Abstract
Common causes of hearing loss in humans - exposure to loud noise or ototoxic drugs and aging - often damage sensory hair cells, reflected as elevated thresholds on the clinical audiogram. Recent studies in animal models suggest, however, that well before this overt hearing loss can be seen, a more insidious, but likely more common, process is taking place that permanently interrupts synaptic communication between sensory inner hair cells and subsets of cochlear nerve fibers. The silencing of affected neurons alters auditory information processing, whether accompanied by threshold elevations or not, and is a likely contributor to a variety of perceptual abnormalities, including speech-in-noise difficulties, tinnitus and hyperacusis. Work described here will review structural and functional manifestations of this cochlear synaptopathy and will consider possible mechanisms underlying its appearance and progression in ears with and without traditional ‘hearing loss’ arising from several common causes in humans.
Keywords: Auditory nerve, Cochlear synaptopathy, Cochlear neuropathy, Hidden hearing loss, Noise-induced hearing loss
1. Overt vs. ‘hidden’ hearing loss
A longstanding view of acquired sensorineural hearing loss (SNHL) has been that cochlear hair cells are among the most vulnerable elements in the cochlea and that, in the vast majority of cases, cochlear nerve fibers degenerate if, and only long after, the loss of their peripheral hair cell targets. This view arose, fundamentally, because of the temporal offset between post-insult degeneration of hair cells and loss of the spiral ganglion cell (SGC) bodies of the primary auditory neurons with which they communicate. In animal models exposed to noise or ototoxic drugs, hair cell loss can be widespread within hours (Bohne and Harding 2000; Lawner et al., 1997; Suzuki et al., 2008; Wang et al., 2002; Webster and Webster, 1978), whereas the loss of SGCs is typically not detectable for weeks to months after insult and can progress for years (Johnsson, 1974; Miller et al., 1997; Sugawara et al., 2005; Webster and Webster, 1978).
Threshold elevations accompany hair cell damage and loss; for human assessments, the behavioral pure tone audiogram is a key metric of this overt hearing loss, providing documentation of the magnitude of the audibility loss, its pattern as a function of frequency, and to some extent underlying site(s) of dysfunction (e.g. middle ear, inner ear). It has long been known, however, that audiometric thresholds do not always reflect reported or demonstrated auditory perceptual difficulties and that thresholds and otopathology are not always well aligned (Bharadwaj et al., 2015; Felder and Schrott-Fischer, 1995; Gordon-Salant, 2005; Grose and Mamo, 2010; Halpin et al., 1994; Moore, 2004; Lobarinas et al., 2013; Ruggles et al., 2011; Schuknecht and Gacek, 1993).
Recent work in animal models has shed new light on this disconnect. It is now clear, at least in the noise-exposed and aging ear, 1) that cochlear neurons are a primary target, 2) that their peripheral synaptic connections are the most vulnerable elements and 3) that cochlear nerve synapses can be destroyed even when hair cells survive. Although threshold shift is a sensitive metric of underlying hair cell damage, it is relatively insensitive to this diffuse loss of inner hair cell (IHC) synapses or of the cochlear nerve fibers they drive; indeed, behavioral detection thresholds for tones are little changed until neural loss exceeds about 80–90% (Schuknecht and Woellner, 1955). Thus, cochlear synaptopathy can be widespread in ears with intact hair cell populations and normal audiograms, where it has been called “hidden” hearing loss (Schaette and McAlpine, 2011).
This basic result has been observed in multiple mammalian species, including compelling preliminary observations in human temporal bones (Viana et al., 2015) and in noise-damage created by both continuous (Rybalko et al., 2015; Singer et al., 2013; Wang and Ren, 2012) and impulsive/blast exposures (Cho et al., 2013) and in ears with, and without permanent threshold shifts (Kujawa et al., 2011). Beyond noise and aging, gentamicin-treated mice (Ruan et al., 2014) and temporal bones of humans who received aminoglycosides in life (Hinojosa and Lerner, 1987; Sone et al., 1998) can display diffuse cochlear neuropathy for treatments not sufficient to cause hair cell loss. To date, findings have been most thoroughly described in mouse models of noise and aging, as discussed in the following sections.
