Abstract
Noise-induced hearing loss (NIHL) affects a large number of military personnel and civilians. Regenerating inner-ear cochlear hair cells (HCs) is a promising strategy to restore hearing after NIHL. In this review, we first summarize recent transcriptome profile analysis of zebrafish lateral lines and chick utricles where spontaneous HC regeneration occurs after HC damage. We then discuss recent studies in other mammalian regenerative systems such as pancreas, heart and central nervous system. Both spontaneous and forced HC regeneration occurs in mammalian cochleae in vivo involving proliferation and direct lineage conversion. However, both processes are inefficient and incomplete, and decline with age. For direct lineage conversion in vivo in cochleae and in other systems, further improvement requires multiple factors, including transcription, epigenetic and trophic factors, with appropriate stoichiometry in appropriate architectural niche. Increasing evidence from other systems indicates that the molecular paths of direct lineage conversion may be different from those of normal developmental lineages. We therefore hypothesize that HC regeneration does not have to follow HC development and that epigenetic memory of supporting cells influences the HC regeneration, which may be a key to successful cochlear HC regeneration. Finally, we discuss recent efforts in viral gene therapy and drug discovery for HC regeneration. We hope that combination therapy targeting multiple factors and epigenetic signaling pathways will provide promising avenues for HC regeneration in humans with NIHL and other types of hearing loss.
Keywords: Hearing loss, hair cell regeneration, development, epigenetics, direct conversion, drug discovery
1. Noise-induced hearing loss and its military relevance
Hearing impairments, including hearing loss and tinnitus, are the most common military service-connected disabilities. There are many sources of potentially damaging noise in military settings, including weapons systems, engines, and explosions. The prevalence of hearing problems in the US military operation in Afghanistan and Iraqi ranged from 7.3% to 26.6% among more than 90,000 veterans who received the Veterans Health Administration health care(Frayne et al., 2011). Hearing loss significantly affects performance, and is one of the top reasons why soldiers cannot be redeployed (Casali, 2010; Hawkins et al., 1980). Given the importance of auditory acuity in perceiving commands and sensing enemy activity, it is clear that even mild hearing loss increases the risk to military personnel. Service-related injuries that result in hearing loss have decreased with the advent of hearing protection, but many military personnel still develop NIHL by the end of their tour. Hearing loss that restricts or prevents continued military service can result in the loss of personnel, including some of the most effective and experienced officers and non-commissioned officers. Furthermore, NIHL is irreversible, resulting in a permanent disability. As a result, 4.1 million US veterans currently receive disability compensation and treatment for service-connected hearing disability at a total cost of more than $2 billion annually (US Department of Veterans Affairs, 2017, Page 53). Among those veterans who suffer from NIHL, a resulting breakdown in communication with caregivers and family members can impede their reintegration into society and exacerbate their depression and anxiety. In view of these consequences, the US Department of the Navy has recently declared “The Naval Global War on Noise” (Naval Safety Center, 2007).
One common but extreme cause of NIHL and tinnitus is explosions. The blast injuries occur frequently in situations where blast exposure cannot be predicted, where the intensity of the blast exceeds the protective capability of the available mechanical protective devices, or where protective devices are simply not available (Arnold et al., 2004; Arnold et al., 2003; Patterson et al., 1997). In wars or terrorist attacks, such blasts are particularly damaging to both military personnel and civilians. In addition to damaging the central nervous system (CNS), blast injuries most commonly cause disruption or damage to the auditory sensory system. The damage to the peripheral auditory sensory system due to the extreme physical force of a blast is often diverse and may include rupture of the tympanic membrane (TM, ear drum), fracture of the middle ear bones, dislocation of sensory hair cells from the basilar membrane, and loss of spiral ganglia that innervate hair cells (Hamernik et al., 1987; Patterson et al., 1997; Roberto et al., 1989). In studies of humans with blast injury, approximately 17% to 35% were afflicted with severe TM rupture (Garth, 1994; Gondusky et al., 2005; Xydakis et al., 2007), and 33% to 78% exhibited moderate to severe sensorineural hearing loss [hair cell (HC) and ganglion loss] as a result of exposure to terrorist or military explosions (Cave et al., 2007; Fausti et al., 2009; Gondusky et al., 2005; Hoffer et al., 2010; Nageris et al., 2008).
Regenerative therapies may provide a means of hearing restoration to allow individuals with NIHL (and other types of hearing loss) to continue in military service, thereby reducing the attrition of the armed forces. This is particularly important when blast injury or post-traumatic stress disorder is associated with the hearing loss.
In this review, we will first discuss the lessons from recent regeneration studies in non-mammalian vertebrates (zebrafish and chicken) and other mammalian regenerative tissues (pancreas, heart and CNS), and then focus on the recent progresses on cochlear HC regeneration in vivo, specifically highlighting the differences between SC-to-HC direct lineage conversion and HC development, and essential roles of epigenetics in HC regeneration. Finally, we will summarize recent development in gene therapy and drug delivery for regenerative purposes.
2. Regeneration in non-mammalian vertebrates and other mammalian tissues
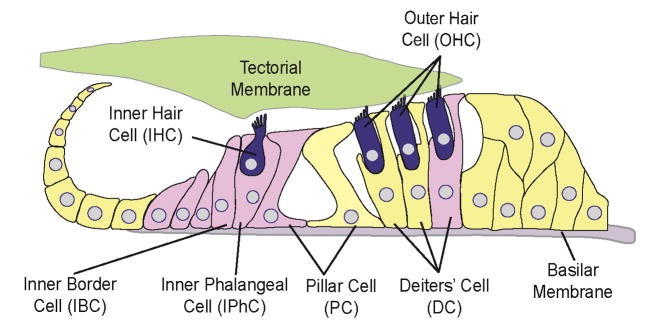
NIHL is primarily caused by damage to mechanosensory HCs in the cochlea (Hamernik et al., 1989; Johnsson et al., 1976; Liberman et al., 1984; Wang et al., 2002). The cochlea is a coiled structure in the inner ear that contains the auditory epithelium known as the organ of Corti. The organ of Corti contains HCs, which detect incoming sound, and supporting cells (SCs) (Fig. 1). HCs are subdivided into inner hair cells (IHCs), which are responsible for transmitting detected sound waves to auditory regions of the brain, and outer hair cells (OHCs), which are responsible for amplifying the sound (Dallos, 2008; Dallos et al., 2008). Only mammals have OHCs, which are unique because they primarily receive efferent signaling from the brain (Dallos, 2008) and express the motor protein prestin, allowing them to be electromotile and resulting in their ability to modulate sound (Dallos, 2008). NIHL results in the preferential loss of OHCs; IHCs are lost only in severe cases of NIHL (Hamernik et al., 1989).
Fig. 1.
Diagram (cross section) of the mature cochlear organ of Corti, illustrating the major sensory and non-sensory cell types. IHC: inner hair cell; OHC: outer hair cell; IPhC: inner phalangeal cell; IBC: inner border cell; PC: pillar cell; DC: Deiters’ cell. Lgr5+ cells at neonatal age are highlighted with pink color.
Unfortunately, humans and other mature mammals cannot replace lost HCs in the organ of Corti, so the loss of HCs can lead to permanent deafness. However, birds, fish, and other non-mammals can recover lost hearing either by proliferating SCs and then converting them into functional HCs or by directly transdifferentiating SCs to HCs (Adler et al., 1996; Baird et al., 1996; Corwin et al., 1988; Jones et al., 1996; Ryals et al., 1988; Warchol et al., 1996). Interestingly, mature mammalian vestibular end organs, such as utricle, exhibit limited HC regeneration after damage, which occurs almost exclusively by transdifferentiation (Golub et al., 2012; Jung et al., 2013; Slowik et al., 2013). Notably, the regeneration of vestibular HCs declines with age (especially in the early developmental stage) and is not sufficient to compensate HC loss (Burns et al., 2012; Gopen et al., 2003; Kirkegaard et al., 2005; Rauch et al., 2001). Nevertheless, these natural processes provide a potential roadmap for mammalian cochlear HC regeneration.
2.1. HC regeneration in non-mammalian vertebrates
Fish, birds, and other nonmammalian vertebrates have the remarkable ability to regenerate cochlear HCs after damage. Zebrafish regenerate lost HCs almost exclusively from mitotic SCs (Ma et al., 2008); in birds, cochlear HC death induces adjacent SCs to proliferate and transdifferentiate into new HCs (Baird et al., 1996; Girod et al., 1989; Raphael, 1992). A detailed assessment of HC regeneration in zebrafish and chickens has been performed using cell- or organ-specific transcriptome profile analysis (Jiang et al., 2014; Steiner et al., 2014), with the aim of explaining why mammalian cochleae lack such regenerative ability. Activation of conserved signaling pathways involved in the spontaneous regeneration could be a key to reprogram the quiescent SCs in mature mammalian cochleae.
2.1.1 The zebrafish lateral line
Two recent studies using genome-wide transcriptome analysis of presumptive HC progenitor cells in the zebrafish lateral line have demonstrated that multiple signaling pathways are necessary to activate the regenerative responses after HC injury in this species (Jiang et al., 2014; Steiner et al., 2014). Here, the zebrafish lateral line makes the high temporal resolution analysis possible because HCs can be killed within 30 min by copper sulfate or neomycin and regeneration occurs rapidly within 24 h. Analysis at multiple time points after HC injury indicated that the correct order of multiple signaling pathways is crucial. For example, Notch signaling and FGF signaling were downregulated immediately after HC loss (within 1 h), whereas cell cycle and Jak1/Stat3 pathways were activated at the same time. In contrast, Wnt/β-catenin was activated much later (at 12 h) (Jiang et al., 2014). It suggests that in the early stage of spontaneous regeneration in zebrafish, the immediate inhibition of Notch signaling downregulates Sox2 and activates Atoh1 to enable SCs for further proliferation and/or differentiation. However, because Notch needs to be reinstated later to specify the fate of SCs and control the organ size, it is reasonable that Notch target genes (i.e., sox2, her4, and notch3) were later upregulated at 3 to 5 h, consistent with the findings of several previous studies at later time points in zebrafish, guinea pigs, and chickens (Hori et al., 2007; Ma et al., 2008; Stone et al., 1999). At the same time, FGF and Jak1/Stat3 pathways may contribute to the early proliferation status as Wnt/β-catenin pathway-regulated proliferation likely occurs in the later stage. In a following study, the same group clarified spatially restricted Notch and Wnt pathways in the zebrafish lateral line -- in distinct tissue compartments, Notch signaling inhibits proliferation through Wnt-dependent and -independent pathways and suppresses differentiation via other, unidentified mechanisms (Romero-Carvajal et al., 2015). According to these results, the authors speculated that the lack of immediate and transient Notch downregulation is the key difference between mammalian cochleae and zebrafish lateral lines with regard to HC regeneration and that the temporal inhibition of Notch would be fruitful for mammalian HC regeneration (Jiang et al., 2014). Indeed, genetic (e.g., by knocking out one core component of Notch signaling pathway, RBP-J) or pharmaceutical (e.g., by using γ-secretase inhibitors) suppressing Notch signal in normal or damaged cochleae induced transdifferentiation from SCs (excluding pillar cells) to HCs (Doetzlhofer et al., 2009; Korrapati et al., 2013; Mizutari et al., 2013; Yamamoto et al., 2006). However, these studies only agree on the effect of Notch in neonatal mice, while HC conversion in adult cochleae still needs more evidence. In contrast to what Mizutari et al (Mizutari et al., 2013) reported (a γ-secretase inhibitor at extremely high doses produced new hair cells and partially restored hearing), Maass et al (Maass et al., 2015) thoroughly investigated the mRNA level of Notch ligands, receptors, and downstream effectors, and found dramatic downregulation of Notch signaling after postnatal day 6. The contradictory findings may result from non-specific effect of the drug used by Mizutari et al. Alternatively, the mouse has only one allele of endogenous Sox2 gene and the haploinsufficiency of Sox2 may genetically interact with Notch signaling (Li et al., 2012; Walters et al., 2015b). Moreover, the ablation of Notch signal is deleterious to differentiating Deiters’ cells (Campbell et al., 2016), and this toxic effect must be considered before any clinical application.
Besides these well-characterized pathways, several less characterized signaling pathways have been implicated in mantle-cell regenerative responses; for example, atg2bl, a putative mediator of autophagy, is upregulated (Steiner et al., 2014). However, further investigation is required to validate their roles in HC regeneration.
2.1.2. The chick utricle
Avian sensory epithelia provide enriched information sources for HC regeneration. The chick utricle culture is believed to resemble the utricle in vivo as regards to its regenerative responses and thus provide important insights into signaling pathways during HC regeneration. After an initial report in which transcriptome in chick utricle cultures were analyzed during the initial 48 h after laser or neomycin damage (Hawkins et al., 2007), Lovett and colleagues further investigated the gene expression profiles using RNA-seq in chick utricle cultures up to 168 h after a one-day aminoglycoside antibiotic treatment (Ku et al., 2014). They uncovered three sequential but overlapping phases of HC regeneration: DNA replication/cell cycle control; conversion; and regenerative proliferation. As expected, the Notch and FGF signaling pathways play critical but complex roles in these time windows. Specifically, Notch signaling was elevated after the damage possibly as a consequence of upregulation of Atoh1, followed by a transient inhibition at 48–72 hour time window when DNA replication signal and the SC-to-HC conversion are at the peak. Meanwhile, the FGF signaling is more complicated – different FGF family members showed variable expression patterns during the regeneration process. With additional experiments (adding exogenous FGF20 or a FGFR inhibitor), the authors concluded that the FGF signaling (especially FGF20) was a negative mediator for regenerative proliferation. Indeed, FGF signaling function depends on cellular context – although mostly demonstrated for their roles in promoting proliferation (e.g. (De Moerlooze et al., 2000)), the FGFR3 signal drives differentiation while suppresses proliferation in bone development (Colvin et al., 1996). Notably, in early developmental stage (i.e. E10.5 – E12.5) of the murine cochlea, FGF20 is required for sensory progenitor cell proliferation (Huh et al., 2015).
Interestingly, some novel regeneration regulator candidates were identified by examining the expression pattern of transcription factor (TF) genes. In the total of 212 differentially expressed TF genes, 8 TF genes including MAMLD1 and RBPJL (which are activated by the Notch signal and increase Hes genes while repress Atoh1 ) were upregulated during the 48- to 72-h window in which phenotypic conversion dominates, while 18 TF genes including BTG1 (a gene that functions as a coactivator of cell differentiation and a suppressor of proliferation) were downregulated at the same time window. Similar to the transcriptome studies in zebrafish, a number of unexpected pathways were also identified, including cytokines, interleukins, and interleukin receptors (Ku et al., 2014). Given the recent findings on the importance of fractalkine signaling and macrophages in the mammalian inner ear (Kaur et al., 2015) and the discovery of complement components associated with innate immune responses after noise trauma (Patel et al., 2013), resident immune cells within the mammalian inner ear may play roles in regenerative responses.
