Abstract
Here, we provide an overview of pathways available upon the gas-phase oxidation of peptides and DNA via ion/ion reactions and explore potential applications of these chemistries. The oxidation of thioethers (i.e., methionine residues and S-alkyl cysteine residues), disulfide bonds, S-nitrosylated cysteine residues, and DNA to the [M+H+O]+ derivative via ion/ion reactions with periodate and peroxymonosulfate anions is demonstrated. The oxidation of neutral basic sites to various oxidized structures, including the [M+H+O]+, [M-H]+, and [M-H-NH3]+ species, via ion/ion reactions is illustrated and the oxidation characteristics of two different oxidizing reagents, periodate and persulfate anions, are compared. Lastly, the highly efficient generation of molecular radical cations via ion/ion reactions with sulfate radical anion is summarized. Activation of the newly generated molecular radical peptide cations results in losses of various neutral side chains, several of which generate dehydroalanine residues that can be used to localize the amino acid from which the dehydroalanine was generated. The chemistries presented herein result in a diverse range of structures that can be used for a variety of applications including the identification and localization of S-alkyl cysteine residues, the oxidative cleavage of disulfide bonds, and the generation of molecular radical cations from even-electron doubly protonated peptides.
Keywords: Ion/ion reaction, oxidation, tandem mass spectrometry, radical cations
Graphical Abstract
INTRODUCTION
The oxidation of peptides and proteins is a ubiquitous event that has been implicated in both normal and damaging cellular processes [1]. For example, protein oxidation has been implicated in signaling [2,3], gene regulation [4,5], aging [6,7], cancer [8,9], diabetes [10,11], atherosclerosis [12,13], and Parkinson’s [14] and Alzheimer’s diseases [15,16]. The oxidation of proteins results from interactions with reactive oxygen and nitrogen species that are either present natively or are generated as byproducts of oxygen metabolism [1,17]. Oxidative modifications can appear in a wide variety of forms ranging from the addition of an oxygen atom on amino acid side chains to protein cross-linking [1]. For example, proline, arginine, and lysine side chains can be oxidized to carbonyl derivatives [7,18], methionine is commonly oxidized to methionine sulfoxide, and less commonly to methionine sulfone [19], and cysteine oxidation can lead to intra- or inter-protein crosslinks [20,21,22]. Oxidative stress in red blood cells has been shown to induce hemoglobin crosslinking to the cytoskeleton, resulting in association of the heme center with the phospholipid membrane and allowing it to act as a local Fenton reagent [23]. Moreover, reactive oxygen and nitrogen species can also interact with and oxidize other bioanalytes, such as DNA, sugars, and lipids [1,24,25,26]. For these and other reasons it is important to study the oxidation of bioanalytes.
Tandem mass spectrometry (MSn) has been used for the analysis of various modifications [27,28,29] of peptides and proteins and is the most informative method currently used in the analysis of oxidative modifications [30]. As most types of oxidation result in a change in the overall mass of the peptide or protein of interest, MS is uniquely equipped to detect these changes and has been used to detect methionine oxidation [31], analyze glutathionylated hemoglobin [32], and determine the total load of oxidative modifications on a protein [33]. Mass spectrometric analysis of oxidation has furthermore been applied in the study of several diseases including Alzheimer’s disease [16], diabetes [34], breast cancer [35], and influenza [36]. Some oxidative modifications can alter the fragmentation of peptides and proteins upon collision-induced dissociation (CID). For example, oxidation of the methionine side chain to the sulfoxide derivative results in highly efficient ejection of methanesulfenic acid (64 Da) [37,38,39,40,41], and cysteine residues oxidized to form intra- or intermolecular disulfide bonds can inhibit sequence information obtained for the regions protected within the disulfide linkage [42,43,44].
Recently, the oxidation of multiply protonated polypeptide cations to various forms, including [M+H+O]+, [M-H]+, and M+• species, in the gas-phase via ion/ion reactions (i.e., the interaction of oppositely charged ions within a mass spectrometer) has been demonstrated [45,46]. Ion/ion reactions have several benefits over traditional solution-phase techniques for generating oxidized species including, for example, much faster reaction times, reduction of side-reactions via mass isolation of both reactants, and control over the extent of modification (i.e., the number of modifications that occur) [47]. Periodate anion (IO4−) selectively oxidizes non-modified peptides containing methionine residues, and to a lesser extent, tryptophan residues, to the oxygen transfer species [M+H+O]+ [45]. Methionine-containing peptides then undergo efficient loss of 64 Da, corresponding to ejection of methanesulfenic acid, upon collision-induced dissociation (CID) of the [M+H+O]+ species, in agreement with activation of methionine sulfoxide-containing peptides generated in solution [37–41,45]. Periodate anion is also capable of oxidizing neutral lysine and arginine residues, predominantly to the hydrogen deficient species [M-H]+, in systems lacking other easily oxidized residues [48]. A suite of reagents generated upon negative nanoelectrospray ionization (nESI) of an aqueous solution of sodium persulfate, i.e., persulfate anion (HS2O8−), peroxymonosulfate anion (HSO5−), and sulfate radical anion (SO4-•), have also been shown to be oxidizing reagent anions for ion/ion reactions [46]. Peroxymonosulfate anion also generates oxygen transfer species upon activation of peroxymonosulfate-peptide complexes, and is a stronger oxidizing reagent anion than periodate as species both containing and lacking methionine/tryptophan residues undergo oxidation. Persulfate anion generates both oxygen transfer and hydrogen deficient species upon activation of the persulfate-peptide complex. Sulfate radical anion abstracts both a proton and a hydrogen atom upon activation of the ion/ion complex to generate a molecular radical cation.
Here, we summarize and illustrate the various chemistries that occur upon oxidation ion/ion reactions and present novel examples of the applications for which these chemistries can be used. The oxygen transfer chemistry is demonstrated here using clusters of periodate anions (i.e., H(IO4)2−), which results in oxidation of two methionine residues in the same peptide in a single ion/ion reaction step. Furthermore, the oxidation of maleimide-protected cysteine residues to the [M+H+O]+ derivative is shown for the first time. The oxidation of a S-nitroso peptide is also demonstrated, though the NO• loss is too facile to detect the intact [M+H+O]+ species. Rather, the [M+H+O-NO•]+• species is observed and undergoes radical-directed cleavages that confirm that the oxidation occurs on the S-nitroso cysteine sulfur atom. The selective oxidation of guanine nucleobases in a doubly protonated DNA oligomer is demonstrated here for the first time. The oxidation of neutral basic sites to [M+H+O]+, [M-H]+, and [M-H-NH3]+ species is demonstrated and the reactivities of periodate and persulfate anions are compared. Lastly, the generation of radical cations via ion/ion reactions with sulfate radical anion is briefly discussed. The oxidation chemistry described here results in the generation of a wide variety of species that can undergo fragmentation pathways dramatically distinct from their non-oxidized counterparts, thereby enabling strategies to facilitate the structural characterization of molecules with readily oxidizable sites.
EXPERIMENTAL SECTION
Materials
Methanol and glacial acetic acid were purchased from Mallinckrodt (Phillipsburg, NJ). ARAMAKA, ARACAKA, HAHAHAA, and GRGMGRGMGRL were synthesized by Pepnome Limited (Shenzhen, China). KAKAKAA was synthesized by NeoBioSci (Cambridge, MA) and RARARAA was synthesized by CHI Scientific (Maynard, MA). KGAILCGIALK was synthesized by CPC Scientific (Sunnyvale, CA, USA). Sodium persulfate, sodium periodate, insulin, pepsin, S-nitrosoglutathione, and N-hydroxymaleimide were purchased from Sigma Aldrich (St. Louis, MO). ACCGAG was purchased from Integrated DNA Technologies (Coraville, IA, USA). All peptide and DNA stock solutions for positive nanoelectrospray were prepared in a 49.5/49.5/1 (vol/vol/vol) solution of methanol/water/acetic acid at an initial concentration of ~1 mg/mL and diluted 100-fold prior to use. The periodate solution was prepared in a 50/50 (vol/vol) solution of methanol/water at a concentration of ~1 mg/mL and diluted 10-fold prior to use. The proton bound dimer of periodate was observed upon negative nESI of a solution of sodium periodate in 50/50 vol/vol water/acetic acid. The persulfate solution was prepared in an aqueous solution at a concentration of ~1 mg/mL and diluted 10-fold prior to use.
Mass Spectrometry
All experiments were performed on a QTRAP 4000 hybrid triple quadrupole/linear ion trap mass spectrometer (AB Sciex, Concord, ON, Canada), previously modified for ion/ion reactions [49]. Multiply protonated peptides and singly charged anion reagent populations were sequentially injected into the instrument via alternately pulsed nano-electrospray (nESI) [50]. The peptide cations and oxidizing reagent anions were independently isolated in the Q1-mass filter prior to injection into the q2 reaction cell. Intact persulfate and sulfate radical anion were observed upon negative nESI of an aqueous solution of sodium persulfate. Peroxymonosulfate anion was also observed at a minor abundance upon negative nESI of the sodium persulfate solution. However, a large nozzle-skimmer voltage difference increased the abundance of this ion species as it is a fragment generated from intact persulfate anion [46]. The ions of opposite polarity were allowed to react for a mutual storage reaction time of 20–1000 ms. Reactions with sulfate radical anion were optimized for time as the radical anion is extremely reactive and can react with neutrals in the trap. Mutual storage times for reactions with sulfate radical anion ranged from 20 – 200 ms. The ion/ion reaction products were then transferred to Q3, where the product ions were subjected to further characterization via MSn and mass analysis using mass-selective axial ejection (MSAE) [51].
Syntheses
S-nitrosylated KGAILCGIALK was synthesized by combining 1 mg peptide with 1 mg S-nitrosoglutathione in 1 mL of water at 37 °C for 1 hr. The S-nitrosylation mixture was then diluted 100 fold in 49.5/49.5/1 vol/vol/vol methanol/water/acetic acid for electrospray. The peptic digest was performed by combining 0.1 mg insulin with 0.025 mg pepsin in 1% acetic acid at 37 °C for 6 hr [52]. The digestion mixture was diluted to 1–5 µM in 49/49/2 vol/vol/vol methanol/water/acetic acid and used without further purification. For the maleimide protection 0.1 mg N-hydroxymaleimide was added to a 1 mg/mL solution of the peptide ARACAKA in methanol. The solution was shaken at room temperature for 10 min, reconstituted in 49.5/49.5/1 vol/vol/vol methanol/water/acetic acid, and sprayed via positive nESI without any further purification.
