increased compared with controls (Figure 1B). Figure 1C and 1D showed that blood glucose concentrations in PSD rats were significantly higher than in control rats at 30, 60, 90 minutes (P < 0.05) and the glucose total AUC of PSD group was increased significantly compared with controls (P < 0.01). Administration of ZBPYR obviously reduced blood glucose levels and enhanced insulin sensitivity. Compared with PSD group, there were significant differences in PDZ (ZDF rats with PSDACD by ZBPYR treatment) group of the OGTT and ITT (P < 0.01), but the blood glucose did not recover to normal at the end point (Figure 1A-1D). The body weight of the rats in three groups was no significant differences (Table 1).
Figure 1. Effects of ZBPYR on glucose tolerance and insulin resistance in ZDF rats.
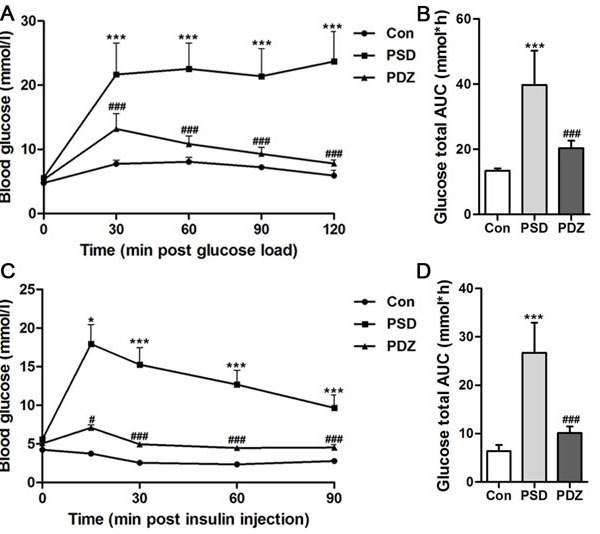
A. B. Oral glucose tolerance tests were examined in 14-hr-fasted rats after 10 weeks with high-fat diet. A: Blood glucose levels, B. Total glucose area under the curve (AUC). C. D. Insulin tolerance tests were conducted on 6-hr-fasted animals. C. Blood glucose levels, D. Total glucose AUC. Black circles, control (n = 3); black squares, PSD group (n = 3); black triangles, PDZ group (n = 3). *P < 0.05, ***P < 0.001 PSD vs. Con; #P < 0.05, ###P < 0.001 PDZ vs. PSD. Con: control ZDF rats; PSD: ZDF rats treated with PS; PDZ: ZDF rats treated with PS combining ZBPYR administration.
Table 1. Mean Body Weight (g) of rats in each group.
| Week | Con | PSD | PDZ |
|---|---|---|---|
| 1W | 144.33±4.89 | 148.48±3.63 | 145.14±8.42 |
| 2W | 192.19±8.91 | 187.53±18.94 | 189.52±6.43 |
| 3W | 236.48±11.60 | 214.93±22.04 | 226.87±12.35 |
| 4W | 275.24±10.42 | 272.86±19.21 | 264.86±2.90 |
| 5W | 304.86±9.50 | 298.38±17.35 | 291.71±1.03 |
| 6W | 324.76±8.16 | 317.24±15.14 | 312.48±2.62 |
| 7W | 340.76±9.70 | 329.62±11.55 | 334.29±3.71 |
| 8W | 350.67±9.99 | 336.57±13.40 | 356.86±2.27 |
| 9W | 354.00±13.00 | 339.33±17.36 | 366.86±5.58 |
Scores on open field activity
ZDF rats exposed to PS exhibited a series of abnormal behaviors, such as piloerection, indolent and fidget, which are typical anxiety- and depression-like behaviors. In the Open Field test, there were significant differences between the rats in Con group and PSD group, but no differences between Con group and PDZ group, indicating that the model of depressive-like behaviors was successfully established in ZDF rats. The PSD rats displayed being lack of physical activity (Figure 2A), less frequency entered into the center (Figure 2B), and shorter time in the center (Figure 2C) compared with controls. Vertical numbers of PSD rats were significantly decreased compared with controls (P < 0.05, Figure 2D). The behavior of rats in group PDZ resumed to normal levels after 8-week treatment of ZBPYR with the exposure to chronic restraint stress.
Figure 2. Parameters of activities in the open field test.