2. Cochlear synaptopathy and neurodegeneration in noise-exposed and aging mice
In recent years, results of a study aiming to investigate whether noise can have delayed or progressive consequences in humans (Gates et al., 2000) motivated a series of experiments in an inbred strain of good-hearing, normally aging mice (CBA/CaJ), where intended exposures could be rigidly specified, unintended exposures avoided, and a variety of other potentially cofounding variables controlled in genetically ~ identical individuals. Mice were exposed at various ages and were held with age-matched controls for varying post-exposure times. Contrary to existing dogma, results demonstrated that noise can cause ongoing changes in cochlear structure and function long after it has ceased. An unanticipated finding of these initial studies was a dramatic loss of cochlear neurons as young-exposed animals aged after a noise exposure that produced moderate, permanent threshold shift (PTS), but no hair cell loss (Kujawa and Liberman, 2006).
To explore this finding of noise-induced primary neuropathy further, and to uncomplicate interpretation, the observations were repeated for an exposure that produced only temporary threshold shift (TTS) in fully adult animals (Kujawa and Liberman, 2009). In this work, mice from the same inbred strain were exposed to a band of noise placed in the region of best threshold sensitivity. The noise was titrated in level and duration to produce a large, acute threshold shift (30–40 dB at 24 h), but one that recovered by 2 weeks, without hair cell loss. Immunostained cochlear whole mounts and plastic-embedded sections (Fig. 1A–D), imaged by confocal and conventional light microscopy, were assessed to quantify hair cells, cochlear neurons, and synaptic structures providing the communication conduits. Hair cell-based distortion product otoacoustic emissions (DPOAEs) and neural-based auditory brainstem responses (ABRs) or compound action potentials (CAPs) of the auditory nerve were used to assess the peripheral consequences of the noise on function (Fig. 3A and B).
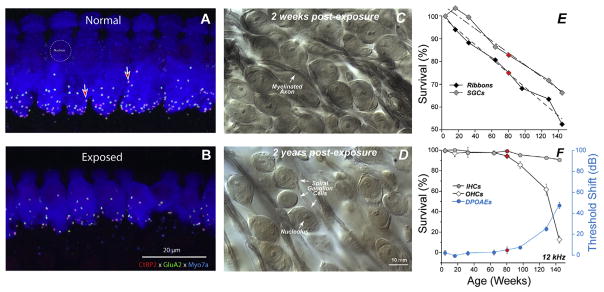
Fig. 1. Noise-induced and age-related loss of synapses and SGNs.
Evaluating synaptopathy by triple-staining cochlear whole mounts for a pre-synaptic marker (CtBP2-red), a postsynaptic marker (GluA2-green) and a hair cell marker (Myosin VIIa-blue). Confocal z-stacks in the IHC area from a control (A) and a noise-exposed mouse (B), 2 wks post exposure. Light micrographs of osmium-stained plastic sections from noise-exposed ears, 2 wks (C) or 2 yrs (D) post exposure. Exposure in B and D was 8–16 kHz, 2 h, 100 dB SPL, delivered at 16 wk to CBA/CaJ mice. (E) In aging ears from the same inbred strain, synaptic counts at IHCs decrease steadily from 4 to 144 wks and parallel ganglion cell loss follows whereas, (F) threshold loss begins comparatively later and accelerates beyond 80 wks, mirrored by accelerating loss of OHCs. IHC loss is trivial at any age. Red symbols flag 80 wk data points for all measurements. After Kujawa and Liberman 2006, 2009; Sergeyenko et al. (2013).
Presaging the ganglion cell losses, results of these studies revealed an acute loss of synapses between IHCs and the peripheral terminals of the spiral ganglion neurons that contact them (Kujawa and Liberman, 2009). Although thresholds recovered, by design, and no hair cells were lost, IHC synaptic losses were greater than 40% in basal cochlear regions, when assessed 24 h post noise, and were stable 2 and 8 weeks later. Losses were proportional in magnitude and cochlear location to the SGC loss observed in the previous series, suggesting that this interruption of IHC-to-neural communication set the stage for the neurodegeneration.