RESULTS AND DISCUSSION
Generation of [M+H+O]+ Species: Thioethers
The first demonstration of the gas-phase oxidation of peptides via ion/ion reactions involved methionine-containing peptides [45]. Doubly protonated peptides were subjected to ion/ion reactions with periodate anion. Upon reaction, all peptides underwent complex formation with periodate anion to generate the ion/ion complex [M+2H+IO4]+. However, only peptides containing methionine, and to a much lesser extent, tryptophan residues, generated an oxidized [M+H+O]+ species upon activation of the ion/ion complex. All other peptides examined underwent loss of periodic acid (HIO4) upon activation of the ion/ion complex to generate the charge-reduced [M+H]+ species. Activation of the [M+H+O]+ species generated in the gas-phase was compared to the [M+H+O]+ species generated via solution-phase oxidation and the resulting spectra were identical suggesting that these two species were of the same primary structure. It was then found that the gas-phase ion/ion oxidation of thioethers was a general phenomenon as S-alkyl cysteine residues were also shown to undergo this oxygen transfer chemistry [53]. This was demonstrated with S-ethyl, S-farnesyl, and S-carbamidomethyl cysteine residues upon ion/ion reactions between doubly protonated peptides and both periodate and peroxymonosulfate anions. Furthermore, thioethers oxidized to the sulfoxide form undergo a facile rearrangement that leads to the loss of alkyl sulfenic acids (RSOH) [37–41,54]. For methionine residues this results in a loss of 64 Da corresponding to the ejection of methanesulfenic acid, a pathway that is well documented by groups investigating the solution-phase oxidation of methionine residues [37–41]. Interestingly, the loss of alkyl sulfenic acids from oxidized S-alkyl cysteine residues results in the generation of a dehydroalanine residue (Dha) at the site of the original modified residue [53,54,55,56]. This is especially useful as Dha residues open a low energy pathway that leads to cleavage of the N-Cα bond of the Dha residue to generate c- and z-ions upon CID [56,57,58]. As c- and z-ions are not generally observed upon CID of protonated even-electron species, these ions can be used to identify the presence of Dha and localize the original S-alkyl cysteine within the peptide.
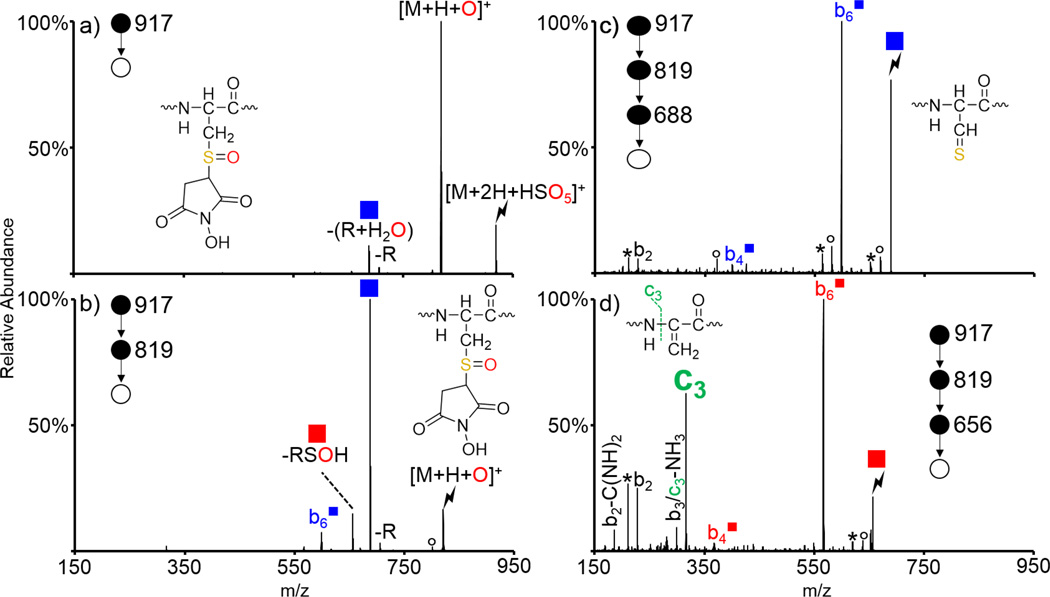
While we have reported on the oxidation of cysteine residues alkylated with common protecting agents (e.g., iodoacetamide and alkyl halides) [53], maleimide chemistry is another common method of protecting cysteine residues, the oxidation behavior of which is related here. The peptide ARACAKA was reacted with N-hydroxymaleimide to generate ARACmAKA (superscript m indicates that the cysteine has been protected with N-hydroxymaleimide) and the doubly protonated species was subjected to ion/ion reactions with an oxidizing reagent anion (Supplemental Figure S-1). Periodate anion (IO4−) and peroxymonosulfate anion (HSO5−) both generate the oxygen transfer [M+H+O]+ species upon activation of ion/ion complexes with peptides containing thioethers, and are largely interchangeable for the examples shown here. A variety of examples using both oxidizing reagent anions are shown to demonstrate the versatility of the chemistry described herein. Activation of the ion/ion complex between doubly protonated ARACmAKA and HSO5− is shown in Figure 1(a) and predominantly generates the oxygen transfer species [M+H+O]+. The structure of the oxidized species is shown in the insets of Figures 1(a) and (b). The [M+H+O]+ species was then isolated and subjected to CID and the resulting spectrum is shown in Figure 1(b). Similar to other S-alkyl cysteine residues, oxidized ARACmAKA loses the maleimide protecting group as an alkyl sulfenic acid to generate a dehydroalanine-containing peptide (indicated by a red square, -RSOH in Figure 1). The mechanism for the loss of alkyl sulfenic acids upon CID of sulfoxide derivatives has been discussed previously [53,54,56]. Unique to this protecting group, however, is the dominant nominal loss of the maleimide group and a water (indicated by a blue square, -(R+H2O) in Figure 1). This loss occurs via abstraction of the β-hydrogen atom on the maleimide group rather than the peptide backbone and has been previously observed and discussed in detail for maleimide-protected cysteine residues oxidized in solution [59]. Activation of the -(R+H2O) species is shown in Figure 1(c) and generates a variety of b-ions, including the b2, 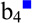 , and
, and 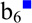 species. The blue square superscript indicates fragments that have lost the (R+H2O) group and can be used to localize the modification. Activation the dehydroalanine-containing species generated by alkyl sulfenic acid loss is shown in Figure 1(d) and generates an abundant c3 ion in addition to the dominant
species. The blue square superscript indicates fragments that have lost the (R+H2O) group and can be used to localize the modification. Activation the dehydroalanine-containing species generated by alkyl sulfenic acid loss is shown in Figure 1(d) and generates an abundant c3 ion in addition to the dominant 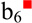 ion (red square superscripts indicate fragments containing a Dha residue whereas peaks shown in green are derived from the dehydroalanine effect). The peak 17 Da below the c3 ion could either correspond to the b3 ion or a sequential ammonia loss from the c3 ion. Previous results have shown that c-ions generated via the dehydroalanine effect oftentimes readily lose ammonia indicating that this fragment is likely sequential in nature and is also derived from the dehydroalanine effect [53,55]. Since the dehydroalanine effect generates c- and z-ions N-terminal to the location of the original S-alkyl cysteine residue, this ion indicates that the modified cysteine residue is the fourth residue in the peptide, which could be useful in de novo sequencing applications. The control spectrum corresponding to CID of the protonated species is shown in Supplemental Figure S-2 and predominantly yields the b6 ion. Some loss of the protecting group is observed along with loss of the protecting group as a thiol to generate the dehydroalanine-containing species. A minor c3 ion derived from sequential fragmentation of the Dha-containing species is also observed.
ion (red square superscripts indicate fragments containing a Dha residue whereas peaks shown in green are derived from the dehydroalanine effect). The peak 17 Da below the c3 ion could either correspond to the b3 ion or a sequential ammonia loss from the c3 ion. Previous results have shown that c-ions generated via the dehydroalanine effect oftentimes readily lose ammonia indicating that this fragment is likely sequential in nature and is also derived from the dehydroalanine effect [53,55]. Since the dehydroalanine effect generates c- and z-ions N-terminal to the location of the original S-alkyl cysteine residue, this ion indicates that the modified cysteine residue is the fourth residue in the peptide, which could be useful in de novo sequencing applications. The control spectrum corresponding to CID of the protonated species is shown in Supplemental Figure S-2 and predominantly yields the b6 ion. Some loss of the protecting group is observed along with loss of the protecting group as a thiol to generate the dehydroalanine-containing species. A minor c3 ion derived from sequential fragmentation of the Dha-containing species is also observed.
Figure 1.
Oxidation of ARACmAKA via ion/ion reactions with HSO5−, where Cm indicates a cysteine residue protected with N-hydroxymaleimide. CID of (a) the ion/ion complex between doubly protonated ARACmAKA and HSO5−, (b) the [M+H+O]+ species, (c) loss of (R+H2O) from the oxidized species, and (d) the dehydroalanine species generated upon loss of the alkyl sulfenic acid from the oxidized species. Lightning bolts indicate the species subjected to CID. Asterisks indicate ammonia losses, degree signs indicate water losses, red and blue squares indicate loss of RSOH and (R+H2O) from the oxidized species, respectively. Red/blue fragments with square superscripts indicate fragment ions that have lost RSOH/(R+H2O).
Generation of [M+H+O]+ Species: Disulfide bonds
Similar to thioethers, disulfide bonds were also found to be susceptible to oxidation via ion/ion reactions [60]. Activation of the ion/ion complexes between intermolecularly disulfide-linked peptides and oxidizing reagent anions generates a combination of species resulting from (i) proton transfer from the peptide dication to the reagent anion to yield the charge-reduced [M+H]+ species, (ii) oxygen transfer from the reagent to the peptide to generate the oxidized thiosulfinate [M+H+O]+ derivative, and (iii) species corresponding to oxidative cleavage of the disulfide bond, likely due to secondary fragmentation of the fragile [M+H+O]+ species. While disulfide bonds are important for the structure and function of proteins, their presence can complicate MSn analysis, as CID of protonated peptides does not typically result in cleavage of the disulfide bonds [42]. Solution-phase oxidation is commonly done to cleave disulfide bonds, but it results in oxidation of the linked cysteine residues all the way to the cysteic acid derivative and may result in oxidation of other sites on the peptide/protein of interest [61].