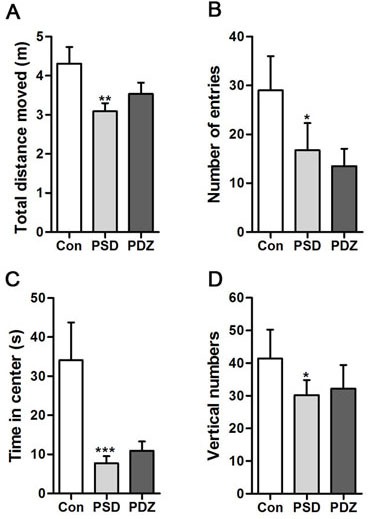
Total distances moved in the open field were measured. Vertical numbers during the experiment and numbers of entries into the center of the open field were counted. Time spent in the center of the open field was calculated. *P < 0.05, **P < 0.01, ***P < 0.001, PSD, PDZ vs. Con. Con: control ZDF rats; PSD: ZDF rats treated with PS; PDZ: ZDF rats treated with PS combining ZBPYR administration.
ZBPYR improves spatial learning and memory performances
Morris water maze test was conducted in the last week (Week 16) to assess the spatial learning and memory performance of the rats. The escape latency of the control group was shorter than model groups from day 3 to 5 (P < 0.05), and progressively decreased over 4 days of training. The performances of the rats in PDZ group were close to control group and significantly strengthened compared with PSD group during days 3 to 5 (P < 0.05 at day 3, P < 0.01 at day 5) (Figure 3A). In the probe test, we observed that PDZ rats swam shorter distance to locate the original platform position than PSD rats (Figure 3B). A high frequency of crossing the target quadrant located with the platform was checked in PDZ group compared with PSD group (Figure 3C). There were no significant differences among the groups in terms of escape latency in the visible platform version (Figure 3D). These results indicated that ZBPYR administration evidently improved cognitive decline of ZDF rats with PSDCAD.
Figure 3. ZBPYR improves the performance of PSDACD rats in the Morris water maze test.
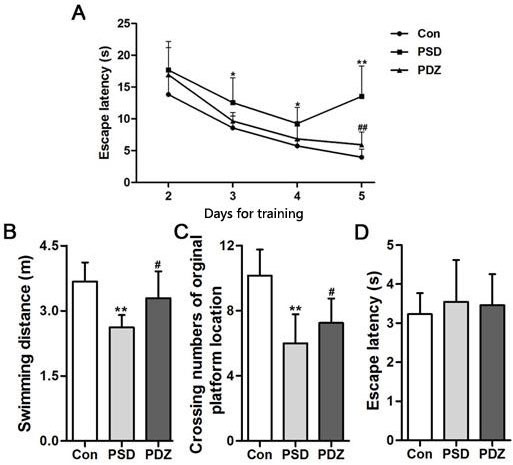
A. Learning performance of the animals was analyzed in the training trials by escape latency. PDZ rats presented shorter escape latency on the 4th and 5th day of training Black circles, control (n = 3); black squares, PSD group (n = 3); black triangles, PDZ (n = 3). B. C. Memory performance was investigated in the probe test. B: The swimming distances in training trials, C. The numbers of crossing over the original platform location. D. Escape latency was evaluated in the visible platform version of the Morris water maze. *P < 0.05, **P < 0.01 PSD vs. Con; #P < 0.05, ##P < 0.01 PDZ vs. PSD. Con: control ZDF rats; PSD: ZDF rats treated with PS; PDZ: ZDF rats treated with PS combining ZBPYR administration.
ZBPYR regulates gut microbiota in ZDF rats with PSDCAD
Gut microbiota provides a promising perspective to explore the pathological mechanism of PSDACD [26–29]. To reveal the link between T2D with PSDCAD and gut microbiota, the colons from three groups were sampled and sequenced. Microbial diversity analysis based on OTUs cluster suggested that the abundances of bacteria taxa even differ in the same anatomical sites. At phyla level, Bacteroidetes and Firmicutes were dominant bacteria among three groups (The total of their relative abundances was 97%, 99% and 91%, respectively), however, the slightly increased Firmicutes and decreased Bacteroidetes indicated that PSDACD may induce the varieties in the compositions of gut microbiota (Figure 4A-4C). The bacterial imbalance could be ameliorated by ZBPYR administration as the Firmicutes/Bacteroidetes ratio was decreased in PDZ group (Con, 1.49:1; PSD, 1.86:1; PDZ, 1.40:1). At class level, we identified a small ‘‘core’’ microbiota (mainly in the colon), including Bacilli, Bacteroidia, and Clostridia, and the relative percentages of these bacteria differ in the different groups shown in Figure 4D-4F.
Figure 4. The “core” of intestinal community among Con, PSD and PDZ groups, respectively.
