Abstract
Introduction
Although the Diabetes Prevention Program and other clinical trials demonstrated the efficacy of intensive lifestyle interventions (ILI) and metformin to prevent Type 2 diabetes, no studies have tested their comparative effects in pragmatic settings. This study was designed to compare the real-world effectiveness of ILI, metformin, and standard care among Hispanic women (Latinas) with prediabetes.
Study design
RCT.
Setting/participants
Ninety-two Latinas, who had a mean hemoglobin A1c of 5.9%, BMI of 33.3 kg/m2, and waist circumference of 97.4 cm, were recruited from an urban community and randomly assigned to ILI, metformin, or standard care using 1:1:1 allocation. Data were collected from 2013–2015 and analyzed in 2016.
Intervention
The 12-month ILI was adapted from the Diabetes Prevention Program’s ILI and delivered by community health workers (promotoras) over 24 sessions. Metformin participants received 850 mg twice daily. Those randomized to standard care continued their regular medical care.
Main outcome measures
Weight and secondary outcomes (waist circumference, blood pressure, hemoglobin A1c, fasting plasma glucose, insulin, and lipids) were assessed at baseline and 12 months.
Results
ILI participants demonstrated significantly greater mean weight loss (−4.0 kg, 5.0%) than metformin (−0.9 kg, 1.1%) and standard care participants (+0.8 kg, 0.9%) (p<0.001). The difference in weight loss between metformin and standard care was not significant. The ILI group experienced a greater reduction in waist circumference than standard care (p=0.001), and a marginal improvement in hemoglobin A1c compared with metformin and standard care (p=0.063).
Conclusions
In the first comparative effectiveness trial of diabetes prevention treatments, a 12-month ILI produced significantly greater weight loss than metformin and standard care among Latinas with prediabetes. These data suggest that ILI delivered by promotoras is an effective strategy for preventing diabetes in this high-risk group, which may be superior to metformin. Future pragmatic trials involving larger samples should examine differences in diabetes incidence associated with these treatments.
INTRODUCTION
Diabetes prevention has become a top public health priority given the large burden of Type 2 diabetes and the availability of effective interventions that lower individuals’ risk of developing it. The U.S. Diabetes Prevention Program (DPP) clinical trial of 3,234 adults with prediabetes found that intensive lifestyle intervention (ILI) and metformin reduced diabetes incidence by 58% and 31%, respectively.1 A large body of translational research has demonstrated the effectiveness of DPP-based ILI in diverse populations and communities,2–4 which has informed the development of a national network of organizations delivering this program, and a recent announcement that Medicare will reimburse its delivery.5,6
Although many studies have translated the DPP’s ILI in diverse settings, none has compared its effectiveness to metformin in such contexts. Previous research demonstrates that pragmatic ILI programs achieve smaller effects than highly controlled efficacy studies.2,3 Though the effectiveness of metformin in the real world may also be lower than that observed in DPP and another similar efficacy study,7 there are no translational studies of metformin for lowering diabetes risk since the publication of these earlier trials. Therefore, it remains unknown whether DPP-based ILI or metformin is superior among U.S. adults with prediabetes in real-world settings. Given that 38% of the U.S. adult population has prediabetes,8 this unanswered question has significant public health implications.
Hispanics in the U.S. experience a disproportionate burden of Type 2 diabetes, with Hispanic women (hereafter called Latinas) having a higher lifetime risk of developing the disease than any other demographic group.9 This population’s high risk and low representation in existing DPP translational research highlights the importance of studying diabetes prevention in Latinas.10–12 Community health workers, or promotoras as lay health educators are often called in Hispanic communities, are an effective workforce for delivering DPP-based ILI.2 Using lay health workers to deliver DPP-based ILI is also considered more responsive to participants’ needs and more cost effective than delivery by health professionals.13,14 For these reasons, promotoras represent a promising model for delivering ILI at scale.
The Promotora Effectiveness Versus Metformin Trial (PREVENT-DM) was designed to test the comparative effectiveness of metformin, a DPP-based ILI delivered by promotoras, and standard care for inducing weight loss among Latinas with prediabetes. This randomized trial evaluated the impact of these interventions on 12-month weight change in this high-risk population. Based on data from the DPP efficacy trial,1 the hypothesis was that weight loss would be significantly greater among ILI participants than those randomized to receive metformin or standard care.
METHODS
The PREVENT-DM study was a three-arm comparative effectiveness trial with a parallel group design that randomized participants in a 1:1:1 ratio to receive either:
a group-based adaptation of the DPP lifestyle intervention delivered by promotoras;
metformin 850 mg twice daily; or
standard care plus written educational materials on diabetes prevention.
The study protocol was approved by the IRB of Temple University and Northwestern University, and was recorded in the National Clinical Trials Registry (NCT02088034). Greater detail on the rationale and design of the study was published previously.15 The trial was conducted in partnership with Puentes de Salud, a Latino-serving community health center in Philadelphia that has operated promotora-led interventions continuously since 2007.16
Study Population
The inclusion criteria for study participants were Latinas aged ≥20 years with prediabetes, defined by impaired fasting glucose (fasting plasma glucose of 100–125 mg/dL), elevated hemoglobin A1c (HbA1c) of 5.7%–6.4% (39–46 mmol/mol), or both. Potential participants were excluded if they had diabetes at baseline, were currently pregnant or planned to become pregnant, or were participating in a supervised weight loss program. In addition, those with any of the following clinical criteria were excluded: blood pressure ≥160/100 mmHg, contraindication to metformin, chronic conditions that could affect a participant’s ability to participate (e.g., severe osteoarthritis), medical comorbidities that could influence body weight (e.g., uncontrolled thyroid disease), or medications that could affect weight or glucose metabolism (e.g., oral corticosteroids).
Women of self-reported Latino ethnicity were recruited during community health fairs and at Latino-serving community health centers in Philadelphia. Women who expressed interest during health fairs completed the American Diabetes Association’s Diabetes Risk Assessment Questionnaire,17 and were invited for subsequent fasting venipuncture if their risk score was ≥4. For clinic-based recruitment efforts, initial eligibility screening was conducted by reviewing electronic health record data after obtaining approval from patients’ primary care provider. Those without fasting plasma glucose or HbA1c measurement within the prior 6 months were invited to complete these tests to determine their prediabetes status. Women with prediabetes were invited for a study physician interview and physical exam to determine the presence of clinical exclusion criteria outlined above. Participants gave written informed consent prior to enrollment. All data were collected at Temple University’s Center for Obesity Research and Education.
