Abstract
Objectives
To explore whether work schedules and physically demanding work were associated with markers of ovarian reserve and response.
Methods
This analysis included women (n=473 and n=313 for ovarian reserve and ovarian response analysis, respectively) enrolled in a prospective cohort study of couples presenting to an academic fertility center (2004–2015). Information on occupational factors was collected on a take-home questionnaire, and reproductive outcomes were abstracted from electronic medical records. Generalized linear models and generalized linear mixed models were used to evaluate the associations.
Results
Women who reported lifting or moving heavy objects at work had 1.0 fewer total oocytes (p-value=0.08), 1.4 fewer mature oocytes (p-value=0.007), and 0.7 fewer antral follicles (p-value=0.06) compared to women who reported never lifting or moving heavy objects at work. The inverse association between heavy lifting and oocyte yield was stronger in women >37 years and with a BMI ≥25 kg/m2. Women who worked evening/night/rotating shifts had 2.3 (p-value<0.001) fewer mature oocytes, on average, compared with women who worked day-only shifts. None of the occupational exposures were associated with day 3 FSH or peak estradiol levels.
Conclusion
Women working non-daytime shifts and those with physically demanding jobs had fewer mature oocytes retrieved after controlled ovarian hyperstimulation. Our results provide insight into possible mechanisms linking these occupational exposures with decreased fecundity.
Keywords: Shift work, physically demanding work, ovarian reserve and response, in vitro fertilization, infertility
INTRODUCTION
Some occupational factors have been suggested to disrupt circadian regulation [1] which could in turn affect reproductive outcomes among women. For instance, shift work, long working hours and physical factors have been found to increase the risk of menstrual cycle disturbances,[2, 3] spontaneous abortion,[4] preterm birth [5, 6] and low birth weight;[6] however the relation of these occupational factors with fecundity is less clear.[7–15]
While the terms fertility and fecundity are often used synonymously, they are separate constructs with fecundity defined as the biologic capacity for reproduction and fertility defined as demonstrated fecundity, usually measured by live births. Given the absence of a gold standard to measure fecundity, previous studies have relied on a variety of different endpoints. For example, in previous publications on occupational factors and fecundity, the main outcome was defined as the time to pregnancy,[7–9, 12–15] while in others it was measured as the pregnancy duration,[5, 11] pregnancy and delivery rate,[11] or time of unprotected intercourse.[10] However, given the nature of these studies, they were unable to measure biomarkers of fecundity such as reproductive hormones or ovarian function, and as such they could only postulate on the possible mechanisms underlying associations between occupational factors and fecundity. By studying a cohort of women undergoing in vitro fertilization (IVF), it is possible to directly observe many biomarkers of fecundity which cannot be observed in couples attempting to conceive naturally. These include markers of ovarian reserve (e.g. number of antral follicles and levels of follicle stimulating hormone (FSH), commonly used as indicators of ovarian age) and ovarian response to stimulation (e.g. number of mature oocytes which are capable of developing into healthy embryos); these measures can thus lend insight into potential biological mechanisms.
To address this gap, we examined whether shift work and physically demanding work are associated with markers of ovarian reserve (i.e. total antral follicle count (AFC) and follicle stimulating hormone (FSH) levels) and ovarian response to controlled ovarian hyperstimulation during IVF (i.e. peak estradiol (E2) levels and oocyte yields) among women attending an academic fertility center in Boston, MA.
METHODS
Study population
Study participants were women enrolled in the Environment and Reproductive Health (EARTH) Study, an ongoing prospective cohort established in 2004 to evaluate environmental and dietary determinants of fertility.[16] Women between 18 and 45 years and who planned to use their own gametes at enrollment were eligible to participate in the study. Approximately 60% of those contacted by the research nurses enrolled. For this analysis, women were eligible if their AFC was determined at the Massachusetts General Hospital (MGH) Fertility Center prior to assisted reproductive technology (ART) cycle initiation between the years of 2004 and 2015 (n=581). Of these, 107 (18%) women were excluded because they did not complete a take-home questionnaire. Women who did not complete the take-home questionnaire did not have significantly different demographic characteristics compared to women included in the analysis. We additionally excluded 1 woman who was missing body mass index (BMI). Of the 473 women included in the AFC analysis, 313 (66%) had completed at least one fresh IVF cycle (n=462 cycles) by December 2015. Women excluded from the second analysis did not have significantly different baseline characteristics compared to women included in the analysis. The study was approved by the Human Studies Institutional Review Boards of the MGH, and the Harvard T.H. Chan School of Public Health. Participants signed an informed consent after the study procedures were explained by trained research study staff and all questions were answered.