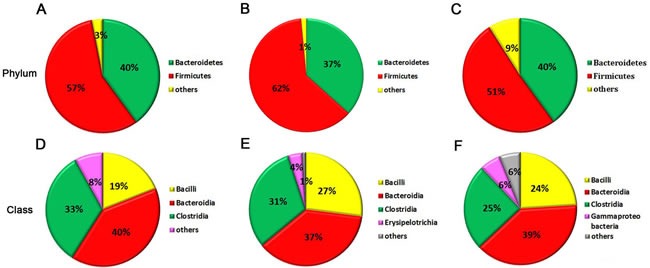
A-C: The distribution of intestinal community at the phyla level. D-F: The main bacterial class and the relative abundance of each bacteria. A and D: Control group; B and E: PSD group; C and F: PDZ group..
Heat map of the relative abundance of microbial species altered by PSDACD and ZBPYR treatment showed the differences of gut bacterial compositions compared among Con group, PSD group and PDZ group at the genus level (Figure 5). Consistent with previous reports, we found that the Firmicutes/ Bacteroidetes ratio was higher in PSD group compared with control group, in addition, it was recovered by ZBPYR administration in PDZ group (Figure 6A). The relative percentage of Roseburia was less in model group than that in control group (0.18% vs. 2.15%, Figure 6B), in contrast greater numbers of Roseburia were detected in PDZ group compared with PSD group (0.53% vs. 0.18%, Figure 6B). Interestingly, the analysis of Coprococcus followed similar trends as the relative percentage of Roseburia. These results confirmed that the homeostasis of the gut microbiota in PSDACD individuals was destroyed. ZBPYR could effectively manipulate the gut microbiota imbalance caused by PSDACD, mainly acting on Roseburia and Coprococcus.
Figure 5. The heat map of 16S rRNA gene sequencing analysis of colonic mucosa at the genus level.
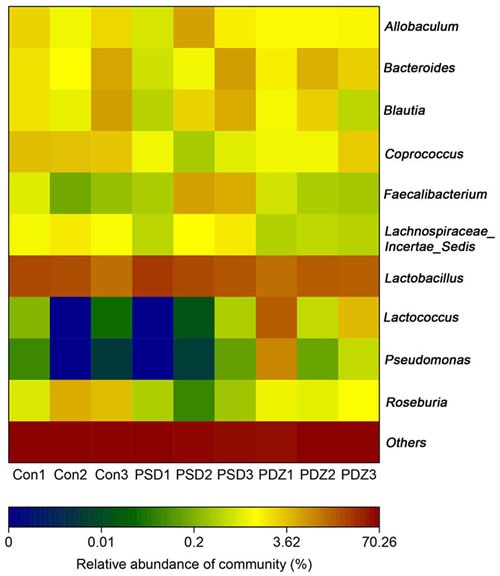
Heat map of the relative abundance of microbial species altered by PSDACD and ZBPYR treatment. Red colors indicate high values, whereas blue colors mean low values for the percent of reads classified at that rank.
Figure 6. Impact of ZBPYR administration on the intestinal micro ecology balance.
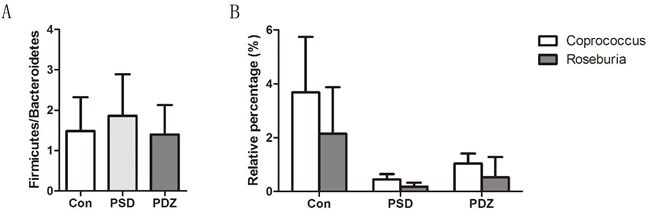
A. Firmicutes/Bacteroidetes (F/B) ratios in three groups. B. 16S rRNA gene sequencing analysis of genus Roseburia and Coprococcus (n = 3, each group). Con: control ZDF rats; PSD: ZDF rats treated with PS; PDZ: ZDF rats treated with PS combining ZBPYR administration.
DISCUSSION
In recent years there is a growing interest in discovering correlations between gut microbiota composition/diversity and stress related disturbances [30]. Nishino et al. reported that the type of commensal microbiota located in the gut has an impact on behavior, such as anxiety-like behavior [31]. The reduction of total number of Xbp1pos lymphocytes in the gut closed to lymphoid tissue of the ileum in rats under induced chronic social stress, assuming the role of Xbp1 expression levels as a trigger of inflammation and potential link between stress and autoimmunity [32]. Recently, Naseribafrouei addressed that an overrepresentation of Bacteroidetes spp. and an underrepresentation of Lachnospiraceae were found in the fecal microbiota of 37 patients with depression [33].