Promotora-led intensive lifestyle intervention
The promotora-led ILI is based on the Group Lifestyle Balance program (copyright 2008, 2010, 2011; University of Pittsburgh), which is an evidence-supported adaptation of the original NIH/National Institute of Diabetes and Digestive and Kidney Diseases–funded DPP.18,19 Findings from formative research informed minimal modifications to the Spanish-language Group Lifestyle Balance program participant handouts to increase their cultural salience for the target population.15,20,21 The 24-session intervention was delivered in Spanish by one promotora to four groups of between five and nine participants, with each session lasting approximately 90 minutes. A second promotora provided logistic support during ILI sessions such as weighing participants and distributing printed materials. There were three part-time promotoras on the intervention team, one of whom led two groups and the other two promotoras each led one group. The first 14 sessions occurred weekly, and the final ten sessions took place biweekly and then monthly. ILI sessions were delivered in a large conference room at the Puentes de Salud health center. The intervention used behavioral strategies such as goal setting, self-monitoring, stimulus control, and problem solving to achieve modest weight loss (5%–7% of initial body weight) by improving dietary patterns (decreasing fat and calorie consumption) and promoting moderate physical activity (≥150 minutes per week). Participants were provided with a digital scale, pedometer, measuring cups, and logs for tracking dietary intake and physical activity. The promotora reviewed participants’ completed logs at each session, providing feedback and accountability for health behavior changes. Prior to implementing ILI, the promotoras received approximately 70 hours of training on the protocol from local and national experts in diabetes prevention. During the PREVENT-DM trial, two members of the investigative team supervised the promotoras’ delivery of the program to monitor fidelity to the intervention protocol. Puentes de Salud’s promotora director provided general oversight and support to the promotoras while implementing ILI. Further details about the team’s structure and training are described elsewhere.15
Metformin
Participants randomized to receive metformin began taking 850 mg daily for the first month and 850 mg twice daily thereafter. This dose was reduced if participants experienced side effects, and then titrated to the highest tolerated dose up to 850 mg twice daily. This metformin dose has been most widely studied in trials aiming to lower diabetes risk,1,22 including DPP. Adherence was assessed monthly by the research coordinator based on pill counts and structured interviews.
Standard care
Those randomized to standard care continued their regular medical care. In addition, the research coordinator gave them educational materials on diabetes prevention from the National Diabetes Education Program and described these materials briefly during quarterly study visits.23
Measures
Data were collected during baseline, 6-month, and 12-month assessments. The primary outcome was the between-group difference in body weight from baseline to 12 months. Weight loss was chosen as the primary outcome because the reduction in diabetes incidence observed with both ILI and metformin in DPP was principally mediated by this mechanism.24,25 Further justification for this primary outcome is provided by the following observations:
Weight loss was the primary outcome in most previously published DPP translation trials.2,26
Weight is routinely measured during clinical encounters where metformin is prescribed.27
Weight can be easily measured in community-based settings where ILI is most often delivered.28
Weight was measured to the nearest 0.1 kg using a Detecto high-capacity digital scale. Weight measurements were used to calculate mean changes in weight, as well as the proportion of participants achieving 5% weight loss from baseline, a threshold that is associated with improvements in diverse cardiometabolic outcomes.29 Height was measured to the nearest 1 mm using a Holtain wall-mounter stadiometer, and used to calculate BMI.
Other clinical measurements included waist circumference and blood pressure (using GE Dinamap anaeroid sphygmomanometer). These measurements were collected by the same staff member throughout the study to eliminate inter-observer variation. After participants fasted for a minimum of 8 hours, venipuncture was performed by a licensed phlebotomist for assessment of HbA1c, glucose, insulin, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides. Fasting glucose and insulin values were used to calculate the homeostasis model assessment of insulin resistance according to the following formula: (fasting insulin [μIU/mL] × fasting glucose [mmol/L]/22.5].30 All plasma specimens were analyzed in the same Quest Diagnostics laboratory that meets the National Glycohemoglobin Standardization Program.31 Participants also completed surveys assessing sociodemographic characteristics, psychosocial factors, and clinical information (prior history of gestational diabetes and family history of diabetes). The cost of ILI per participant year was calculated by summing the total costs of supplies and personnel effort with an indirect rate of 20%, then dividing by the number of ILI participants.
Statistical Analysis
Data from a previous pilot study of the promotora-led ILI provided estimates for participant retention at 12-month follow-up (90%) and 12-month weight loss (4.9 kg, SD=4.9 kg).32 Based on these assumptions, the enrollment target was 30 participants per study arm in order to retain 27 in each group at 12 months. These assumptions allowed for >80% power to detect a mean weight loss difference of at least 4.9 kg (SD=4.9 kg) between groups, which was lower than that observed in DPP, at the overall 5% significance level. Power calculations adjusted for three pairwise comparisons, using a 1.7% significance level for each. Treatment allocation employed a permuted-block design with randomly varying block sizes of six, nine, and 12 that were unknown to the investigators. This method achieves balanced allocation of participants among treatment groups if the final block is filled, while reducing the potential for selection bias by randomly varying block sizes.33 There was no stratification of the permuted block design according to baseline participant characteristics. The random allocation sequence was generated independently by a statistician and concealed in individually sealed envelopes accessible only to the research coordinator, who ultimately assigned participants to the study interventions. The nature of the study design precluded blinding participants or promotoras to treatment assignments.
Descriptive statistics were used to characterize the study cohort at baseline. Analysis of outcome data followed a modified intent-to-treat principle where all participants who completed data collection were analyzed according to their randomized treatment assignment, regardless of their level of intervention adherence. According to the protocol, follow-up data were not collected on participants who became pregnant during the trial to avoid a biased assessment of the primary outcome (12-month weight change). In this modified intent-to-treat analysis, mean 12-month differences among the three groups in weight and secondary cardiometabolic outcomes were assessed using ANCOVA models, adjusting for baseline measurement of each biomarker. For outcomes suggesting significant or marginally significant intervention effects in ANCOVA models, post-hoc comparisons between each treatment arm were conducted using Tukey’s tests for multiple pairwise comparisons. All analyses assumed a 5% level of significance and were conducted using Stata, version 13. Study data were collected from 2013–2015 and analyzed in 2016.