As mentioned earlier, many pathways identified in the zebrafish lateral line and the chick utricle have been validated in mammalian cochleae. For example, the inhibition of Notch signaling initiated HC regeneration in mammalian cochleae (Korrapati et al., 2013; Mizutari et al., 2013; Tona et al., 2014), while enforced Wnt/β-catenin signaling increased the proliferative responses (Chai et al., 2012; Kuo et al., 2015; Shi et al., 2013). However, signaling pathways identified by these transcriptome studies may be limited by the usage of a mixed cell population. The two studies in zebrafish showed varied results due to their using different sources for the purified SCs, i.e., mantle cells and inner SCs (Jiang et al., 2014) versus mantle cells alone (Steiner et al., 2014). These two cell types play different roles during lateral line regeneration (Romero-Carvajal et al., 2015). Similarly, the analysis using the entire chicken utricle culture also diluted the HC conversion signals in a small population of cells with the dominant proliferation signals (Ku et al., 2014). Moreover, it remains to be determined whether mantle cells and inner SCs in zebrafish and SCs in avian utricles are indeed the equivalent of SCs or progenitors in the mammalian inner ear. The transcriptome studies on zebrafish inner ear and chick basilar papilla, as well as single-cell RNA-seq could be necessary to address these questions. In addition, forcedly regenerated HCs in mammalian cochleae are far away from native HCs or spontaneously regenerated HCs in fish and chicken in term of morphology and function. These limitations may result from the loss of stemness for mature SCs or the epigenetic barriers. Moreover, studies using different genetic models raised controversy (e.g. different effects of the Notch inhibitor, proliferation vs. differentiation induced by the Wnt signal). We will discuss these in sections 3 and 4.
2.2. Regeneration in other mammalian systems
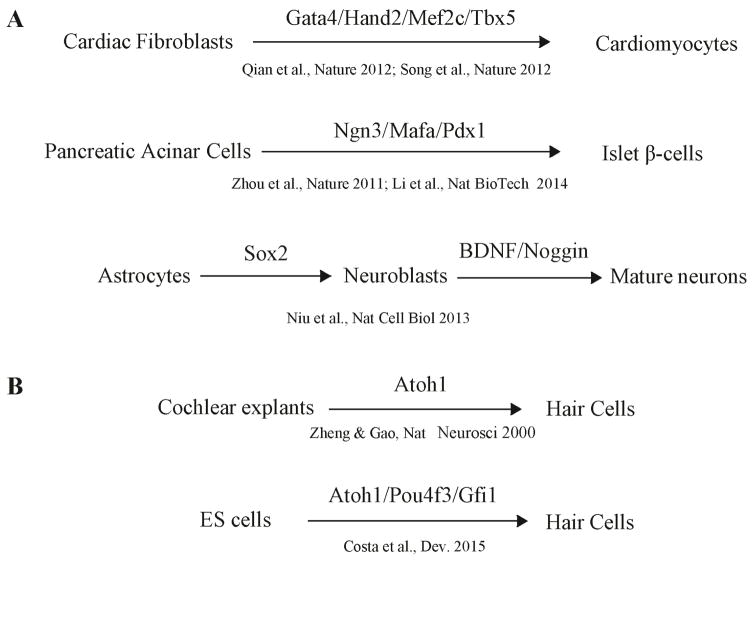
Cardiac, pancreatic, and CNS regeneration have been well studied (Fig. 2A). Here, we will outline similarities among these regenerative systems with the aim of deriving principles to guide our efforts in the field of cochlear HC regeneration.
Fig. 2.
Examples of direct lineage conversion in other mammalian regenerative systems (A) and in the cochlea (B). The original differentiated cells (at left) were converted to another differentiated cell type (at right). The lineage-specific transcription factors are listed for each conversion.
2.2.1. The heart
In spite of different origins from the cochlea (endoderm vs. ectoderm), the mammalian heart presents several similarities during regeneration, including inefficient cell reprograming, age-dependence, immature/nonfunctional cells, etc. After injury, the neonatal mouse cardiomyocytes have already exited the cell cycle normally and exhibit little proliferation, but they start to proliferate after injury (Porrello et al., 2011). In the adult heart, only very limited turnover of cardiomyocytes (1% per year) can be detected (Bergmann et al., 2009). A massive loss of cardiomyocytes due to an infarction cannot be spontaneously repaired; instead, a scar is formed to prevent further damage (Sutton et al., 2000).
Because of the limited regenerative capacity of the heart, several approaches were used to regenerate cardiomyocytes, including transplantation of cells differentiated from induced pluripotent stem cells (iPSCs) or adult stem cell therapy, and direct conversion of other cell types in the heart. The latter strategy may avoid complications of cell transplantation including failure to engraft appropriately, short survival period, and cardiac arrhythmias (Lambers et al., 2016). In fact, several groups have succeeded in converting cardiac fibroblasts to functional cardiomyocytes with an efficiency of 7% to 20% by using a combination of multiple cardiac-lineage TFs (Gata4, Hand2, Mef2c, and Tbx5 or GHMT) (Qian et al., 2012; Song et al., 2012)(Fig. 2A). Recently, progress has been made toward increasing the conversion efficiency three to five folds (Wang et al., 2015) by using polycistronic vectors, indicating the stoichiometry of the reprogramming TFs is essential for the conversion efficiency. Epigenetic factors also play important roles in cardiac regeneration. BAF60c, a chromatin remodeler, when combined with TFs (Gata4 and Tbx5), transdifferentiates mouse mesoderm cells into cardiac myocytes (Takeuchi et al., 2009); microRNAs (miRNA-1, -133, -208, and -499) convert fibroblasts to cardiomyocytes, and the conversion efficiency has been increased to 10-fold by a JAK inhibitor (Jayawardena et al., 2012).
Notably, although iPSC differentiation and direct conversion are promising, the resulting cardiomyocytes, either in vitro or in vivo, still possess immature features of embryonic cardiomyocytes, namely higher proliferation rates, smaller and more rounded morphology lacking t-tubules and binucleation, and abnormal potassium currents that are responsible for arrhythmias (Lambers et al., 2016).
2.2.2. The pancreas
Pancreatic β-cells secrete insulin and play a central role in controlling blood glucose levels and metabolism. Direct lineage conversion from non-β-cells in situ has produced results of considerable interest to researchers investigating cochlear HC regeneration: the regeneration efficiency can be determined by reprograming resources, combined TFs, age, and injury-induced signals. However, full functional restoration of a mechanosensory cell, whose structure is highly-ordered, may be different from that of an insulin secreting cell.
The efficiency of conversion in vitro and in vivo depends on the starting cell types and the TFs used. One TF, Pdx1, could convert 0.3% to 6% of transduced hepatocytes (which start with the same endodermal lineage) to insulin secreting cells (Ferber et al., 2000), but adding other TFs (NeuroD, Ngn3/betacellulin, Mafa, and Pax4) substantially enhanced the conversion efficiency (Berneman-Zeitouni et al., 2014; Yang et al., 2013). In contrast, pancreatic non-β-cells, like acinar cells, are closer to the β-cell lineage and more advantageous to be converted. A set of three TFs (Ngn3, Pdx1, and Mafa) has been effective for converting non-β-cells to β-cells and improving insulin secretion in the adult pancreas, a process that occurs rapidly within the first 10 days with an efficiency of 10% to 20% (Zhou et al., 2008) (Fig. 2A). Using a polycistronic vector to control the stoichiometry, the conversion efficiency has been increased to 50% and conversion occurs rapidly within the first 10 days and gradually progresses for more than 7 months at the global transcriptome and DNA methylation levels so that the converted cells begin to resemble endogenous β-cells (Li et al., 2014). Interestingly, even at 7 months, converted β-cell–like cells, though fully functional in normalizing glucose levels in diabetic mice, remain hybrid-like cells that are part-way between acinar and β-cells, expressing only 70% of β-cell–specific genes and still retaining a significant number (> 1,209) of acinar cell–specific genes. Another interesting feature of this in vivo conversion is that islet-like structures surrounding newly converted β-cells play a key role in their continued maturation; the ability to form such islet-like structures is lost when native β-cells are dissociated, thereby losing their function, and can be facilitated by inter-islet signaling pathways (i.e., Eph/ephrin). Additionally, treating adult mice with EGF and CNTF led to the appearance of β-cell–like cells in diabetic mice, suggesting that these factors could play a role in reprogramming and/or maturation during β-cell conversion, at least from acinar cells (Baeyens et al., 2014).
Another aspect of β-cell conversion from pancreatic alpha cells is the dependency of the process on age and the amount of damage incurred. A severe loss of β-cells could stimulate the conversion of alpha cells to β-cells -- the near complete loss of β-cells caused by genetic expression of diphtheria toxin fragment A (DTA) triggered proliferation and co-expression of glucagon and insulin in alpha cells. The mass and the hybrid feature of these islet cells maintained for more than 10 months (Thorel et al., 2010). However, β-cell loss caused by streptozocin expression invoked no such conversion (Cavelti-Weder et al., 2013), suggesting that the mode of β-cell death could determine whether the conversion of alpha cells to β-cells is triggered. Interestingly, DTA-induced extreme β-cell loss in juveniles before puberty invoked a different type of conversion: from delta cells to β-cells (Chera et al., 2014). It would be interesting to identify the signals that trigger the cell conversion in these different β-cell loss scenarios.
2.2.3. The CNS
The CNS is one of the most difficult systems to regenerate because the vast majority of cells in the adult brain are postmitotic and the cells are extremely diverse, with only a small number of each cell type being present. These two features are shared by the cochlea. Moreover, the neurons and the hair cells are both derived from ectoderm, so the regeneration may also share similar molecular mechanisms.
As in the pancreas and heart, iPSCs, embryonic stem cells (ESCs), and adult stem cells (i.e., mesenchymal stem cells) can differentiate into neurons in vitro; however, the drawbacks of stem cell–based therapy include its potential tumorigenicity, the difficulty of generating diverse neuronal cell types, and the lack of full functionality in the induced neurons (Huang et al., 2015; Petersen et al., 2016). The direct conversion of glia/astrocytes to neuron-like cells in situ offers an alternative and promising strategy. This strategy is largely based on the successful conversion of fibroblasts into neuron-like cells in vitro: the most prominent set of TFs for converting fibroblasts into neurons is designated BAM (Brn2, Ascl1, and Myt1l), and the conversion efficiency in vitro is approximately 20% from mouse cells (Vierbuchen et al., 2010). When applied to human cells, although BAM efficiently promoted ESC differentiation, they only convert very small population of human postnatal fibroblasts (HPFs) to immature neurons. Addition of another TF, NeuroD, however, doubled the conversion efficiency (to 2–4%) to neurons (Pang et al., 2011). Interestingly, although the conversion with different factors from different original cells has various efficiencies, additional factors, especially epigenetic factors or small molecules targeting certain pathways, in general achieve higher efficiency. For example, miR-9/9* and miR-124 suppress the BAF53a subunit of the SWI/SNF-like BAF chromatin remodeling complex and control multiple genes regulating neuronal differentiation (Yoo et al., 2011). Thus, addition of miR-9/9* and miR-124 to TF Ascl1, Myt1l and NeuroD1 increased the conversion efficiency from human fibroblasts to neurons to 80% (Yoo et al., 2011); while applying a GSK-3β inhibitor and a SMAD pathway inhibitor together with TFs Ascl1 and Ngn2 successfully converted about 85% HPFs to neurons (Ladewig et al., 2012). On the other hand, direct conversion of residual glia/astrocytes into neurons may be advantageous due to the native physiologic environment. In fact, several such conversions have been achieved experimentally: from astrocytes to striatum neurons by BAM expression (Torper et al., 2013), from neonatal callosal projection neurons to corticofugal projection neurons by Fezf2 expression (Rouaux et al., 2013), and from astrocytes to striatum neuroblasts by Sox2 expression and subsequently to mature neurons by BDNF and Noggin expression (Niu et al., 2013). The last example is particularly important, as it suggests that a single-lineage TF is insufficient for the full maturation of converted neuron-like cells and that multiple steps involving trophic factors are required.
In most cases, it remains to be demonstrated whether direct lineage conversion to neurons in vivo can be completed or whether the converted neurons are identical to endogenous neurons. Recent genome-wide transcriptome analyses of isolated converted neurons have demonstrated that, as in pancreatic β-cell conversion, converted neuron-like cells are not identical to endogenous neurons (Li et al., 2014; Ye et al., 2015). It is likely that the surrounding microenvironment/niche plays a key role in the maturation of converted cells.
3. Recent progress in HC regeneration in mammalian cochleae
Because of the lack of HC regeneration in mature mammalian cochleae, it is difficult to directly compare their morphologic changes and transcriptome profiles with those for HC generation in zebrafish or chickens. It has, therefore, been difficult to pinpoint the “missing” keys for mammalian cochlear HC regeneration. However, from studies in zebrafish, chicken, mammalian heart, pancreas, and CNS, we have learned 1) that most regenerated cells retain the certain transcriptomic and epigenetic profiles of the cell sources, while they can still be functional; 2) that resident progenitor or stem cells in situ are the likely sources of HC regeneration; and 3) that multiple factors are required for efficient conversion and maturation of converted cells, including TFs, epigenetic factors, trophic factors, the stoichiometry of the factors, and the architectural integrity of the organ of Corti.
In the last decades, significant progress has been made towards HC regeneration in mammalian cochleae. Here, we focus on recent developments (mainly within the past 2–3 years) in the field of HC regeneration in mammalian cochleae in vivo, with some mention of ex vivo or in vitro studies that support in vivo conclusions. It becomes notable that results of ex vivo and in vitro studies using cochlear explants, stem cells, or other cell lines are sometimes inconsistent with those of in vivo experiments. One line of striking evidence in this regard is that when Wnt/β-catenin is overexpressed in neonatal mouse cochlear SCs, both proliferation and conversion occur ex vivo, whereas only proliferation occurs in vivo using the same genetic model (Chai et al., 2012). Comparably, in CNS studies, ex vivo studies of cerebellar granule cell migration concluded that the weaver mutation was non–cell autonomous in nature, whereas it was subsequently proven in vivo to be a true cell–autonomous effect (Goldowitz et al., 1995).
3.1. Spontaneous regeneration in mammalian cochleae
In contrast to what had been commonly believed, neonatal cochleae exhibited spontaneous HC regeneration in vivo after being damaged (Cox et al., 2014b). In two independent mouse models, cochlear HCs were ablated by ectopic expression of a toxin at birth (Postnatal day 0 or P0); surprisingly, a wave of newly regenerated HCs formed between P3–P6. However, most regenerated HCs did not survive and a 2nd wave of death occurred after P7 (Cox et al., 2014b). This transient wave of HC regeneration was further demonstrated to be derived from SCs within the cochlear sensory epithelium by genetic fate mapping. Moreover, both transdifferentiation and mitotic regeneration were observed for the spontaneous HC regeneration, demonstrating the mechanistic conservation of HC regeneration in mammalian cochleae and utricles, zebrafish, and chickens. However, such spontaneous regenerative capacity declines after P7 in vivo. It is important to note that the results from ex vivo studies are consistent with those obtained in vivo (Bramhall et al., 2014). Many more questions remain, however: Why do newly regenerated HCs die eventually? Why do cochleae older than P7 lose their spontaneous regenerative capacity? What mechanisms govern such regeneration? Molecular analysis of this spontaneous regeneration is, therefore, warranted. Interestingly, deletion of Rb induces proliferation of neonatal HCs, while the proliferating cells undergo apoptosis rapidly (Sage et al., 2005; Weber et al., 2008). Thus, the Rb pathway may be involved in the self-regeneration and apoptosis process.