It is well known that chronic psychological stress is associated with enhanced vulnerability to metabolic disturbance (obesity, insulin resistance and T2DM) through hypothalamic-pituitary-adrenal (HPA) axis hyperactivity [11, 34]. Over the past decade, an abundance of evidence indicated that diabetes and obesity are two metabolic diseases with insulin resistance and a low-grade inflammation [35–40]. Several studies showed that metabolic endotoxemia controls the inflammatory tone, obesity and diabetes, and high-fat diet alters gut microbiota and the plasma concentration of lipopolysaccharide (LPS) [41–43]. Despite these efforts attempted to clarify the correlation of microbiota, nervous system and chronic inflammatory diseases, the mechanism is still lacking.
Our previous study explored the effects of the ZBPYR on diabetes-related cognitive decline in db/db mice. ZBPYR improved learning and memory performance impairments, enhanced brain leptin, stimulating insulin signaling and prevented GSK3β overactivity, which disclosed that the benefit of ZBPYR to DACD may be by increasing dendritic spine density and reducing the injury factors of brain leptin and insulin signaling pathway [15]. In this study, Zucker diabetic fatty (ZDF) rat harboring a missense mutation (fatty, fa) in the leptin receptor gene (LEPR) [24] was used to determine whether ZBPYR improves cognitive decline by regulating gut microbiota. We found that ZBPYR reduced blood glucose concentration and promoted insulin sensitivity in ZDF rats with PSDACD (Figure 1A-D), and also enhanced learning and memory performance in PSDACD/ZBPYR rats (Figures 2-3). Recent research reported that the gut microbiota in T2DM patients is characterized by a decrease in the abundance of some universal butyrate-producing bacteria and greater number of opportunistic pathogens [44, 45]. Butyrate produced by microbial fermentation is a short-chain fatty acid, which can stimulate the pancreatic insulin secretion, increase insulin sensitivity and alter insulin signaling [46–48]. It exhibits remarkable functions, such as anti-obesity, alleviation of metabolic stress, protection from inflammatory response and improvement of cognition impairment [49, 50]. Roseburia, as a major butyrate producer, has a positive correlation with mental health [30]. And Roseburia could be increased by healthy diets to improve insulin sensitivity in obese population [51]. Moreover, decreased Coprococcus was found in obese patients with type 2 diabetes [52]. Coprococcus is significantly negatively correlated with LPS in plasma [53]. LPS can stimulate the secretion of proinflammatory cytokines, which ultimately impairs insulin sensitivity and promotes insulin resistance-related metabolic disorders [42]. Meanwhile, the abundance of Coprococcus was lower in mice exposed to social stressor compared to controls [54]. Interestingly, our findings pointed that PSDACD was correlated to the changes of gut microbiota characterized by reduced abundance of Roseburia and Coprococcus (Figure 6B), which were consistent with previous studies [44, 51–53, 55]. ZBPYR improved PSDCAD by increasing the relative abundance of beneficial bacteria, i.e. Roseburia and Coprococcus (Figure 6B).
In summary, our work provides evidences to support that the modulation of gut microbiota is associated with the development of PSDACD. We have successfully identified bacterial genus involved in the development of PSDACD, and defined intervention targets of ZBPYR in intestinal microecology for the first time. Inspiringly, these results highlight ZBPYR is a promising anti-PSDACD drug in adults with type 2 diabetes.
METHODS AND MATERIALS
Animals
Male 5-week-old obese Zuker diabetes fatty (ZDF) rats were purchased from Vital River Laboratories (VRL) (Beijing, China) and housed in the specific pathogen-free (SPF) animal experiment center at Dalian Medical University. The animals were fed a high-fat diet and water ad libitum and housed at 24°C ± 2°C with 65% ± 5% humidity with a 12 h light-dark cycle. All animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals at Dalian Medical University (Dalian, China), which were approved by the Animal Ethics Committee of Dalian Medical University (Permit Number: SYXK (Liao) 2008-0002).
Experimental protocol
After 1 week acclimatization, the obese ZDF rats were randomly distributed in 3 groups (n=3 each): ZDF control group, psychological-stress-induced Diabetes-Associated Cognitive Decline (PSD) group, and PS combined ZBPYR administration (PDZ) group. The PSD group and PDZ group were subjected to three stress stimulations: restriction, rotation, and congest. In the experiment of restricting stress, the animals were placed in the homemade rotating device rotating 15 min at 30 rpm with the interval ranging from 40-150 min. Four cycles were performed in each experiment every other day. During the rotation stress experiment, the rats were put in the opening bottles. The size of bottle is appropriate that the rat cannot be freely turned over. Whole experiment time was limited to 2 h, and was employed every other day. In the congest stress experiment, to analog crowded environment, 5 rats were kept in the same cage. It should be noted that rotation stress and restriction stress tests could not be carried out on the same day.