RESULTS
From November 2013 to February 2015, a total of 573 Latinas were screened, 92 of whom were eligible and enrolled (Figure 1). Table 1 presents baseline data from the randomized cohort. PREVENT-DM participants had a mean age of 45 years with generally low levels of educational attainment and mean household income. All participants were Spanish speaking and the majority was foreign born. Most participants had a first-degree family member with diabetes, and almost 20% reported a prior history of gestational diabetes. At baseline, the sample had elevated mean levels of the following cardiometabolic markers: BMI (33.3 kg/m2), waist circumference (97.4 cm), and HbA1c (5.9%, 41 mmol/mol). The mean values for other biomarkers were within normal ranges. Though all participants had prediabetes by HbA1c or fasting plasma glucose criteria, more participants qualified for the study based on elevated HbA1c alone (n=53, 57.6% of total participants). Among the remaining 39 participants, 12 (13.0%) qualified by having impaired fasting glucose alone, and 27 (29.3%) met both glycemic criteria for prediabetes (data not shown). This resulted in a mean baseline fasting glucose concentration below the diagnostic threshold. The treatment arms were comparable with respect to all variables at baseline.
Figure 1. Flow of participants through the trial.
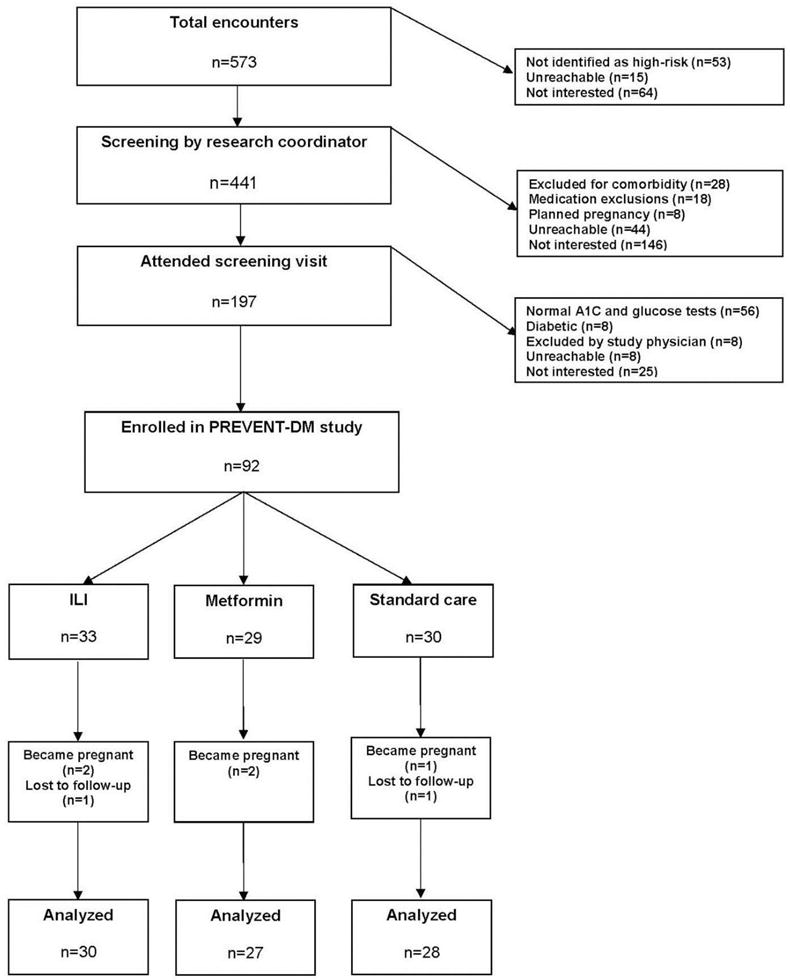
Notes: Boxes and arrows indicate the flow of participants in PREVENT-DM including eligibility, enrollment, randomization, follow-up, and analysis; side arrows provide reasons for ineligibility and non-enrollment.
ILI, Intensive Lifestyle Intervention; PREVENT-DM, Promotora Effectiveness Versus Metformin Trial
Table 1.
Baseline Characteristics of PREVENT-DM Participants by Treatment Assignmenta
| Characteristic | Overall (n=92) |
ILI (n=33) |
Metformin (n=29) |
Standard care (n=30) |
|---|---|---|---|---|
| Age, years | 45.1 ± 12.5 | 45.5 ± 12.3 | 45.8 ± 11.7 | 44.0 ± 13.6 |
| Education, years | 9.7 ± 3.6 | 10.2 ± 3.5 | 9.5 ± 3.6 | 9.2 ± 3.7 |
| Household income, dollars | 15,527 ± 9,922 | 14,905 ± 7,518 | 17,315 ± 11,293 | 14,482 ± 10,894 |
| Foreign born, n (%) | 86 (93.5) | 30 (90.9) | 26 (89.7) | 30 (100) |
| Country/region of origin, n (%) | ||||
| U.S. | 6 (6.5) | 3 (9.1) | 3 (10.3) | 0 (0) |
| Mexico | 41 (44.6) | 14 (42.4) | 11 (37.9) | 16 (53.3) |
| Caribbean | 33 (35.9) | 12 (36.4) | 13 (44.8) | 8 (26.7) |
| South America | 7 (7.6) | 2 (6.1) | 1 (3.4) | 4 (13.3) |
| Central America | 5 (5.4) | 2 (6.1) | 1 (3.4) | 2 (6.7) |
| Duration of U.S. residence, years | 18.2 ± 13.0 | 20.3 ± 13.3 | 17.8 ± 14.2 | 16.5 ± 11.7 |
| Family history of diabetes, n (%) | 65 (70.7) | 25 (75.8) | 21 (72.4) | 19 (63.3) |
| History of gestational diabetes, n (%) | 18 (19.6) | 9 (27.2) | 6 (20.7) | 3 (10.0) |
| Weight, kg | 81.3 ± 17.9 | 85.4 ± 23.0 | 79.7 ± 13.0 | 78.2 ± 15.0 |
| BMI, kg/m2 | 33.3 ± 6.5 | 34.4 ± 7.9 | 33.2 ± 5.5 | 32.2 ± 5.7 |
| Waist circumference, cm | 97.4 ± 11.1 | 101.4 ± 13.0 | 95.6 ± 9.1 | 94.9 ± 9.8 |
| Hemoglobin A1c, % | 5.9 ± 0.2 | 5.9 ± 0.2 | 6.0 ± 0.2 | 5.9 ± 0.3 |
| Fasting plasma glucose, mg/dL | 96.1 ± 9.5 | 97.5 ± 7.5 | 94.6 ± 10.1 | 96.0 ± 10.7 |
| Fasting plasma insulin, μIU/mL | 10.4 ± 5.7 | 10.4 ± 5.8 | 10.6 ± 5.5 | 10.2 ± 6.0 |
| HOMA-IRb | 2.49 ± 1.44 | 2.52 ± 1.36 | 2.49 ± 1.43 | 2.45 ± 1.57 |
| Total cholesterol, mg/dL | 186.6 ± 37.0 | 192.8 ± 34.8 | 186.2 ± 43.6 | 180.3 ± 32.3 |
| LDL cholesterol, mg/dL | 110.3 ± 32.3 | 114.3 ± 30.6 | 110.7 ± 38.8 | 105.6 ± 27.4 |
| HDL cholesterol, mg/dL | 51.0 ± 12.2 | 49.5 ± 13.2 | 52.2 ± 10.7 | 51.3 ± 12.8 |
| Triglycerides, mg/dL | 125.1 ± 56.9 | 144.6 ± 73.7 | 110.6 ± 30.5 | 117.6 ± 50.6 |
| Systolic blood pressure, mmHg | 119.6 ± 17.0 | 118.4 ± 13.9 | 122.2 ± 19.8 | 118.3 ± 17.6 |
| Diastolic blood pressure, mmHg | 74.5 ± 9.6 | 74.5 ± 9.8 | 75.9 ± 10.2 | 73.3 ± 9.0 |
Data are presented as means ± SD or n (%).