Assessment of the exposure
Information on work schedule and physically demanding work was collected on a take-home questionnaire. Women reported how often they lifted or moved heavy objects in their current job with response options of “never”, “sometimes”, or “often”. Women also reported the level of physical exertion in their current job using the following categories: light (e.g. most time spent sitting, office work), moderate (e.g. lifting/pushing light loads, long periods of walking), and heavy (e.g. lifting, pushing heavy loads, heavy manual labor). To assess the effect of shift timing, women reported whether their typical work shift was day, evening, night, or rotating.
Assessment of the outcomes
Markers of Ovarian Reserve
All women participating in the study underwent an evaluation of ovarian AFC through transvaginal ultrasonography. All of these transvaginal ultrasounds were performed in the year prior to the IVF cycle by one of the MGH reproductive endocrinology and infertility physicians on the 3rd day of an unstimulated menstrual cycle or on the 3rd day of a progesterone withdrawal bleed. FSH was measured in serum, collected on the third day of the menstrual cycle, using an automated electrochemiluminescence immunoassay at the MGH Core Laboratory as previously described.[17] No fertility medications were used in the cycle preceding either the ultrasonographic determination of the AFC or the FSH measurements. Women with a diagnosis of polycystic ovarian syndrome (PCOS) as noted in their medical records were not included in this analysis (n=48), however, of the 473 women, 11 (2.3%) women had AFC>30 and to reduce the influence of these very high values, we truncated AFC at 30.
Markers of Ovarian Response
Women who completed an IVF cycle in our study underwent one of three controlled ovarian stimulation protocols on day 3 of induced menses after completing a cycle of oral contraceptives: 1) luteal phase gonadotropin-releasing hormone (GnRH)-agonist protocol, 2) follicular phase GnRH-agonist/Flare protocol, or 3) GnRH-antagonist protocol. Lupron dose was reduced at, or shortly after, the start of ovarian stimulation with FSH/hMG in the luteal phase GnRH-agonist protocol. FSH/hMG and GnRH-agonist or GnRH-antagonist was continued to the day of trigger with human chorionic gonadotropin (hCG). During gonadotropin treatment, women were monitored for serum E2 levels (Elecsys Estradiol II reagent kit, Roche Diagnostics), follicle size measurements and counts, and endometrial thickness and pattern. hCG was administered intramuscularly approximately 35–36 hours before the scheduled egg retrieval procedure to induce oocyte maturity. Details of egg retrieval have been previously described.[17] In brief, peak serum E2 concentration was defined as the highest level of E2 preceding the oocyte retrieval and obtained on the day of hCG administration. Following egg retrieval, embryologists determined the total number of oocytes retrieved and classified them as germinal vesicle, metaphase I, metaphase II (MII) or degenerated.
Assessment of covariates
The participant’s date of birth was collected at entry, and weight and height were measured by trained study staff. BMI was calculated as weight (in kilograms) per height (in meters) squared. The detailed take-home questionnaire also contained additional questions on lifestyle factors, reproductive health, and medical history. Time spent in leisure time physical and sedentary activities was assessed using a validated questionnaire.[18] Specifically, women reported the average time per week during the preceding year spent on any of the following activities: walking, jogging, running, biking, swimming, tennis, squash, weightlifting, aerobics or aerobic exercise equipment, and moderate (e.g. yard work, gardening), heavy (e.g. digging, chopping) outdoor work, sitting at work, sitting while driving, and sitting at home. Each activity question had 13 categories for response ranging from “never” to “40+ hours per week”. The duration of activity was assigned using the median value for each category. We calculated total physical and sedentary activity (hrs/week) by summing across all physical and sedentary activities. Clinical information including infertility diagnosis and protocol type was abstracted from electronic medical records.
Statistical analysis
Demographic and baseline reproductive characteristics of the women were presented using median ± interquartile ranges (IQRs) or percentages. Women’s exposures to each occupational factor were categorized into groups based on the questionnaire responses, although when appropriate, categories were collapsed due to small sample sizes. Associations between frequency of moving heavy objects at work and typical work shift, with demographic and reproductive characteristics were evaluated using Kruskal–Wallis tests for continuous variables and chi-squared tests for categorical variables (or Fisher’s exact test where appropriate). We fit multivariable generalized linear models to estimate the association of occupational factors with AFC (Poisson distribution and log link) and FSH level (normal distribution and identity link). For the IVF outcomes, multivariable generalized linear mixed models with were used to evaluate the association of occupational factors with oocyte counts (Poisson distribution and log link) and peak E2 level (normal distribution and identity link), with a random intercept to account for correlation in outcomes across multiple IVF cycles per woman. To allow for better interpretation of the results, population marginal means [19] are presented adjusting for all the covariates in the model (with covariates at their average values).