Preparation and administration of ZBPYR
The ZBPYR consists of 12 herbs: Salvia miltiorrhiza Bge., Polygala tenuifolia Willd., Panax ginseng C.A.Mey., Dioscorea opposite Thunb., Poria cocos (Schw.) Wolf, Paronia lactiflora Pall., Dolichos lablab L., Acorus tatarinowii Schott, Santalum album L., Citrus reticulate Blanco., Nelumbo nucifera Gaertn. and Glycyrrhiza uralensis Fisch. All herbs were purchased from Dalian Lao Weixie outpatient department (Dalian, China). The mixtures were soaked in 8 volumes (v/w) of distilled water for 30 min and subsequently boiled for 90 min, then sandalwood was added. The decoction was filtered and concentrated before stored at 4°C. During a period of 8 weeks, ZBPYR was administered by oral gavage at a dose of 0.1 mL/10 g body weight in the treatment group, while PS and control group were orally administered an equal dose of ultrapure water (Milli-Q Integral Water Purification System, Millipore Corporation, Billerica, MA, USA).
Oral glucose tolerance test and Insulin tolerance test
The control and ZDF rats were fasted for 14 h (overnight) followed by conducting an oral glucose tolerance test (OGTT) with a glucose solution in saline at 2 g/kg. Rats were fasted for 6 h before insulin tolerance test (ITT) and injected intraperitoneally with regular human insulin (0.75 U/kg body weight; Novolin, Novo Nordisk (China) Pharmaceutical Co., Ltd., Tianjin, China). Tail blood was sampled at 0, 30, 60 and 120 min after the glucose administration and at 0, 15, 30, 60, and 90 min after insulin injection, respectively. Blood glucose was then determined from tail blood using a glucometer (Roche, Mannheim, Germany).
Behavioral experiments
Behavioral tests, including Open field tests and Morris water maze tests, were performed during the last 7 days of treatment according to the following schedule: Day 1 Open field test and Day 2–7 Morris water maze test. All procedures were conducted as previously described in detail [15, 56–58].
Sample preparation
All rats were anesthetized with ether and decapitated. The colon samples were immediately dissected on ice, weighed and then rapidly frozen in liquid nitrogen. All samples were stored at −80°C.
DNA Purification, 16S rRNA Gene Amplification and Illumina MiSeq sequencing
Genomic DNA was extracted from colon segments, the V1-V3 region of the bacteria 16S rRNA gene were amplified by PCR as previously described [18]. The barcode (an eight-base sequence) is unique to each sample. PCR reactions were performed as described previously [18]. Electrophoresis was used to isolate the PCR products on 2% agarose gels, and then the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, U.S.) was applied to purify separated products followed by quantifying DNA using QuantiFluor™-ST (Promega, U.S.). The purified pooled products were sequenced on an Illumina MiSeq platform (Lingen Biotechnology Co., Ltd., Shanghai, China). The reads were denoised into the NCBI Sequence Read Archive (SRA) database, and sequences were further analyzed as previously described in detail [18, 49]. A total of 226054 high quality gene sequences were obtained from 9 rat colon samples with an average of 25117 sequences per sample (13736 to 30954 sequences).
Statistical analyzes
All data were presented as means ± SD. Statistical analysis was evaluated by one-way ANOVA, followed by LSD (against all groups) post hoc with SPSS 17.0 (SPSS, Chicago, IL, USA). For data comparison, variables with non-Gaussian distribution were ASIN-square-root-transformed. P-values < 0.05 were considered statistically significant.
Acknowledgments
This study was supported by grants from the National Nature Science Foundation of China (No. 81230084).
Footnotes
CONFLICTS OF INTERESTS
None declared.