Homeostasis model assessment of insulin resistance (HOMA-IR) is calculated according to the following formula: [fasting insulin (μIU/mL) × fasting glucose (mmol/L)/22.5].
PREVENT-DM, Promotora Effectiveness Versus Metformin Trial; ILI, intensive lifestyle intervention; LDL, low density lipoprotein; HDL, high density lipoprotein
Five participants (5.4%) became pregnant during the study, which precluded continuation of interventions and assessment of outcomes according to the protocol. Two participants (2.2%) were lost to follow-up at 12 months. Those randomized to ILI attended an average of 14.2 (SD=8.4, 59.2%) of the 24 sessions. Three participants (9.1%) in the ILI group did not attend any of the lifestyle sessions, and 23 (69.6%) attended at least nine sessions, which is an attendance threshold associated with clinically meaningful weight loss.34 Session attendance in the ILI group was significantly associated with 12-month weight loss (r =0.37, p=0.046). There were no adverse events reported among ILI participants.
In the metformin arm, three participants (10.3%) never took the medication. Including those who never took metformin, the mean adherence over the duration of the study was 66.4% of dispensed doses. Among the 29 participants randomized to receive metformin, 11 (37.9%) took at least 80% of dispensed doses, which was the threshold used in DPP to define high adherence.1 Ten metformin participants (34.4%) reported side effects: gastrointestinal (27.6%), dizziness/vertigo (3.4%), and headache (3.4%). One of these participants (3.4%) discontinued the medication because of side effects. There were no adverse events reported by participants in the standard care arm.
A total of 85 participants (92.4%) completed 12-month study assessments. Tables 2 and 3 present the 12-month difference in cardiometabolic outcomes within and between treatment groups, respectively. There was a significant difference among the groups in 12-month weight loss (ILI, −4.0 kg; metformin, −0.9 kg; standard care, +0.8 kg; p<0.001). (Table 2) Post-hoc pairwise comparisons were significant for ILI versus standard care (−4.8 kg, p<0.001) and ILI versus metformin (−3.1 kg, p=0.013), but not for metformin versus standard care (−1.7 kg, p=0.276) (Table 3). Six- and 12-month weight changes in the three treatment arms are displayed in Appendix 1.
Table 2.
12-Month Outcomes Among PREVENT-DM Participants by Treatment Assignment
| Outcome | Mean 12-month difference (95% CI) within each groupa | |||
|---|---|---|---|---|
| ILI (n=30) |
Metformin (n=27) |
Standard care (n=28) |
p-valueb | |
| Weight, kg | −4.0 (−5.5, −2.6) | −0.9 (−2.4, 0.6) | 0.8 (−0.8, 2.3) | <0.001 |
| Weight change, %c | −5.0 (−6.8, −3.2) | −1.1 (−3.0, 0.7) | 0.9 (−0.9, 2.8) | <0.001 |
| BMI, kg/m2 | −1.6 (−2.3, −1.0) | −0.4 (−1.0, 0.3) | 0.3 (−0.3, 1.0) | <0.001 |
| Waist circumference, cm | −4.0 (−5.5, −2.6) | −1.8 (−3.3, −0.3) | −0.2 (−1.7, 1.3) | 0.002 |
| Hemoglobin A1c, % | −0.06 (−0.14, 0.02) | 0.06 (−0.03, 0.14) | 0.06 (−0.03, 0.13) | 0.063 |
| Fasting plasma glucose, mg/dL | −2.8 (−5.7, 0.1) | −2.3 (−5.4, 0.7) | −3.6 (−6.6, −0.6) | 0.830 |
| Fasting plasma insulin, μIU/mL | 1.4 (−1.0, 3.9) | −0.4 (−2.9, 2.1) | 1.5 (−0.9, 3.9) | 0.484 |
| HOMA-IRd | 0.27 (−0.31, 0.84) | −0.13 (−0.73, 0.47) | 0.26 (−0.31, 0.83) | 0.556 |
| Total cholesterol, mg/dL | −1.9 (−10.2, 6.4) | −6.8 (−15.6, 1.9) | −2.7 (−11.3, 5.9) | 0.690 |
| LDL cholesterol, mg/dL | −1.6 (−8.9, 5.7) | −3.0 (−10.7, 4.6) | −2.0 (−9.5, 5.5) | 0.963 |
| HDL cholesterol, mg/dL | 1.4 (−1.2, 4.0) | −2.3 (−5.0, 0.5) | 0.6 (−2.1, 3.3) | 0.133 |
| Triglycerides, mg/dL | −6.3 (−21.8, 9.1) | −4.8 (−20.9, 11.3) | −5.9 (−21.6, 9.8) | 0.990 |
| Systolic blood pressure, mmHg | −8.4 (−12.9, − 3.9) | −4.5 (−9.4, 0.3) | −3.8 (−8.5, 0.8) | 0.320 |
| Diastolic blood pressure, mmHg | −2.6 (−5.7, 0.4) | −0.6 (−3.9, 2.6) | −0.9 (−4.1, 2.2) | 0.620 |
The 12-month difference in outcome measures within each treatment group was determined using analysis of covariance (ANCOVA) models adjusted for baseline levels of each biomarker, and is expressed as the baseline value subtracted from the 12-month value.
p-values reflect the intervention arm effect across all study arms from ANCOVA models. Boldface indicates statistical significance (p<0.05).