The gas-phase oxidation approach described here allows greater control over the extent of oxidation and enables the addition of exactly one oxygen atom and the cleavage of one disulfide bond. More oxygen atoms can be added via sequential ion/ion reactions with multiply charged species if needed. Other methods which selectively cleave disulfide bonds in the gas-phase, including irradiation at 266 nm [62], ion/ion reactions with transition metal-containing species [63,64,65], CID of peptides/proteins with limited proton mobility [66], and electron capture [67], electron transfer [68], and electron detachment dissociation [69], have also been described. The oxidation chemistry described here has the benefit of labeling disulfide bonds via the addition of an oxygen atom, which has been used to identify peaks of interest derived from enzymatic digestion mixtures of lysozyme and insulin without isolation or prior chromatographic separation [60]. All ions resulting from positive nESI of the digestion mixtures were subjected to ion/ion reaction experiments with an oxidizing reagent anion (IO4−) followed by a short period of dipolar DC excitation, a broad-band activation technique, to fragment the resulting ion/ion complexes. This experiment was then repeated using a proton transfer reagent anion (IO3−). The resulting proton transfer spectrum (generated via reaction with IO3−) was subtracted from the resulting oxidation spectrum (generated via reaction with IO4−), leaving only species that had undergone oxidation and resulted in a mass shift. This labeling procedure eliminates the need for reduction and alkylation in traditional disulfide-mapping experiments as well as pre-MS chromatographic separations, which are time-consuming.
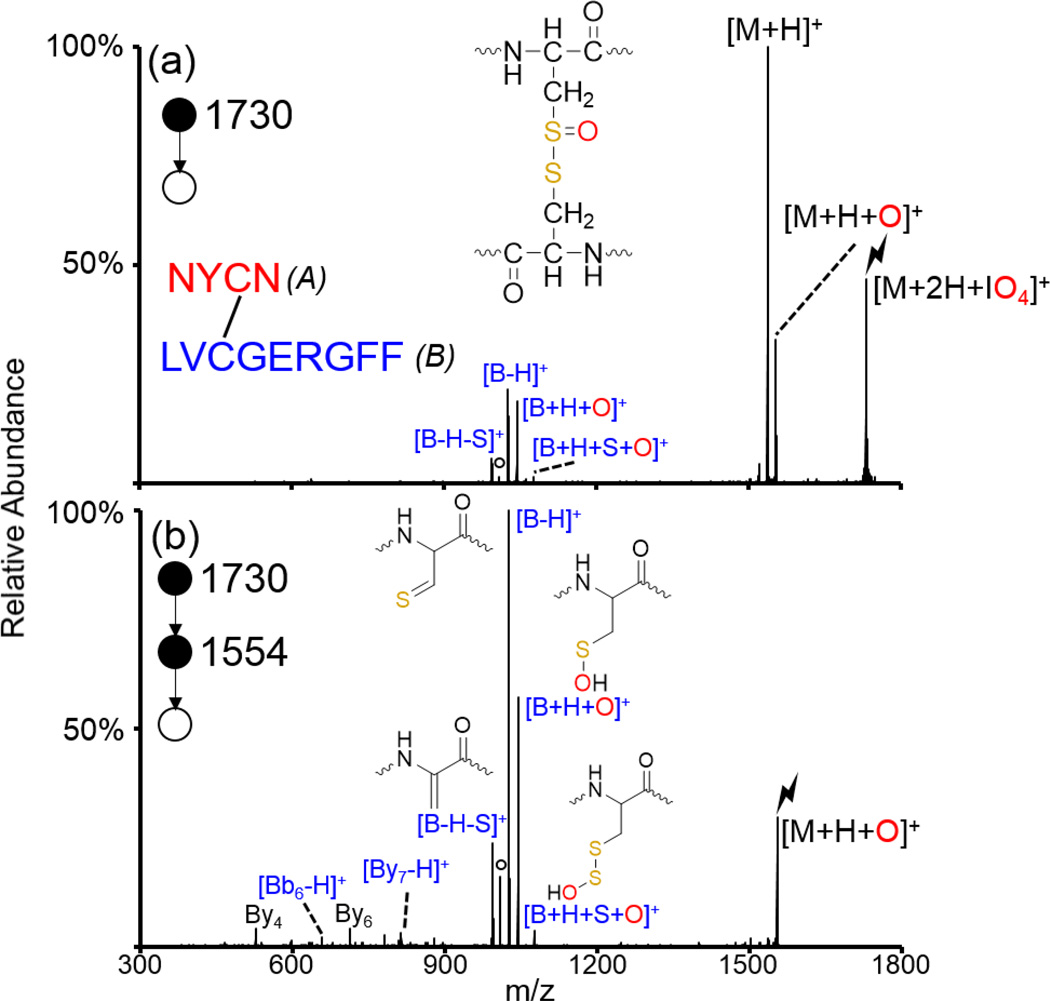
The peptide NYCN/LVCGERGFF (slashes indicate two distinct peptides connected via intermolecular disulfide bond, the peptide on the left is referred to herein as ‘A’ while the peptide on the right is referred to as ‘B’) was generated from a peptic digest of insulin, a peptide hormone consisting of one intramolecular and two intermolecular disulfide bonds. Activation of the protonated peptide is shown in Supplemental Figure S-3 and no fragments corresponding to cleavage of the disulfide bond are observed. Doubly protonated NYCN/LVCGERGFF was subjected to ion/ion reactions with IO4− and the resulting ion/ion complex was subjected to CID (Figure 2(a)). Generation of the proton transfer product, the oxygen transfer [M+H+O]+ species, and fragments corresponding to oxidative cleavage of the disulfide bond are observed. Isolation and activation of the [M+H+O]+ species is shown in Figure 2(b) and almost exclusively results in the generation of fragments corresponding to the oxidative cleavage of the disulfide bond (indicated in blue). The dominant peak observed is the [B-H]+ species, indicating that the two chains have separated and the terminal carbon and sulfur atoms in the cysteine residue are connected by a double bond (similar in structure to the maleimide-protected cysteine after the loss of R+H2O). The cluster of peaks centered around the [B-H]+ ion (i.e., the [B+H+O]+, [B+H+S+O]+, and [B-H-S]+ ions) are a signature pattern for the oxidative cleavage of intermolecular disulfide bonds and can be used to indicate one of the two originally disulfide-linked peptides [60]. This cluster of peaks is derived from abstraction of various hydrogen atoms by the oxygen or sulfur atoms as discussed previously [60]. There are also some smaller fragment ions corresponding to backbone cleavages along the B-chain (viz., By4, By6, Bb6-H, and By7-H). The Bb6-H and By7-H fragments are both secondary fragments derived from sequential cleavage of the disulfide bond and the peptide backbone. The By6 and By7-H ions localize the cysteine residue within the peptide chain. No fragments derived from the A-chain are observed, likely due to the lesser proton affinity of the A-chain as compared to the B-chain. The oxidative cleavage of disulfide bonds is useful in the analysis of peptides, proteins, and post-translational modifications, such as S-glutathionylation and S-cysteinylation, in which low molecular weight thiols are disulfide-linked to peptides/proteins [60].
Figure 2.
Oxidation of an intermolecularly disulfide-linked peptide generated from a peptic digest of insulin. CID of (a) the ion/ion complex between doubly protonated NYCN/LVCGERGFF and IO4−, and (b) CID of the oxidized species. The sequence of the peptide is shown inset in (a) along with the structure of the [M+H+O]+ species. Structures of the [B+H+S+O]+, [B+H+O]+, [B-H]+, and [B-H-S]+ species are shown in (b). Fragments derived from cleavage of the disulfide bond are indicated in blue as all fragments observed correspond to fragments from the B-chain (indicated in blue in inset). Lightning bolts indicate species subjected to CID and degree signs indicate water losses.
Generation of [M+H+O]+ Species: S-Nitroso Peptides
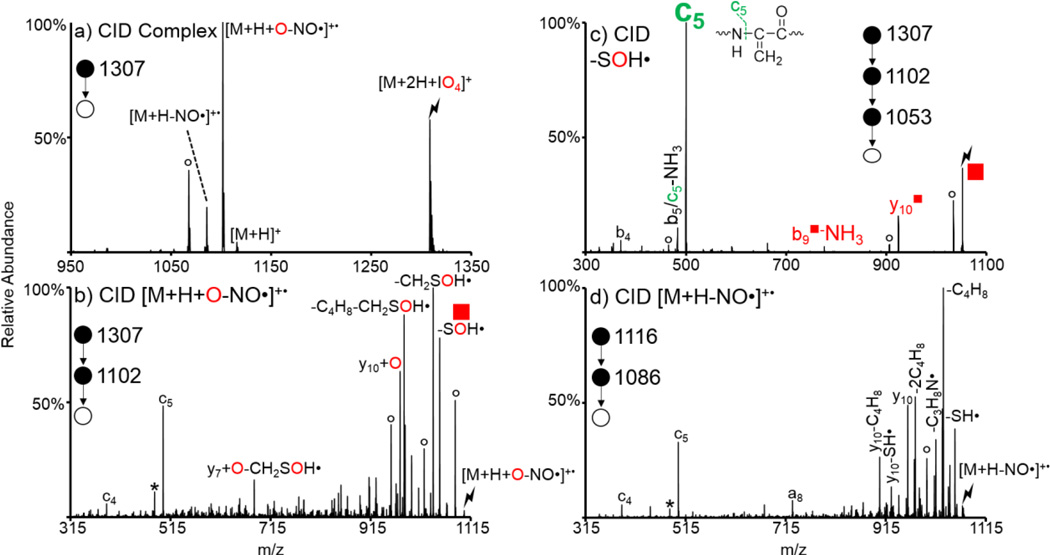
In addition to alkylation and the formation of disulfide bonds, the nitrosylation of sulfur atoms in cysteine residue is a common post-translational modification [70]. Activation of these S-nitroso compounds predominantly results in loss of NO• to generate a radical peptide cation [71]. As the modification is immediately lost upon CID it can be difficult to determine the site of original modification. S-nitroso cysteine residues are also susceptible to oxidation upon ion/ion reactions with oxidizing reagent anions, as is shown in Figure 3 for the reaction between doubly protonated S-NO KGAILCGIALK and IO4−. Activation of the ion/ion complex [M+2H+IO4]+ is shown in Figure 3(a) and generates a variety of peaks, the most prominent of which is the sequential loss of NO• from the oxidized species, i.e., [M+H+O-NO•]+•. Interestingly, no intact [M+H+O]+ is observed; presumably due to the facile nature of NO• loss. Furthermore, the proton transfer charge-reduced [M+H]+ species is observed, along with the sequential NO• loss. Activation of the [M+H+O-NO•]+• species is shown in Figure 3(b) and results in a variety of neutral side chain losses and peptide backbone fragments. This spectrum is very similar to the control, viz., CID of the [M+H-NO•]+•, shown in Figure 3(d), with the exception of the losses of SOH• and CH2SOH• instead of SH• and CH2SH• and the presence of the y10+O as opposed to the y10. These peaks indicate that the oxidation has occurred on the sulfur atom of the original S-NO cysteine residue. An additional step of activation on the SOH• loss, which generates a Dha selectively at the site of the original S-nitrosylated cysteine, was done to localize the site of the S-nitrosylation (Figure 3(c)). The c5 ion is derived from the dehydroalanine effect and is the most dominant fragment observed in Figure 3(c). Since unmodified cysteine residues do not undergo oxidation the SOH• loss only occurs from, and selectively generates dehydroalanine at, modified (i.e., S-nitrosylated) cysteine residues. The enhanced generation of c- and z-ions N-terminal to Dha residues makes localization of the S-nitrosylation within the peptide straightforward.