REFERENCES
- 1.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–94. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanhanen M, Koivisto K, Karjalainen L, Helkala EL, Laakso M, Soininen H, Sr, Riekkinen P. Risk for non-insulin-dependent diabetes in the normoglycaemic elderly is associated with impaired cognitive function. Neuroreport. 1997;8:1527–30. doi: 10.1097/00001756-199704140-00041. [DOI] [PubMed] [Google Scholar]
- 3.Gold SM, Dziobek I, Sweat V, Tirsi A, Rogers K, Bruehl H, Tsui W, Richardson S, Javier E, Convit A. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 2007;50:711–9. doi: 10.1007/s00125-007-0602-7. [DOI] [PubMed] [Google Scholar]
- 4.Spauwen PJ, Kohler S, Verhey FR, Stehouwer CD, van Boxtel MP. Effects of type 2 diabetes on 12-year cognitive change: results from the Maastricht Aging Study. Diabetes Care. 2013;36:1554–61. doi: 10.2337/dc12-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biessels GJ, Deary IJ, Ryan CM. Cognition and diabetes: a lifespan perspective. Lancet Neurol. 2008;7:184–90. doi: 10.1016/S1474-4422(08)70021-8. [DOI] [PubMed] [Google Scholar]
- 6.Li R, Zang A, Zhang L, Zhang H, Zhao L, Qi Z, Wang H. Chrysin ameliorates diabetes-associated cognitive deficits in Wistar rats. Neurol Sci. 2014;35:1527–32. doi: 10.1007/s10072-014-1784-7. [DOI] [PubMed] [Google Scholar]
- 7.Mijnhout GS, Scheltens P, Diamant M, Biessels GJ, Wessels AM, Simsek S, Snoek FJ, Heine RJ. Diabetic encephalopathy: A concept in need of a definition. Diabetologia. 2006;49:1447–8. doi: 10.1007/s00125-006-0221-8. [DOI] [PubMed] [Google Scholar]
- 8.Sharma AN, Elased KM, Garrett TL, Lucot JB. Neurobehavioral deficits in db/db diabetic mice. Physiol Behav. 2010;101:381–8. doi: 10.1016/j.physbeh.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trofimiuk E, Braszko JJ. Concomitant docosahexaenoic acid administration ameliorates stress-induced cognitive impairment in rats. Physiol Behav. 2013;118:171–7. doi: 10.1016/j.physbeh.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Kelly SJ, Ismail M. Stress and type 2 diabetes: a review of how stress contributes to the development of type 2 diabetes. Annu Rev Public Health. 2015;36:441–62. doi: 10.1146/annurev-publhealth-031914-122921. [DOI] [PubMed] [Google Scholar]
- 11.Sanghez V, Razzoli M, Carobbio S, Campbell M, McCallum J, Cero C, Ceresini G, Cabassi A, Govoni P, Franceschini P, de Santis V, Gurney A, Ninkovic I, et al. Psychosocial stress induces hyperphagia and exacerbates diet-induced insulin resistance and the manifestations of the Metabolic Syndrome. Psychoneuroendocrinology. 2013;38:2933–42. doi: 10.1016/j.psyneuen.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Bergmann N, Gyntelberg F, Faber J. The appraisal of chronic stress and the development of the metabolic syndrome: a systematic review of prospective cohort studies. Endocr Connect. 2014;3:R55–80. doi: 10.1530/EC-14-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eriksson AK, Ekbom A, Granath F, Hilding A, Efendic S, Ostenson CG. Psychological distress and risk of pre-diabetes and Type 2 diabetes in a prospective study of Swedish middle-aged men and women. Diabet Med. 2008;25:834–42. doi: 10.1111/j.1464-5491.2008.02463.x. [DOI] [PubMed] [Google Scholar]
- 14.Liang LN, Hu SY, Zhan LB. [Effects of zibu piyin recipe on the insulin resistance in the hippocampus of pi-yin deficiency diabetic rats]. [Article in Chinese] Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32:356–61. [PubMed] [Google Scholar]
- 15.Chen J, Liang L, Zhan L, Zhou Y, Zheng L, Sun X, Gong J, Sui H, Jiang R, Zhang F, Zhang L. ZiBuPiYin recipe protects db/db mice from diabetes-associated cognitive decline through improving multiple pathological changes. PLoS One. 2014;9:e91680. doi: 10.1371/journal.pone.0091680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhan LB, Niu XP, Sui H, Gong XY. Protective effect of spleen-yin-nourishing recipe on amyloid beta-peptide-induced damage of primarily cultured rat hippocampal neurons and its mechanism. Zhong Xi Yi Jie He Xue Bao. 2009;7:242–8. doi: 10.3736/jcim20090309. [DOI] [PubMed] [Google Scholar]
- 17.Zhan LB, Sui H, Lu XG, Sun CK, Zhang J, Ma H. Effects of Zibu Piyin recipe on SNK-SPAR pathway in neuron injury induced by glutamate. Chin J Integr Med. 2008;14:117–22. doi: 10.1007/s11655-008-0117-1. [DOI] [PubMed] [Google Scholar]
