Since the early 1990s, investigators have toiled to establish the transfer of genes to human somatic cells as a valid therapy (fig 1). The potential of gene transfer was highlighted by three trials, involving participants with haemophilia B and two types of severe combined immunodeficiency—X linked and adenosine deaminase deficient.1-3 Yet even the most successful trials of gene transfer have engendered questions about its prospects. In the haemophilia B trial the detection of vector—the agent which carries genes to cells (fig 2)—in participants' semen raised concerns about modifications of the germline.4 The results of the X linked severe combined immunodeficiency trial were offset by unexpected, vector induced leukaemia in two participants.5
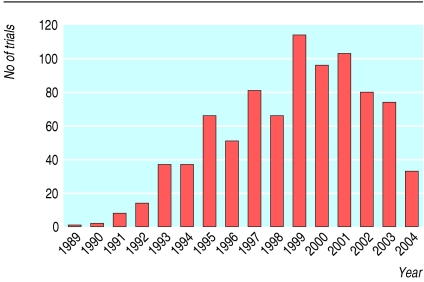
Fig 1.
Number of gene transfer trials approved worldwide has increased since 1989; 77% have been conducted in the United States and the United Kingdom. Most trials have used virus based vectors (70%), are phase 1 (63%), and involve investigational treatments for cancer (66%). Adapted from Wiley Gene Therapy Clinical Trial Database (www.wiley.co.uk/wileychi/genmed/clinical/)
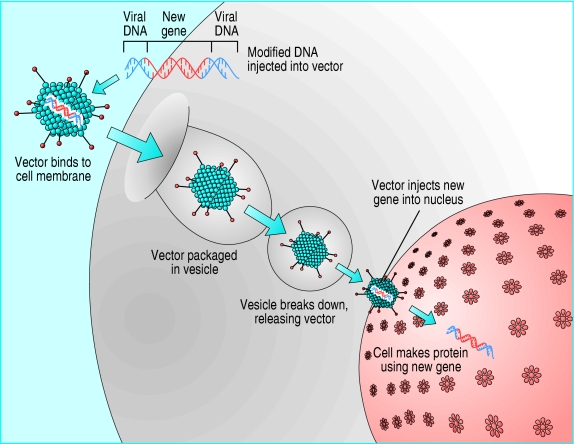
Fig 2.
In a gene transfer, therapeutic DNA is combined with a vector (often of viral origin). Vectors can be injected into recipient's tissue directly or used to modify cells ex vivo for transplantation to the recipient
Several hazards associated with gene transfer have been verified by clinical experience and others are predicted on theoretical grounds. It therefore may be worth considering whether risks shown in studies of human gene transfer present any unusual ethical and social challenges and, if so, what should be done to tackle them. In this article I review several matters relating to human gene transfer—safety features that distinguish traditional drugs from agents used to transfer genes, ethical issues raised by uncertainties about risk and toxicological properties, and studies on safety.
Sources and selection criteria
I searched for relevant articles in Medline through PubMed using the terms “gene transfer”, “gene therapy”, “risk”, “safety”, “toxicity”, and “ethics”. I also reviewed the minutes of the meetings of the Recombinant DNA Advisory Committee, US gene therapy policy conferences, and the UK Gene Transfer Advisory Committee, and websites of various other national review committees and scientific societies dealing with human gene transfer.
Summary points
Risks associated with gene transfer in humans present several conceptual and methodological challenges to toxicology
Uncertainties of risks demand central ethical review of all trial protocols and high scientific standards for trials
Latency and uncertainties of risks may disfavour the use of healthy trial participants
Ethical guidance on managing potential occupational hazards and risks to the public is limited
Trials generally enrol severely ill participants, therefore the characterisation of risks may depend heavily on postmarketing surveillance
The United Kingdom and Australia are exceptional for having implemented measures to track long term health of trial participants
Somatic human gene transfer and risk
The risk associated with human gene transfer has five conceptual characteristics that, although not unique, arise more often with this type of therapy (see box). Firstly, active agents rather than chemicals are used to transfer genetic material. These vectors (usually retroviruses or adenoviruses) are therefore potentially capable of propagating themselves, recombining with other viruses, or carrying out complex programmes.6 Secondly, gene transfer functions on the basis of genetic information rather than on chemical structure. Thus, whereas chemical structures indirectly affect genetic functions, gene transfer directly participates in gene expression. Although this partly accounts for its therapeutic potential, it also underscores the possible potency of risks. Thirdly, many agents act simultaneously as delivery devices through their vector as well as pharmacological agents through their transgene. Not only does this complicate the assessment of risk but each component can cooperate to worsen risks. For instance, the leukaemia that occurred in the X linked severe combined immunodeficiency trial may be attributable to the combined toxicity of the vector (which integrated near an oncogene) and the transgene (which may have helped transform T cells).7 Fourthly, gene transfer agents that stably modify a person's tissues can involve risks with long latencies. Continuous, life long exposure to transgenes or vectors increases the probability of subtle toxic properties becoming manifest over the long term. Finally, much of the toxicity related to gene transfer is mediated through the immune system. Immune over reaction resulted in the death of a participant in a trial of an adenovirus vector in 1999.8 Induced immunity against standard treatments (for example, factor IX protein in participants in the haemophilia B trial) or autoimmunity are also concerns with human gene transfer.