Neonatal mouse cochlear SCs can proliferate with appropriate genetic modifications. Specifically, SCs can proliferate when cell cycle inhibitors (i.e. p27kip1 and Rb) are removed (Liu et al., 2012b; Oesterle et al., 2011; Yu et al., 2010) or when canonical Wnt signaling is increased by stabilizing β-catenin (Chai et al., 2012; Shi et al., 2013). It should also be emphasized that mature cochleae exhibit no proliferative effects when these same genes are manipulated in a similar manner (Cai et al., 2014; Cai et al., 2015; Kelly et al., 2012; Liu et al., 2012a; Walters et al., 2013).
Apart from the SC proliferation for HC regeneration, some neonatal SCs can themselves be regenerated after damage (Mellado Lagarde et al., 2014). Inner phalangeal cells (IPhCs) and inner border cells (IBCs) (Fig. 1) can be regenerated, after toxin-induced autonomous cell loss, via the direct conversion of neighboring SCs in the GER regions medial to IPhCs/IBCs (Mellado Lagarde et al., 2014), whereas the loss of Deiters’ cells (DCs) or pillar cells (PCs) at similar neonatal ages cannot (Mellado Lagarde et al., 2013). Not surprisingly, such regenerative capacity is lost again after P7 in vivo. That such a remarkable regenerative capacity is exhibited only in neonatal mouse cochleae suggests the immature and stem cell–like nature of these SCs, a feature that is also supported by single-cell RNA-seq profile analysis (Burns et al., 2015; Waldhaus et al., 2015).
Notably, in neonates, certain cell populations of the organ of Corti are multipotent; they can proliferate and generate otic spheres, as well as differentiate and express HC markers, after being mechanically isolated and grown in culture in vitro. Stem cell markers, including p27Kip1, Sox2, Lgr5, and Axin2, are expressed consistently in the developing neonatal cochlea. By using genetically modified mouse models carrying fluorescent protein labeling, cells expressing p27Kip1, Sox2, Lgr5, and Axin2 were purified from neonatal mouse cochleae, which displayed significant potency for self-renewal (Chai et al., 2012; Jan et al., 2013; Kiernan et al., 2005; Shi et al., 2012; Shi et al., 2013; White et al., 2006). Moreover, the decrease in the level of these markers during aging (for example, Lgr5 is broadly expressed in GER cells, IPhs, IPCs, and the third DCs after birth but becomes restricted to the third DCs in adulthood; see Fig. 1) correlates with the reduced regeneration ability in SCs. However, more evidence is needed to prove cells expressing these genes are indeed cochlear progenitor cells. For example, only about 30% of Lgr5-positive cells purified from neonatal murine cochleae expressed HC markers under the differentiation condition, suggesting the heterogeneity of these cells (Chai et al., 2012). Recently, the application of single-cell PCR and RNA-seq has allowed the high-resolution analysis of different cochlear cell populations. For example, Waldhaus et al. compared apical inner pillar cells (IPCs) and outer pillar cells (OPCs) (Fig. 1) and revealed that decreased Notch signaling and increased Wnt signaling may account for the different potencies of IPCs and OPCs for regeneration (Waldhaus et al., 2015). However, the epigenetic signature for these potential progenitor cells is still lacking, simply because of the technical barrier posed by the small number of cells. ESCs or progenitor cells have quite different chromatin properties and DNA methylation status compared with differentiated cells (see Fig. 3 for the epigenetic landscape) (Layman et al., 2014). These epigenetic features can be keys to the lineage differentiation and regeneration in mature cochleae.
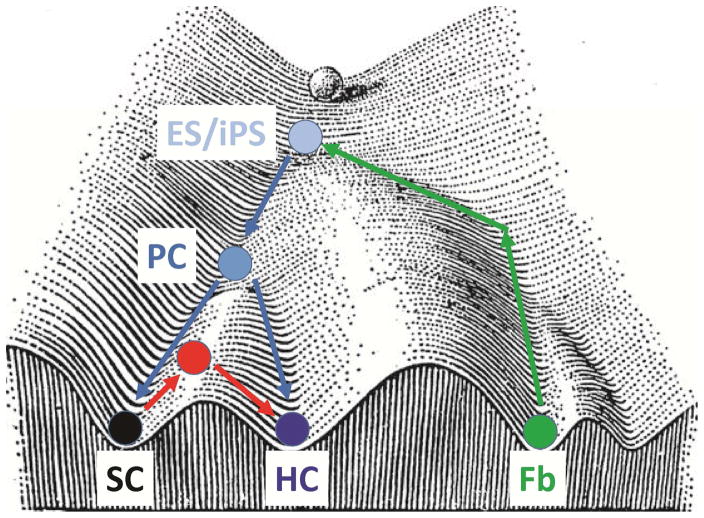
Fig. 3.
Modified “Waddington landscape” for cochlear HC development and regeneration. The original (shaded) cell at the top of the “mountain” represents a zygotic cell. Below, along the “valley,” is an ES/iPS cell representing a pluripotent embryonic stem cell or induced pluripotent stem cell that can give rise to an otic progenitor cell (PC), which in turn gives rise to either a supporting cell (SC) or a hair cell (HC) by following the normal developmental paths (blue arrows). A differentiated fibroblast (Fb) can be reversely converted to iPS by the four Yamanaka factors, following an “uphill” path (green arrows) as opposed to the normal “downhill” developmental path. Importantly, the direct conversion of an SC to an HC takes a path (red arrows) that differs from that of indirect conversion in order to avoid the PC state; there is, therefore, a unique, hybrid cell type (red cell) that is different from a PC or ES/iPS.
3.2. Multiple factors for HC regeneration
Embryonic, neonatal, and juvenile SCs can be converted to HCs by the forced expression of Atoh1 in vivo (Atkinson et al., 2014; Gubbels et al., 2008; Izumikawa et al., 2008; Izumikawa et al., 2005; Kawamoto et al., 2003; Kelly et al., 2012; Kraft et al., 2013; Kuo et al., 2015; Liu et al., 2014b; Liu et al., 2012a; Pan et al., 2013; Wu et al., 2013). Such conversion was first described in a pioneering report in which ectopic expression of Atoh1 successfully converted SCs in the GER region to HCs in rat cochlear explant culture (Fig. 2B) (Zheng et al., 2000). Together with a study in which fibroblasts were converted to skeletal muscle cells by MyoD in vitro (Davis et al., 1987), this study was one of the first on direct lineage conversion by lineage specific TFs. However, the effect of Atoh1 in HC regeneration has been controversial. In several reports, Atoh1 overexpression is capable to convert SCs to functional HCs in the cochlea of mice and guinea pigs by in utero gene transfer or viral delivery to adult inner ears (Gubbels et al., 2008; Izumikawa et al., 2005; Kawamoto et al., 2003; Kraft et al., 2013). In contrast, we and others showed that ectopic expression of Atoh1 in SCs in neonates and juveniles elicited only partial conversion, leading to immature HCs in vivo (Kelly et al., 2012; Liu et al., 2014b; Liu et al., 2012a). The immature features of these HCs included the lack of terminal differentiation markers (i.e., prestin, oncomodulin, or vGlut3), abnormal morphology (i.e., a larger cell body size), immature electrophysiologic responses, and immature hair-bundle morphology (Liu et al., 2014b; Liu et al., 2012a). Moreover, the efficiency of Atoh1-mediated conversion was low (6%–20%), and the mature cochlear SCs did not respond to ectopic expression of Atoh1 (Kelly et al., 2012; Liu et al., 2014b; Liu et al., 2012a). Recently, Atkinson et al. showed that transduction with adenoviruses containing Atoh1, even when combined with neurotrophin-3 (Nt3) or BDNF, resulted in an increase in immature HCs but no increase in synaptic ribbons after aminoglycoside treatment in adult guinea pigs (Atkinson et al., 2014). This contradiction may be partially attributed to the varied responsiveness to Atoh1 in SC subtypes.
A clinical trial of this Atoh1 gene therapy was approved by the FDA for HC regeneration (CGF166 by Novartis and GenVec). It was paused for further review of the trial’s Data Safety Monitoring Board (GenVec Press Release Jan. 20, 2016) and continued afterwards (GenVec Press Release May. 2, 2016). Referring to the regeneration in other systems, we believe additional factors are required to facilitate more efficient and fully functional HC conversion in vivo. One example is that co-activation of canonical Wnt signaling with Atoh1 overexpression increased the number of converted HCs in vivo by nearly 10 folds (Kuo et al., 2015). Because proliferation and conversion are both involved in zebrafish and chicken HC regeneration, it is reasonable to combine Atoh1 and β-catenin overexpression in SCs in vivo. Indeed, both proliferation and conversion of SCs to HCs were detected in neonates, and not surprisingly, new HCs were found in the apical cochlear turns in mature animals—at least 10-fold more than were observed with Atoh1 or β-catenin ectopic expression alone (Kuo et al., 2015). Interestingly, the authors only detected β-catenin-regulated proliferation but not differentiation, whereas a previous report demonstrated that high levels of β-catenin increased cell proliferation and low levels facilitated HC differentiation (Shi et al., 2013). These controversies may result from the different models used by the two groups. Shi et al. used the Sox2-CreER line to turn on stabilized β-catenin, while Kuo et al. used Lgr5-CreER and PLP-CreER lines. One possibility is that β-catenin activates negative feedback signals (e.g. Notch signaling) and suppresses HC conversion, while this suppression does not exist in the Sox2-CreER line due to Sox2 haplodeficiency (Kuo et al., 2015; Shi et al., 2013). Notably, these synergistic effects of Atoh1 and β-catenin did not improve HC maturation and hearing in vivo. Whether Wnt signaling is active in normal or injured mature cochleae remains debatable, but active studies in this area may lead to increased regeneration of HCs. Given that Notch inhibition occurs early in zebrafish HC regeneration (Jiang et al., 2014), Atoh1 is a target for both Notch and β-catenin (Lanford et al., 2000; Shi et al., 2010), and upregulation of Wnt signaling activates Notch pathway in cochleae (Kuo et al., 2015), it is reasonable to further investigate the roles of Notch, Wnt, and Atoh1 in mature HC regeneration, especially their synergistic effect.
What could be the underlying mechanism for that Atoh1 plus additional factors induce direct lineage conversion to functional HCs? According to the literature, most cell fate conversion requires more than one factor (Fig. 2) (One exception is a single TF, MyoD, induced conversion from fibroblasts to myoblasts (Davis et al., 1987)). Genome-wide analysis of TF occupation showed that TFs physically interact with only a low percentage of their consensus binding sites, suggesting that the chromatin structure is a stronger effector than the DNA sequence (Carr et al., 1999; Iyer et al., 2001; Kaplan et al., 2011; Yang et al., 2006). One or a few TFs (namely pioneer factors) usually bind the genomic DNA first, modify the chromatin structure, and facilitate the interaction of other factors(Davis et al., 1987; Liang et al., 2008; Soufi et al., 2012; Vierbuchen et al., 2010). As a result, these factors together generate the lineage-specific transcriptome. For example, FoxA, a TF, is critical for hepatocyte differentiation and regeneration. FoxA resembles the linker histone structure, and the binding of FoxA increases the accessibility of the DNA to the other TFs (Sekiya et al., 2009; Zaret et al., 2011). In HC development and regeneration, Atoh1 displayed some pioneer factor features like spontaneous binding (Abdolazimi et al., 2016). However, it is not clear whether the binding of Atoh1 to DNA facilitates the DNA-protein interaction of other TFs. Interestingly, recent studies identified GATA3 and TFE2 as co-factors of Atoh1 to promote HC conversion from GER cells in explant (Masuda et al., 2012), and earlier studies showed GATA3 and TFE2 to be involved in increasing DNAase I hypersensitivity and mediating nucleosome positioning (i.e. features of pioneer factors) (Eeckhoute et al., 2007; Ishii et al., 2004). It would be interesting to clarify whether GATA3 and TFE2 are necessary for Atoh1 to bind more target sites or Atoh1 forms a pioneer-factor complex with GATA3 and/or TFE2 and, consequently, initiates HC regeneration.
Could other factors contribute to cell proliferation and conversion to HCs in vivo? One interesting TF is Pou4f3, which has been recognized as a survival promoter rather than a differentiation factor (Erkman et al., 1996; Xiang et al., 1997). However, overexpression of Eya1 and Six1 can convert SCs to HC-like cells in embryonic cochlear explants (Ahmed et al., 2012), in which a significant portion (> 50%) of newly converted Myo7a-expressing HCs did not express Atoh1 but did express Pou4f3. This observation suggests that Pou4f3 may play an equally important role as Atoh1 in HC regeneration. In support of this hypothesis, the overexpression of a combination of three TFs (Atoh1, Pou4f3, and Gfi1) can convert mouse embryoid body (mEB) cells to HC-like cells in vitro (Costa et al., 2015) (Fig. 2B). In addition to Pou4f3, Lin28b is an Atoh1 target that, when overexpressed, can itself induce HC formation in explant culture (Golden et al., 2015). The overexpression of Sox4/11 can similarly convert SCs to HC-like cells in utricle explants, whereas their activity in the cochlea is unknown (Gnedeva et al., 2015). The lack of ephrin-B2 signaling led to transdifferentiation from SCs to HCs in vivo, which was possibly due to SC translocation to HC layer and conversion to HC fate (Defourny et al., 2015).
Overall, the HC regeneration research has made significant accomplishments in identifying new factors and improving regeneration efficiency in vivo. Especially, the genome-wide and single-cell resolution studies provide invaluable information to investigate the cochlea development and regeneration (Burns et al., 2015; Jiang et al., 2014; Ku et al., 2014; Liu et al., 2014a; Scheffer et al., 2015; Steiner et al., 2014; Waldhaus et al., 2015). It is the time now to revisit several conceptually important questions related HC regeneration in more detailed.
4. Differences between HC regeneration and HC development
It has long been debated whether HC regeneration needs to follow a molecular path similar to that in normal HC development. Significant effort has been made in studying HC developmental programs in the hope of providing guidelines for cochlear HC regeneration, but the difference between regeneration and development is becoming more acceptable (Atkinson et al., 2015; Srivastava et al., 2016). In the best example, mouse ESCs or otic progenitor cells were redirected, in multiple steps, toward the HC fate in vitro by mimicking the natural HC developmental environment in vivo (Koehler et al., 2013; Oshima et al., 2010). Although a recent study showed organoid HCs achieved from a 3D stem cell culture system recapitulated electrophysiological properties of non-mammalian or mammalian vestibular hair cells (Liu et al., 2016), most of the resulting HC-like cells exhibited diverse electrophysiologic and morphologic properties of HCs, and never phenocopied cochlear HCs (Koehler et al., 2013; Liu et al., 2016; Oshima et al., 2010). However, a combination of four TFs (Oct3/4, Sox2, Klf4, and c-Myc, also known as Yamanaka factors) successfully reversed the development and generated ESC-like cells from terminally differentiated fibroblasts (Takahashi et al., 2006). This breakthrough discovery makes us to reconsider the regeneration strategy compared with normal development.
4.1. The Waddington landscape model for development and regeneration
Historically, the cell fate lineage specification between different cell states has been modeled as the “Waddington landscape” (Waddington, 1957), where “peaks” with higher energy represent more pluripotent cell states, whereas “valleys” represent paths of normal developmental cell differentiation (Fig. 3). The Yamanaka factors converted cells (i.e. fibroblasts) at their low-valley points to more progenitor-like, high-peak states (iPS in Fig. 3) through a reverse path in the mountainous developmental landscape (Takahashi et al., 2015). Similarly, cochlear SCs are terminally differentiated cells at low-valley points, whereas otic progenitor cells (PC in Fig. 3) are supposed to be downstream from, but closer to, ESCs (ES in Fig. 3) at higher peaks. Therefore, SCs in the low-energy starting state may not have to dedifferentiate to the higher-energy state of progenitor/stem cells and thus save energy by taking different paths to reach the common final destination of mature, differentiated HCs (Takahashi et al., 2015) (Fig. 3). Thus, a new hybrid cell state between the SC and HC states may exist in the direct conversion path (indicated by the red circle and path in Fig. 3). These two paths (direct conversion and normal development) may or may not converge before reaching the final destination of mature HCs.