Figure 3.
Oxidation of S-NO KGAILCGIALK via ion/ion reactions with IO4−. Ion trap CID of (a) the ion/ion complex between doubly protonated S-NO KGAILCGIALK and IO4−, (b) the oxidized [M+H+O-NO•]+• species, (c) the SOH• loss species ( ) from (b) to generate the Dha species, and (d) the NO• loss from the protonated peptide (control spectrum). Lightning bolts indicate species subjected to CID, degree signs indicate water losses, and asterisks indicate ammonia losses.
) from (b) to generate the Dha species, and (d) the NO• loss from the protonated peptide (control spectrum). Lightning bolts indicate species subjected to CID, degree signs indicate water losses, and asterisks indicate ammonia losses.
Generation of [M+H+O]+ Species Using Clusters
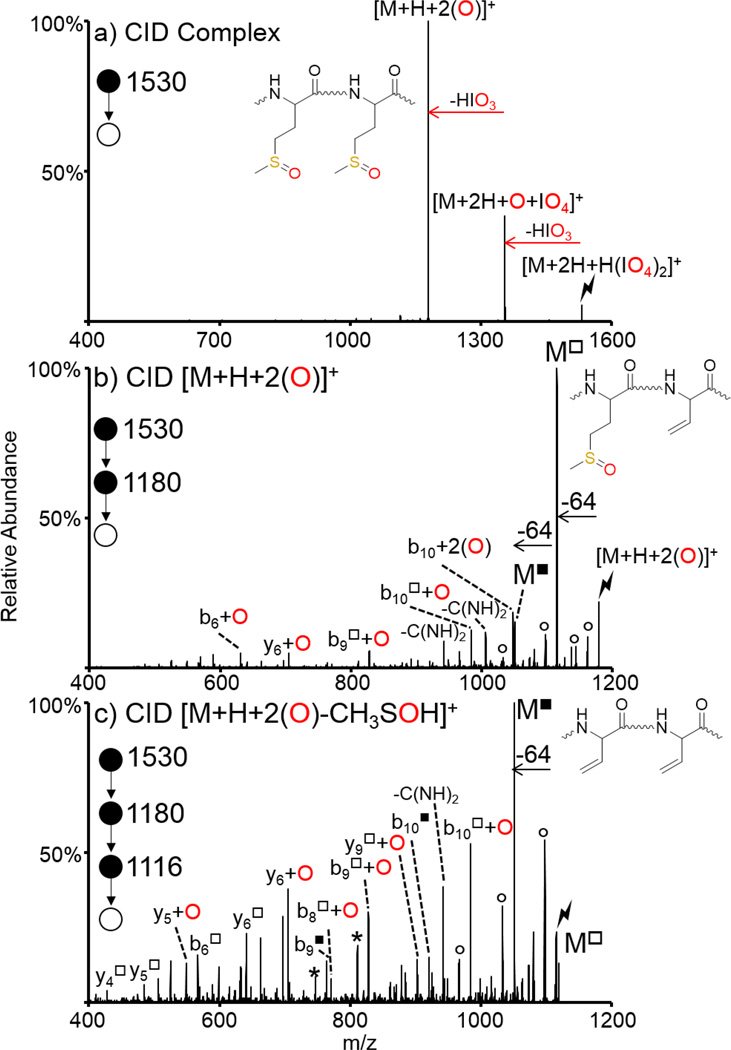
All gas-phase ion/ion oxidation experiments described to date have used a single oxidizing reagent anion to oxidize a peptide once. While sequential ion/ion reactions can be performed to add multiple oxidizing reagent anions and thus add multiple oxygen atoms to a peptide, this requires the use of a more highly charged peptide precursor to avoid neutralization, which is not always practical. One way to avoid this is the use of reagent clusters, as has been previously shown for the modification of multiple amine sites in peptides [72]. Here, we show the ion/ion reaction between a doubly protonated peptide containing two methionine residues, GRGMGRGMGRL, and a proton bound dimer of two periodate anions, H(IO4)2−. Activation of the ion/ion complex, [M+2H+H(IO4)2]+ is shown in Figure 4(a) and results in two dominant sequential losses of HIO3 to generate [M+2H+O+IO4]+ and [M+H+2(O)]+, respectively. Activation of the [M+H+2(O)]+ species is shown in Figure 4(b) and predominantly results in loss of 64 Da corresponding to the ejection of methane sulfenic acid, as discussed previously (fragments that have lost one methanesulfenic acid moiety are denoted with a hollow square superscript). Further activation of the 64 Da loss from the [M+H+2(O)]+ species is shown in Figure 4(c) and results in another dominant loss of 64 Da corresponding to ejection of another methanesulfenic acid (fragments that have lost two methanesulfenic acid moieties are indicated with a solid square superscript in Figure 4) from the second methionine residue, indicating that both methionine residue side chains have undergone oxidation. There is also a variety of backbone fragments in Figure 4 (b) and (c) that can be used to localize the oxidation sites to the two methionine side chains. The triply charged precursor of this peptide has previously been subjected to sequential ion/ion reactions with IO4− to generate the doubly adducted [M+3H+2IO4]+ species [45]. This species reacts almost identically to the one generated here using reagent cluster anions, however, it required a more highly charged precursor and multiple reagent anion adduction steps. The use of cluster reagent anions is a useful way to oxidize a peptide multiple times while minimizing the ‘charge tax’ associated with ion/ion reactions.
Figure 4.
Oxidation of both methionine residues in GRGMGRGMGRL via a single ion/ion reaction with a periodate cluster. CID of (a) ion/ion complex between doubly protonated GRGMGRGMGRL and H(IO4)2−, (b) the doubly oxidized [M+H+2(O)]+ species, and (c) the 64 Da loss (CH3SOH) from the doubly oxidized species. Insets show methionine residues in GRGMGRGMGRL where (a) both Met residues are oxidized, (b) one of the Met residues has undergone loss of CH3SOH (64 Da), and (c) both Met residues have lost CH3SOH. Lightning bolts indicate species subjected to CID, asterisks indicate ammonia losses, and degree signs indicate water losses. Hollow square superscripts indicate species that have lost one CH3SOH group while solid square superscripts indicate species that have lost two CH3SOH groups.
Generation of [M+H+O]+ Species: DNA
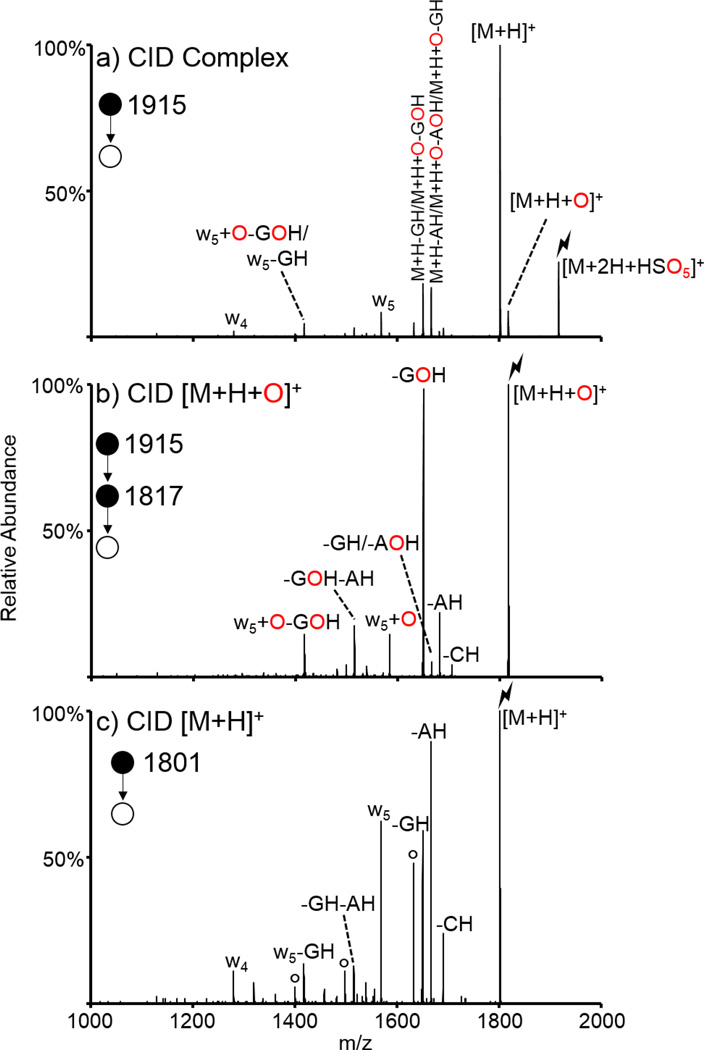
All ion/ion oxidation experiments described to this point involved doubly protonated peptides and oxidizing reagent anions. Here, we show the oxidation of another type of bioanalyte of interest, DNA, via ion/ion reactions. The doubly protonated oligonucleotide ACCGAG was subjected to ion/ion reactions with HSO5−. The resulting ion/ion complex was subjected to CID and generated the spectrum shown in Figure 5(a). Activation of the [M+2H+HSO5]+ species resulted in proton transfer, oxygen transfer, and some secondary fragments, likely due to energetic transfer conditions between q2 and Q3. Further isolation and activation of the oxygen transfer species resulted in the spectrum shown in Figure 5(b), while activation of the proton transfer species generated the spectrum shown in Figure 5(c). The most striking difference between the spectra of Figures 5(b) and 5(c) is the presence of the prominent loss of the oxidized guanine base (-GOH) upon CID of the oxidized species. This species both indicates that one of the two guanine nucleobases undergoes oxidation upon reaction with peroxymonosulfate anion and demonstrates that the loss of an oxidized guanine is a more energetically favorable pathway than the loss of any other base. The small peak 16 Da above the GOH loss can correspond to either the loss of non-oxidized guanine, i.e., GH, or the loss of oxidized adenine, i.e., AOH. Ion/ion reactions were done between the poly-A 6-mer and peroxymonosulfate (data not shown) and the oxidized species was observed upon CID of the ion/ion complex, along with some loss of AOH upon CID of the oxidized species, indicating that this GH/AOH loss peak observed for ACCGAG likely consists of a combination of the two structures. However, the dominant loss of GOH from the precursor as well as smaller sequential losses of GOH from fragment ions indicate that the vast majority of the oxidation occurs selectively on the guanine. Also present in the spectrum corresponding to CID of the oxidized species are sequential base losses (i.e., -GOH-AH), and the oxidized w5 ion, which also undergoes loss of GOH. This is almost identical to the control spectrum except for the addition of oxygen to the w5 ion and the lack of the w4 ion. Furthermore, guanine is the most readily oxidized nucleobase in condensed-phase experiments [73,74], supporting the observations from this gas-phase oxidation experiment. This is the first demonstration of the oxidation of DNA via ion/ion reactions.