Several methodological issues need consideration when testing the safety of gene transfer. Animal models, for example, present major problems because viruses that are pathogenic in humans often have different risk profiles in animals.9 A person's prior exposure to viruses similar to the vector can influence their response to gene transfer. This observation, combined with a non-linear dose-toxicity curve, led investigators in the 1999 adenovirus trial to conclude that the evaluation of vector safety was problematic in a traditional phase 1 trial design.8
Few of these features are unique to gene transfer. For example, trials of live vaccines involve active agents, conventional drugs can be immunotoxic, and animal models often fail to predict toxicity. What makes the risk with gene transfer distinctive is the frequency with which these hazards arise in trials, their co-occurrence in a trial, and our limited experience of assessing and managing such hazards.
Ethics of risk in clinical trials
Does the character of risk with gene transfer generate new or major ethical challenges? This question may be best approached in three parts: what issues arise from immaturity of knowledge about risk, what ethical questions derive from toxicological characteristics, and what issues are raised by research aimed at improving the knowledge of risk?
Uncertainty and risk
While the toxicological properties of gene transfer agents remain obscure, ethics committees face formidable challenges in prosecuting two mandates for clinical trials10: evaluating the proportionality of risk and possible benefit, and overseeing risk disclosure during consent. The novelty of risks related to gene transfer means that uncertainties are more radical than those for conventional therapeutics, where a century of pharmacology provides a modicum of predictability.
The complexity of risk from gene transfer militates against the practice of using only local ethics committees to review trials (for example, in Canada or in some privately funded US trials, but not in the United Kingdom).11 Ethics committees that encounter such protocols in jurisdictions that do not mandate central review should consider requiring the protocol's submission to a review body such as the Recombinant DNA Advisory Committee. In disclosing risk, investigators should go beyond simply mentioning the possibility of unforeseen consequences. Enough adverse events have occurred that investigators should state outright that previous human gene transfer trials involved unforeseen consequences.
A second set of ethical issues concerns how ethics committees assure the value of trials. All major ethics codes require that clinical research be capable of generating valuable medical knowledge.10 In the past, gene transfer trials have often failed to do so.12 Ethics committees and investigators should be attentive to the scientific quality of proposed trials. Firstly, gene transfer exposes participants to the possibility of serious, unforeseeable, and latent harms. Because trials, especially those in the early phases (accounting for most trials), are aimed at generalisable knowledge rather than therapy, such risks can only be justified if the trial can make major contributions to the advancement of scientific and medical knowledge. Demanding that trials maximise their gathering of information on toxicology improves a protocol's balance of risk and social value. Secondly, ethics committees should bear in mind that, after 15 years, no gene therapy has been commercialised. Owing to the uncertainties surrounding gene transfer, most trials should be conceptualised less as testing an agent's prospect of commercialisation and more as producing information that can be applied to the development of gene transfer. This is especially true because data on toxicity in humans are scarce and can only be gathered through trials. Ethics committees and investigators can fortify the value of gene transfer studies by considering plans for long term follow up and disseminating toxicological findings.
Important features of risk associated with gene transfer
Conceptual features
Uses active agents rather than chemicals
Composed of genetic material that directly affects gene expression
Functions simultaneously as delivery devices (vectors) and pharmacological agents (transgenes)
Stable genetic modification has risks with long latencies
Certain viral vectors present risks to public health and occupational risks
Methodological features
Limited number of animal models for predicting vector safety
Wide variability in humans' response to some vectors
Possible non-linear dose-response curves with gene transfer vectors
Character of toxicity
Although the mechanisms of risk related to gene transfer may be different from those for drugs composed of small molecules, its clinical end points are more or less familiar. Nevertheless, they have implications for the ethics of gene transfer trials. In recent years there has been considerable debate about whether such trials should, to maximise beneficence, enrol people who have exhausted all other treatment options, or whether, to secure autonomy, they should avoid the coercive influence of illness on informed consent and instead enrol relatively healthy people.13 The possibility of latent reactions erodes the ethical cogency of using healthy volunteers in trials because they are more likely to live long enough to experience latent adverse events. For similar reasons, latency also heightens the hazards of conducting gene transfer trials in children who have non-life threatening diseases. Moreover, children may be more sensitive to the long term hazards of gene transfer because their tissues are still developing; indeed, age may have been a factor in the leukaemia observed in the X linked severe combined immunodeficiency trial.14
Some risks from gene transfer, namely those to third parties, are uncommon in clinical research. Firstly, risks to the descendants of trial participants because of the inadvertent modification of germ cells are not identical to those for chemical mutagens. Whereas inadvertent modification can occur in existing genes, insertional mutagenesis adds new genetic elements that can have activity independent of where they are inserted. Moreover, gene transfer vectors contain a series of potentially functional genes (for example, promoters, markers, and transgenes) and therefore can cause a broader range of effects. Secondly, trials using viral vectors occasionally present risks to the public through transmission of transgenes or contagion. Concerns about contagion may increase as investigators pursue vectors (for example, lentivirus vectors and those that are replication competent) for which recombination or transmission are major safety issues.