Confounding was assessed using prior knowledge based on biological relevance and through descriptive statistics from our study population. The variables considered as potential confounders included factors previously related to reproductive outcomes in this and other studies, and factors associated with occupational factors and reproductive outcomes in this study. Covariates were retained in the final models if they resulted in a change in the exposure-outcomes effect estimate >10%. Final models were adjusted for age (continuous), BMI (continuous), education (less than college graduate, college graduate and graduate degree) and infertility diagnosis (male, female and unexplained). Additional models were run co-adjusting for other occupational factors to account for any correlations between occupational exposures. Effect modification by age (<37 yrs vs. ≥37 yrs) and BMI (<25 kg/m2 vs. ≥25 kg/m2) was tested by adding a cross product term to the final multivariate model. Statistical analyses were performed with SAS (version 9.4; SAS Institute Inc., Cary, NC, USA).
RESULTS
The median (IQR) age and BMI of the 473 women was 35 years (32, 38) and 23.2 kg/m2 (21.2, 26.2), respectively (Table 1). Participants were mostly Caucasian (85%), with a college degree or higher (92%), and most had never smoked (73%). The majority of women (80%) had undergone a previous fertility exam and less than half of the women (48%) had been treated for infertility before the study. Unexplained infertility was the primary infertility diagnosis at enrollment (44%). 40% of women reported lifting or moving heavy objects at work, 22% reported moderate or heavy physical exertion at work, and 91% worked day-only shifts. Women who reported moving or lifting heavy objects at work, were less educated on average, compared with women who reported never moving or lifting heavy objects at work (49% vs 66% with graduate school education). No other baseline characteristics differed substantially across these two groups (Table 1). Women who worked evening/night or rotating shifts were less educated and engaged in more leisure-time physical activity on average, compared with women who worked day-only shifts (38% vs 62% with graduate school education, and 9 vs. 5 hr/week) (Supplemental Table 1). The median (IQR) AFC among the 473 women was 12 (8, 17), and the median (IQR) number of mature oocyte yields in the 313 women who underwent IVF was 9 (6, 12).
Table 1.
Baseline characteristicsa of 473 women in the Environment and Reproductive Health (EARTH) Study by frequency of lifting heavy objects at work.
| Total Cohort | Lifting heavy objects at work | |||
|---|---|---|---|---|
|
|
||||
| (n=473) | Never (n=283) |
Sometime/Often (n=190) |
P-valueb | |
| Baseline characteristics | ||||
| Age, years | 35.0 (32.0, 38.0) | 35.0 (32.0, 38.0) | 35.0 (32.0, 38.0) | 0.56 |
| Race/Ethnic group, n (%) | 0.32 | |||
| White/Caucasian | 400 (84.6) | 239 (84.4) | 161 (84.7) | |
| Black | 8 (1.7) | 3 (1.1) | 5 (2.6) | |
| Asian | 44 (9.3) | 30 (10.6) | 14 (7.4) | |
| Other | 21 (4.4) | 11 (3.9) | 10 (5.3) | |
| Body Mass Index, kg/m2 | 23.2 (21.2, 26.2) | 23.2 (21.0, 26.2) | 23.2 (21.3, 26.2) | 0.63 |
| Smoking status, nc (%) | 0.77 | |||
| Never smoked | 345 (73.1) | 208 (73.8) | 137 (72.1) | |
| Former smoker | 115 (24.4) | 68 (24.1) | 47 (24.7) | |
| Current smoker | 12 (2.5) | 6 (2.1) | 6 (3.2) | |
| Education, n (%) | 0.001 | |||
| < College graduate | 39 (8.3) | 17 (6.0) | 22 (11.6) | |
| College graduate | 153 (32.4) | 79 (27.9) | 74 (39.0) | |
| Graduate degree | 281 (59.4) | 187 (66.1) | 94 (49.5) | |
| Total physical activity (hr/week) | 5.4 (2.7, 9.5) | 5.0 (2.7, 9.2) | 6.2 (2.7, 10.5) | 0.23 |
| Reproductive characteristics, n (%) | ||||
| History of ever been pregnant | 188 (39.8) | 107 (37.8) | 81 (42.6) | 0.29 |
| History of been treated for infertility | 226 (47.8) | 137 (48.4) | 89 (46.8) | 0.74 |
| Previous infertility exam | 379 (80.1) | 230 (81.3) | 149 (78.4) | 0.45 |
| Initial infertility diagnosis | 0.53 | |||
| Male factor | 124 (26.2) | 70 (24.7) | 54 (28.4) | |
| Female factor | 141 (29.8) | 78 (27.6) | 63 (33.2) | |
| Diminished Ovarian Reserve | 46 (9.7) | 23 (8.1) | 23 (12.1) | |
| Endometriosis | 26 (5.5) | 15 (5.3) | 11 (5.8) | |
| Ovulation Disorders | 35 (7.4) | 21 (7.4) | 14 (7.4) | |
| Tubal | 25 (5.3) | 14 (5.0) | 11 (5.8) | |
| Uterine | 9 (1.9) | 5 (1.8) | 4 (2.1) | |
| Unexplained | 208 (44.0) | 135 (47.7) | 73 (38.4) | |
Values are presented as median (IQR) unless otherwise noted.