In fact, there are opposing views on whether conversion bypasses progenitor cell state. During the retina regeneration in zebrafish, Müller glia cells first dedifferentiate to a progenitor stage before these injury-induced progenitor cells proliferate and differentiate to rod receptors following the developmental path (Fausett et al., 2006; Powell et al., 2013); however, this assertion is only based on expression of several progenitor cell markers in converting Müller cells. In contrast, the mature glia cells can be induced to neurons without dedifferentiating to progenitor cells (Vierbuchen et al., 2010) and direct conversion occurs without returning to progenitor cell states in pancreas, heart and other regenerative systems as described above.
In the auditory system, a recent study in the zebrafish lateral line showed that fish with a retroviral integration-disrupted gene, Pho, exhibited normal HC development but defective HC regeneration (Behra et al., 2009). Specifically, the retroviral insertion into the first exon of Pho results in a null mutation zebrafish line. The fish developed normal lateral line, but after damage induced by copper or neomycin, the pho mutant showed significantly lower proliferation rate of support cells and severely impaired HC regeneration. The distinct roles of Pho in HC development and regeneration are one of the first lines of genetic evidence to highlight different molecular pathways between HC development and HC regeneration. The same investigators recently screened more than 200 gene-targeted mutant zebrafish lines and discovered nine that also exhibited specific defects in HC regeneration but showed no obvious developmental defects and morphological defects (Pei and Burgess, unpublished; (Burgess et al., 2016)). In addition, in zebrafish, retinoic acid pathway regulates FGF activity during HC development but they have no crosstalk in regeneration (Rubbini et al., 2015). These results strongly favor different paths of HC regeneration and HC development.
4.2. Breaking the epigenetic barriers
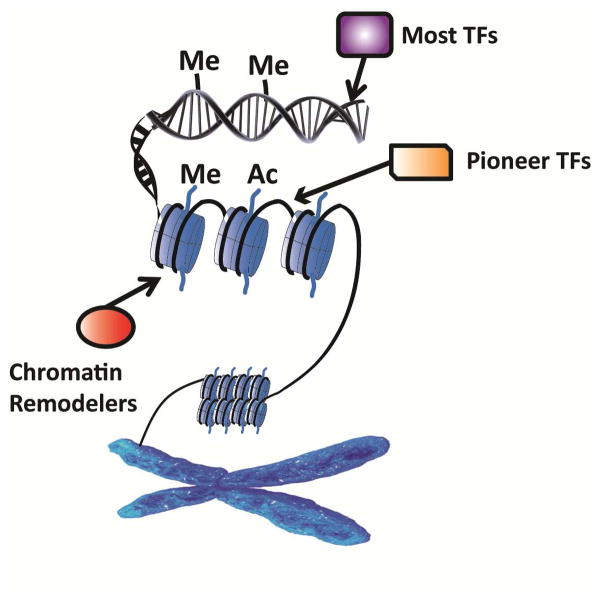
At molecular levels, terminally differentiated cells such as SCs have different genome-wide transcriptome and methylation profiles from those of progenitor/stem cells and differentiated final cell state such as mature HCs. Theoretically, either directly converting SCs to HCs or dedifferentiating SCs to progenitors is energetically unfavorable. One of energy barriers is epigenetic that separates the lineage fates, including histone modification (methylation, acetylation, and phosphorylation), DNA methylation and hydroxymethylation, and chromatin remodeling (Fig. 4).
Fig. 4.
Possible epigenetic regulatory mechanisms during HC regeneration. The compact chromatin structure becomes accessible to transcriptional mechanisms by histone modification, including methylation (Me) and acetylation (Ac), and chromatin remodeler binding. Pioneer TFs are able to interact with the condensed DNA and facilitate the targeting of other TFs. DNA methylation also controls the initiation of transcription.
For example, histone acetylation has been broadly demonstrated in cell conversion and regeneration. Histone deacetylase (HDAC) inhibition dramatically facilitated iPSC induction by Yamanaka factors (Huangfu et al., 2008). In regenerative liver, an altered pattern of histone acetylation was found decades ago (Pogo et al., 1968). By using pharmacologic and genetic methods, investigators determined that zinc-dependent HDACs are required for liver regeneration (Huang et al., 2013) and that overexpressing SIRT1 (a Class III HDAC) also suppressed hepatocyte proliferation by impairing farnesoid X receptor activity (Garcia-Rodriguez et al., 2014). Similarly, it has been thoroughly reviewed that epigenetic factors regulate the development and regeneration of pancreas (Avrahami et al., 2012; Migliorini et al., 2014), heart (Quaife-Ryan et al., 2016), and CNS (Arlotta et al., 2014; Petersen et al., 2016).
Our increasing knowledge of epigenetic regulation provides new insights into HC differentiation and regeneration. Class I histone deacetylases are expressed in otic vesicles in mouse embryos and in the organ of Corti in neonatal animals (Layman et al., 2013; Murko et al., 2010). Interestingly, histone methyltransferase EZH2, histone demethylase KDM1A, and NuRD complex core CHD4 all show a similar developmental expression pattern in the murine organ of Corti. The coordinated changes in epigenetic regulators and critical genes (including Atoh1 and mTRET) during HC development indicate that HC differentiation and maturation may be under epigenetic control (Layman et al., 2013). Indeed, HDAC1 knockdown disturbed inner ear development in zebrafish, and the pharmacologic inhibition of LSD1 resulted in fewer sensory HCs in zebrafish neuromasts during lateral line development (He et al., 2016; He et al., 2013). In mammals, a recent study on the epigenetic status of the Atoh1 locus revealed that histone modifications at that locus contribute to the changes in the expression level of Atoh1 mRNA during development (Stojanova et al., 2015). During the progression from E14.5 otic precursors to E17.5 HCs, the bivalent chromatin structure markers H3K4me3 and H3K27me3 were downregulated at the Atoh1 enhancer region, indicating a poised status for Atoh1 expression. During the same period, the acetylation of the active enhancer marker H3K27 increased and elevated Atoh1 expression, which correlated with HC differentiation. Afterwards, H3K9me3 suppressed Atoh1 expression until the HCs were mature at P6. Although in vivo evidence is still lacking as to whether histone modifications are sufficient and necessary for Atoh1-mediated HC development, this report is the first to show that the epigenetic regulation of a specific gene could play a role in HC differentiation.
Are epigenetic factors also involved in HC regeneration? A series of studies by He et al. suggest that LSD1 and HDACs are required for HC regeneration in zebrafish. Small-molecule inhibitors of LSD1 or HDAC reduced HC regeneration by suppressing cell proliferation (He et al., 2014; He et al., 2016). However, because SCs in adult mammalian cochleae lose their proliferation ability, HDAC and LSD1 may then play different roles than during development. Although HDAC inhibitors facilitate the conversion of MEFs to iPSCs, the application of HDAC inhibitors consistently, and surprisingly, had no effect on Atoh1-mediated conversion in adult mouse cochleae in vivo (Layman et al., 2015). Thus, although HDAC inhibitors are yet to be tested in younger mouse cochleae, it is possible that less dynamic epigenetic regulatory mechanisms (e.g., DNA methylation) dominantly regulate lineage-specific genes for HC fate.
Moreover, post-transcriptional regulation, especially microRNA-induced gene silence, has been implicated in ear development and HC regeneration. MicroRNAs are broadly expressed in mouse cochleae (Weston et al., 2006), and conditional knockout mice for dicer (which processes pre-miRNA to functional miRNA) showed impaired HC development and premature cell death (Friedman et al., 2009). Further studies identified the specific miRNAs and their downstream targets. In these studies, the miRNA 183 family, miRNA 124, miRNA 210, and let7 were shown to play essential roles in HC formation during inner ear development (Golden et al., 2015; Huyghe et al., 2015; Li et al., 2010; Weston et al., 2011). The miRNA 183 family also facilitates Atoh1-promoted HC regeneration in vitro (Ebeid et al., 2016).
Notably, although the majority of TF-induced conversion cannot totally erase the epigenetic memory (Hiler et al., 2015; Polo et al., 2010), a recent study indicates TF-induced conversion may be capable to modify the epigenetic landscape. The investigators profiled the DNA methylation of converted pancreatic β-cells from acinar cells in vivo over a period of up 12 months (Li et al., 2014). The results clearly demonstrated that the different DNA methylation regions compared to those of endogenous β-cells rapidly decreased within the first 10 days. Indeed, besides the direct manipulation on the global epigenetic regulators (e.g. HDACs), pioneer factors, as reviewed above, can modify chromosome structure and help overcome the epigenetic barriers. Identification of proper pioneer factors could be essential to improve the conversion for HC regeneration.
5. Clinical applications
With much progress having been made toward understanding the molecular genetics of HC regeneration in various species, the next question is how to use this knowledge to benefit people who have lost their cochlear HCs and are already hearing impaired, such as those with NIHL. To address this important translational question, we will review the current status of therapeutics for inner ear diseases.
5.1. Viral delivery of factors for HC regeneration
Many recent studies have successfully used viral vectors to deliver genes in vivo in order to correct genetic defects within the cochlea, thus making viral gene therapy a promising avenue for HC regeneration.
One of the first such studies used AAV1 to deliver vGlut3 (a gene necessary for IHC synaptic ribbon structure and function) into the cochlea of vGlut3-knockout deaf mice, thereby successfully restoring hearing and reversing the morphologic changes in the afferent nerve innervation to the IHCs (Akil et al., 2012). The AAV1-vGlut3 expression is specific to neonatal IHCs in the knockout mice, despite the broad viral uptake within the cochlea, and the hearing tests used included ABR, DPOAE, and startle behavioral response tests. Similarly, Chien et al. used AAV8-whirlin (a gene involved in hair-bundle structure and function) to infect neonatal whirlin-deficient deaf mice, resulting in stereocillia development in infected HCs and the enhanced survival of IHCs in vivo; however, the hearing of infected whirlin-deficient mice was not improved (Chien et al., 2016). In another study using AAV2/1-MsrB3, Kim et al. successfully infected embryonic otocysts of MsrB3-deficient mice at E12.5 and thereby restored hearing and hair-bundle morphology in adults (Kim et al., 2016). Similar approaches have been successful in vivo for Gjb2 (Iizuka et al., 2015; Yu et al., 2014), Cx30 (Gjbx) (Miwa et al., 2013), and (Askew et al., 2015).
Given such successes having been achieved with gene therapy in animal models, one would expect viral delivery of HC regeneration genes into the organ of Corti in vivo to have a bright future. However, despite the robust effect reported in the original report (Izumikawa et al., 2005), researchers (even including the same group) either failed to convert SCs in mature cochleae or only observed immature and nonfunctional HCs after Atoh1 overexpression using either viral infection or transgenic lines (Izumikawa et al., 2008; Kawamoto et al., 2003; Kelly et al., 2012; Kraft et al., 2013; Liu et al., 2012a; Yang et al., 2012a). Since Atoh1 is able to repair or regenerate the damaged or lost stereocilia of the HC, the improved-hearing and observed “regenerated” HCs may came from this repair effect (Yang et al., 2012b). Another explanation is that Atoh1 has different impact on different types of SCs. For example, adult IPh and IB cells are more responsive to Atoh1 induction than mature DCs and PCs (Walters et al, unpublished). Another complication is about the role of Atoh1 in HC survival: Atoh1 is required for differentiated HC survival (Cai et al., 2013; Pan et al., 2012), but continuously high level of Atoh1 in converted HCs may result in cell death (Kelly et al., 2012; Liu et al., 2012a).
Clearly, many questions remain regarding effective and safe gene therapy for HC regeneration. For example, how effective and specific are various viruses, including adenovirus, lentivirus, and many subtypes of AAVs, in infecting the mature organ of Corti? Many studies showed inconsistent transduced cell groups and varied transduction efficiency (Akil et al., 2012; Duan et al., 2010; Han et al., 1999; Kho et al., 2000; Kilpatrick et al., 2011; Konishi et al., 2008; Liu et al., 2005; Luebke et al., 2001; Mondain et al., 1998; Stone et al., 2005), resulting from different ex vivo or in vivo conditions, different animal models, different expressing vectors, different viral diffusion rates, etc. Moreover, will the virus affect other cells and cause permanent genetic changes? Although AAV has minimal toxicity and rarely causes inflammation, its vector has smaller gene packaging capacities. Furthermore, since AAVs do not pass the round window membrane (RWM), a surgery is required to instill the virus into the cochlea, increasing the risk of hearing loss associated with surgical trauma and inflammation. The gelatin sponge or RWM digestion may be a potential good solution for this (Jero et al., 2001; Wang et al., 2012). Nevertheless, viral gene therapy remains promising as one of the main methods for HC regeneration. Recent advances in CASPR/Cas9 and NgAgo offer alternative promising avenues to correct genetic deafness mutations (Gao et al., 2016; Zuris et al., 2015). Notably, as an alternative delivery method, researchers are developing non-viral vector transfer approaches. Recent studies use nanoparticles to deliver plasmids into the cochlea and greatly improve the efficiency compared to the old lipid-based method (Zhang et al., 2011; Zuris et al., 2015).
5.2. Drug discovery for HC regeneration
Recently, the pharmacologic conversion of fibroblasts into neural stem cells has become feasible (Zhang et al., 2016). A cocktail of nine drugs targeting several known signaling pathways has been demonstrated to induce the conversion in vitro and in vivo, thus giving a reason to expect that a cocktail of different drugs could help regenerate HCs in vitro and in vivo.
Commonly used screening systems include cell lines, stem cells, primary cells, explants, and zebrafish -- each has its advantages and disadvantages. Cell lines (e.g., HeLa and HEI-OC1) are easy to culture and expand, but they are far away from real cochlear cells. Screening for p27kip1 inhibitors, for example, was undertaken via two different approaches. The first approach was performed at the transcriptional level by directly screening for p27kip1 transcriptional inhibitors in Hela cells (Walters et al., 2014). By using a p27kip1 promoter–driven luciferase reporter assay, Walters et al. screened a library of 4,350 bioactive compounds, including 835 FDA-approved compounds, and discovered four inhibitors (Alsterpaullone, 2-Cyanoethyl or A2CE, Cerivastatin, Resveratrol, and Triacetylresveratrol) that can inhibit p27kip1 transcription in various cell lines and cochlear explants. One compound, A2CE, was shown to inhibit the p27kip1 upstream regulator Sirt2. It remains to be determined whether these compounds function in vivo when delivered to animal models. The second approach was to screen p27kip1 protein inhibitors (Iconaru et al., 2015). Traditionally, this approach had been considered impossible because p27 normally behaves as an intrinsically disordered protein (IDP) that takes various forms under different conditions. Iconaru et al. utilized a novel method (a NMR-based fragment screening) to identify fragment binding hits (which are much smaller than small-molecule compounds but bind more easily to disordered proteins with lower affinity). They discovered two groups of fragments from which they hope to develop better compounds with higher affinity for p27 protein in cells.