Subsequent studies showed that cochlear synaptopathy also precedes hair cell loss and threshold shift in the aging mouse ear (Sergeyenko et al., 2013). In the same normally aging inbred strain, IHC synapse counts decline steadily throughout life, with losses reaching ~50% in oldest ears and beginning well before significant loss of threshold sensitivity or outer hair cells (OHCs) (compare Fig. 1E and F). SGC losses follow, ultimately reaching about 40% although IHC losses are only ~5% in oldest ears. SGC losses also are closely parallel to those reported in an age-graded series of human temporal bones with preserved hair cells (Makary et al., 2011; see Fig. 6). Thus, the neural loss in these aging ears, as in the TTS ears, is primary rather than a secondary consequence of the loss of their IHC targets. Moreover, when animals received a single, TTS- and synaptopathy-producing exposure as young adults, ongoing synaptic and neural losses were larger than those that otherwise occurred in aging ears (Fernandez et al., 2015).
3. Glutamate excitotoxicity as an instigating factor
The IHC - cochlear nerve synapse is the primary conduit through which information about the acoustic environment is transmitted to the auditory nervous system. In the normal ear, 95% of cochlear nerve fibers make synaptic connection only with IHCs (Spoendlin, 1972). Each cochlear nerve fiber has a cell body in the spiral ganglion, a peripheral axon in the osseous spiral lamina (OSL) and an unmyelinated terminal dendrite in the organ of Corti, with a terminal bouton that forms a synapse with the IHC. The synapse is comprised of a presynaptic ribbon surrounded by a halo of neurotransmitter-containing vesicles within the IHC (Nouvian et al., 2006) and a postsynaptic active zone on the cochlear nerve terminal, with glutamate (AMPA-type) receptors for the released neurotransmitter (Puel, 1995; Glowatzki and Fuchs, 2002). Collectively, these synapses convey information about stimulus intensity and temporal properties over a wide dynamic range (Moser et al., 2006). As summarized in a recent review (Reijntjes and Pyott, 2016), the mechanisms supporting the diversity and breadth of afferent firing are likely resident within this complex, determining the intrinsic excitability of the neural elements, and the modulation of this excitability by chemical transmitters.
The time course of the initial events after exposure suggested a role for an excitotoxic process. Work by Puel and colleagues has shown that local application of glutamate receptor (GluR) agonists can produce dose-dependent swelling of cochlear nerve terminals contacting IHCs, as shown in Fig. 2. The dendritic swelling is observed under IHCs, but not OHCs, and is prevented by prior intracochlear perfusion of glutamate antagonists (see Ruel et al., 2007 for review).
Fig. 2. Excitotoxic swelling in the cochlea.
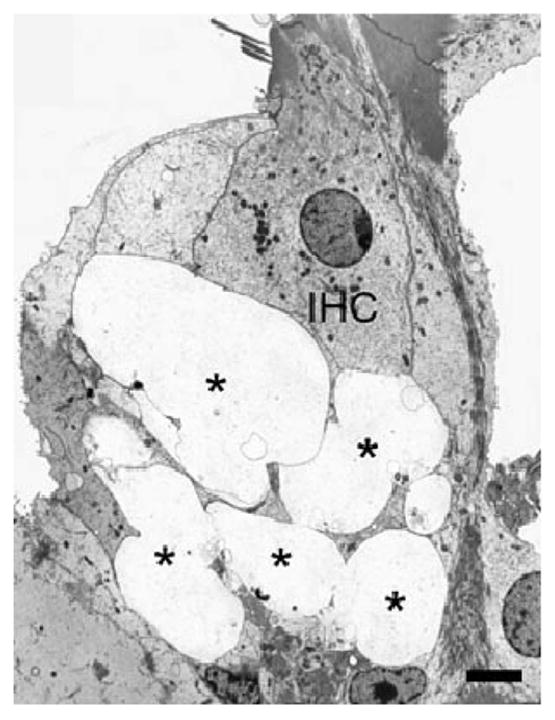
Infusion of AMPA (200 μM) in the cochlea triggers massive swelling of afferent endings (*) underneath the inner hair cell (IHC). Scale bar = 1 μM. From Ruel et al. (2007), with permission.