From Kruskal-Wallis test for continuous variables and chi-squared tests (or Fisher’s exact test where appropriate) for categorical variables.
This variable has 1 missing.
Follicular phase GnRH-agonist/Flare protocol.
Luteal phase GnRH-agonist protocol.
Abbreviations: IQR, interquartile range; N, number.
There was a marginal association between frequency of lifting or moving heavy objects at work and total AFC after adjusting for age, BMI, education and infertility diagnosis (Table 2). Women who reported lifting or moving heavy objects at work had a lower AFC (adjusted difference = -0.7) compared with women who reported never lifting or moving heavy objects at work (p-value=0.06). This association remained after adjustment for level of physical exertion but became attenuated when adjusted for typical work shift (data not shown). Frequency of moving heavy objects was unrelated with day 3 FSH levels. Typical work schedule and level of physical exertion at work were not associated with total AFC and day 3 FSH levels (Table 2).
Table 2.
Day 3 FSH levels and total antral follicle counts by frequency of moving heavy objects, level of physical exertion and typical work shift among 473 women in the Environment and Reproductive Health (EARTH) Study.
| Occupational Characteristics (number of women) |
Day 3 FSH Levels, IU/L | Total Antral Follicle Counts, n | ||
|---|---|---|---|---|
| Unadjusted | Adjusteda | Unadjusted | Adjusteda | |
| Moving or lifting heavy objects | ||||
|
| ||||
| Never (n=283) | 7.4 (7.0, 7.7) | 7.4 (7.0, 7.7) | 13.5 (13.1, 14.0) | 13.4 (12.9, 13.8) |
| Sometimes/Often (n=190) | 7.8 (7.3, 8.2) | 7.8 (7.4, 8.2) | 13.0 (12.5, 13.5) | 12.7 (12.2, 13.2) |
| p-value | 0.17 | 0.11 | 0.13 | 0.06 |
|
| ||||
| Level of physical exertion | ||||
|
| ||||
| Light (n=368) | 7.5 (7.2, 7.8) | 7.4 (7.1, 7.7) | 13.2 (12.9, 13.6) | 13.1 (12.7, 13.5) |
| Moderate/Heavy (n=105) | 7.7 (7.1, 8.3) | 7.9 (7.3, 8.5) | 13.7 (13.0, 14.4) | 13.1 (12.4, 13.8) |
| p-value | 0.46 | 0.14 | 0.25 | 0.99 |
|
| ||||
| Typical work shift | ||||
|
| ||||
| Day-shift (n=431) | 7.5 (7.2, 7.8) | 7.5 (7.2, 7.8) | 13.3 (13.0, 13.7) | 13.2 (12.8, 13.5) |
| Evening/Night/Rotating-shift (n=42) | 7.7 (6.7, 8.6) | 7.8 (6.9, 8.7) | 13.2 (12.1, 14.3) | 12.5 (11.5, 13.6) |
| p-value | 0.80 | 0.53 | 0.77 | 0.27 |
Data are presented as predicted marginal means (95% CI) adjusted for age (continuous), BMI (continuous), education (less than college graduate, college graduate and graduate degree) and infertility diagnosis (male, female and unexplained).