Figure 5.
Oxidation of DNA via ion/ion reactions with HSO5−. CID of (a) the ion/ion complex between ACCGAG2+ and HSO5−, (b) the oxidized species, and (c) the protonated (control) species. Lightning bolts indicate species subjected to CID, degree signs indicate water losses.
Generation of [M-H]+ and [M-H-NH3]+ species
Intact persulfate anion (HS2O8−) is a stronger oxidizing reagent than periodate anion (IO4−) as it (i) oxidizes peptides both containing and lacking easily oxidized residues (i.e., Met and Trp) to the [M+H+O]+ species, and (ii) generates a variety of oxidized products, i.e., [M+H+O]+ and [M-H]+, upon activation of the ion/ion complex regardless of the amino acid composition. Furthermore, the [M+H+O]+ species generated upon CID of the persulfate-peptide ion/ion complex may be different in structure from that obtained for CID of the periodate-peptide ion/ion complex [46]. For example, activation of the ion/ion complex between doubly protonated ARAMAKA and periodate exclusively yielded the sulfoxide oxidation product whereas activation of the complex between the same species and persulfate resulted in a mixture of structures corresponding to the hydrogen deficient [M-H]+ species, oxidation of the Met sulfur atom to the sulfoxide form, and oxidation of the terminal carbon atom in the methionine side chain.
The oxidation of neutral basic residues to [M+H+O]+ and [M-H]+ derivatives was first demonstrated with persulfate anion [46]. Activation of persulfate/peptide ion/ion complexes can undergo several pathways to generate oxidized or charge-reduced species. Loss of H2SO5 from the ion/ion complex, viz., [M+2H+HS2O8]+, generates a species of the form [M+H+SO3]+. Further activation of this ion exclusively returns the charge-reduced species via loss of SO3. Loss of SO3 from the ion/ion complex generates a species of the form [M+2H+HSO5]+, which is identical to the ion/ion complex generated upon reaction between a doubly protonated peptide and peroxymonosulfate anion (HSO5−), which can yield either the oxygen transfer [M+H+O]+ or the proton transfer [M+H]+ species upon activation as discussed in the previous sections. Loss of H2SO4 from the ion/ion complex generates a species of the form [M+H+SO4]+. Activation of this species can either undergo loss of SO3 to generate the oxygen transfer [M+H+O]+ species (which is not necessarily the same structure as the [M+H+O]+ species generated from CID of the SO3 loss) or loss of H2SO4 to generate the [M-H]+ species. Potential mechanisms and structures for these pathways have been discussed in detail previously [46]. Recently, we showed that periodate is also capable of oxidizing neutral basic sites in certain systems [48]. Peptides lacking easily oxidized residues (i.e., Met, Trp, disulfide bonds, etc.) and containing neutral basic sites can generate one or more of three species upon activation of periodate-peptide ion/ion complexes. The loss of iodic acid, HIO3, to generate the oxygen transfer [M+H+O]+ species can occur, though this pathway is always very minor in abundance. The loss of H3IO4 (while this may correspond to the loss of H2O and HIO3, these losses are not observed sequentially) to generate the [M-H]+ species can occur, and is often dominant for arginine-containing systems. Lastly, the loss of I(OH)4NH2 to generate the [M-H-NH3]+ species has been observed from neutral lysine residues and is often dominant for lysine-containing peptides. While peaks corresponding to this mass are also observed for arginine-containing species they are derived from a sequential ammonia loss from the [M-H]+ species instead of directly from the complex, in contrast with the case for lysine-containing peptides. (Comparison of the spectra derived from MS/MS of the [M-H-NH3]+ species directly from the complex and MS3 of the ammonia loss from the [M-H]+ species are identical for arginine-containing species but different for lysine-containing species, as discussed in detail previously [48].) Furthermore, while the oxidation of lysine residues to the [M-H-NH3]+ derivative was first noticed in ion/ion reactions with periodate anion, these species are also observed in reactions with persulfate anion where they are likely derived from a similar mechanism.
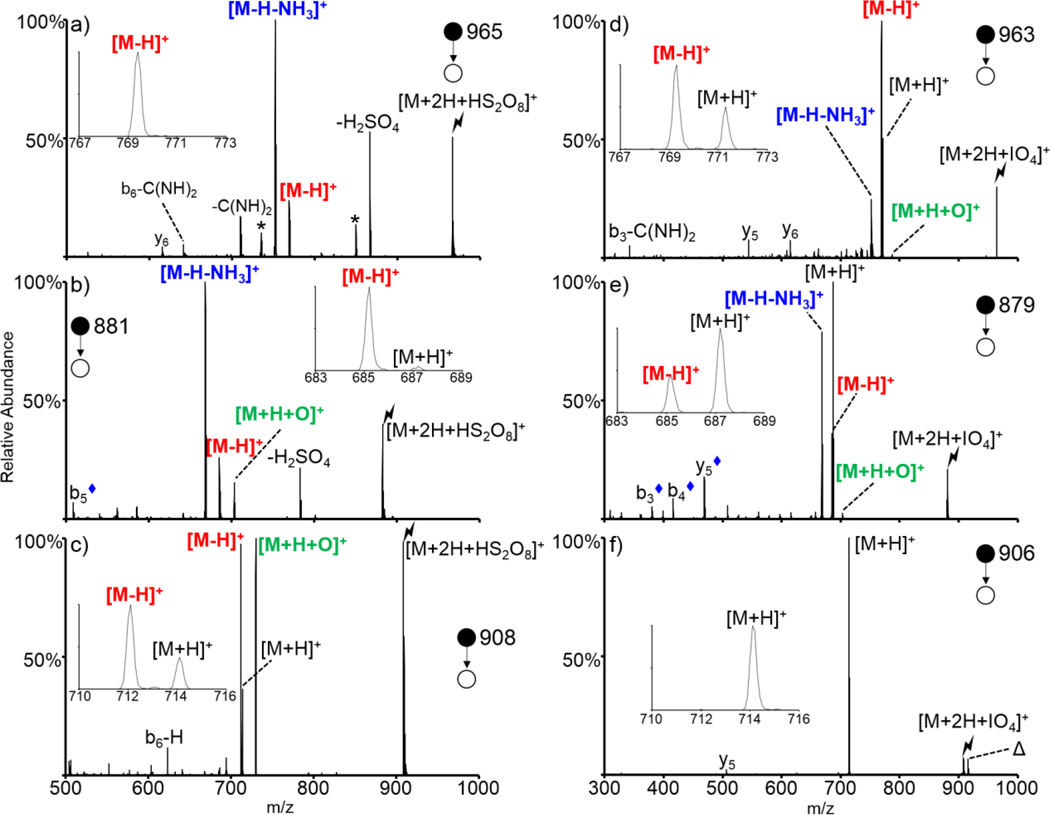
A direct comparison between the oxidation of various peptides containing neutral basic residues with periodate and persulfate anions is shown in Figure 6. A series of doubly protonated peptides of the form XAXAXAA, where X = Arg, Lys, or His, was subjected to ion/ion reactions with both persulfate (left of Figure 6) and periodate (right of Figure 6). The top panels show the CID spectra of the complex formed via ion/ion reactions between doubly protonated RARARAA and HS2O8− (Figure 6(a)) and IO4− (Figure 6(d)). A small amount of oxygen transfer product [M+H+O]+ is observed upon activation of the periodate-RARARAA complex, though this pathway is extremely minor. This pathway was not observed for the persulfate-RARARAA complex. The [M-H]+ product was observed upon activation of both ion/ion complexes, though it was observed at a much higher abundance for the periodate-RARARAA complex. The insets in Figure 6 show a zoom-in of the mass region around the [M-H]+ and [M+H]+ region. No proton transfer is observed for the persulfate oxidation reaction whereas a minor amount of proton transfer is observed for the periodate oxidation reaction indicating the high efficiency with which this peptide is oxidized with each of these reagents. Furthermore, the [M-H-NH3]+ species is the dominant peak observed upon persulfate oxidation and is present at a lower abundance upon periodate oxidation, but as mentioned previously, likely corresponds to a sequential loss of ammonia from the [M-H]+ peak for this arginine-containing system.
Figure 6.
Comparison of the oxidation of neutral basic sites via ion/ion reactions with HS2O8−(left) and IO4− (right). CID of the ion/ion complex between doubly protonated RARARAA and (a) HS2O8−, (d) IO4−, KAKAKAA and (b) HS2O8−, (e) IO4−, and HAHAHAA and (c) HS2O8−, (f) IO4−. Lightning bolts indicate species subjected to CID, asterisks indicate loss of ammonia, delta signs indicate contaminants present in the isolation, and blue diamonds indicate [b/y-H-NH3]+ fragments. Insets show the zoomed in mass region around the [M-H]+ and [M+H]+ species.
The oxidation of doubly protonated KAKAKAA with persulfate and periodate is shown in Figure 6(b) and (e), respectively. Activation of the persulfate-peptide complex generated a small but significant amount of the oxygen transfer species [M+H+O]+, whereas CID of the periodate-peptide complex generated an extremely minor amount of this species. The [M-H]+ species is generated at a similar ratio upon activation of the complexes between the peptide and both persulfate and periodate anion. The zoomed-in regions in the insets show that proton transfer is a dominant pathway upon activation of the complex with periodate whereas it is not observed for the complex with persulfate, reflecting the apparently stronger oxidation capability of persulfate. The [M-H-NH3]+ pathway, which is a unique oxidation pathway exclusively observed for lysine residues (i.e., while masses nominally corresponding to [M-H-NH3]+ species are observed for peptides lacking lysine residues, these species correspond to a sequential loss of ammonia from the [M-H]+ species whereas it corresponds to a unique structure for lysine-containing peptides), is the dominant peak observed upon activation of the persulfate complex and is the dominant oxidation product observed for the periodate complex (secondary in abundance only to the proton transfer species).