Percent weight change is based on the weight change from baseline to 12 months, determined using the following formula: [(12-month weight − baseline weight)/baseline weight × 100].
Homeostasis model assessment of insulin resistance (HOMA-IR) is calculated according to the following formula: [fasting insulin (μIU/mL) × fasting glucose (mmol/L)/22.5].
PREVENT-DM, Promotora Effectiveness Versus Metformin Trial; ILI, intensive lifestyle intervention; LDL, low density lipoprotein; HDL, high density lipoprotein
Table 3.
Pairwise Comparisons of Selected Outcomes Between Treatment Groupsa
| Outcome | Mean 12-month difference (95% CI) between groupsb p-valuec |
||
|---|---|---|---|
| ILI vs. standard care | Metformin vs. standard care | ILI vs. metformin | |
| Weight, kg | −4.8 (−7.3, −2.2) <0.001 |
−1.7 (−4.2, 0.9) 0.276 |
−3.1 (−5.7, −0.6) 0.013 |
| Weight change, %d | −6.0 (−9.1, −2.8) <0.001 |
−2.1 (−5.2, 1.1) 0.261 |
−3.9 (−7.0, −0.7) 0.011 |
| BMI, kg/m2 | −2.0 (−3.0, −0.9) <0.001 |
−0.7 (−1.8, 0.4) 0.254 |
−1.2 (−2.3, −0.2) 0.018 |
| Waist circumference, cm | −3.8 (−6.4, −1.3) 0.001 |
−1.7 (−4.2, 0.8) 0.259 |
−2.2 (−4.7, 0.3) 0.100 |
| Hemoglobin A1c, % | −0.12 (−0.26, 0.02) 0.108 |
0.00 (−0.14, 0.14) 0.999 |
−0.12 (−0.26, 0.02) 0.106 |
Comparisons of between-group mean differences in outcomes were conducted with post-hoc Tukey’s tests for multiple pairwise comparisons.
Between-group differences in outcome measures are expressed as the mean 12-month change in one treatment arm minus the mean 12-month change in the other treatment arm.
p-values express the significance of pairwise comparisons in outcome measures between treatment groups. Boldface indicates statistical significance (p<0.05).
Percent weight change is based on the weight change from baseline to 12 months, determined using the following formula: [(12-month weight − baseline weight)/baseline weight × 100].
ILI, intensive lifestyle intervention
On average, participants in the ILI group lost 5.0% of their initial body weight, compared with 1.1% weight loss among metformin participants and 0.9% weight gain in the standard care group (p<0.001). The number of participants who achieved 5% weight loss by treatment assignment was 15 (50.0%) for ILI, four (14.8%) for metformin, and two (7.1%) for standard care. Pairwise comparisons in percent weight loss revealed statistical significance for ILI versus standard care (6.0%, p<0.001) and metformin (3.9%, p=0.011), but not for metformin versus standard care (2.1%, p=0.261). (Table 3) There were also statistically significant differences across treatment groups in the 12-month change in waist circumference (p=0.002). The reduction in waist circumference was significantly greater in ILI than the standard care group (p=0.001), but other pairwise comparisons failed to demonstrate significant differences. The differences among groups in the 12-month change in HbA1c did not reach statistical significance (p=0.063). In general, the 12-month changes in other cardiometabolic markers improved or were stable, but there were no significant differences across groups (Table 2).
Only one participant, who was randomized to standard care, developed diabetes during the study period. A total of 12 participants (13.0%) reverted to normoglycemia during the trial based on HbA1c values, with seven of 30 (23.3%) in the ILI group, three of 27 (11.1%) in the metformin group, and two of 28 (7.1%) in the standard care group.
DISCUSSION
In PREVENT-DM, Latina participants with prediabetes who received a promotora-led ILI lost significantly more weight than either metformin or standard care participants. There was no significant difference in weight loss between the metformin and standard care arms. On average, those randomized to ILI achieved a mean weight loss equivalent to 5.0% of their initial body weight. ILI participants demonstrated significantly greater reductions in waist circumference than standard care participants. Although small in magnitude and not statistically significant, there was a reduction in HbA1c among ILI participants, compared with slight increases in the metformin and standard care groups.
This is the first DPP translational study to test the comparative effectiveness of ILI and metformin in a community-based setting. Two efficacy studies that tested ILI versus metformin in adults with prediabetes reported divergent findings about the relative risk reduction associated with these treatments. Although DPP reported a nearly twofold reduction in diabetes incidence with ILI compared with metformin,1 the Indian Diabetes Prevention Programme (IDPP) found that these treatments were equivalent at preventing or delaying diabetes.7 The DPP and IDPP differed in several important ways that may have contributed to this discrepancy. First, IDPP presented little information about its lifestyle intervention, which precludes comparison of the behavioral approaches used. Second, IDPP participants assigned to metformin received a total daily dose of 500 mg, which is substantially lower than the 1,700-mg daily dose studied in DPP. Third, the Asian Indian participants in IDDP were substantially leaner than those enrolled in DPP (baseline BMI of 25.8 vs 34.0, respectively) with higher diabetes risk (baseline HbA1c of 6.2% [42 mmol/mol) vs 5.9% [41 mmol/mol], respectively], which may be explained by greater rates of β-cell dysfunction observed in this population relative to other racial/ethnic groups.35 Though weight loss mediated the observed treatment effects observed in DPP,24,25 there was no weight loss associated with active treatment in IDPP.