In regards to markers of ovarian response, peak E2 level was unrelated to the occupational exposures. However, women who reported lifting or moving heavy objects at work had 1.0 (p-value=0.08) and 1.4 (p-value=0.007) fewer total and mature oocyte counts compared to women who reported never lifting or moving heavy objects at work, reflecting decreases of 8.8% and 14.4%, respectively (Table 3). Level of physical exertion at work and typical work schedule were also related to number of total and mature oocyte yields in our study population (Table 3). In adjusted models, women who had moderate to heavy physical exertion at work had a 1.3 (p-value=0.02) fewer mature oocytes on average than woman with light physical exertion at work. In addition, women who worked evening/night/rotating shifts had 2.3 (p-value<0.001) fewer mature oocytes, on average, compared with women who worked day only shifts. This association was stronger when evening/night shift workers were considered as a separate category (3.2 fewer mature oocytes compared with women working day shifts, n=8) (data not shown).
Table 3.
Total and mature oocyte yields and peak E2 levels by frequency of moving heavy objects, level of physical exertion and typical work among 313 women contributing 462 fresh IVF cycles in the Environment and Reproductive Health (EARTH) Study.
| Occupational Characteristics (number of fresh IVF cycles) |
Total Oocyte Yield, n | Mature (MII) Oocyte Yield, n | Peak E2 Levels, pmol/L | |||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | Unadjusted | Adjusteda | Unadjusted | Adjusteda | |
| Moving or lifting heavy objects | ||||||
| Never (n=276) | 11.6 (10.8, 12.4) | 11.4 (10.7, 12.2) | 9.8 (9.2, 10.5) | 9.7 (9.1, 10.3) | 2153 (1424, 2882) | 2133 (1427, 2840) |
| Sometimes/Often (n=186) | 10.5 (9.7, 11.5) | 10.4 (9.5, 11.3) | 8.5 (7.8, 9.2) | 8.3 (7.7, 9.0) | 2072 (1177, 2968) | 2069 (1199, 2939) |
| p-value | 0.10 | 0.08 | 0.007 | 0.004 | 0.89 | 0.73 |
| Level of physical exertion | ||||||
| Light (n=356) | 11.5 (10.8, 12.2) | 11.3 (10.7, 12.0) | 9.5 (9.0, 10.1) | 9.4 (8.9, 10.0) | 2153 (1911, 2394) | 2134 (1897, 2371) |
| Moderate/Heavy (n=106) | 10.1 (9.0, 11.3) | 9.9 (8.8, 11.1) | 8.3 (7.4, 9.3) | 8.1 (7.3, 9.1) | 2099 (1648, 2550) | 2103 (1656, 2551) |
| p-value | 0.05 | 0.04 | 0.03 | 0.02 | 0.70 | 0.82 |
| Typical work shift | ||||||
| Day-shift (n=426) | 11.3 (10.7, 11.9) | 11.2 (10.6, 11.8) | 9.4 (8.9, 10.0) | 9.3 (8.9, 9.8) | 2138 (1941, 2335) | 2425 (1935, 2315) |
| Evening/Night/Rotating-shift (n=36) | 9.3 (7.7, 11.2) | 8.7 (7.3, 10.5) | 7.5 (6.2, 9.0) | 7.0 (5.8, 8.4) | 1917 (1422, 2411) | 1890 (1404, 2376) |
| p-value | 0.05 | 0.01 | 0.02 | <0.001 | 0.26 | 0.23 |
Data are presented as predicted marginal means (95% CI) adjusted for age (continuous), BMI (continuous), education (less than college graduate, college graduate and graduate degree) and infertility diagnosis (male, female and unexplained).
The associations between frequency of moving or lifting heavy objects and typical work shift and oocyte yields remained similar when the individual models were further adjusted for the other occupational characteristics (Supplemental Table 2); however, the inverse association between level of physical exertion at work and mature oocyte yield became attenuated after adjustment for either work schedule or frequency of moving or lifting a heavy load. Further adjustment for total leisure-time physical activity (hr/week) and smoking status (never vs. ever smoker) did not affect the results (data not shown).
Finally, we explored whether BMI or age modified the associations between occupational characteristics and markers of ovarian reserve and response. The association between frequency of moving heavy objects at work and number of mature oocyte yield appeared to be stronger among overweight/obese women compared to lean women and stronger among older women compared with young women (Figure 1); however, the tests for interaction were not significant (p-value for interaction=0.19 and 0.26, respectively). For example, women with a BMI≥25 who reported moving or lifting heavy objects at work had 2.2 fewer mature oocytes (p-value=0.008) compared to women with a BMI≥25 who never reported moving or lifting heavy objects at work, whereas this difference in lean women was only 1.1 fewer mature oocytes (p-value= 0.10). Similarly, women who were ≥37 years old and reported moving or lifting heavy objects at work, on average, had 1.9 fewer mature oocytes (p-value=0.02) compared women who were ≥37 years old and never reported moving or lifting heavy objects at work. The analogous difference in younger women was 1.2 fewer mature oocytes (p-value= 0.07) (Figure 1). None of the other associations were modified by age or BMI.