Although the primary otic progenitor is a good model to study HC differentiation and regeneration, it has a limited number of cells and passages, and is not ideal for large-scale screening (Oshima et al., 2010). Thus, methods have recently been developed to overcome this. Conditionally reprogrammed otic stem cells (CR-OSCs), “pseudo-immortalized” cochlear progenitor cells, have been developed using the “conditional reprogramming” technique (Walters et al., 2015c). These cells can bypass the senescence that is inherent to cochlear progenitor cells without genetic alteration, and more than 15 million such cells can be generated from a single cochlea. The CR-OSCs can be further differentiated in vitro with feeder cells, and they upregulate both early and terminal differentiation genes associated with HCs, including prestin. CR-OSCs also respond to known HC cues, including the upregulation of HC genes in response to Atoh1 overexpression and the upregulation of prestin expression after thyroid hormone administration. A hair cell line capable of regulated expression of HC genes can easily be recreated in any laboratory from any mouse strain of interest. These CR-OSCs, which are now available upon request (licensed and distributed via Applied Biological Materials, Inc.), make it feasible to test candidate compounds or genes for HC regeneration ability. It is also possible that future refinements of CR-OSCs will allow large-scale drug screenings for HC regeneration.
Similarly, immortalized multipotent otic progenitor (iMOP) cells, a fate-restricted cell type, were created by the transient expression of c-Myc in Sox2-expressing otic progenitor cells from mouse embryonic cochleae (Kwan et al., 2015). Subsequently, the endogenous c-Myc was activated, and the existing Sox2-dependent transcripts were amplified to promote self-renewal and even retain the ability of the cells to differentiate into functional HCs and neurons. This progenitor-derived immortalized cell line are also suitable to screen and test drugs and factors for HC regeneration.
Inducing the expression of Atoh1, Pou4f3, and Gfi1 together in mouse embryoid body cells generated cells with certain HC markers, and some of the induced cells exhibit stereociliary bundles (Costa et al., 2015). This system also provides a potential platform for regeneration drug screening, but further studies are needed to investigate the molecular landscape of HC induction from stem cells by TFs, which may be quite different from that of HC conversion from SCs as reviewed above.
By using zebrafish lateral line models, Namdaran et al. identified compounds that modulate HC regeneration (Namdaran et al., 2012). From a total of 1,680 compounds screened, two enhancers and six inhibitors have been identified for HC regeneration in this model. It remains to be determined whether these promising compounds also work as expected in mammalian cochleae, considering fundamental differences between zebrafish and mammals.
To validate the effect of drugs from high-throughput screening or genetic studies of p27Kip1, Atoh1, and Notch in HC regeneration, several groups have characterized small-molecule compounds (inhibitors of p27 or Notch and activators of Atoh1 or Wnt) using mammalian deaf models including mice and guinea pigs. However, it is notable that different methods that induce hearing loss also have different impacts on SCs. For example, diphtheria toxin (DT) induces HC death (through the artificially expressed DT receptors in HCs) and triggers robust Wnt signal, which facilitates spontaneous HC regeneration in the neonatal cochlea, while neomycin treatment (which is toxic to many cells in the cochlea) also leads to HC ablation, but only turns on weak Wnt signal, which fails to regenerate HCs (Hu et al., 2016). Moreover, although noise, aminoglycoside, and cisplatin trigger similar apoptotic pathways in HCs (Cheng et al., 2003; Devarajan et al., 2002; Nicotera et al., 2003; Wang et al., 2004), it remains largely unknown whether the SCs endure similar signals upon damage and whether this affects their regeneration ability.
The best-known in vivo study found that using the Notch inhibitor LY411575 (a γ-secretase inhibitor at a dose of 4 mM) in noise-damaged mouse cochleae resulted in the activation of Atoh1 and the induction of new HC formation, leading to an 8-dB improvement in hearing (Mizutari et al., 2013). However, as we discussed previously, this effect is still under debate. Using the Notch inhibitor DAPT (an analogue of LY411575) or a blocking antibody to the Notch1 receptor, Maass et al only detected HC regeneration in neonatal mice but not in adult animals. Considering the slightly different drugs used in the two studies, as well as the other distinct experimental conditions (i.e. the Sox2-CreER mouse vs. the wild type mouse), additional Notch inhibitors must be tested by lineage tracing of SC conversion to HCs followed by noise damage in vivo (Tona et al., 2014).
Since Notch inhibition results in different outcomes (e.g. regeneration or no effect, purely differentiation or proliferation) (Daudet et al., 2009; Maass et al., 2015; Mizutari et al., 2013; Wu et al., 2016). Efforts have also been made to screen for activators of Atoh1 (Edge et al, 2012, US Patent #: US8188131B2) that could complement Atoh1 viral gene therapy and consolidate Notch inhibitor effects because activation of Atoh1 is considered as the potential mechanism of Notch inhibition (Doetzlhofer et al., 2009).
It is now conceivable that the development of appropriate drugs will enable effective HC regeneration in vivo. However, many hurdles remain. For example, how can these reagents be delivered effectively to the mature organ of Corti in vivo? Drugs are normally difficult to pass through the blood-labyrinth barrier (BLB). In general, many drug candidates are poorly characterized in terms of their ability to cross the BLB and their pharmacokinetics and pharmacodynamics (PK/PD) in the inner ear fluids. For example, doxycycline cannot effectively penetrate into the cochlea of adults (Cox et al., 2014a; Walters et al., 2015a), despite several reports to the contrary (Kelly et al., 2012; Tarang et al., 2015). Moreover, Dox is not easily cleared by the inner ear fluids after it does get in (Cox et al., 2014a; Walters et al., 2015a). The most effective method for drug delivery is local delivery (by intra- or trans-tympanic or intracochlear injection), which is the most commonly used and effective method of drug delivery in patients with middle or inner ear diseases (El Kechai et al., 2015). Such a method resembles the intravitreal injections into the eyes that are used to treat certain ophthalmic diseases. For effective drug delivery to stimulate HC regeneration in vivo, it is highly desirable that these obstacles be further studied and overcome.
Highlights.
Spontaneous and forced hair cell regeneration occurs in mammalian cochleae in vivo;
Multiple factors can enhance hair cell regeneration;
Paths of regeneration may be different from those of development;
Epigenetic memory is a key to hair cell regeneration;
Drug discovery holds promise in clinical therapy for hearing loss.
Acknowledgments
We thank Drs. Brandon Cox, Zhiyong Liu, and Wanda Layman for comments and Keith Laycock for editing. The authors regret that space limitations prevent their acknowledging the many valuable contributions of colleagues.
Funding: This work was supported by the National Institutes of Health [grant numbers 2R01DC006471 (to J.Z.), 1R01DC015010-01A1 (to J.Z.), 1R01DC01544401 (to J.Z.), 1R21DC013879-01 (to J.Z.), and P30CA21765 (to St. Jude)], ALSAC, the Office of Naval Research [grant numbers N000140911014, N000141210191, N000141210775, and N000141612315 (to J.Z.)], and The Hartwell Foundation [Individual Biomedical Research Award to J.Z.].
Abbreviations
- NIHL
noise induced hearing loss
- HC
hair cell
- SC
supporting cell
- OHC
outer hair cell
- IHC
inner hair cell
- CNS
central nervous system
- TM
tympanic membrane
- RNA-seq
RNA sequencing
- TF
transcription factor
- iPSC
induced pluripotent stem cell
- ESC
embryonic stem cell
- HPF
human postnatal fibroblast
- mEB
mouse embryoid body
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdolazimi Y, Stojanova Z, Segil N. Selection of cell fate in the organ of Corti involves the integration of Hes/Hey signaling at the Atoh1 promoter. Development. 2016;143:841–50. doi: 10.1242/dev.129320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler HJ, Raphael Y. New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear. Neuroscience letters. 1996;205:17–20. doi: 10.1016/0304-3940(96)12367-3. [DOI] [PubMed] [Google Scholar]
- Ahmed M, Wong EY, Sun J, Xu J, Wang F, Xu PX. Eya1-Six1 interaction is sufficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox2. Developmental cell. 2012;22:377–90. doi: 10.1016/j.devcel.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akil O, Seal RP, Burke K, Wang C, Alemi A, During M, Edwards RH, Lustig LR. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron. 2012;75:283–93. doi: 10.1016/j.neuron.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlotta P, Berninger B. Brains in metamorphosis: reprogramming cell identity within the central nervous system. Current opinion in neurobiology. 2014;27:208–14. doi: 10.1016/j.conb.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JL, Halpern P, Tsai MC, Smithline H. Mass casualty terrorist bombings: a comparison of outcomes by bombing type. Annals of emergency medicine. 2004;43:263–73. doi: 10.1016/s0196-0644(03)00723-6. [DOI] [PubMed] [Google Scholar]
- Arnold JL, Tsai MC, Halpern P, Smithline H, Stok E, Ersoy G. Mass-casualty, terrorist bombings: epidemiological outcomes, resource utilization, and time course of emergency needs (Part I) Prehospital and disaster medicine. 2003;18:220–34. doi: 10.1017/s1049023x00001096. [DOI] [PubMed] [Google Scholar]
- Askew C, Rochat C, Pan B, Asai Y, Ahmed H, Child E, Schneider BL, Aebischer P, Holt JR. Tmc gene therapy restores auditory function in deaf mice. Science translational medicine. 2015;7:295ra108. doi: 10.1126/scitranslmed.aab1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson PJ, Huarcaya Najarro E, Sayyid ZN, Cheng AG. Sensory hair cell development and regeneration: similarities and differences. Development. 2015;142:1561–71. doi: 10.1242/dev.114926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson PJ, Wise AK, Flynn BO, Nayagam BA, Richardson RT. Hair cell regeneration after ATOH1 gene therapy in the cochlea of profoundly deaf adult guinea pigs. PloS one. 2014;9:e102077. doi: 10.1371/journal.pone.0102077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avrahami D, Kaestner KH. Epigenetic regulation of pancreas development and function. Seminars in cell & developmental biology. 2012;23:693–700. doi: 10.1016/j.semcdb.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeyens L, Lemper M, Leuckx G, De Groef S, Bonfanti P, Stange G, Shemer R, Nord C, Scheel DW, Pan FC, Ahlgren U, Gu G, Stoffers DA, Dor Y, Ferrer J, Gradwohl G, Wright CV, Van de Casteele M, German MS, Bouwens L, Heimberg H. Transient cytokine treatment induces acinar cell reprogramming and regenerates functional beta cell mass in diabetic mice. Nature biotechnology. 2014;32:76–83. doi: 10.1038/nbt.2747. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Baird RA, Steyger PS, Schuff NR. Mitotic and nonmitotic hair cell regeneration in the bullfrog vestibular otolith organs. Annals of the New York Academy of Sciences. 1996;781:59–70. doi: 10.1111/j.1749-6632.1996.tb15693.x. [DOI] [PubMed] [Google Scholar]
- Behra M, Bradsher J, Sougrat R, Gallardo V, Allende ML, Burgess SM. Phoenix is required for mechanosensory hair cell regeneration in the zebrafish lateral line. PLoS genetics. 2009;5:e1000455. doi: 10.1371/journal.pgen.1000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berneman-Zeitouni D, Molakandov K, Elgart M, Mor E, Fornoni A, Dominguez MR, Kerr-Conte J, Ott M, Meivar-Levy I, Ferber S. The temporal and hierarchical control of transcription factors-induced liver to pancreas transdifferentiation. PloS one. 2014;9:e87812. doi: 10.1371/journal.pone.0087812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall NF, Shi F, Arnold K, Hochedlinger K, Edge AS. Lgr5-positive supporting cells generate new hair cells in the postnatal cochlea. Stem cell reports. 2014;2:311–22. doi: 10.1016/j.stemcr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S, Pei W, Xu L, Varshney G, Huang S, Idol J. A systems biology approach to understanding hearing regeneration in zebra fish. ARO Midwinter Meeting; 2016. p. 308. [Google Scholar]
- Burns JC, On D, Baker W, Collado MS, Corwin JT. Over half the hair cells in the mouse utricle first appear after birth, with significant numbers originating from early postnatal mitotic production in peripheral and striolar growth zones. Journal of the Association for Research in Otolaryngology : JARO. 2012;13:609–27. doi: 10.1007/s10162-012-0337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JC, Kelly MC, Hoa M, Morell RJ, Kelley MW. Single-cell RNA-Seq resolves cellular complexity in sensory organs from the neonatal inner ear. Nature communications. 2015;6:8557. doi: 10.1038/ncomms9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai T, Groves AK. The Role of Atonal Factors in Mechanosensory Cell Specification and Function. Molecular neurobiology. 2014 doi: 10.1007/s12035-014-8925-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai T, Seymour ML, Zhang H, Pereira FA, Groves AK. Conditional deletion of Atoh1 reveals distinct critical periods for survival and function of hair cells in the organ of Corti. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:10110–22. doi: 10.1523/JNEUROSCI.5606-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai T, Jen HI, Kang H, Klisch TJ, Zoghbi HY, Groves AK. Characterization of the transcriptome of nascent hair cells and identification of direct targets of the Atoh1 transcription factor. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:5870–83. doi: 10.1523/JNEUROSCI.5083-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DP, Chrysostomou E, Doetzlhofer A. Canonical Notch signaling plays an instructive role in auditory supporting cell development. Scientific reports. 2016;6:19484. doi: 10.1038/srep19484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr A, Biggin MD. A comparison of in vivo and in vitro DNA-binding specificities suggests a new model for homeoprotein DNA binding in Drosophila embryos. The EMBO journal. 1999;18:1598–608. doi: 10.1093/emboj/18.6.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali JG. Powered Electronic Augmentations in Hearing Protection Technology Circa 2010 including Active Noise Reduction, Electronically-Modulated Sound Transmission, and Tactical Communications Devices: Review of Design, Testing, and Research. Int J Acoust Vib. 2010;15:168–186. [Google Scholar]
- Cave KM, Cornish EM, Chandler DW. Blast injury of the ear: clinical update from the global war on terror. Military medicine. 2007;172:726–30. doi: 10.7205/milmed.172.7.726. [DOI] [PubMed] [Google Scholar]