In this study, participants randomized to ILI demonstrated significantly greater weight loss (−4.0 kg) than the metformin (−0.9 kg) and standard care (0.8 kg) groups. Although smaller in magnitude, this weight loss pattern was similar to that observed among Latinas in DPP. The corresponding 12-month weight loss for this subgroup in DPP was −5.8 kg for ILI, −2.2 kg for metformin, and −1.1 kg for placebo.36 Two recent meta-analyses of DPP-based ILI, including a total of 45 unique studies, highlight the clinical significance of weight loss found in PREVENT-DM. The first meta-analysis, published in 2012, reported a pooled mean weight loss of 3.99% from baseline to 12 months across 28 studies translating the DPP’s ILI.2 This analysis further stratified weight loss by the type of interventionist delivering the program, finding a mean weight loss of 3.15% for ILI delivered by lay health workers like the promotoras in PREVENT-DM. A second meta-analysis of 25 trials, which included more recent studies of ILI and those published outside the U.S., reported a pooled mean weight loss of 2.1 kg at 12 months.3 The weight loss observed among ILI participants in PREVENT-DM was at least comparable to three other DPP translation studies conducted in similar low-income, Hispanic populations.10–12
Previous efficacy trials provide context for interpreting current findings from the metformin group. In DPP, metformin participants lost 2.6 kg at 12 months, corresponding to 2.7% of initial body weight.37 The greater weight loss observed in DPP relative to PREVENT-DM is likely related to higher metformin adherence reported in DPP.37 There was no weight loss observed among metformin participants in IDPP, which may be related to the lower dose of metformin and substantially leaner sample relative to the current study and DPP. A meta-analysis of other efficacy trials testing metformin in adults with elevated diabetes risk found a mean BMI reduction of 5.3% from baseline to follow-up.22 The greater BMI reduction in these trials may also result from higher adherence compared with PREVENT-DM, in addition to differences in the studied populations (e.g., women with polycystic ovary syndrome).22
In contrast to previous efficacy studies of ILI and metformin, the authors did not observe significant improvements in glycemic measures. This may partly be explained by the glycemic inclusion criteria in PREVENT-DM. As mentioned above, the majority of participants qualified for the trial based on the HbA1c criterion alone, resulting in a mean baseline fasting glucose value below the diagnostic threshold for prediabetes (96.1 mg/dL). Data from the placebo group in DPP demonstrate that adults with higher baseline fasting glucose values (110–125 mg/dL) have an almost fourfold increased risk of developing diabetes relative to those with lower fasting glucose (95–109 mg/dL).1 However, DPP participants with higher baseline glucose experienced greater reductions in diabetes incidence with both ILI and metformin than those who had lower fasting glucose at baseline.1 Therefore, the absence of significant reductions in fasting glucose or HbA1c in PREVENT-DM may partly result from studying a population that was at lower risk of developing diabetes, and therefore less likely to experience glycemic improvements from ILI and metformin. The authors did not assess impaired fasting glucose because oral glucose tolerance tests are burdensome and unlikely to be performed in real-world diabetes prevention efforts, as evidenced by their low uptake in routine clinical practice.27,38
To the authors’ knowledge, PREVENT-DM provides the first evidence describing the comparative weight loss effectiveness of ILI and metformin in a real-world setting. The randomized design of PREVENT-DM distinguishes this trial from many previous DPP translational studies that used non-experimental designs.2,3 Only two participants (2.2%) were lost to follow-up during the 12-month study, which represents another strength. This trial focused on a high-risk, yet understudied population in the existing literature. PREVENT-DM included mostly Mexican and Caribbean Latinas (80.5% of participants), who have a higher diabetes risk than other Hispanic subgroups.39 These groups comprise 76.9% of U.S. Hispanics, suggesting that these data may inform diabetes prevention efforts in many Hispanic communities nationwide.40 The ILI intervention cost $287.22 per participant year (data not shown), which may also inform future implementation and dissemination efforts.
Limitations
This study also has some notable weaknesses. Although enrollment and retention targets were achieved, the study was underpowered to detect a modest but clinically meaningful weight difference between the metformin and standard care arms. However, PREVENT-DM had sufficient power to demonstrate significantly larger weight loss associated with ILI than both standard care and metformin. The 12-month follow-up period precluded assessment of weight loss maintenance beyond the study’s completion. This study was a community-based translation trial with fewer eligibility criteria than DPP, a less intensive screening process, and no run-in period. The proportion of those screened who participated in PREVENT-DM was either similar or higher than that reported in previous translational trials of ILI.41–43 However, PREVENT-DM still enrolled motivated study volunteers, potentially limiting the external validity of the findings among all Latinas with prediabetes. Generalizability is also limited in men and other racial/ethnic groups. Like many other pragmatic and behavioral intervention trials, the inability to blind participants or promotoras to treatment assignment was inherent to the study design, and could have introduced bias.
CONCLUSIONS
The PREVENT-DM trial makes an important contribution to the literature. This study of Latinas with prediabetes suggests that ILI delivered by promotoras is superior to metformin and standard care at inducing clinically and statistically significant weight loss. Relative to standard care, the ILI also produced a significantly greater reduction in waist circumference, which is associated with lower risk of developing diabetes among women with prediabetes.44 These findings support the ongoing development of infrastructure to deliver ILI programs at scale, which is underway.45 There is a need for future pragmatic research that will definitively test the comparative effectiveness of ILI, metformin, and standard care in adults with prediabetes. Such trials should examine long-term differences in treatment adherence and weight loss, as well as the development of diabetes and cardiovascular disease associated with each intervention. By presenting evidence on the real-world effectiveness of ILI, metformin, and standard care for inducing weight loss and lowering diabetes risk, this study and larger subsequent trials can inform shared decision making about prediabetes management between patients and healthcare providers. In addition, these studies may influence diabetes prevention policy by enabling analysis of differences in each intervention’s uptake, effectiveness, and cost. Future work is also needed to elucidate the barriers that hinder adoption of diabetes prevention treatments, and to design effective strategies that promote their use in practice.