Figure 1.
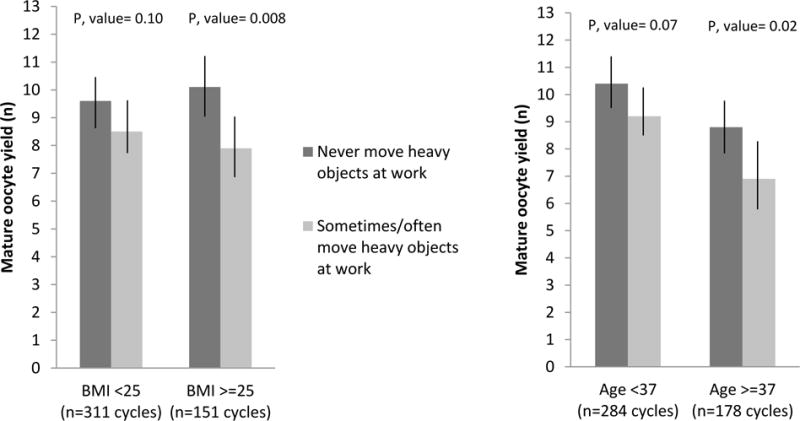
Effect of BMI and age on the association between mature oocyte yield and frequency of moving heavy objects at work among 313 women contributing 462 fresh IVF cycles in the Environment and Reproductive Health (EARTH) Study.
DISCUSSION
As far as we are aware, this is the first study exploring the association between occupational factors and markers of ovarian reserve and response among women of reproductive age. We found inverse associations between moving or lifting heavy objects at work and total and mature oocyte yields and possibly AFC. Moreover, the inverse association between heavy lifting and mature oocyte yield was slightly stronger among overweight and obese women and women ≥37 years. In addition, non-day work schedules were inversely related to oocyte yields, independent of physical work demands. These findings have clinical implications, as women with fewer mature oocytes would have fewer eggs which are capable of developing into healthy embryos. None of the occupational factors, however, were associated with day 3 FSH or peak E2 levels following controlled ovarian hyperstimulation. These results suggest that occupational factors may be more specifically affecting oocyte production and quality, rather than accelerating ovarian aging, in this study population of women attending a fertility center.
Our findings that women who reported moving or lifting heavy objects at work had lower oocyte yields is consistent with a previous study which examined the effect of physical factors at work on menstrual cycle characteristics and fecundity among female nurses in the US.[3, 7] In these studies, nurses who reported lifting or moving a heavy load >15 times/day had had a 34% higher prevalence of irregular cycles and a 43% (95% CI 10, 83%) longer median duration of pregnancy attempt (even after further adjustment for current menstrual cycle regularity) compared to women who never lifted heavy loads. Also similar to us, Gaskins and coworkers found that the inverse association between moving heavy objects at work and fecundity was modified by BMI, being much stronger in overweight and obese women.[7] However, they did not find any effect modification by age. Our findings are also in agreement a study conducted among 260 nonmedical female hospital workers employed at 39 Dutch hospitals who were planning a pregnancy.[5, 15] In this study, women who worked jobs with high intensity and fatigue had shorter pregnancy durations (up to 18 days shorter) [5] and a longer time to pregnancy.[15] Taken together with our results, it appears that lower oocyte quality could be one pathway mediating the relationship between high frequency of moving or lifting heavy loads at work and reduced fecundity. However, the specific mechanism by which moving or lifting heavy loads at work affects oocyte quality is still unknown.
We also found that women who worked rotating, night, and evening shifts had lower oocyte yields, compared with women who worked day shift. Our finding that working non-day shifts tends to have negative reproductive consequences is consistent with two papers which shown that rotating shift work was associated with a higher prevalence of irregular cycles.[2, 3] Irregular work shift has been also related to reduced fecundity in European [9, 10, 12] and Asian women.[11] For instance, Zhu and colleagues found that fixed evening workers and fixed night workers had a longer time to pregnancy among 39,913 women enrolled in the National Birth Danish Cohort between 1998 and 2000.[12] Based on our results, working non-day shifts may affect oocyte quality and fecundity through circadian rhythm disruption. However, Gaskins and coworkers found no relation between work shift and fecundity in women enrolled in the NHS3.[7] Similarly to them, other previous publications found no associations between work shift and fecundity [13, 14] and a recently published meta-analysis found a non-significant effect of shift work compared with no shift work on infertility (adjusted odds ratio: 1.12; 95% confidence interval (95% CI), 0.86, 1.43). [8] Given the mixed results of previous studies, clearly more research is needed.