- Cavelti-Weder C, Shtessel M, Reuss JE, Jermendy A, Yamada T, Caballero F, Bonner-Weir S, Weir GC. Pancreatic duct ligation after almost complete beta-cell loss: exocrine regeneration but no evidence of beta-cell regeneration. Endocrinology. 2013;154:4493–502. doi: 10.1210/en.2013-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai R, Kuo B, Wang T, Liaw EJ, Xia A, Jan TA, Liu Z, Taketo MM, Oghalai JS, Nusse R, Zuo J, Cheng AG. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:8167–72. doi: 10.1073/pnas.1202774109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AG, Cunningham LL, Rubel EW. Hair cell death in the avian basilar papilla: characterization of the in vitro model and caspase activation. Journal of the Association for Research in Otolaryngology : JARO. 2003;4:91–105. doi: 10.1007/s10162-002-3016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chera S, Baronnier D, Ghila L, Cigliola V, Jensen JN, Gu G, Furuyama K, Thorel F, Gribble FM, Reimann F, Herrera PL. Diabetes recovery by age-dependent conversion of pancreatic delta-cells into insulin producers. Nature. 2014;514:503–7. doi: 10.1038/nature13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien WW, Isgrig K, Roy S, Belyantseva IA, Drummond MC, May LA, Fitzgerald TS, Friedman TB, Cunningham LL. Gene Therapy Restores Hair Cell Stereocilia Morphology in Inner Ears of Deaf Whirler Mice. Molecular therapy : the journal of the American Society of Gene Therapy. 2016;24:17–25. doi: 10.1038/mt.2015.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nature genetics. 1996;12:390–7. doi: 10.1038/ng0496-390. [DOI] [PubMed] [Google Scholar]
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–4. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- Costa A, Sanchez-Guardado L, Juniat S, Gale JE, Daudet N, Henrique D. Generation of sensory hair cells by genetic programming with a combination of transcription factors. Development. 2015;142:1948–59. doi: 10.1242/dev.119149. [DOI] [PubMed] [Google Scholar]
- Cox BC, Dearman JA, Brancheck J, Zindy F, Roussel MF, Zuo J. Generation of Atoh1-rtTA transgenic mice: a tool for inducible gene expression in hair cells of the inner ear. Scientific reports. 2014a;4:6885. doi: 10.1038/srep06885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BC, Chai R, Lenoir A, Liu Z, Zhang L, Nguyen DH, Chalasani K, Steigelman KA, Fang J, Rubel EW, Cheng AG, Zuo J. Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development. 2014b;141:816–29. doi: 10.1242/dev.103036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P. Cochlear amplification, outer hair cells and prestin. Current opinion in neurobiology. 2008;18:370–6. doi: 10.1016/j.conb.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P, Wu X, Cheatham MA, Gao J, Zheng J, Anderson CT, Jia S, Wang X, Cheng WH, Sengupta S, He DZ, Zuo J. Prestin-based outer hair cell motility is necessary for mammalian cochlear amplification. Neuron. 2008;58:333–9. doi: 10.1016/j.neuron.2008.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudet N, Gibson R, Shang J, Bernard A, Lewis J, Stone J. Notch regulation of progenitor cell behavior in quiescent and regenerating auditory epithelium of mature birds. Developmental biology. 2009;326:86–100. doi: 10.1016/j.ydbio.2008.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- De Moerlooze L, Spencer-Dene B, Revest JM, Hajihosseini M, Rosewell I, Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127:483–92. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- Defourny J, Mateo Sanchez S, Schoonaert L, Robberecht W, Davy A, Nguyen L, Malgrange B. Cochlear supporting cell transdifferentiation and integration into hair cell layers by inhibition of ephrin-B2 signalling. Nature communications. 2015;6:7017. doi: 10.1038/ncomms8017. [DOI] [PubMed] [Google Scholar]
- Devarajan P, Savoca M, Castaneda MP, Park MS, Esteban-Cruciani N, Kalinec G, Kalinec F. Cisplatin-induced apoptosis in auditory cells: role of death receptor and mitochondrial pathways. Hearing research. 2002;174:45–54. doi: 10.1016/s0378-5955(02)00634-2. [DOI] [PubMed] [Google Scholar]
- Doetzlhofer A, Basch ML, Ohyama T, Gessler M, Groves AK, Segil N. Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Developmental cell. 2009;16:58–69. doi: 10.1016/j.devcel.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan M, Mi Q. Local delivery of reporter gene to the cochlea does not spread to brain tissue in an animal model. Acta oto-laryngologica. 2010;130:25–30. doi: 10.3109/00016480902963053. [DOI] [PubMed] [Google Scholar]
- Ebeid M, Sripal P, Pecka J, Beisel K, Soukup G. Transcriptome-wide Comparison of the Impact of Atoh1/miR-183 Family on Pluripotent Stem Cells and Multipotent Otic Progenitors. ARO Mid-Winter Meeting; 2016. p. 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeckhoute J, Keeton EK, Lupien M, Krum SA, Carroll JS, Brown M. Positive cross-regulatory loop ties GATA-3 to estrogen receptor alpha expression in breast cancer. Cancer research. 2007;67:6477–83. doi: 10.1158/0008-5472.CAN-07-0746. [DOI] [PubMed] [Google Scholar]
- El Kechai N, Agnely F, Mamelle E, Nguyen Y, Ferrary E, Bochot A. Recent advances in local drug delivery to the inner ear. International journal of pharmaceutics. 2015;494:83–101. doi: 10.1016/j.ijpharm.2015.08.015. [DOI] [PubMed] [Google Scholar]
- Erkman L, McEvilly RJ, Luo L, Ryan AK, Hooshmand F, O’Connell SM, Keithley EM, Rapaport DH, Ryan AF, Rosenfeld MG. Role of transcription factors Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature. 1996;381:603–6. doi: 10.1038/381603a0. [DOI] [PubMed] [Google Scholar]
- Fausett BV, Goldman D. A role for alpha1 tubulin-expressing Muller glia in regeneration of the injured zebrafish retina. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:6303–13. doi: 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausti SA, Wilmington DJ, Gallun FJ, Myers PJ, Henry JA. Auditory and vestibular dysfunction associated with blast-related traumatic brain injury. Journal of rehabilitation research and development. 2009;46:797–810. doi: 10.1682/jrrd.2008.09.0118. [DOI] [PubMed] [Google Scholar]
- Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, Barshack I, Seijffers R, Kopolovic J, Kaiser N, Karasik A. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nature medicine. 2000;6:568–72. doi: 10.1038/75050. [DOI] [PubMed] [Google Scholar]
- Frayne SM, Chiu VY, Iqbal S, Berg EA, Laungani KJ, Cronkite RC, Pavao J, Kimerling R. Medical care needs of returning veterans with PTSD: their other burden. Journal of general internal medicine. 2011;26:33–9. doi: 10.1007/s11606-010-1497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman LM, Dror AA, Mor E, Tenne T, Toren G, Satoh T, Biesemeier DJ, Shomron N, Fekete DM, Hornstein E, Avraham KB. MicroRNAs are essential for development and function of inner ear hair cells in vertebrates. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7915–20. doi: 10.1073/pnas.0812446106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Shen XZ, Jiang F, Wu Y, Han C. DNA-guided genome editing using the Natronobacterium gregoryi Argonaute. Nature biotechnology. 2016 doi: 10.1038/nbt.3547. [DOI] [PubMed] [Google Scholar]
- Garcia-Rodriguez JL, Barbier-Torres L, Fernandez-Alvarez S, Gutierrez-de Juan V, Monte MJ, Halilbasic E, Herranz D, Alvarez L, Aspichueta P, Marin JJ, Trauner M, Mato JM, Serrano M, Beraza N, Martinez-Chantar ML. SIRT1 controls liver regeneration by regulating bile acid metabolism through farnesoid X receptor and mammalian target of rapamycin signaling. Hepatology. 2014;59:1972–83. doi: 10.1002/hep.26971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garth RJ. Blast injury of the auditory system: a review of the mechanisms and pathology. The Journal of laryngology and otology. 1994;108:925–9. doi: 10.1017/s0022215100128555. [DOI] [PubMed] [Google Scholar]
- Girod DA, Duckert LG, Rubel EW. Possible precursors of regenerated hair cells in the avian cochlea following acoustic trauma. Hearing research. 1989;42:175–94. doi: 10.1016/0378-5955(89)90143-3. [DOI] [PubMed] [Google Scholar]
- Gnedeva K, Hudspeth AJ. SoxC transcription factors are essential for the development of the inner ear. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:14066–71. doi: 10.1073/pnas.1517371112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden EJ, Benito-Gonzalez A, Doetzlhofer A. The RNA-binding protein LIN28B regulates developmental timing in the mammalian cochlea. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E3864–73. doi: 10.1073/pnas.1501077112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldowitz D, Smeyne RJ. Tune into the weaver channel. Nat Genet. 1995;11:107–9. doi: 10.1038/ng1095-107. [DOI] [PubMed] [Google Scholar]
- Golub JS, Tong L, Ngyuen TB, Hume CR, Palmiter RD, Rubel EW, Stone JS. Hair cell replacement in adult mouse utricles after targeted ablation of hair cells with diphtheria toxin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:15093–105. doi: 10.1523/JNEUROSCI.1709-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondusky JS, Reiter MP. Protecting military convoys in Iraq: an examination of battle injuries sustained by a mechanized battalion during Operation Iraqi Freedom II. Military medicine. 2005;170:546–9. doi: 10.7205/milmed.170.6.546. [DOI] [PubMed] [Google Scholar]
- Gopen Q, Lopez I, Ishiyama G, Baloh RW, Ishiyama A. Unbiased stereologic type I and type II hair cell counts in human utricular macula. The Laryngoscope. 2003;113:1132–8. doi: 10.1097/00005537-200307000-00007. [DOI] [PubMed] [Google Scholar]
- Gubbels SP, Woessner DW, Mitchell JC, Ricci AJ, Brigande JV. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature. 2008;455:537–41. doi: 10.1038/nature07265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamernik RP, Patterson JH, Salvi RJ. The effect of impulse intensity and the number of impulses on hearing and cochlear pathology in the chinchilla. The Journal of the Acoustical Society of America. 1987;81:1118–29. doi: 10.1121/1.394632. [DOI] [PubMed] [Google Scholar]
- Hamernik RP, Patterson JH, Turrentine GA, Ahroon WA. The quantitative relation between sensory cell loss and hearing thresholds. Hearing research. 1989;38:199–211. doi: 10.1016/0378-5955(89)90065-8. [DOI] [PubMed] [Google Scholar]
- Han JJ, Mhatre AN, Wareing M, Pettis R, Gao WQ, Zufferey RN, Trono D, Lalwani AK. Transgene expression in the guinea pig cochlea mediated by a lentivirus-derived gene transfer vector. Human gene therapy. 1999;10:1867–73. doi: 10.1089/10430349950017545. [DOI] [PubMed] [Google Scholar]
- Hawkins DB, Wightman FL. Interaural time discrimination ability of listeners with sensorineural hearing loss. Audiology : official organ of the International Society of Audiology. 1980;19:495–507. doi: 10.3109/00206098009070081. [DOI] [PubMed] [Google Scholar]
- Hawkins RD, Bashiardes S, Powder KE, Sajan SA, Bhonagiri V, Alvarado DM, Speck J, Warchol ME, Lovett M. Large scale gene expression profiles of regenerating inner ear sensory epithelia. PloS one. 2007;2:e525. doi: 10.1371/journal.pone.0000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Cai C, Tang D, Sun S, Li H. Effect of histone deacetylase inhibitors trichostatin A and valproic acid on hair cell regeneration in zebrafish lateral line neuromasts. Frontiers in cellular neuroscience. 2014;8:382. doi: 10.3389/fncel.2014.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Tang D, Cai C, Chai R, Li H. LSD1 is Required for Hair Cell Regeneration in Zebrafish. Molecular neurobiology. 2016;53:2421–34. doi: 10.1007/s12035-015-9206-2. [DOI] [PubMed] [Google Scholar]
- He Y, Yu H, Sun S, Wang Y, Liu I, Chen Z, Li H. Trans-2-phenylcyclopropylamine regulates zebrafish lateral line neuromast development mediated by depression of LSD1 activity. The International journal of developmental biology. 2013;57:365–73. doi: 10.1387/ijdb.120227hl. [DOI] [PubMed] [Google Scholar]
- Hiler D, Chen X, Hazen J, Kupriyanov S, Carroll PA, Qu C, Xu B, Johnson D, Griffiths L, Frase S, Rodriguez AR, Martin G, Zhang J, Jeon J, Fan Y, Finkelstein D, Eisenman RN, Baldwin K, Dyer MA. Quantification of Retinogenesis in 3D Cultures Reveals Epigenetic Memory and Higher Efficiency in iPSCs Derived from Rod Photoreceptors. Cell stem cell. 2015;17:101–15. doi: 10.1016/j.stem.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffer ME, Balaban C, Gottshall K, Balough BJ, Maddox MR, Penta JR. Blast exposure: vestibular consequences and associated characteristics. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2010;31:232–6. doi: 10.1097/MAO.0b013e3181c993c3. [DOI] [PubMed] [Google Scholar]
- Hori R, Nakagawa T, Sakamoto T, Matsuoka Y, Takebayashi S, Ito J. Pharmacological inhibition of Notch signaling in the mature guinea pig cochlea. Neuroreport. 2007;18:1911–4. doi: 10.1097/WNR.0b013e3282f213e0. [DOI] [PubMed] [Google Scholar]
- Hu L, Lu J, Chiang H, Wu H, Edge AS, Shi F. Diphtheria Toxin-Induced Cell Death Triggers Wnt-Dependent Hair Cell Regeneration in Neonatal Mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2016;36:9479–89. doi: 10.1523/JNEUROSCI.2447-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Barr E, Rudnick DA. Characterization of the regulation and function of zinc-dependent histone deacetylases during rodent liver regeneration. Hepatology. 2013;57:1742–51. doi: 10.1002/hep.26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Tan S. Direct lineage conversion of astrocytes to induced neural stem cells or neurons. Neurosci Bull. 2015;31:357–67. doi: 10.1007/s12264-014-1517-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Maehr R, Guo W, Eijkelenboom A, Snitow M, Chen AE, Melton DA. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nature biotechnology. 2008;26:795–7. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh SH, Warchol ME, Ornitz DM. Cochlear progenitor number is controlled through mesenchymal FGF receptor signaling. eLife. 2015:4. doi: 10.7554/eLife.05921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyghe A, Van den Ackerveken P, Sacheli R, Prevot PP, Thelen N, Renauld J, Thiry M, Delacroix L, Nguyen L, Malgrange B. MicroRNA-124 Regulates Cell Specification in the Cochlea through Modulation of Sfrp4/5. Cell reports. 2015;13:31–42. doi: 10.1016/j.celrep.2015.08.054. [DOI] [PubMed] [Google Scholar]
- Iconaru LI, Ban D, Bharatham K, Ramanathan A, Zhang W, Shelat AA, Zuo J, Kriwacki RW. Discovery of Small Molecules that Inhibit the Disordered Protein, p27(Kip1) Scientific reports. 2015;5:15686. doi: 10.1038/srep15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka T, Kamiya K, Gotoh S, Sugitani Y, Suzuki M, Noda T, Minowa O, Ikeda K. Perinatal Gjb2 gene transfer rescues hearing in a mouse model of hereditary deafness. Human molecular genetics. 2015;24:3651–61. doi: 10.1093/hmg/ddv109. [DOI] [PubMed] [Google Scholar]
- Ishii H, Sen R, Pazin MJ. Combinatorial control of DNase I-hypersensitive site formation and erasure by immunoglobulin heavy chain enhancer-binding proteins. The Journal of biological chemistry. 2004;279:7331–8. doi: 10.1074/jbc.M308973200. [DOI] [PubMed] [Google Scholar]
- Iyer VR, Horak CE, Scafe CS, Botstein D, Snyder M, Brown PO. Genomic binding sites of the yeast cell-cycle transcription factors SBF and MBF. Nature. 2001;409:533–8. doi: 10.1038/35054095. [DOI] [PubMed] [Google Scholar]
- Izumikawa M, Batts SA, Miyazawa T, Swiderski DL, Raphael Y. Response of the flat cochlear epithelium to forced expression of Atoh1. Hearing research. 2008;240:52–6. doi: 10.1016/j.heares.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nature medicine. 2005;11:271–6. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Jan TA, Chai R, Sayyid ZN, van Amerongen R, Xia A, Wang T, Sinkkonen ST, Zeng YA, Levin JR, Heller S, Nusse R, Cheng AG. Tympanic border cells are Wnt-responsive and can act as progenitors for postnatal mouse cochlear cells. Development. 2013;140:1196–206. doi: 10.1242/dev.087528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawardena TM, Egemnazarov B, Finch EA, Zhang L, Payne JA, Pandya K, Zhang Z, Rosenberg P, Mirotsou M, Dzau VJ. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circulation research. 2012;110:1465–73. doi: 10.1161/CIRCRESAHA.112.269035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jero J, Mhatre AN, Tseng CJ, Stern RE, Coling DE, Goldstein JA, Hong K, Zheng WW, Hoque AT, Lalwani AK. Cochlear gene delivery through an intact round window membrane in mouse. Human gene therapy. 2001;12:539–48. doi: 10.1089/104303401300042465. [DOI] [PubMed] [Google Scholar]