Supplementary Material
Acknowledgments
The authors would like to thank the promotoras and study participants for their dedicated participation. We acknowledge the University of Pittsburgh’s Diabetes Prevention Support Center for their technical assistance and support. We also thank Maria Vargas, MPH, of Northwestern University for her help in preparing the manuscript. We thank the many study volunteers for their efforts, in addition to Stella Ouelette, MPP and Erica Bagwell (Temple University Center for Obesity Research and Education) for their significant administrative support. This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (K23-DK095981). The promotoras who delivered the intensive lifestyle intervention were supported by the study’s clinic partner, Puentes de Salud, through grants from the Kynett Foundation, Claneil Foundation, and Connelly Foundation. The research presented in this paper is that of the authors and does not reflect the official policy of NIH. The study sponsors played no role in the study design.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author responsibilities were as follows: MJO wrote the manuscript; RCW and GDF contributed to the study design; MJO, AS, and JDC researched data; and AP, AS, VAA, RCW, GDF, RTA, JDC, and CH reviewed/edited the manuscript.
Clinical trial registration number: NCT02088034.
No financial disclosures were reported by the authors of this paper.
References
- 1.Knowler W, Barrett-Connor E, Fowler S, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. https://doi.org/10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali MK, Echouffo-Tcheugui J, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff (Millwood) 2012;31(1):67–75. doi: 10.1377/hlthaff.2011.1009. https://doi.org/10.1377/hlthaff.2011.1009. [DOI] [PubMed] [Google Scholar]
- 3.Dunkley AJ, Bodicoat DH, Greaves CJ, et al. Diabetes Prevention in the Real World: Effectiveness of Pragmatic Lifestyle Interventions for the Prevention of Type 2 Diabetes and of the Impact of Adherence to Guideline Recommendations A Systematic Review and Meta-analysis. Diabetes Care. 2014;37(4):922–923. doi: 10.2337/dc13-2195. https://doi.org/10.2337/dc13-2195. [DOI] [PubMed] [Google Scholar]
- 4.Balk EM, Earley A, Raman G, Avendano EA, Pittas AG, Remington PL. Combined Diet and Physical Activity Promotion Programs to Prevent Type 2 Diabetes Among Persons at Increased Risk: A Systematic Review for the Community Preventive Services Task Force. Ann Intern Med. 2015;163(6):437–451. doi: 10.7326/M15-0452. https://doi.org/10.7326/M15-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albright AL, Gregg EW. Preventing type 2 diabetes in communities across the U.S.: the National Diabetes Prevention Program. Am J Prev Med. 2013;44(4):S346–S351. doi: 10.1016/j.amepre.2012.12.009. https://doi.org/10.1016/j.amepre.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pear R. Medicare Proposal Takes Aim at Diabetes. New York Times; 2016. www.nytimes.com/2016/03/23/us/politics/medicare-proposal-takes-aim-at-diabetes.html?_r=0. Accessed May 16, 2016. [Google Scholar]
- 7.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar A, Vijay V. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia. 2006;49(2):289–297. doi: 10.1007/s00125-005-0097-z. https://doi.org/10.1007/s00125-005-0097-z. [DOI] [PubMed] [Google Scholar]
- 8.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. JAMA. 2015;314(10):1021–1029. doi: 10.1001/jama.2015.10029. https://doi.org/10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 9.Narayan KMV, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290(14):1884–1890. doi: 10.1001/jama.290.14.1884. https://doi.org/10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 10.Ockene IS, Tellez TL, Rosal MC, et al. Outcomes of a Latino community-based intervention for the prevention of diabetes: the Lawrence Latino Diabetes Prevention Project. Am J Public Health. 2012;102(2):336–342. doi: 10.2105/AJPH.2011.300357. https://doi.org/10.2105/AJPH.2011.300357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vincent D, McEwen MM, Hepworth JT, Stump CS. The effects of a community-based, culturally tailored diabetes prevention intervention for high-risk adults of Mexican descent. Diabetes Educ. 2014;40(2):202–213. doi: 10.1177/0145721714521020. https://doi.org/10.1177/0145721714521020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Name MA, Camp AW, Magenheimer EA, et al. Effective Translation of an Intensive Lifestyle Intervention for Hispanic Women With Prediabetes in a Community Health Center Setting. Diabetes Care. 2016;39(4):525–531. doi: 10.2337/dc15-1899. https://doi.org/10.2337/dc15-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Brien MJ, Squires AP, Bixby RA, Larson SC. Role development of community health workers: an examination of selection and training processes in the intervention literature. Am J Prev Med. 2009;37(6 Suppl 1):S262–269. doi: 10.1016/j.amepre.2009.08.011. https://doi.org/10.1016/j.amepre.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katula JA, Vitolins MZ, Rosenberger EL, et al. Healthy Living Partnerships to Prevent Diabetes (HELP PD): design and methods. Contemp Clin Trials. 2010;31(1):71–81. doi: 10.1016/j.cct.2009.09.002. https://doi.org/10.1016/j.cct.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez A, Alos VA, Scanlan A, et al. The rationale, design, and baseline characteristics of PREVENT-DM: A community-based comparative effectiveness trial of lifestyle intervention and metformin among Latinas with prediabetes. Contemp Clin Trials. 2015;45(Part B):320–327. doi: 10.1016/j.cct.2015.10.011. https://doi.org/10.1016/j.cct.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Brien MJ, Halbert CH, Bixby R, Pimentel S, Shea JA. Community health worker intervention to decrease cervical cancer disparities in Hispanic women. J Gen Intern Med. 2010;25(11):1186–1192. doi: 10.1007/s11606-010-1434-6. https://doi.org/10.1007/s11606-010-1434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association. Diabetes Risk Test. 2011 http://main.diabetes.org/dorg/PDFs/risk-test-paper-version.pdf. Accessed June 28, 2016.
- 18.Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP) description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–2171. doi: 10.2337/diacare.25.12.2165. https://doi.org/10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kramer MK, Kriska AM, Venditti EM, et al. Translating the Diabetes Prevention Program: a comprehensive model for prevention training and program delivery. Am J Prev Med. 2009;37(6):505–511. doi: 10.1016/j.amepre.2009.07.020. https://doi.org/10.1016/j.amepre.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien MJ, Davey A, Alos VA, Whitaker RC. Diabetes-related behaviors in Latinas and non-Latinas in California. Diabetes Care. 2013;36(2):355–361. doi: 10.2337/dc12-0548. https://doi.org/10.2337/dc12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Brien MJ, Shuman SJ, Barrios DM, Alos VA, Whitaker RC. A qualitative study of acculturation and diabetes risk among urban immigrant Latinas: implications for diabetes prevention efforts. Diabetes Educ. 2014;40(5):616–625. doi: 10.1177/0145721714535992. https://doi.org/10.1177/0145721714535992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salpeter SR, Buckley NS, Kahn JA, Salpeter EE. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121(2):149–157. doi: 10.1016/j.amjmed.2007.09.016. https://doi.org/10.1016/j.amjmed.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 23.National Diabetes Education Program. Publications. 2014 http://ndep.nih.gov/publications/index.aspx. Accessed April 8, 2016.