Our study has limitations worth noting. Due to its design, it may not be possible to generalize our findings to couples conceiving without medical intervention. However, these findings may be applicable to other women seeking infertility treatment which is a sizeable population.[20] Also, as is true for all observational studies, misclassification of occupational exposure is possible, however because we collected the occupational information when the women joined the study and all outcomes were collected subsequent to this, we would expect any misclassification to be non-differential. In addition, only current shift work and physically demanding work were assessed in our questionnaire. Thus, we were not able to assess the effect of long work hours on markers of ovarian reserve or response and we were not able to control for other work characteristics that might be correlated with shift and physically demanding work. Because we did not explicitly define rotating shifts, but rather left this up to the interpretation of the woman, this was likely a heterogeneous category including women working rotating day shifts and those switching between day and night shifts. We also did not collect information on duration of shift or duration of physically demanding work and thus it is hard to tease out the short- vs. long-term effects of these exposures. Future work in other studies is needed to further disentangle the effects of rotating shift work with and without nights and current versus lifetime exposure to shift work on fecundity. Finally, the amount and type of work a woman does could be related with many aspects of her life, such as socioeconomic status and financial pressure, which are hard to quantify and could result in residual confounding. It is also possible that women who perform heavy lifting at work are exposed to other occupational exposures and this residual confounding may still be driving some of the results we observed. Strengths of our study include its prospective design which minimizes the possibility of reverse causation and avoids recall bias and our ability to measure well-accepted markers of fertility and fecundity. By studying a population of women undergoing infertility treatment we were also able to circumvent a major limitation of prior studies on occupational exposures and fecundity which is the lack of information on frequency or timing of sexual intercourse which is suspected to be a major confounder of the work schedule and fecundity associations. Finally, our comprehensive adjustment for other lifestyle factors that could be confounding the association between occupational factors and fecundity was a strength.
In conclusion, women working non-day shifts and those who had more physically demanding jobs had fewer mature oocytes retrieved after controlled ovarian hyperstimulation. Our results provide insight into possible mechanisms linking these occupational exposures with decreased fecundity.
Supplementary Material
WHAT THIS PAPER ADDS.
-
-
While previous studies have demonstrated a link between work schedule and physical factors and fecundity, none have been able to directly measure biomarkers of fecundity such as reproductive hormones or ovarian function, and as such they could only postulate on the possible mechanisms underlying the associations between occupational factors and fecundity.
-
-
In this prospective cohort study of women presenting to an academic fertility center, moving or lifting heavy objects at work was inversely associated with total and mature oocyte yields and antral follicle counts. The inverse association between heavy lifting and mature oocyte yield was stronger among overweight/obese women and women ≥37 years. Non-daytime work schedules were inversely related to oocyte yields.
-
-
Our results provide insight into possible mechanisms linking these occupational exposures with decreased fecundity.
Acknowledgments
We would like to acknowledge all members of the EARTH study team, specifically the Harvard T. H. Chan School of Public Health research nurse Myra G. Keller, research staff Ramace Dadd and Patricia Morey, physicians and staff at Massachusetts General Hospital fertility center and a special thanks to all the study participants.
STUDY FUNDING: This work was supported by NIH grants R01ES022955, R01ES009718, and R01ES000002 from the National Institute of Environmental Health Sciences (NIEHS) and L50-HD085359 from the National Institute of Child Health and Human Development (NICHD).
Footnotes
CONFLICT OF INTEREST: None of the authors has any conflicts of interest to declare.
AUTHOR’S CONTRIBUTION TO MANUSCRIPT: R.H. J.E.C., A.J.G. were involved in study concept and design, and critical revision for important intellectual content of the manuscript; P.L.W contributed to method modification and provided statistical expertise; L.M.A analyzed data, drafted the manuscript and had a primary responsibility for final content; L.M.A, P.L.W. J.E.C, R.H. and A.J.G. interpreted the data; A.J.G. reviewed the statistical analysis; I.S and J.B.F were involved in acquisition of the data. All authors were involved in the critical revision of the manuscript and approved the final manuscript.