- Jiang L, Romero-Carvajal A, Haug JS, Seidel CW, Piotrowski T. Gene-expression analysis of hair cell regeneration in the zebrafish lateral line. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E1383–92. doi: 10.1073/pnas.1402898111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson LG, Hawkins JE., Jr Degeneration patterns in human ears exposed to noise. The Annals of otology, rhinology, and laryngology. 1976;85:725–39. doi: 10.1177/000348947608500603. [DOI] [PubMed] [Google Scholar]
- Jones JE, Corwin JT. Regeneration of sensory cells after laser ablation in the lateral line system: hair cell lineage and macrophage behavior revealed by time-lapse video microscopy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:649–62. doi: 10.1523/JNEUROSCI.16-02-00649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JY, Avenarius MR, Adamsky S, Alpert E, Feinstein E, Raphael Y. siRNA targeting Hes5 augments hair cell regeneration in aminoglycoside-damaged mouse utricle. Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21:834–41. doi: 10.1038/mt.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan T, Li XY, Sabo PJ, Thomas S, Stamatoyannopoulos JA, Biggin MD, Eisen MB. Quantitative models of the mechanisms that control genome-wide patterns of transcription factor binding during early Drosophila development. PLoS genetics. 2011;7:e1001290. doi: 10.1371/journal.pgen.1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur T, Zamani D, Tong L, Rubel EW, Ohlemiller KK, Hirose K, Warchol ME. Fractalkine Signaling Regulates Macrophage Recruitment into the Cochlea and Promotes the Survival of Spiral Ganglion Neurons after Selective Hair Cell Lesion. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:15050–61. doi: 10.1523/JNEUROSCI.2325-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:4395–400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MC, Chang Q, Pan A, Lin X, Chen P. Atoh1 directs the formation of sensory mosaics and induces cell proliferation in the postnatal mammalian cochlea in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:6699–710. doi: 10.1523/JNEUROSCI.5420-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kho ST, Pettis RM, Mhatre AN, Lalwani AK. Safety of adeno-associated virus as cochlear gene transfer vector: analysis of distant spread beyond injected cochleae. Molecular therapy : the journal of the American Society of Gene Therapy. 2000;2:368–73. doi: 10.1006/mthe.2000.0129. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KS. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434:1031–5. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- Kilpatrick LA, Li Q, Yang J, Goddard JC, Fekete DM, Lang H. Adeno-associated virusmediated gene delivery into the scala media of the normal and deafened adult mouse ear. Gene therapy. 2011;18:569–78. doi: 10.1038/gt.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MA, Cho HJ, Bae SH, Lee B, Oh SK, Kwon TJ, Ryoo ZY, Kim HY, Cho JH, Kim UK, Lee KY. Methionine Sulfoxide Reductase B3-Targeted In Utero Gene Therapy Rescues Hearing Function in a Mouse Model of Congenital Sensorineural Hearing Loss. Antioxidants & redox signaling. 2016;24:590–602. doi: 10.1089/ars.2015.6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard M, Nyengaard JR. Stereological study of postnatal development in the mouse utricular macula. The Journal of comparative neurology. 2005;492:132–44. doi: 10.1002/cne.20736. [DOI] [PubMed] [Google Scholar]
- Koehler KR, Mikosz AM, Molosh AI, Patel D, Hashino E. Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature. 2013;500:217–21. doi: 10.1038/nature12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M, Kawamoto K, Izumikawa M, Kuriyama H, Yamashita T. Gene transfer into guinea pig cochlea using adeno-associated virus vectors. The journal of gene medicine. 2008;10:610–8. doi: 10.1002/jgm.1189. [DOI] [PubMed] [Google Scholar]
- Korrapati S, Roux I, Glowatzki E, Doetzlhofer A. Notch signaling limits supporting cell plasticity in the hair cell-damaged early postnatal murine cochlea. PloS one. 2013;8:e73276. doi: 10.1371/journal.pone.0073276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft S, Hsu C, Brough DE, Staecker H. Atoh1 induces auditory hair cell recovery in mice after ototoxic injury. The Laryngoscope. 2013;123:992–9. doi: 10.1002/lary.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku YC, Renaud NA, Veile RA, Helms C, Voelker CC, Warchol ME, Lovett M. The transcriptome of utricle hair cell regeneration in the avian inner ear. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:3523–35. doi: 10.1523/JNEUROSCI.2606-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo BR, Baldwin EM, Layman WS, Taketo MM, Zuo J. In Vivo Cochlear Hair Cell Generation and Survival by Coactivation of beta-Catenin and Atoh1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:10786–98. doi: 10.1523/JNEUROSCI.0967-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Shen J, Corey DP. C-MYC transcriptionally amplifies SOX2 target genes to regulate self-renewal in multipotent otic progenitor cells. Stem cell reports. 2015;4:47–60. doi: 10.1016/j.stemcr.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladewig J, Mertens J, Kesavan J, Doerr J, Poppe D, Glaue F, Herms S, Wernet P, Kogler G, Muller FJ, Koch P, Brustle O. Small molecules enable highly efficient neuronal conversion of human fibroblasts. Nature methods. 2012;9:575–8. doi: 10.1038/nmeth.1972. [DOI] [PubMed] [Google Scholar]
- Lambers E, Kume T. Navigating the labyrinth of cardiac regeneration. Developmental dynamics : an official publication of the American Association of Anatomists. 2016 doi: 10.1002/dvdy.24397. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, Shailam R, Norton CR, Gridley T, Kelley MW. Expression of Math1 and HES5 in the cochleae of wildtype and Jag2 mutant mice. Journal of the Association for Research in Otolaryngology : JARO. 2000;1:161–71. doi: 10.1007/s101620010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layman WS, Zuo J. Epigenetic regulation in the inner ear and its potential roles in development, protection, and regeneration. Frontiers in cellular neuroscience. 2014;8:446. doi: 10.3389/fncel.2014.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layman WS, Sauceda MA, Zuo J. Epigenetic alterations by NuRD and PRC2 in the neonatal mouse cochlea. Hearing research. 2013;304:167–78. doi: 10.1016/j.heares.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layman WS, Williams DM, Dearman JA, Sauceda MA, Zuo J. Histone deacetylase inhibition protects hearing against acute ototoxicity by activating the Nf-B pathway. Cell death discovery 1. 2015 doi: 10.1038/cddiscovery.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Kloosterman W, Fekete DM. MicroRNA-183 family members regulate sensorineural fates in the inner ear. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:3254–63. doi: 10.1523/JNEUROSCI.4948-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Collado M, Villasante A, Matheu A, Lynch CJ, Canamero M, Rizzoti K, Carneiro C, Martinez G, Vidal A, Lovell-Badge R, Serrano M. p27(Kip1) directly represses Sox2 during embryonic stem cell differentiation. Cell stem cell. 2012;11:845–52. doi: 10.1016/j.stem.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Cavelti-Weder C, Zhang Y, Clement K, Donovan S, Gonzalez G, Zhu J, Stemann M, Xu K, Hashimoto T, Yamada T, Nakanishi M, Zhang Y, Zeng S, Gifford D, Meissner A, Weir G, Zhou Q. Long-term persistence and development of induced pancreatic beta cells generated by lineage conversion of acinar cells. Nature biotechnology. 2014;32:1223–30. doi: 10.1038/nbt.3082. [DOI] [PubMed] [Google Scholar]
- Liang HL, Nien CY, Liu HY, Metzstein MM, Kirov N, Rushlow C. The zinc-finger protein Zelda is a key activator of the early zygotic genome in Drosophila. Nature. 2008;456:400–3. doi: 10.1038/nature07388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Dodds LW. Single-neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves. Hearing research. 1984;16:55–74. doi: 10.1016/0378-5955(84)90025-x. [DOI] [PubMed] [Google Scholar]
- Liu H, Pecka JL, Zhang Q, Soukup GA, Beisel KW, He DZ. Characterization of transcriptomes of cochlear inner and outer hair cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014a;34:11085–95. doi: 10.1523/JNEUROSCI.1690-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XP, Koehler KR, Mikosz AM, Hashino E, Holt JR. Functional development of mechanosensitive hair cells in stem cell-derived organoids parallels native vestibular hair cells. Nature communications. 2016;7:11508. doi: 10.1038/ncomms11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Okada T, Sheykholeslami K, Shimazaki K, Nomoto T, Muramatsu S, Kanazawa T, Takeuchi K, Ajalli R, Mizukami H, Kume A, Ichimura K, Ozawa K. Specific and efficient transduction of Cochlear inner hair cells with recombinant adeno-associated virus type 3 vector. Molecular therapy : the journal of the American Society of Gene Therapy. 2005;12:725–33. doi: 10.1016/j.ymthe.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Liu Z, Fang J, Dearman J, Zhang L, Zuo J. In vivo generation of immature inner hair cells in neonatal mouse cochleae by ectopic Atoh1 expression. PloS one. 2014b;9:e89377. doi: 10.1371/journal.pone.0089377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Dearman JA, Cox BC, Walters BJ, Zhang L, Ayrault O, Zindy F, Gan L, Roussel MF, Zuo J. Age-dependent in vivo conversion of mouse cochlear pillar and Deiters’ cells to immature hair cells by Atoh1 ectopic expression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012a;32:6600–10. doi: 10.1523/JNEUROSCI.0818-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Walters BJ, Owen T, Brimble MA, Steigelman KA, Zhang L, Mellado Lagarde MM, Valentine MB, Yu Y, Cox BC, Zuo J. Regulation of p27Kip1 by Sox2 maintains quiescence of inner pillar cells in the murine auditory sensory epithelium. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012b;32:10530–40. doi: 10.1523/JNEUROSCI.0686-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke AE, Foster PK, Muller CD, Peel AL. Cochlear function and transgene expression in the guinea pig cochlea, using adenovirus- and adeno-associated virus-directed gene transfer. Human gene therapy. 2001;12:773–81. doi: 10.1089/104303401750148702. [DOI] [PubMed] [Google Scholar]
- Ma EY, Rubel EW, Raible DW. Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:2261–73. doi: 10.1523/JNEUROSCI.4372-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass JC, Gu R, Basch ML, Waldhaus J, Lopez EM, Xia A, Oghalai JS, Heller S, Groves AK. Changes in the regulation of the Notch signaling pathway are temporally correlated with regenerative failure in the mouse cochlea. Frontiers in cellular neuroscience. 2015;9:110. doi: 10.3389/fncel.2015.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda M, Pak K, Chavez E, Ryan AF. TFE2 and GATA3 enhance induction of POU4F3 and myosin VIIa positive cells in nonsensory cochlear epithelium by ATOH1. Developmental biology. 2012;372:68–80. doi: 10.1016/j.ydbio.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellado Lagarde MM, Cox BC, Fang J, Taylor R, Forge A, Zuo J. Selective ablation of pillar and deiters’ cells severely affects cochlear postnatal development and hearing in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:1564–76. doi: 10.1523/JNEUROSCI.3088-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellado Lagarde MM, Wan G, Zhang L, Gigliello AR, McInnis JJ, Zhang Y, Bergles D, Zuo J, Corfas G. Spontaneous regeneration of cochlear supporting cells after neonatal ablation ensures hearing in the adult mouse. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:16919–24. doi: 10.1073/pnas.1408064111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliorini A, Bader E, Lickert H. Islet cell plasticity and regeneration. Molecular metabolism. 2014;3:268–74. doi: 10.1016/j.molmet.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa T, Minoda R, Ise M, Yamada T, Yumoto E. Mouse otocyst transuterine gene transfer restores hearing in mice with connexin 30 deletion-associated hearing loss. Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21:1142–50. doi: 10.1038/mt.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutari K, Fujioka M, Hosoya M, Bramhall N, Okano HJ, Okano H, Edge AS. Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron. 2013;77:58–69. doi: 10.1016/j.neuron.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondain M, Restituito S, Vincenti V, Gardiner Q, Uziel A, Delabre A, Mathieu M, Bousquet J, Demoly P. Adenovirus-mediated in vivo gene transfer in guinea pig middle ear mucosa. Human gene therapy. 1998;9:1217–21. doi: 10.1089/hum.1998.9.8-1217. [DOI] [PubMed] [Google Scholar]
- Murko C, Lagger S, Steiner M, Seiser C, Schoefer C, Pusch O. Expression of class I histone deacetylases during chick and mouse development. The International journal of developmental biology. 2010;54:1527–37. doi: 10.1387/ijdb.092971cm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nageris BI, Attias J, Shemesh R. Otologic and audiologic lesions due to blast injury. Journal of basic and clinical physiology and pharmacology. 2008;19:185–91. doi: 10.1515/jbcpp.2008.19.3-4.185. [DOI] [PubMed] [Google Scholar]
- Namdaran P, Reinhart KE, Owens KN, Raible DW, Rubel EW. Identification of modulators of hair cell regeneration in the zebrafish lateral line. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:3516–28. doi: 10.1523/JNEUROSCI.3905-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera TM, Hu BH, Henderson D. The caspase pathway in noise-induced apoptosis of the chinchilla cochlea. Journal of the Association for Research in Otolaryngology : JARO. 2003;4:466–77. doi: 10.1007/s10162-002-3038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu W, Zang T, Zou Y, Fang S, Smith DK, Bachoo R, Zhang CL. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nature cell biology. 2013;15:1164–75. doi: 10.1038/ncb2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterle EC, Chien WM, Campbell S, Nellimarla P, Fero ML. p27(Kip1) is required to maintain proliferative quiescence in the adult cochlea and pituitary. Cell cycle. 2011;10:1237–48. doi: 10.4161/cc.10.8.15301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima K, Shin K, Diensthuber M, Peng AW, Ricci AJ, Heller S. Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell. 2010;141:704–16. doi: 10.1016/j.cell.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan N, Jahan I, Kersigo J, Duncan JS, Kopecky B, Fritzsch B. A novel Atoh1 “self-terminating” mouse model reveals the necessity of proper Atoh1 level and duration for hair cell differentiation and viability. PloS one. 2012;7:e30358. doi: 10.1371/journal.pone.0030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan S, Wan J, Liu S, Zhang S, Xiong H, Zhou J, Xiong W, Yu K, Fu Y. Lentivirus carrying the Atoh1 gene infects normal rat cochlea. Neural Regen Res. 2013;8:1551–9. doi: 10.3969/j.issn.1673-5374.2013.17.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Sudhof TC, Wernig M. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–3. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, Hu Z, Bard J, Jamison J, Cai Q, Hu BH. Transcriptome characterization by RNA-Seq reveals the involvement of the complement components in noise-traumatized rat cochleae. Neuroscience. 2013;248:1–16. doi: 10.1016/j.neuroscience.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JH, Jr, Hamernik RP. Blast overpressure induced structural and functional changes in the auditory system. Toxicology. 1997;121:29–40. doi: 10.1016/s0300-483x(97)03653-6. [DOI] [PubMed] [Google Scholar]