- 24.Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102–2107. doi: 10.2337/dc06-0560. https://doi.org/10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lachin JM, Christophi CA, Edelstein SL, et al. Factors associated with diabetes onset during metformin versus placebo therapy in the diabetes prevention program. Diabetes. 2007;56(4):1153–1159. doi: 10.2337/db06-0918. https://doi.org/10.2337/db06-0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mudaliar U, Zabetian A, Goodman M, et al. Cardiometabolic Risk Factor Changes Observed in Diabetes Prevention Programs in U.S. Settings: A Systematic Review and Meta-analysis. PLoS Med. 2016;13(7):e1002095. doi: 10.1371/journal.pmed.1002095. https://doi.org/10.1371/journal.pmed.1002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Brien MJ, Lee JY, Carnethon MR, et al. Detecting Dysglycemia Using the 2015 United States Preventive Services Task Force Screening Criteria: A Cohort Analysis of Community Health Center Patients. PLoS Med. 2016;13(7):e1002074. doi: 10.1371/journal.pmed.1002074. https://doi.org/10.1371/journal.pmed.1002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.YMCA’s Diabetes Prevention Program. Diabetes Prevention Program FACT SHEET: November 2015. hicago, IL: YMCA of the USA; 2015. [Google Scholar]
- 29.Look AHEAD Research Group. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring, Md) 2006;14(5):737–752. doi: 10.1038/oby.2006.84. https://doi.org/10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. https://doi.org/10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 31.Little RR. Glycated hemoglobin standardization–National Glycohemoglobin Standardization Program (NGSP) perspective. Clin Chem Lab Med. 2003;41(9):1191–1198. doi: 10.1515/CCLM.2003.183. https://doi.org/10.1515/CCLM.2003.183. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien MJ, Perez A, Alos VA, et al. The feasibility, acceptability, and preliminary effectiveness of a promotora-led diabetes prevention program (PL-DPP) in Latinas: a pilot study. Diabetes Educ. 2015;41(4):485–494. doi: 10.1177/0145721715586576. https://doi.org/10.1177/0145721715586576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matts JP, Lachin JM. Properties of permuted-block randomization in clinical trials. Control Clin Trials. 1988;9(4):327–344. doi: 10.1016/0197-2456(88)90047-5. https://doi.org/10.1016/0197-2456(88)90047-5. [DOI] [PubMed] [Google Scholar]
- 34.Ackermann RT, Liss DT, Finch EA, et al. A Randomized Comparative Effectiveness Trial for Preventing Type 2 Diabetes. Am J Public Health. 2015;105(11):2328–2334. doi: 10.2105/AJPH.2015.302641. https://doi.org/10.2105/AJPH.2015.302641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanaya AM, Herrington D, Vittinghoff E, et al. Understanding the high prevalence of diabetes in U.S. south Asians compared with four racial/ethnic groups: the MASALA and MESA studies. Diabetes Care. 2014;37(6):1621–1628. doi: 10.2337/dc13-2656. https://doi.org/10.2337/dc13-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.West DS, Elaine Prewitt T, Bursac Z, Felix HC. Weight loss of black, white, and Hispanic men and women in the Diabetes Prevention Program. Obesity (Silver Spring) 2008;16(6):1413–1420. doi: 10.1038/oby.2008.224. https://doi.org/10.1038/oby.2008.224. [DOI] [PubMed] [Google Scholar]
- 37.Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–737. doi: 10.2337/dc11-1299. https://doi.org/10.2337/dc11-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ealovega MW, Tabaei BP, Brandle M, Burke R, Herman WH. Opportunistic screening for diabetes in routine clinical practice. Diabetes Care. 2004;27(1):9–12. doi: 10.2337/diacare.27.1.9. https://doi.org/10.2337/diacare.27.1.9. [DOI] [PubMed] [Google Scholar]
- 39.Schneiderman N, Llabre M, Cowie CC, et al. Prevalence of diabetes among Hispanics/Latinos from diverse backgrounds: the Hispanic community health study/study of Latinos (HCHS/SOL) Diabetes Care. 2014;37(8):2233–2239. doi: 10.2337/dc13-2939. https://doi.org/10.2337/dc13-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pew Hispanic Center. Statistical Portrait of Hispanics in the United States. 2013 http://assets.pewresearch.org/wp-content/uploads/sites/7/2015/05/2015-05-12_statistical-portrait-of-hispanics-in-the-united-states-2013_final.pdf. Accessed October 17, 2016.
- 41.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community. The DEPLOY Pilot Study. Am J Prev Med. 2008;35(4):357–363. doi: 10.1016/j.amepre.2008.06.035. https://doi.org/10.1016/j.amepre.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ackermann RT, Finch EA, Schmidt KK, et al. Rationale, design, and baseline characteristics of a community-based comparative effectiveness trial to prevent type 2 diabetes in economically disadvantaged adults: The RAPID Study. Contemp Clin Trials. 2014;37(1):1–9. doi: 10.1016/j.cct.2013.10.003. https://doi.org/10.1016/j.cct.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katula JA, Vitolins MZ, Rosenberger EL, et al. One-year results of a community-based translation of the diabetes prevention program. Diabetes Care. 2011;34(7):1451–1457. doi: 10.2337/dc10-2115. https://doi.org/10.2337/dc10-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song X, Qiu M, Zhang X, et al. Gender-related affecting factors of prediabetes on its 10-year outcome. BMJ Open Diabetes Res Care. 2016;4:e000169. doi: 10.1136/bmjdrc-2015-000169. https://doi.org/10.1136/bmjdrc-2015-000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.CDC. National Diabetes Prevention Program: Registry of Recognized Programs. 2016 https://nccd.cdc.gov/DDT_DPRP/State.aspx?STATE=ALL. Accessed April 15, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