Considerations for investigators and ethics committees
Risk review—expertise of local ethics committees is generally insufficient to the task of ethics and risk review
Risk disclosure—that unexpected adverse events have occurred in previous trials should be disclosed, regardless of vector
Informed consent—severely ill participants tend to misconstrue trials as “therapy” rather than scientific experiments; consent procedures should aim to correct such misconceptions
Study design—trials should maximise their social utility by gathering and disseminating information, including results of long term follow up and autopsy
Eligibility criteria—the possibility of latent adverse events should be considered when selecting eligibility criteria for trials involving stable genetic modification
Additional educational resources
Websites
UK Department of Health (www.advisorybodies.doh.gov.uk/genetics/gtac/publications)—recommendations and annual reports of UK's Gene Therapy Advisory Committee
National Institutes of Health (www4.od.nih.gov/oba/RAC/meeting)—transcripts of meetings, protocol reviews, guidance for writing consent documents, and safety symposia of the US Recombinant DNA Advisory Committee
National Health and Medical Research Council (www.nhmrc.gov.au/research/gtrap/about.htm)—recommendations, trial registry, and reports from Australia's Gene and Related Therapy Research Advisory Panel
Georgetown University (www.georgetown.edu/research/nrcbl/publications/scopenotes/sn24.htm)—history, overview, and literature survey on the ethics of human gene transfer
Information for patients
US National Library of Medicine Genetics Home Reference (ghr.nlm.nih.gov/info=gene_therapy/show/alltopics)—provides a general overview of gene therapy, addressing safety and ethical issues
National Institutes of Health Genetic Modification Clinical Research Information System (www.gemcris.od.nih.gov)—database on gene transfer protocols and safety information for investigators, ethics committees, and trial participants
Many countries have mechanisms for reviewing such concerns about safety. These are, however, generally not well suited for detailed deliberation on the ethical issues raised by risks to third parties. Such hazards complicate the risk assessment and consent process in part because major codes of research ethics, as presently written, centre on the protection of participants and do not directly consider the safety of third parties. Also, there is a general void in ethical scholarship (apart from the xenotransplantation literature15) about how to approach several important ethical questions concerning risk to third parties. For example, should trials that present theoretical risks from transmission seek the consent of third parties? How should benefits to trial participants be weighed against speculative risks to third parties?
Research on toxicology
Efforts to gather toxicological information raise important ethical issues. Animal models may only provide limited safety information for gene transfer, and toxicology data are therefore likely to derive largely from experiments in humans. Indeed, two important adverse events related to gene transfer (death8 and leukaemia16) were not predicted by animal studies using similar doses of vector.
One set of questions on toxicology related to gene transfer arises because most studies in humans—as with many other trials of hazardous agents—enrol participants with advanced illness. Such participants are likely to misinterpret the purpose of the trial as providing therapy rather than producing generalisable knowledge.17 Enrolment in studies on the safety of gene transfer is therefore susceptible to being based on “misinformed” consent. Also, participants who perceive a trial as providing therapy may be less willing to comply with intrusive procedures (for example, long term follow up and autopsy) that are aimed at testing safety. By policing consent procedures for language that promotes misconceptions about therapy, investigators may encourage participants to cooperate with a trial's toxicological aspects.18
Premarketing studies of drugs often have insufficient power to expose rare adverse events19; the collection of toxicity data is further hampered because gene transfer trials generally enrol participants with severe illness. For instance, attributing causes for adverse events is confounded by underlying medical conditions. Moreover, such populations are unlikely to survive and experience theoretically predicted latent adverse events. Therefore, many risks will only be characterised once gene transfer extends to populations with less severe medical conditions; patients and the public (rather than trial participants) will likely bear many of the risks involved in characterising latent toxicity.