- Petersen GF, Strappe PM. Generation of diverse neural cell types through direct conversion. World journal of stem cells. 2016;8:32–46. doi: 10.4252/wjsc.v8.i2.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo BG, Pogo AO, Allfrey VG, Mirsky AE. Changing patterns of histone acetylation and RNA synthesis in regeneration of the liver. Proceedings of the National Academy of Sciences of the United States of America. 1968;59:1337–44. doi: 10.1073/pnas.59.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY, Apostolou E, Stadtfeld M, Li Y, Shioda T, Natesan S, Wagers AJ, Melnick A, Evans T, Hochedlinger K. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nature biotechnology. 2010;28:848–55. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–80. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell C, Grant AR, Cornblath E, Goldman D. Analysis of DNA methylation reveals a partial reprogramming of the Muller glia genome during retina regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:19814–9. doi: 10.1073/pnas.1312009110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–8. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaife-Ryan GA, Sim CB, Porrello ER, Hudson JE. Resetting the epigenome for heart regeneration. Seminars in cell & developmental biology. 2016 doi: 10.1016/j.semcdb.2015.12.021. [DOI] [PubMed] [Google Scholar]
- Raphael Y. Evidence for supporting cell mitosis in response to acoustic trauma in the avian inner ear. Journal of neurocytology. 1992;21:663–71. doi: 10.1007/BF01191727. [DOI] [PubMed] [Google Scholar]
- Rauch SD, Velazquez-Villasenor L, Dimitri PS, Merchant SN. Decreasing hair cell counts in aging humans. Annals of the New York Academy of Sciences. 2001;942:220–7. doi: 10.1111/j.1749-6632.2001.tb03748.x. [DOI] [PubMed] [Google Scholar]
- Roberto M, Hamernik RP, Turrentine GA. Damage of the auditory system associated with acute blast trauma. The Annals of otology, rhinology & laryngology. Supplement. 1989;140:23–34. doi: 10.1177/00034894890980s506. [DOI] [PubMed] [Google Scholar]
- Romero-Carvajal A, Navajas Acedo J, Jiang L, Kozlovskaja-Gumbriene A, Alexander R, Li H, Piotrowski T. Regeneration of Sensory Hair Cells Requires Localized Interactions between the Notch and Wnt Pathways. Developmental cell. 2015;34:267–82. doi: 10.1016/j.devcel.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouaux C, Arlotta P. Direct lineage reprogramming of post-mitotic callosal neurons into corticofugal neurons in vivo. Nature cell biology. 2013;15:214–21. doi: 10.1038/ncb2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubbini D, Robert-Moreno A, Hoijman E, Alsina B. Retinoic Acid Signaling Mediates Hair Cell Regeneration by Repressing p27kip and sox2 in Supporting Cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:15752–66. doi: 10.1523/JNEUROSCI.1099-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240:1774–6. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- Sage C, Huang M, Karimi K, Gutierrez G, Vollrath MA, Zhang DS, Garcia-Anoveros J, Hinds PW, Corwin JT, Corey DP, Chen ZY. Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science. 2005;307:1114–8. doi: 10.1126/science.1106642. [DOI] [PubMed] [Google Scholar]
- Scheffer DI, Shen J, Corey DP, Chen ZY. Gene Expression by Mouse Inner Ear Hair Cells during Development. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:6366–80. doi: 10.1523/JNEUROSCI.5126-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya T, Muthurajan UM, Luger K, Tulin AV, Zaret KS. Nucleosome-binding affinity as a primary determinant of the nuclear mobility of the pioneer transcription factor FoxA. Genes & development. 2009;23:804–9. doi: 10.1101/gad.1775509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Kempfle JS, Edge AS. Wnt-responsive Lgr5-expressing stem cells are hair cell progenitors in the cochlea. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:9639–48. doi: 10.1523/JNEUROSCI.1064-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Hu L, Edge AS. Generation of hair cells in neonatal mice by beta-catenin overexpression in Lgr5-positive cochlear progenitors. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:13851–6. doi: 10.1073/pnas.1219952110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Cheng YF, Wang XL, Edge AS. Beta-catenin up-regulates Atoh1 expression in neural progenitor cells by interaction with an Atoh1 3′ enhancer. The Journal of biological chemistry. 2010;285:392–400. doi: 10.1074/jbc.M109.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slowik AD, Bermingham-McDonogh O. Hair cell generation by notch inhibition in the adult mammalian cristae. Journal of the Association for Research in Otolaryngology : JARO. 2013;14:813–28. doi: 10.1007/s10162-013-0414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufi A, Donahue G, Zaret KS. Facilitators and impediments of the pluripotency reprogramming factors’ initial engagement with the genome. Cell. 2012;151:994–1004. doi: 10.1016/j.cell.2012.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava D, DeWitt N. In Vivo Cellular Reprogramming: The Next Generation. Cell. 2016;166:1386–96. doi: 10.1016/j.cell.2016.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AB, Kim T, Cabot V, Hudspeth AJ. Dynamic gene expression by putative hair-cell progenitors during regeneration in the zebrafish lateral line. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E1393–401. doi: 10.1073/pnas.1318692111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanova ZP, Kwan T, Segil N. Epigenetic regulation of Atoh1 guides hair cell development in the mammalian cochlea. Development. 2015;142:3529–36. doi: 10.1242/dev.126763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone IM, Lurie DI, Kelley MW, Poulsen DJ. Adeno-associated virus-mediated gene transfer to hair cells and support cells of the murine cochlea. Molecular therapy : the journal of the American Society of Gene Therapy. 2005;11:843–8. doi: 10.1016/j.ymthe.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Stone JS, Rubel EW. Delta1 expression during avian hair cell regeneration. Development. 1999;126:961–73. doi: 10.1242/dev.126.5.961. [DOI] [PubMed] [Google Scholar]
- Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–8. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. A developmental framework for induced pluripotency. Development. 2015;142:3274–85. doi: 10.1242/dev.114249. [DOI] [PubMed] [Google Scholar]
- Takeuchi JK, Bruneau BG. Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature. 2009;459:708–11. doi: 10.1038/nature08039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarang S, Doi SM, Gurumurthy CB, Harms D, Quadros R, Rocha-Sanchez SM. Generation of a Retinoblastoma (Rb)1-inducible dominant-negative (DN) mouse model. Frontiers in cellular neuroscience. 2015;9:52. doi: 10.3389/fncel.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorel F, Nepote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464:1149–54. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tona Y, Hamaguchi K, Ishikawa M, Miyoshi T, Yamamoto N, Yamahara K, Ito J, Nakagawa T. Therapeutic potential of a gamma-secretase inhibitor for hearing restoration in a guinea pig model with noise-induced hearing loss. BMC neuroscience. 2014;15:66. doi: 10.1186/1471-2202-15-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torper O, Pfisterer U, Wolf DA, Pereira M, Lau S, Jakobsson J, Bjorklund A, Grealish S, Parmar M. Generation of induced neurons via direct conversion in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:7038–43. doi: 10.1073/pnas.1303829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–41. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhaus J, Durruthy-Durruthy R, Heller S. Quantitative High-Resolution Cellular Map of the Organ of Corti. Cell reports. 2015;11:1385–99. doi: 10.1016/j.celrep.2015.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters B, Lin W, Diao S, Brimble M, Iconaru L, Dearman J, Goktug A, Chen T, Zuo J. High-throughput screening reveals Alsterpaullone, 2-Cyanoethyl as a potent p27Kip1 transcriptional inhibitor. PloS one. 2014 doi: 10.1371/journal.pone.0091173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters BJ, Zuo J. Postnatal development, maturation and aging in the mouse cochlea and their effects on hair cell regeneration. Hearing research. 2013;297:68–83. doi: 10.1016/j.heares.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters BJ, Zuo J. A Sox10(rtTA/+) Mouse Line Allows for Inducible Gene Expression in the Auditory and Balance Organs of the Inner Ear. Journal of the Association for Research in Otolaryngology : JARO. 2015a;16:331–45. doi: 10.1007/s10162-015-0517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters BJ, Yamashita T, Zuo J. Sox2-CreER mice are useful for fate mapping of mature, but not neonatal, cochlear supporting cells in hair cell regeneration studies. Scientific reports. 2015b;5:11621. doi: 10.1038/srep11621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters BJ, Diao S, Zheng F, Walters BJ, Layman WS, Zuo J. Pseudo-immortalization of postnatal cochlear progenitor cells yields a scalable cell line capable of transcriptionally regulating mature hair cell genes. Scientific reports. 2015c;5:17792. doi: 10.1038/srep17792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Murphy R, Taaffe D, Yin S, Xia L, Hauswirth WW, Bance M, Robertson GS, Wang J. Efficient cochlear gene transfection in guinea-pigs with adeno-associated viral vectors by partial digestion of round window membrane. Gene therapy. 2012;19:255–63. doi: 10.1038/gt.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ladrech S, Pujol R, Brabet P, Van De Water TR, Puel JL. Caspase inhibitors, but not c-Jun NH2-terminal kinase inhibitor treatment, prevent cisplatin-induced hearing loss. Cancer research. 2004;64:9217–24. doi: 10.1158/0008-5472.CAN-04-1581. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu Z, Yin C, Asfour H, Chen O, Li Y, Bursac N, Liu J, Qian L. Stoichiometry of Gata4, Mef2c, and Tbx5 influences the efficiency and quality of induced cardiac myocyte reprogramming. Circulation research. 2015;116:237–44. doi: 10.1161/CIRCRESAHA.116.305547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. Journal of the Association for Research in Otolaryngology : JARO. 2002;3:248–68. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol ME, Corwin JT. Regenerative proliferation in organ cultures of the avian cochlea: identification of the initial progenitors and determination of the latency of the proliferative response. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:5466–77. doi: 10.1523/JNEUROSCI.16-17-05466.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T, Corbett MK, Chow LM, Valentine MB, Baker SJ, Zuo J. Rapid cell-cycle reentry and cell death after acute inactivation of the retinoblastoma gene product in postnatal cochlear hair cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:781–5. doi: 10.1073/pnas.0708061105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston MD, Pierce ML, Rocha-Sanchez S, Beisel KW, Soukup GA. MicroRNA gene expression in the mouse inner ear. Brain research. 2006;1111:95–104. doi: 10.1016/j.brainres.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Weston MD, Pierce ML, Jensen-Smith HC, Fritzsch B, Rocha-Sanchez S, Beisel KW, Soukup GA. MicroRNA-183 family expression in hair cell development and requirement of microRNAs for hair cell maintenance and survival. Developmental dynamics : an official publication of the American Association of Anatomists. 2011;240:808–19. doi: 10.1002/dvdy.22591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441:984–7. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- Wu J, Li W, Lin C, Chen Y, Cheng C, Sun S, Tang M, Chai R, Li H. Co-regulation of the Notch and Wnt signaling pathways promotes supporting cell proliferation and hair cell regeneration in mouse utricles. Scientific reports. 2016;6:29418. doi: 10.1038/srep29418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Li M, Chen ZT, Zhang XB, Liu HZ, Li Z, Guo WW, Zhao LD, Ren LL, Li JN, Yi HJ, Han D, Yang WY, Wu Y, Yang SM. In vivo delivery of Atoh1 gene to rat cochlea using a dendrimer-based nanocarrier. Journal of biomedical nanotechnology. 2013;9:1736–45. doi: 10.1166/jbn.2013.1684. [DOI] [PubMed] [Google Scholar]
- Xiang M, Gan L, Li D, Chen ZY, Zhou L, O’Malley BW, Jr, Klein W, Nathans J. Essential role of POU-domain factor Brn-3c in auditory and vestibular hair cell development. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:9445–50. doi: 10.1073/pnas.94.17.9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xydakis MS, Bebarta VS, Harrison CD, Conner JC, Grant GA, Robbins AS. Tympanic-membrane perforation as a marker of concussive brain injury in Iraq. The New England journal of medicine. 2007;357:830–1. doi: 10.1056/NEJMc076071. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Tanigaki K, Tsuji M, Yabe D, Ito J, Honjo T. Inhibition of Notch/RBP-J signaling induces hair cell formation in neonate mouse cochleas. Journal of molecular medicine. 2006;84:37–45. doi: 10.1007/s00109-005-0706-9. [DOI] [PubMed] [Google Scholar]
- Yang A, Zhu Z, Kapranov P, McKeon F, Church GM, Gingeras TR, Struhl K. Relationships between p63 binding, DNA sequence, transcription activity, and biological function in human cells. Molecular cell. 2006;24:593–602. doi: 10.1016/j.molcel.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Yang J, Bouvron S, Lv P, Chi F, Yamoah EN. Functional features of trans-differentiated hair cells mediated by Atoh1 reveals a primordial mechanism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012a;32:3712–25. doi: 10.1523/JNEUROSCI.6093-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SM, Chen W, Guo WW, Jia S, Sun JH, Liu HZ, Young WY, He DZ. Regeneration of stereocilia of hair cells by forced Atoh1 expression in the adult mammalian cochlea. PloS one. 2012b;7:e46355. doi: 10.1371/journal.pone.0046355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Akinci E, Dutton JR, Banga A, Slack JM. Stage specific reprogramming of mouse embryo liver cells to a beta cell-like phenotype. Mechanisms of development. 2013;130:602–12. doi: 10.1016/j.mod.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Mostajo-Radji MA, Brown JR, Rouaux C, Tomassy GS, Hensch TK, Arlotta P. Instructing Perisomatic Inhibition by Direct Lineage Reprogramming of Neocortical Projection Neurons. Neuron. 2015;88:475–83. doi: 10.1016/j.neuron.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476:228–31. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Wang Y, Chang Q, Wang J, Gong S, Li H, Lin X. Virally expressed connexin26 restores gap junction function in the cochlea of conditional Gjb2 knockout mice. Gene therapy. 2014;21:71–80. doi: 10.1038/gt.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Weber T, Yamashita T, Liu Z, Valentine MB, Cox BC, Zuo J. In vivo proliferation of postmitotic cochlear supporting cells by acute ablation of the retinoblastoma protein in neonatal mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:5927–36. doi: 10.1523/JNEUROSCI.5989-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret KS, Carroll JS. Pioneer transcription factors: establishing competence for gene expression. Genes & development. 2011;25:2227–41. doi: 10.1101/gad.176826.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Lin YH, Sun YJ, Zhu S, Zheng J, Liu K, Cao N, Li K, Huang Y, Ding S. Pharmacological Reprogramming of Fibroblasts into Neural Stem Cells by Signaling-Directed Transcriptional Activation. Cell stem cell. 2016;18:653–67. doi: 10.1016/j.stem.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhang Y, Lobler M, Schmitz KP, Ahmad A, Pyykko I, Zou J. Nuclear entry of hyperbranched polylysine nanoparticles into cochlear cells. International journal of nanomedicine. 2011;6:535–46. doi: 10.2147/IJN.S16973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nature neuroscience. 2000;3:580–6. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–32. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuris JA, Thompson DB, Shu Y, Guilinger JP, Bessen JL, Hu JH, Maeder ML, Joung JK, Chen ZY, Liu DR. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nature biotechnology. 2015;33:73–80. doi: 10.1038/nbt.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]