References
- 1.Gamble KL, Resuehr D, Johnson CH. Shift work and circadian dysregulation of reproduction. Front Endocrinol (Lausanne) 2013;4:92. doi: 10.3389/fendo.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawson CC, Whelan EA, Lividoti Hibert EN, et al. Rotating shift work and menstrual cycle characteristics. Epidemiology. 2011;22:305–12. doi: 10.1097/EDE.0b013e3182130016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawson CC, Johnson CY, Chavarro JE, et al. Work schedule and physically demanding work in relation to menstrual function: the Nurses’ Health Study 3. Scand J Work Environ Health. 2015;41:194–203. doi: 10.5271/sjweh.3482. [DOI] [PubMed] [Google Scholar]
- 4.Bonde JP, Jorgensen KT, Bonzini M, et al. Miscarriage and occupational activity: a systematic review and meta-analysis regarding shift work, working hours, lifting, standing, and physical workload. Scand J Work Environ Health. 2013;39:325–34. doi: 10.5271/sjweh.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Florack EI, Pellegrino AE, Zielhuis GA, et al. Influence of occupational physical activity on pregnancy duration and birthweight. Scand J Work Environ Health. 1995;21:199–207. doi: 10.5271/sjweh.28. [DOI] [PubMed] [Google Scholar]
- 6.Palmer KT, Bonzini M, Harris EC, et al. Work activities and risk of prematurity, low birth weight and pre-eclampsia: an updated review with meta-analysis. Occup Environ Med. 2013;70:213–22. doi: 10.1136/oemed-2012-101032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaskins AJ, Rich-Edwards JW, Lawson CC, et al. Work schedule and physical factors in relation to fecundity in nurses. Occup Environ Med. 2015;72:777–83. doi: 10.1136/oemed-2015-103026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stocker LJ, Macklon NS, Cheong YC, et al. Influence of Shift Work on Early Reproductive Outcomes: A Systematic Review and Meta-analysis. Obstet Gynecol. 2014;124:99–110. doi: 10.1097/AOG.0000000000000321. [DOI] [PubMed] [Google Scholar]
- 9.Ahlborg G, Jr, Axelsson G, Bodin L. Shift work, nitrous oxide exposure and subfertility among Swedish midwives. Int J Epidemiol. 1996;25:783–90. doi: 10.1093/ije/25.4.783. [DOI] [PubMed] [Google Scholar]
- 10.Bisanti L, Olsen J, Basso O, et al. Shift work and subfecundity: a European multicenter study. European Study Group on Infertility and Subfecundity. J Occup Environ Med. 1996;38:352–8. doi: 10.1097/00043764-199604000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Uehata T, Sasakawa N. The fatigue and maternity disturbances of night workwomen. J Hum Ergol (Tokyo) 1982;11(Suppl):465–74. [PubMed] [Google Scholar]
- 12.Zhu JL, Hjollund NH, Boggild H, et al. Shift work and subfecundity: a causal link or an artefact? Occup Environ Med. 2003;60:E12. doi: 10.1136/oem.60.9.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spinelli A, Figa-Talamanca I, Osborn J. Time to pregnancy and occupation in a group of Italian women. Int J Epidemiol. 1997;26:601–9. doi: 10.1093/ije/26.3.601. [DOI] [PubMed] [Google Scholar]
- 14.Tuntiseranee P, Olsen J, Geater A, et al. Are long working hours and shiftwork risk factors for subfecundity? A study among couples from southern Thailand. Occup Environ Med. 1998;55:99–105. doi: 10.1136/oem.55.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Florack EI, Zielhuis GA, Rolland R. The influence of occupational physical activity on the menstrual cycle and fecundability. Epidemiology. 1994;5:14–8. doi: 10.1097/00001648-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Hauser R, Meeker JD, Duty S, et al. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology. 2006;17:682–91. doi: 10.1097/01.ede.0000235996.89953.d7. [DOI] [PubMed] [Google Scholar]
- 17.Mok-Lin E, Ehrlich S, Williams PL, et al. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int J Androl. 2010;33:385–93. doi: 10.1111/j.1365-2605.2009.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. International journal of epidemiology. 1994;23:991–9. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 19.Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least square means. Am Stat. 1980;34:216–21. [Google Scholar]
- 20.Thoma ME, McLain AC, Louis JF, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99:1324–31.e1. doi: 10.1016/j.fertnstert.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