Owing to the uncertainties and inexperience surrounding risks from gene transfer, systems may need to be established for postmarketing surveillance (for example, registries) and the long term follow up of trial participants. In the United States, such long term follow up is not mandatory, and anecdotal evidence indicates that it is not widely practised.18 In contrast, the United Kingdom20 and Australia (www7.health.gov.au/nhmrc/research/gtrap.htm) track the medical records of recipients of gene transfer. Follow up and postmarketing surveillance are potentially costly, can medicalise people's lives, and infringe on their privacy. Nevertheless, spontaneous reporting of adverse events is unreliable for detecting latent adverse events,19 and more active measures may be necessary to protect the public, and patients and their descendants, should gene transfer expand to milder medical conditions.
Although recent trials confirm the feasibility of gene therapy, they also highlight that its risks are poorly understood. The task for researchers in gene transfer will be to characterise these risks while attending to the complex ethical challenges of conducting gene transfer studies in humans.
JK is the sole contributor to this paper.
Funding: Canadian Institutes of Health Research.
Competing interests: None declared.
References
- 1.Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet 2000;24: 257-61. [DOI] [PubMed] [Google Scholar]
- 2.Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay JP, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med 2002;346: 1185-93. [DOI] [PubMed] [Google Scholar]
- 3.Aiuti A, Slavin S, Aker M, Ficara F, Deola S, Mortellaro A, et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science 2002;296: 2410-3. [DOI] [PubMed] [Google Scholar]
- 4.Marshall E. Gene therapy. Viral vectors still pack surprises. Science 2001;294: 1640. [DOI] [PubMed] [Google Scholar]
- 5.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 2003;302: 415-9. [DOI] [PubMed] [Google Scholar]
- 6.Williams D. Clarity and risk: the challenges of the new technologies. Med Device Technol 2001;12: 12-4. [PubMed] [Google Scholar]
- 7.Baum C, Dullmann J, Li Z, Fehse B, Meyer J, Williams DA, et al. Side effects of retroviral gene transfer into hematopoietic stem cells. Blood 2003;101: 2099-14. [DOI] [PubMed] [Google Scholar]
- 8.Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab 2003;80: 148-58. [DOI] [PubMed] [Google Scholar]
- 9.Dettweiler U, Simon P. Points to consider for ethics committees in human gene therapy trials. Bioethics 2001;15: 491-500. [DOI] [PubMed] [Google Scholar]
- 10.Emanuel EJ, Wendler D, Grady C. What makes clinical research ethical? JAMA 2000;283: 2701-11. [DOI] [PubMed] [Google Scholar]
- 11.Kimmelman J. Protection at the cutting edge: the case for central review of human gene transfer research. CMAJ 2003;169: 781-2. [PMC free article] [PubMed] [Google Scholar]
- 12.Orkin SH, Motulsky AG. Report of the National Institutes of Health ad hoc committee. Report and recommendations of the panel to assess the NIH investment in research on gene therapy. Bethesda, MD: National Institutes of Health, 7 Dec 1995. Available at www4.od.nih.gov/oba/rac/panelrep.htm
- 13.King NMP. Defining and describing benefit appropriately in clinical trials. J Law Med Ethics 2000;28: 332-43. [DOI] [PubMed] [Google Scholar]
- 14.Kohn DB, Sadelain M, Glorioso JC. The occurrence of leukemia following gene therapy. Nat Rev Cancer 2003;3: 477-88. [DOI] [PubMed] [Google Scholar]
- 15.Welin S. Starting clinical trials of xenotransplantation—reflections on the ethics of the early phase. J Med Ethics 2000;26: 231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohn DB, Sadelain M, Dunbar C, Bodine D, Kiem HP, Candotti F, et al. American Society of Gene Therapy (ASGT) ad hoc subcommittee on retroviral-mediated gene transfer to hematopoietic stem cells. Mol Ther 2003;8, 180-7. [DOI] [PubMed] [Google Scholar]
- 17.Lidz CW, Appelbaum PS. The therapeutic misconception: problems and solutions. Med Care 2002;40: V55-63. [DOI] [PubMed] [Google Scholar]
- 18.King NMP. Accident and desire: inadvertent germline effects in clinical research. Hastings Cent Rep 2003;33: 23-30. [PubMed] [Google Scholar]
- 19.Brewer T, Colditz GA. Postmarketing surveillance and adverse drug reactions. JAMA 1999;281: 824-9. [DOI] [PubMed] [Google Scholar]
- 20.Nevin NC, Spink J. Gene therapy advisory committee: long-term monitoring of patients participating in gene therapy: health departments of the United Kingdom. Hum Gene Ther 2000;11: 1253-5. [DOI] [PubMed] [Google Scholar]