Lastly, doubly protonated HAHAHAA was subjected to ion/ion reactions with persulfate and periodate anions and activation of the resulting ion/ion complexes is shown in Figures 6(c) and (f), respectively. Activation of the periodate-peptide complex exclusively results in loss of HIO4 to generate the charge-reduced [M+H]+ species. No oxidation is observed for this species, though previous reactions between periodate anion and other histidine-containing peptides have shown evidence for a very minor extent of oxygen transfer to the His side chain [48]. Activation of the HAHAHAA-persulfate complex, however, results in dominant formation of the oxygen transfer [M+H+O]+ and hydrogen deficient [M-H]+ species. Proton transfer is observed at a minor abundance. Ion trap CID of each of the oxidized HAHAHAA species is shown in Supplemental Figure S-4. Overall, persulfate anion is a stronger oxidizing reagent anion than periodate anion as it results in a higher degree of oxidation for all three of these systems (the proton transfer pathway is of moderate abundance upon activation of periodate-peptide complexes whereas it is of extremely minor abundance/not observed upon activation of persulfate-peptide complexes). Furthermore, additional steps of MSn on the various oxidized species, with the exception of [KAKAKAA+H+O]+, generates different spectra for peptides oxidized with periodate and persulfate (Supplemental Figures S-5 through S-8), indicating that the structures resulting from oxidation with the two reagents may be different. The oxidation of neutral basic sites is important in certain systems like proteins and cell-penetrating peptides, both of which contain a high density of unprotonated basic residues under traditional nESI conditions [48].
Generation of molecular radical cations
The generation of radical peptide cations is an area of ongoing research as odd-electron peptides yield fragments, such as those derived from side chain losses, upon CID that are complementary to those generated from their even-electron counterparts [75,76]. Several methods of generating radical peptide cations for tandem MS analysis have been demonstrated. Collisional activation of ternary metal-ligand peptide complexes [77,78,79,80], free radical-initiated reactions [81,82], CID of nitroso peptides [71,83], and photolysis of peptides with iodinated tyrosine residues or electrostatic complexes [84] have all been shown to generate radical molecular cations. Furthermore, several methods of generating radical cations via ion/ion reactions have recently been reviewed [85]. The most efficient means of generating a peptide radical cation via ion/ion reaction is through ion/ion reactions between a multiply protonated peptide of interest and sulfate radical anion [46]. Activation of the resulting ion/ion complex results in generation of the molecular radical cation via highly efficient loss of sulfuric acid. Similar to the loss of alkyl sulfenic acids from oxidized S-alkyl cysteine side chains, some odd-electron neutral losses from various amino acids upon CID of peptide radical cations generate dehydroalanine residues [86]. This chemistry has recently been used to selectively cleave and localize various amino acids within peptides via fragments derived from the dehydroalanine effect [55].
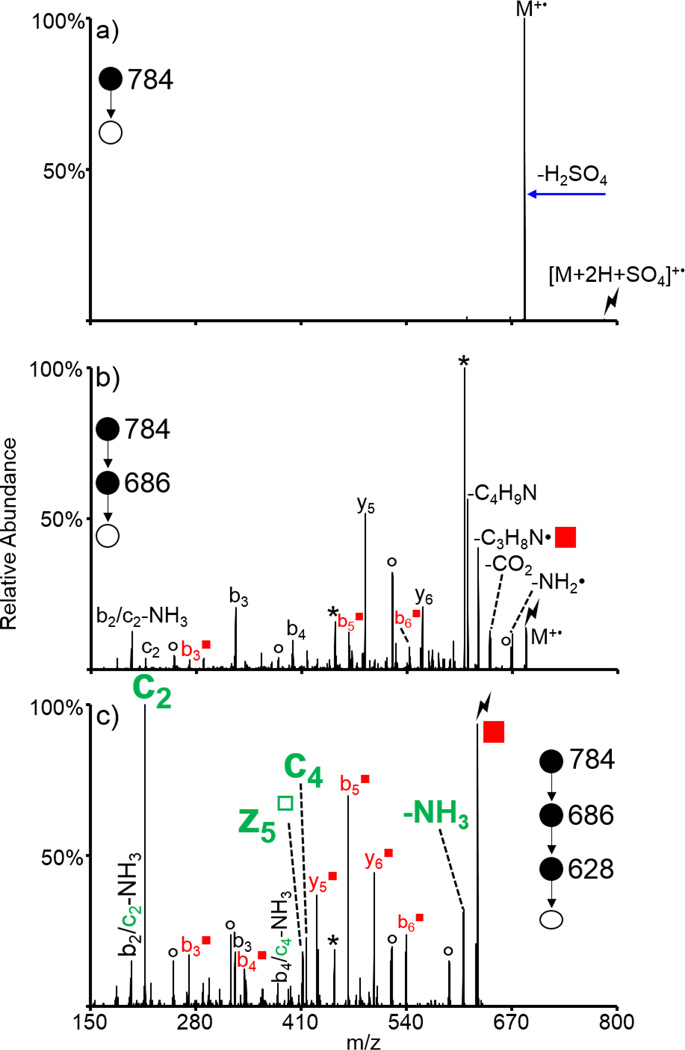
Doubly protonated KAKAKAA was subjected to ion/ion reactions with sulfate radical anion (SO4−•). Activation of the resulting ion/ion complex [M+2H+SO4]+• is shown in Figure 7(a) and results exclusively in loss of H2SO4 to generate the molecular radical cation, viz., M+•. Activation of the complex is a highly efficient method for generating the molecular radical cation. However, it is often already observed upon ion/ion reaction, without the need for an additional activation step for the complex. Results from activation of the molecular radical cation are shown in Figure 7(b), which show a variety of peaks corresponding to backbone fragments and neutral side chain losses characteristic of CID of odd-electron peptide cations. Of particular interest is the loss of C3H8N• (58 Da, indicated with a red square in Figure 7(b) and (c)), which generates a dehydroalanine residue in the place of one of the three lysine residues. Further isolation and activation of this species is shown in Figure 7(c). Notably, the c2 ion is the most dominant peak in the spectrum. This ion is generated via the cleavage of the N-Cα bond of the second lysine residue (indicating conversion to Dha upon loss of C3H8N•) and is derived from the dehydroalanine effect as well. Also observed are the z5□, c4, c4-NH3, and c2-NH3 ions, which are all derived from the dehydroalanine effect. The hollow square indicates the z-type ion generated by the dehydroalanie effect. (While the c4-NH3 and c2-NH3 species could theoretically correspond to the b4 and b2 species, respectively, previous experiments indicate that these peaks are likely sequential losses from the c4 and c2 ions [53,55].) Furthermore, the loss of ammonia from the parent ion may also be derived from the dehydroalanine effect when the lysine at the N-terminus has been converted into Dha (i.e., an ammonia loss can also be considered a z7□ ion). Other fragments both containing (b3■, b4■, b5■, b6■, y5■, and y6■) and lacking (b3) modified lysine residues are also observed. Ions derived from the dehydroalanine effect are observed next to all three of the lysine residues (though the ammonia loss from the precursor is somewhat ambiguous as peptides commonly lose ammonia upon CID), and can be used to localize the lysine residues within the peptide. This approach has also been used to localize methionine residues in the peptide ARAMAKA, both methionine and arginine residues in the peptide GRGMGRGMGRL, and leucine residues in a melittin system with mixed cation sites (sequence: GIGAVLKVLTTGLPALISWIKRKRQQ) [55]. Furthermore, the loss of SH• from the cysteine side chain observed upon CID of the molecular radical cation generated upon NO• loss from an S-NO peptide was used to localize cysteine residues [55].
Figure 7.
Generation of a molecular radical cation of KAKAKAA via ion/ion reactions with sulfate radical anion. CID of (a) the ion/ion complex between doubly protonated KAKAKAA and SO4−•, (b) the molecular radical cation of KAKAKAA, and (c) the loss of C3H8N• from (b) to generate a Dha residue out of one of the lysine residues. Lightning bolts indicate species subjected to CID, asterisks indicate loss of ammonia, degree signs indicate loss of water, red squares indicate Dha-containing species, and fragments derived from the dehydroalanine effect are indicated in green.
CONCLUSIONS
Here, we have drawn together a variety of gas-phase oxidation reactions recently discovered via ion/ion chemistry and described some possible applications for these reactions that are already apparent. We have discussed the oxidation of thioethers in methionine and S-alkyl cysteine residues and demonstrated a new example illustrating the oxidation of a cysteine residue protected with N-hydroxymaleimide. Similar to previous examples demonstrating the oxidation of S-alkyl cysteine residues, this system underwent loss of the alkyl sulfenic acid to generate the dehydroalanine species, which was then used to localize the modified cysteine residue via the generation of c- and z-ions derived from the dehydroalanine effect. Unique to the hydroxymaleimide protecting group, however, was the dominant loss of the maleimide plus the elements of water, which was previously observed for maleimide-protected cysteine residues oxidized in solution. Furthermore, we demonstrated the simultaneous oxidation of multiple methionine residues in one peptide via ion/ion reactions with reagent clusters of the form H(IO4)2−. We demonstrated the oxidation of an S-nitrosylated peptide, which could aid in the localization of the nitrosylation to a specific cysteine side chain, and an intermolecularly disulfide-linked peptide, which underwent highly efficient oxidative cleavage of the disulfide bond upon oxidation. We also demonstrated the oxidation of DNA to the [M+H+O]+ derivative. This species predominantly lost the oxidized guanine base, indicating that the majority of the oxidation chemistry likely occurred on the guanine nucleobase, in analogy with solution-phase observations. We also compared the oxidation of neutral basic residues to [M+H+O]+, [M-H]+, and [M-H-NH3]+ species via ion/ion reactions with persulfate and periodate anions. Lastly, we demonstrated the generation of molecular radical cations via ion/ion reactions with sulfate radical anion, a reagent derived upon negative nESI of an aqueous solution of sodium persulfate. This is especially useful as molecular radical cations undergo fragmentation distinct from their even-electron counterparts upon CID, some of which generate Dha residues that can be used to selectively localize amino acids within the peptide of interest. Overall, we have demonstrated a variety of oxidation ion/ion reactions and identified applications in which they may be useful. The applications of these chemistries are diverse and range from mapping disulfide bonds to obtaining complementary sequence information from molecular radical cations.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health under Grant GM R37-45372. Graduate student support for A.L.P. provided by Emerson Kampen and Bilsland Dissertation Fellowships.