There is similar longstanding evidence that cochlear neurons are directly targeted by noise, through excess sound-induced release of the endogenous neurotransmitter. Morphological studies have documented similar swelling of type I cochlear nerve terminals in the region of their synaptic contact with IHCs (Spoendlin, 1971; Robertson, 1983; Puel et al., 1998). Such terminal swelling can be seen for exposures that produce PTS or TTS, including the exposure producing the neuropathy described here (8–16 kHz at 100 dB SPL for 2 h; Kujawa and Liberman, 2009). As for the glutamate agonist-induced excitotoxicity, the ultrastructural pathology in cochlear nerve terminals immediately after noise exposure is dramatic. Protection against the noise-induced swelling is provided by cochlear perfusion of the AMPA/kainate antagonist, kynurenate and by Riluzole, which may protect by inhibiting glutamate release (Ruel et al., 2005).
One working hypothesis (Kujawa and Liberman, 2009) is that this excitotoxicity is a primary initial event in the degenerative cascade observed after noise: 1) in the hours and days immediately post exposure, some unmyelinated terminal dendrites of SGCs degenerate back to the habenula as a direct effect of glutamate excitotoxicity, associated dendritic swelling and possible terminal rupture; 2) the loss of these peripheral terminals interrupts the neurotrophin signaling required for normal development and maintenance of the cochlear innervation (Fritzsch et al., 2004; Ramekers et al., 2012; Stankovic et al., 2004) by removing the intimate association between cochlear supporting cells (or hair cells) and the neuronal Trk receptors for the neurotrophins; and 3) this interruption of neurotrophin signaling compromises the long-term viability of those neurons, essentially sealing their fate at an early stage of the process (though the subsequent intracellular balancing act between cell death and cell survival pathways may take months to resolve).
A key test of the hypothesized role of neurotrophins in the neurodegeneration that follows synaptic and terminal loss in these ears is provided by rescue experiments demonstrating synaptogenesis and recovery of function in a noise model (Wan et al., 2014; Suzuki et al., 2016). Given that loss of SGCs and their central projections is very slow after such insults, and IHC targets often survive, results suggest the exciting possibility of hair cell – neuron reconnection over a long therapeutic window in human application.
Although it is easy to imagine excess glutamate release resulting from prolonged, high-level acoustic stimulation, the glutamate excitotoxicity hypothesis must be reconciled with recent studies suggesting that IHC synaptopathy is also a primary effect of aminoglycoside antibiotics. As we have reported for noise exposure, others have shown that when aminoglycoside doses are titrated to levels below those causing hair cell loss, there can nevertheless be significant loss of synaptic terminals on IHCs (Ruan et al., 2014) and basal turn IHC synapses and SGCs (Oishi et al., 2015). Classic studies of aminoglycoside ototoxicity focused on the hair cells as primary targets and considered neural losses to be a secondary consequence of hair cell loss (McFadden et al., 2004; Takeno et al., 1998; Bae et al., 2008; Dodson and Mohuiddin, 2000). However, aminoglycoside-induced excitotoxic swelling of nerve terminals also has been reported in both cochlear and vestibular end organs (Basile et al., 1996; Duan et al., 2000; Sedo-Cabezon et al., 2014; Smith, 1999), suggesting direct, excitotoxic effects of these drugs on neural elements.
Recent studies also suggest that IHC synaptopathy may result from impulse noise exposure (Cho et al., 2013). Again, although it is easy to imagine high-level impulsive stimuli damaging by direct mechanical effects, it is not obvious why a stimulus lasting only microseconds should lead to over-release of neurotransmitter. Clearly, more research is necessary to understand whether all these elicitors of synaptopathy act via the same mechanism.