- 18.Gu C, Yang Y, Xiang H, Li S, Liang L, Sui H, Zhan L, Lu X. Deciphering bacterial community changes in zucker diabetic fatty rats based on 16S rRNA gene sequences analysis. Oncotarget. 2016;7:48941–48952. doi: 10.18632/oncotarget.10597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bienenstock J, Kunze W, Forsythe P. Microbiota and the gut-brain axis. Nutr Rev. 2015;73(Suppl 1):28–31. doi: 10.1093/nutrit/nuv019. [DOI] [PubMed] [Google Scholar]
- 20.Dinan TG, Cryan JF. Melancholic microbes: a link between gut microbiota and depression? Neurogastroenterol Motil. 2013;25:713–9. doi: 10.1111/nmo.12198. [DOI] [PubMed] [Google Scholar]
- 21.Geurts L, Neyrinck AM, Delzenne NM, Knauf C, Cani PD. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: novel insights into molecular targets and interventions using prebiotics. Benef Microbes. 2014;5:3–17. doi: 10.3920/BM2012.0065. [DOI] [PubMed] [Google Scholar]
- 22.Bercik P, Collins SM, Verdu EF. Microbes and the gut-brain axis. Neurogastroenterol Motil. 2012;24:405–13. doi: 10.1111/j.1365-2982.2012.01906.x. [DOI] [PubMed] [Google Scholar]
- 23.Tomaro-Duchesneau C, Saha S, Malhotra M, Jones ML, Labbe A, Rodes L, Kahouli I, Prakash S. Effect of orally administered L. fermentum NCIMB 5221 on markers of metabolic syndrome: an in vivo analysis using ZDF rats. Appl Microbiol Biotechnol. 2014;98:115–26. doi: 10.1007/s00253-013-5252-8. [DOI] [PubMed] [Google Scholar]
- 24.Phillips MS, Liu Q, Hammond HA, Dugan V, Hey PJ, Caskey CJ, Hess JF. Leptin receptor missense mutation in the fatty Zucker rat. Nat Genet. 1996;13:18–9. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- 25.Yamashita T, Murakami T, Iida M, Kuwajima M, Shima K. Leptin receptor of Zucker fatty rat performs reduced signal transduction. Diabetes. 1997;46:1077–80. doi: 10.2337/diab.46.6.1077. [DOI] [PubMed] [Google Scholar]
- 26.Mittal R, Debs LH, Patel AP, Nguyen D, Patel K, O’Connor G, Grati M, Mittal J, Yan D, Eshraghi AA, Deo SK, Daunert S, Liu XZ. Neurotransmitters: The Critical Modulators Regulating Gut-Brain Axis. J Cell Physiol. 2016. [DOI] [PMC free article] [PubMed]
- 27.Leung K, Gut Thuret S. Microbiota: A Modulator of Brain Plasticity and Cognitive Function in Ageing. Healthcare (Basel) 2015;3:898–916. doi: 10.3390/healthcare3040898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daulatzai MA. Role of stress, depression, and aging in cognitive decline and Alzheimer’s disease. Curr Top Behav Neurosci. 2014;18:265–96. doi: 10.1007/7854_2014_350. [DOI] [PubMed] [Google Scholar]
- 29.Caracciolo B, Xu W, Collins S, Fratiglioni L. Cognitive decline, dietary factors and gut-brain interactions. Mech Ageing Dev. 2014;136-137:59–69. doi: 10.1016/j.mad.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Li L, Su Q, Xie B, Duan L, Zhao W, Hu D, Wu R, Liu H. Gut microbes in correlation with mood: case study in a closed experimental human life support system. Neurogastroenterol Motil. 2016;28:1233–40. doi: 10.1111/nmo.12822. [DOI] [PubMed] [Google Scholar]
- 31.Nishino R, Mikami K, Takahashi H, Tomonaga S, Furuse M, Hiramoto T, Aiba Y, Koga Y, Sudo N. Commensal microbiota modulate murine behaviors in a strictly contamination-free environment confirmed by culture-based methods. Neurogastroenterol Motil. 2013;25:521–8. doi: 10.1111/nmo.12110. [DOI] [PubMed] [Google Scholar]
- 32.Topol IA, Kamyshny AM, Abramov AV, Kolesnik YM. Expression of XBP1 in lymphocytes of the small intestine in rats under chronic social stress and modulation of intestinal microflora composition. Fiziol Zh. 2014;60:38–44. [PubMed] [Google Scholar]
- 33.Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linlokken A, Wilson R, Rudi K. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26:1155–62. doi: 10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- 34.Belanger A, Lavoie N, Trudeau F, Massicotte G, Gagnon S. Preserved LTP and water maze learning in hyperglycaemic-hyperinsulinemic ZDF rats. Physiol Behav. 2004;83:483–94. doi: 10.1016/j.physbeh.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 35.Lee J. Adipose tissue macrophages in the development of obesity-induced inflammation, insulin resistance and type 2 diabetes. Arch Pharm Res. 2013;36:208–22. doi: 10.1007/s12272-013-0023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koduru SR, Achuthankutty S, Ghanim H, Dandona P, et al. Comment on: Lassenius Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care. 2011;34:1809–1815. doi: 10.2337/dc10-2197. Diabetes Care 2012 35 e17 author reply e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhardwaj S, Misra A, Khurana L, Gulati S, Shah P, Vikram NK. Childhood obesity in Asian Indians: a burgeoning cause of insulin resistance, diabetes and sub-clinical inflammation. Asia Pac J Clin Nutr. 2008;17(Suppl 1):172–5. [PubMed] [Google Scholar]