REFERENCES
- 1.Barelli S, Canellini G, Thadikkaran L, Crettaz D, Quadroni M, Rossier JS, Tissot J-D, Lion N. Oxidation of proteins: Basic principles and perspectives for blood proteomics. Proteomics Clin. Appl. 2008;2:142–157. doi: 10.1002/prca.200780009. [DOI] [PubMed] [Google Scholar]
- 2.Herrlich P, Boehmer FD. Redox regulation of signal transduction in mammalian cells. Biochem. Pharmacol. 2000;59:35–41. doi: 10.1016/s0006-2952(99)00298-1. [DOI] [PubMed] [Google Scholar]
- 3.Finkel T. Redox-dependent signal transduction. FEBS Letters. 2000;476:52–54. doi: 10.1016/s0014-5793(00)01669-0. [DOI] [PubMed] [Google Scholar]
- 4.Morel Y, Barouki R. Repression of gene expression by oxidative stress. Biochem. J. 1999;342:481–496. [PMC free article] [PubMed] [Google Scholar]
- 5.Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic. Biol. Med. 2000;28:463–499. doi: 10.1016/s0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 6.Sohal RS. Role of oxidative stress and protein oxidation in the aging process. Free Radic. Biol. Med. 2002;33:37–44. doi: 10.1016/s0891-5849(02)00856-0. [DOI] [PubMed] [Google Scholar]
- 7.Levine RL, Stadtman ER. Oxidative modification of proteins during aging. Exp. Gerontol. 2001;36:1495–1502. doi: 10.1016/s0531-5565(01)00135-8. [DOI] [PubMed] [Google Scholar]
- 8.Cerutti PA. Oxidant stress and carcinogenesis. Eur. J. Clin. Invest. 1991;21:1–5. doi: 10.1111/j.1365-2362.1991.tb01350.x. [DOI] [PubMed] [Google Scholar]
- 9.Ceruti PA. Oxyradicals and cancer. Lancet. 1994;344:862–863. doi: 10.1016/s0140-6736(94)92832-0. [DOI] [PubMed] [Google Scholar]
- 10.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 11.Wolff SP, Jiang ZY, Hunt JV. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic. Biol. Med. 1991;10:339–352. doi: 10.1016/0891-5849(91)90040-a. [DOI] [PubMed] [Google Scholar]
- 12.Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD, Lusis AJ. Atherosclerosis: Basic mechanisms: Oxidation, inflammation, and genetics. Circulation. 1995;91:2488–2496. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- 13.Jurgens G, Hoff HF, Chisolm GM, III, Esterbauer H. Modification of human serum low density lipoprotein by oxidation - Characterization and pathophysiological implications. Chem. Phys. Lipids. 1987;45:315–336. doi: 10.1016/0009-3084(87)90070-3. [DOI] [PubMed] [Google Scholar]
- 14.Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc. Natl. Acad. Sci. USA. 1996;93:2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butterfield DA. Proteomics: A new approach to investigate oxidative stress in Alzheimer’s disease brain. Brain Res. 2004;1000:1–7. doi: 10.1016/j.brainres.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Choi J, Malakowsky CA, Talent JM, Conrad CC, Gracy RW. Identification of oxidized plasma proteins in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2002;293:1566–1570. doi: 10.1016/S0006-291X(02)00420-5. [DOI] [PubMed] [Google Scholar]
- 17.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and the ugly. Am. J. Physiol. 1996;271:40–45. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 18.Nystrom T. Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 2005;24:1311–1317. doi: 10.1038/sj.emboj.7600599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogt W. Oxidation of methionyl residues in proteins - tools, targets, and reversal. Free Radical Biology and Medicine. 1995;18:93–105. doi: 10.1016/0891-5849(94)00158-g. [DOI] [PubMed] [Google Scholar]
- 20.Leonard SE, Carroll KS. Chemical ‘omics’ approaches for understanding protein cysteine oxidation in biology. Curr. Opin. Chem. Biol. 2011;15:88–102. doi: 10.1016/j.cbpa.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Hochgräfe F, Mostertz J, Pöther D-C, Becher D, Helmann JD, Hecker M. S-cysteinylation is a general mechanism for thiol protection of Bacillus subtilis proteins after oxidative stress. J. Biol. Chem. 2007;282:25981–25985. doi: 10.1074/jbc.C700105200. [DOI] [PubMed] [Google Scholar]
- 22.Fratelli M, Gianazza E, Ghezzi P. Redox proteomics: identification and functional role of glutathionylated proteins. Expert Rev. Proteomics. 2004;1:365–376. doi: 10.1586/14789450.1.3.365. [DOI] [PubMed] [Google Scholar]
- 23.Sadrzadeh S, Graf MH, Panter E, Hemoglobin SS. A biologic Fenton reagent. J. Biol. Chem. 1984;259:14354–14356. [PubMed] [Google Scholar]
- 24.Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. The FASEB J. 2003;17:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 25.González-Siso MI, García-Leiro A, Tarrío N, Cerdán ME. Sugar metabolism, redox balance and oxidative stress response in the respiratory yeast Kluyveromyces lactis. 2009;8:46–62. doi: 10.1186/1475-2859-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayala A, Muñoz MF, Argüelles S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Medicine and Cellular Longevity. 2014;2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biemann K, Scoble HA. Characterization by Tandem Mass Spectrometry of Structural Modifications in Proteins. Science. 1987;237:992–998. doi: 10.1126/science.3303336. [DOI] [PubMed] [Google Scholar]
- 28.Qin J, Chait BT. Identification and Characterization of Posttranslational Modifications of Proteins by MALDI Ion Trap Mass Spectrometry. Anal. Chem. 1997;69:4002–4009. doi: 10.1021/ac970489n. [DOI] [PubMed] [Google Scholar]
- 29.Mann M, Jensen ON. Proteomic Analysis of Post-Translational Modifications. Nat. Biotechnol. 2003;21:255–261. doi: 10.1038/nbt0303-255. [DOI] [PubMed] [Google Scholar]
- 30.Verrastro I, Pasha S, Jensen KT, Pitt AR, Spickett CM. Mass Spectrometry-Based Methods for Identifying Oxidized Proteins in Disease: Advances and Challenges. Biomolecules. 2015;5:378–411. doi: 10.3390/biom5020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo S, Uehara H, Shacter E. Taurine chloramine-induced inactivation of cofilin protein through methionine oxidation. Free Radic. Biol. Med. 2014;75:84–94. doi: 10.1016/j.freeradbiomed.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 32.Ehrmann DC, Rose K, Calcutt MW, Beller AB, Hill S, Rogers TJ, Steele SD, Hachey DL, Aschner JL. Glutathionylated gammag and gammaa subunits of hemoglobin F: A novel post-translational modifications found in extremely premature infants by LC-MS and nanoLC-MS/MS. J. Mass Spectrom. 2014;49:178–183. doi: 10.1002/jms.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mouls L, Silajdzic E, Haroune N, Spickett CM, Pitt AR. Development of novel mass spectrometric methods for identifying HOCL-induced modifications to proteins. Proteomics. 2009;9:1617–1631. doi: 10.1002/pmic.200800391. [DOI] [PubMed] [Google Scholar]
- 34.Madian AG, Myracle AD, Diaz-Maldonado N, Rochelle NS, Janle EM, Regnier FE. Differential carbonylation of proteins as a function of in vivo oxidative stress. J. Proteome Res. 2011;10:3959–3972. doi: 10.1021/pr200140x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madian AG, Regnier FE. Profiling carbonylated proteins in human plasma. J. Proteome Res. 2010;9:1330–1343. doi: 10.1021/pr900890k. [DOI] [PubMed] [Google Scholar]
- 36.Kumar Y, Liang C, Limmon GV, Liang L, Engelward BP, Ooi EE, Chen J, Tannenbaum SR. Molecular analysis of serum and bronchoalveolar lavage in a mouse model of influenza reveals markers of disease severity that can be clinically useful in humans. PLOS ONE. 2014;9:e86912. doi: 10.1371/journal.pone.0086912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lagerwerf FM, van de Weert M, Heerma W, Haverkamp J. Oxidation of Oxidized Methionine Peptides. Rapid Commun. Mass Spectrom. 1996;10:1905–1910. doi: 10.1002/(SICI)1097-0231(199612)10:15<1905::AID-RCM755>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 38.Reid GE, Roberts KD, Kapp EA, Simpson RJ. Statistical and Mechanistic Approaches to Understanding the Gas-Phase Fragmentation Behavior of Methionine Sulfoxide Containing Peptides. J. Proteome Res. 2004;3:751–759. doi: 10.1021/pr0499646. [DOI] [PubMed] [Google Scholar]
- 39.Schey KL, Finley EL. Identification of Peptide Oxidation by Tandem Mass Spectrometry. Acc. Chem. Res. 2000;33:299–306. doi: 10.1021/ar9800744. [DOI] [PubMed] [Google Scholar]
- 40.Qin J, Chait BT. Identification and characterization of posttranslational modifications of proteins by MALDI ion trap mass spectrometry. Anyl. Chem. 1997;69:3995–4001. doi: 10.1021/ac970489n. [DOI] [PubMed] [Google Scholar]
- 41.Jiang XY, Smith JB, Abraham EC. Identification of a MS-MS fragment diagnostic for methionine sulfoxide. J. Mass Spectrom. 1996;31:1309–1310. [Google Scholar]
- 42.Gorman JJ, Wallis TP, Pitt JJ. Protein Disulfide Bond Determination by Mass Spectrometry. Mass Spectrom. Rev. 2002;21:183–216. doi: 10.1002/mas.10025. [DOI] [PubMed] [Google Scholar]
- 43.Hogan JM, McLuckey SA. Charge State Dependent Collision-induced Dissociation of Native and Reduced Porcine Elastase. J. Mass Spectrom. 2003;38:245–246. doi: 10.1002/jms.458. [DOI] [PubMed] [Google Scholar]