4. Functional effects of synaptopathy
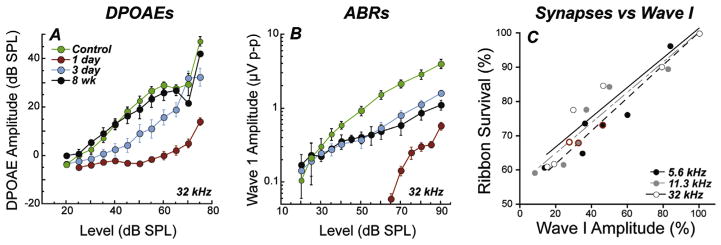
The diffuse synaptic and neural loss observed in both noise-exposed and aging ears does not elevate thresholds. However, if DPOAE responses return to normal (after TTS-producing noise; Kujawa and Liberman, 2009, Fig. 3A) or have not yet deteriorated (in aging; Sergeyenko et al., 2013), the suprathreshold amplitude of ABR wave 1 (Fig. 3B) can be highly predictive of the degree of cochlear synaptopathy (Fig. 3C), as affected neurons are silenced with the loss of their synaptic connection to the IHC. Consistent with the innervation schema of a single auditory neuron communicating with a single IHC via a single synapse (Stamataki et al., 2006), and the basic idea that each fiber contributes a tiny current to ensemble far-field potentials (Antoli-Candela and Kiang, 1978; Buchwald and Huang, 1975), the fractional decrease in ABR wave 1 amplitude scales linearly with the fractional loss of synaptic connections in aging mice (Sergeyenko et al., 2013, Fig. 3C). And, demonstrating the specificity as well as the sensitivity of the wave 1 assay, such permanent neural response amplitude declines are not seen after noise exposures that fail to produce synapse loss (Fernandez et al., 2015). The robustness of the correlation in inbred mice, reviewed here, is likely enhanced by low inter-subject variability due to genetic homogeneity, as well as strict experimental control of intended and untended exposures. These variables will introduce challenges to the study of primary neurodegeneration in the human. Moreover, this correspondence is only straightforward if uncomplicated by hair cell damage, since disruption of mechanoelectric transduction also will reduce the ABR amplitudes.
Fig. 3. Response amplitudes and synapse counts.
Permanent reductions in ABR, but not DPOAE amplitudes in ears with recovered thresholds after noise. Shown are DPOAE (A) and ABR wave 1 (B) response growth functions in the region of maximum acute TTS 1 d and 2 wk after exposure (as in Fig. 1) to 16 wk CBA/CaJ mice; unexposed controls shown for comparison. Neural response amplitude declines are proportional to synaptic and neural losses in aging CBA/CaJ, where synapses are plotted vs mean wave 1 amplitudes (at 80 dB SPL in 4–128 wk animals (C). Panels A,B from Fernandez et al., 2015; Panel C from Sergeyenko et al., 2013.
5. Cochlear neurodegeneration and SR types: special vulnerability of low-SR neurons
In all studies completed thus far, neural loss has been subtotal, raising the possibility that cochlear insults are targeting a subpopulation of cochlear neurons. Auditory nerve fibers (ANFs) contacting IHCs differ in spontaneous rates (SR) of firing (low, medium, high), and their sound-driven firing rates vary over different ranges to support a large dynamic range of neural response (Liberman, 1978). Threshold sensitivity of ANFs is inversely correlated with SR; high-SR fibers have low thresholds, but saturate at levels where high threshold, low-SR fibers continue to code level with increasing firing rate (Winter et al., 1990). In addition to their higher pure-tone thresholds, low-SR ANFs tend to have larger dynamic ranges (Schalk and Sachs, 1980) and reduced susceptibility to excitatory masking by continuous noise stimuli (Costalupes et al., 1984). Thus, although low-SR fibers are not needed for threshold detection, they are likely important for hearing in noise and for fine temporal precision at suprathreshold levels.
Two findings in work presented thus far suggested that the primary neural degeneration that inevitably follows noise-induced and age-related synapse loss might be biased toward the low-SR subgroup, which comprises roughly 40% of the ANF population (Liberman, 1978; Tsuji and Liberman, 1997). First, maximum neuronal loss is roughly 40–50% for a broad range of noise exposures (Kujawa et al., 2011) and in the unexposed, aged ear before hair cell loss is significant (Sergeyenko et al., 2013). Second, a selective loss of high-threshold fibers would provide a natural explanation for the full recovery of thresholds in ears with persistent suprathreshold neural amplitude declines after TTS. Subsequent studies have probed these relationships, as described below.