- 38.Zhuge F, Ni Y, Nagashimada M, Nagata N, Xu L, Mukaida N, Kaneko S, Ota T. DPP-4 inhibition by linagliptin attenuates obesity-related inflammation and insulin resistance by regulating M1/M2 macrophage polarization. Diabetes. 2016. [DOI] [PubMed]
- 39.Revelo XS, Ghazarian M, Chng MH, Luck H, Kim JH, Zeng K, Shi SY, Tsai S, Lei H, Kenkel J, Liu CL, Tangsombatvisit S, Tsui H, et al. Nucleic Acid-Targeting Pathways Promote Inflammation in Obesity-Related Insulin Resistance. Cell Rep. 2016;16:717–30. doi: 10.1016/j.celrep.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–9. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 42.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 43.Boutagy NE, McMillan RP, Frisard MI, Hulver MW. Metabolic endotoxemia with obesity: Is it real and is it relevant? Biochimie. 2016;124:11–20. doi: 10.1016/j.biochi.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–9. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 45.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 46.Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–17. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Backhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 48.Wolin MJ, Miller TL, Yerry S, Zhang Y, Bank S, Weaver GA. Changes of Fermentation Pathways of Fecal Microbial Communities Associated with a Drug Treatment That Increases Dietary Starch in the Human Colon. Applied and Environmental Microbiology. 1999;65:2807–12. doi: 10.1128/aem.65.7.2807-2812.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li HP, Chen X, Li MQ. Butyrate alleviates metabolic impairments and protects pancreatic beta cell function in pregnant mice with obesity. Int J Clin Exp Pathol. 2013;6:1574–84. [PMC free article] [PubMed] [Google Scholar]
- 50.Valvassori SS, Varela RB, Arent CO, Dal-Pont GC, Bobsin TS, Budni J, Reus GZ, Quevedo J. Sodium butyrate functions as an antidepressant and improves cognition with enhanced neurotrophic expression in models of maternal deprivation and chronic mild stress. Curr Neurovasc Res. 2014;11:359–66. doi: 10.2174/1567202611666140829162158. [DOI] [PubMed] [Google Scholar]
- 51.Haro C, Montes-Borrego M, Rangel-Zuniga OA, Alcala-Diaz JF, Gomez-Delgado F, Perez-Martinez P, Delgado-Lista J, Quintana-Navarro GM, Tinahones FJ, Landa BB, Lopez-Miranda J, Camargo A, Perez-Jimenez F. Two Healthy Diets Modulate Gut Microbial Community Improving Insulin Sensitivity in a Human Obese Population. J Clin Endocrinol Metab. 2016;101:233–42. doi: 10.1210/jc.2015-3351. [DOI] [PubMed] [Google Scholar]
- 52.Graessler J, Qin Y, Zhong H, Zhang J, Licinio J, Wong ML, Xu A, Chavakis T, Bornstein AB, Ehrhart-Bornstein M, Lamounier-Zepter V, Lohmann T, Wolf T, et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J. 2013;13:514–22. doi: 10.1038/tpj.2012.43. [DOI] [PubMed] [Google Scholar]
- 53.Xie G, Wang X, Liu P, Wei R, Chen W, Rajani C, Hernandez BY, Alegado R, Dong B, Li D, Jia W. Distinctly altered gut microbiota in the progression of liver disease. Oncotarget. 2016;7:19355–66. doi: 10.18632/oncotarget.8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Le KA Li Y, Xu X, Yang W, Liu T, Zhao X, Tang YG, Cai D, Go VL, Pandol S, Hui H. Alterations in fecal Lactobacillus and Bifidobacterium species in type 2 diabetic patients in Southern China population. Front Physiol. 2012;3:496. doi: 10.3389/fphys.2012.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bjorntorp P, Holm G, Rosmond R. Hypothalamic arousal, insulin resistance and Type 2 diabetes mellitus. Diabet Med. 1999;16:373–83. doi: 10.1046/j.1464-5491.1999.00067.x. [DOI] [PubMed] [Google Scholar]
- 57.Christakis DA, Ramirez JS, Ramirez JM. Overstimulation of newborn mice leads to behavioral differences and deficits in cognitive performance. Sci Rep. 2012;2:546. doi: 10.1038/srep00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]