- 44.Stephenson JL, Cargile BJ, McLuckey SA. Ion Trap Collisional Activation of Disulfide Linkage Intact and Reduced Multiply Protonated Polypeptides. Rapid Commun. Mass Spectrom. 1999;13:2040. doi: 10.1002/(SICI)1097-0231(19991030)13:20<2040::AID-RCM754>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 45.Pilo AL, McLuckey SA. Oxidation of Methionine Residues in Polypeptide Ions via Gas-Phase Ion/Ion Chemistry. J. Am. Soc. Mass Spectrom. 2014;25:1049–1057. doi: 10.1007/s13361-014-0861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pilo AL, Bu J, McLuckey SA. Transformation of [M+2H]2+ Peptide Cations to [M-H]+, [M+H+O]+, and M+• Cations via Ion/Ion Reactions: Reagent Anions Derived from Persulfate. J. Am. Soc. Mass Spectrom. 2015;26:1103–1114. doi: 10.1007/s13361-015-1125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prentice BM, McLuckey SA. Gas-Phase Ion/Ion Reactions of Peptides and Proteins: Acid/Base, Redox, and Covalent Chemistries. Chem. Commun. 2013;49:947–965. doi: 10.1039/c2cc36577d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pilo AL, Bu J, McLuckey SA. Gas-Phase Oxidation of Neutral Basic Residues in Polypeptide Cations by Periodate. J. Am. Soc. Mass Spectrom. 2016 doi: 10.1007/s13361-016-1491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xia Y, Wu J, Londry FA, Hager JW, McLuckey SA. Mutual Storage Mode Ion/Ion Reactions in Hybrid Linear Ion Trap. J. Am. Soc. Mass Spectrom. 2005;16:71–81. doi: 10.1016/j.jasms.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 50.Liang W, Xia Y, McLuckey SA. Alternately Pulsed Nano-electrospray Ionization/Atmospheric Pressure Chemical Ionization for Ion/Ion Reactions in an Electrodynamic Ion Trap. Anal. Chem. 2006;78:3208–3212. doi: 10.1021/ac052288m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Londry FA, Hager JW. Mass selective axial ion ejection from a linear quadrupole ion trap. J. Am. Chem. Soc. 2003;14:1130–1147. doi: 10.1016/S1044-0305(03)00446-X. [DOI] [PubMed] [Google Scholar]
- 52.Kim HI, Beauchamp JL. Mapping disulfide bonds in insulin with the Route 66 Method: selective cleavage of S-C bonds using alkali and alkaline earth metal enolate complexes. J. Am. Soc. Mass Spectrom. 2009;20:157–166. doi: 10.1016/j.jasms.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Pilo AL, Zhao F, McLuckey SA. Selective Gas-Phase Oxidation and Localization of Alkylated Cysteine Residues in Polypeptide Ions via Ion/Ion Chemistry. J. Prot. Res. 2016;15:3139–3146. doi: 10.1021/acs.jproteome.6b00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Froelich JM, Reid GE. Mechanisms for the Proton Mobility Dependent Gas-Phase Fragmentation Reactions of S-alkyl Cysteine Sulfoxide-Containing Peptide Ions. J. Am. Soc. Mass Spectrom. 2007;18:1690–1705. doi: 10.1016/j.jasms.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 55.Pilo AL, Peng Z, McLuckey SA. The Dehydroalanine Effect in the Fragmentation of Ions Derived from Polypeptides. J. Mass Spectrom. 2016;51:857–866. doi: 10.1002/jms.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steen H, Mann M. Similarity between condensed phase and gas phase chemistry: Fragmentation of peptides containing oxidized cysteine residues and its implications for proteomics. J. Am. Soc. Mass Spectrom. 2001;12:228–232. doi: 10.1016/S1044-0305(00)00219-1. [DOI] [PubMed] [Google Scholar]
- 57.Chrisman PA, McLuckey SA. Dissociations of Disulfide-linked Gaseous Polypeptide/Protein Anions: Ion Chemistry with Implications for Protein Identification and Characterization. J. Proteome Res. 2002;1:549–557. doi: 10.1021/pr025561z. [DOI] [PubMed] [Google Scholar]
- 58.Wells JM, Stephenson JL, Jr, McLuckey SA. Charge dependence of protonated insulin decompositions. Int. J. Mass Spectrom. 2000;203:A1–A9. [Google Scholar]
- 59.Chowdhury SM, Munske GR, Ronald RC, Bruce JE. Evaluation of low energy CID and ECD fragmentation behavior of mono-oxidized thio-ether bonds in peptides. J. Am. Soc. Mass Spectrom. 2007;18:493–501. doi: 10.1016/j.jasms.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pilo AL, McLuckey SA. Selective Gas-Phase Ion/Ion Reactions: Enabling Disulfide Mapping via Oxidation and Cleavage of Disulfide Bonds in Intermolecularly-Linked Polypeptide Ions. Anal. Chem. 2016;88:8972–8979. doi: 10.1021/acs.analchem.6b01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun Y, Smith DL. Identification of disulfide-containing peptides by performic acid oxidation and mass spectrometry. Anal. Biochem. 1988;172:130–138. doi: 10.1016/0003-2697(88)90421-6. [DOI] [PubMed] [Google Scholar]
- 62.Agarwal A, Diedrich JK, Julian RR. Direct elucidation of disulfide bond partners using ultraviolet photodissociation mass spectrometry. Anal. Chem. 2011;83:6455–6458. doi: 10.1021/ac201650v. [DOI] [PubMed] [Google Scholar]
- 63.Payne AH, Glish GL. Gas-phase ion/ion interactions between peptides or proteins and iron ions in a quadrupole ion trap. Int. J. Mass Spectrom. 2001;204:47–54. [Google Scholar]
- 64.Mentinova M, McLuckey SA. Cleavage of multiple disulfide bonds in insulin via gold cationization and collision-induced dissociation. Int. J. Mass Spectrom. 2011;308:133–136. doi: 10.1016/j.ijms.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gunawardena HP, O’Hair RAJ, McLuckey SA. Selective Disulfide Bond Cleavage in Gold(I) Cationized Polypeptide Ions Formed via Gas-Phase Ion/Ion Cation Switching. J. Proteome Res. 2006;5:2087–2092. doi: 10.1021/pr0602794. [DOI] [PubMed] [Google Scholar]
- 66.Wells JM, Stephenson JL, Jr, McLuckey SA. Charge Dependence of Protonated Insulin Decompositions. Int. J. Mass Spectrom. 2000;203:A1–A9. [Google Scholar]
- 67.Zubarev RA, Kruger NA, Fridriksson MA, Lewis MA, Horn DM, Carpenter BK, McLafferty FW. Electron Capture Dissociation of Gaseous Multiply-Charged Proteins Is Favored at Disulfide Bonds and Other Sites of High Hydrogen Atom Affinity. J. Am. Chem. Soc. 1999;121:2857–2862. [Google Scholar]
- 68.Chrisman PA, Pitteri SJ, Hogan JM, McLuckey SA. SO2-* electron transfer ion/ion reactions with disulfide linked polypeptide ions. J. Am. Soc. Mass Spectrom. 2005;16:1020–1030. doi: 10.1016/j.jasms.2005.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kalli A, Hakansson K. Preferential cleavage of S-S and C-S bonds in electron detachment dissociation and infrared multiphoton dissociation of disulfide-linked peptide anions. Int. J. Mass Spectrom. 2007;263:71–81. [Google Scholar]
- 70.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-Nitrosylation: Purview and Parameters. Nat. Rev. Mol. Cell. Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 71.Hao G, Gross SS. Electrospray Tandem Mass Spectrometry Analysis of S- and N-Nitrosopeptides: Facile Loss of NO and Radical-Induced Fragmentation. J. Am. Soc. Mass Spectrom. 2006;17:1725–1730. doi: 10.1016/j.jasms.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 72.Prentice BM, Stutzman JR, McLuckey SA. Reagent Cluster Anions for Multiple Gas-Phase Covalent Modifications of Peptide and Protein Cations. J. Am. Soc. Mass Spectrom. 2013;24:1045–1052. doi: 10.1007/s13361-013-0637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steenken S, Jovanovic SV. How easily oxidizable is DNA? One-Electron reduction potentials of adenosine and guanosine radicals in aqueous solution. J. Am. Chem. Soc. 1997;119:617–618. [Google Scholar]
- 74.Morikawa M, Kino K, Oyoshi T, Suzuki M, Kobayashi T, Miyazawa H. Analysis of Guanine Oxidation Products in Double-Stranded DNA and Proposed Guanine Oxidation Pathways in Single-Stranded, Double-Stranded or Quadruplex DNA. Biomolecules. 2014;140–159:140–159. doi: 10.3390/biom4010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tureček F. Peptide Radicals and Cation Radicals in the Gas Phase. Chem. Rev. 2013;113:6691–6733. doi: 10.1021/cr400043s. [DOI] [PubMed] [Google Scholar]
- 76.Oh HB, Moon B. Radical-Driven Peptide Backbone Dissociation Tandem Mass Spectrometry. Mass Spectrom. Rev. 2014;9999:1–17. doi: 10.1002/mas.21426. [DOI] [PubMed] [Google Scholar]
- 77.Chu IK, Rodriguez CF, Lau TC, Hopkinson AC, Siu KWM. Molecular radical cations of oligopeptides. J. Phys. Chem. B. 2000;104:3393–3397. [Google Scholar]
- 78.Bagheri-Madji E, Ke YY, Orlova G, Chu IK, Hopkinson AC, Siu KWM. Copper-mediated peptide radical ions in the gas-phase. J. Phys. Chem. B. 2004;108:11170–11181. [Google Scholar]
- 79.Barlow CK, Moran D, Radom L, McFadyen WD, O’Hair RAJ. Metal-mediated formation of gas-phase amino acid radical cations. J. Phys. Chem. A. 2006;110:8304–8315. [Google Scholar]
- 80.Laskin J, Yang Z, Chu IK. Energetics and dynamics of electron transfer and proton transfer in dissociation of MetalIII(salen)-peptide complexes in the gas phase. J. Am. Chem. Soc. 2008;130:3218–3230. doi: 10.1021/ja077690s. [DOI] [PubMed] [Google Scholar]
- 81.Masterson DS, Yin H, Chacon A, Hachey DL, Norris JL, Porter NA. Lysine peroxycarbamates: Free radical-promoted peptide cleavage. J. Am. Chem. Soc. 2004;126:720–721. doi: 10.1021/ja038615u. [DOI] [PubMed] [Google Scholar]
- 82.Hodyss R, Cox HA, Beauchamp JL. Bioconjugates for tunable peptide fragmentation: Free radical initiated peptide sequencing (FRIPS) J. Am. Chem. Soc. 2005;127:12436–12437. doi: 10.1021/ja052042z. [DOI] [PubMed] [Google Scholar]
- 83.Knudsen ER, Julian RR. Fragmentation chemistry observed in hydrogen deficient radical peptides generated from N-nitrosotryptophan residues. Int. J. Mass Spectrom. 2010;294:83–87. [Google Scholar]
- 84.Ly T, Julian RR. Residue-Specific Radical-Directed Dissociation of Whole Proteins in the Gas Phase. J. Am. Chem. Soc. 2008;130:351–358. doi: 10.1021/ja076535a. [DOI] [PubMed] [Google Scholar]
- 85.Gilbert JD, Fisher CM, Bu J, Prentice BM, Redwine JG, McLuckey SA. Strategies for generating peptide radical cations via ion/ion reactions. J. Mass Spectrom. 2014;50:418–426. doi: 10.1002/jms.3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun QY, Nelson H, Ly T, Stoltz BM, Julian RR. Side chain chemistry mediates backbone fragmentation in hydrogen deficient peptide radicals. J. Proteome Res. 2009;8:958–966. doi: 10.1021/pr800592t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.