5.1. Single unit evidence for low-SR vulnerability
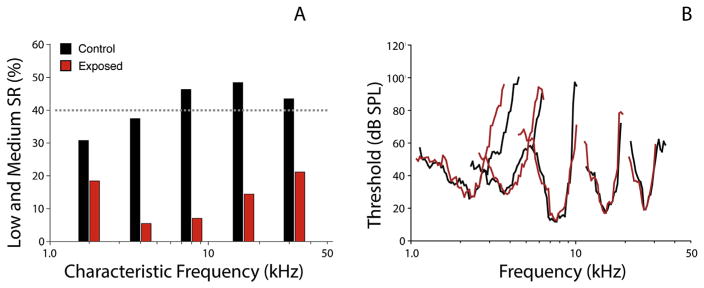
Neurophysiological studies suggest that neurons from the different SR classes are not equally represented in the noise-induced neuropathy (Furman et al., 2013). In these studies, recordings were obtained from single ANFs in guinea pigs after a noise exposure known to produce temporary threshold shifts with acute loss of synapses, as in the mouse model (Kujawa and Liberman, 2009). The proportion of fibers with low SR was significantly smaller in exposed than in control ears, particularly in cochlear frequency regions relevant to the exposure (Fig. 4A). Surviving high-SR fibers showed normal response properties, including normal thresholds and tuning (Fig. 4B), supporting the notion that OHCs were functionally normal and that low-SR neurons with high thresholds were selectively eliminated. Studies in gerbil provide two additional observations of the particular vulnerability of low SR neurons; to aging (Schmiedt et al., 1996) and to ouabain-induced neuropathy (Bourien et al., 2014). In the latter, the dose-response relation revealed first effects on low-SR neurons followed by medium- and then high-SR with increasing drug dose. The apparent vulnerability of low-SR neurons remains unexplained. Low- and high-SR neurons and their synapses distribute differently at IHCs; we speculate that different distributions of glutamate receptor subtypes may contribute to differences in the excitotoxic response to noise. Additionally, low-SR fibers are poor in mitochondria, which are important in buffering intracellular Ca2+; this characteristic might also increase their vulnerability to damage.
Fig. 4. Low-SR neuron loss after noise.
Single unit recordings were made in guinea pigs 10 days after a TTS-producing noise exposure that resulted in permanent ABR amplitude declines and synapse loss but no hair cell loss. Spontaneous rate distributions suggest selective loss of low-SR fibers in the high-frequency region of maximum noise-induced injury (A). In the same animals, thresholds and tuning of surviving nerve fibers, matched for CF, were not altered in noise exposed ears compared to controls (B). The single-fiber database included 367 fibers from 14 control animals, and 382 fibers from 9 exposed animals. After Furman et al., 2013.
5.2. Morphology of synaptic vulnerability
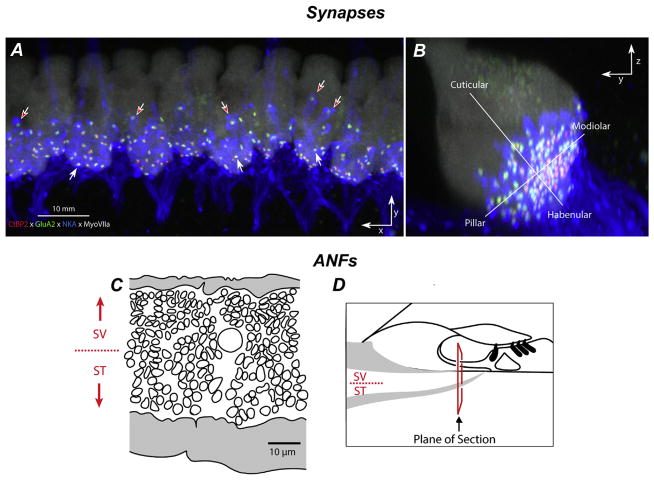
Morphologic support for the preferential loss of low-SR neurons comes from studies in which 1) SR-related spatial distributions of ANFs at IHCs (Liberman, 1980, 1982), 2) presynaptic ribbons and postsynaptic glutamate receptor patches (Yin et al., 2014) and 3) post-noise reorganization of synaptic locations (Liberman et al., 2015) all suggest preferential vulnerability of low-SR neurons and their synapses after noise. Low- and high-SR fibers differ in synaptic position on the IHC and in the size of synaptic ribbons and associated AMPA-receptor patches (Liberman et al., 2011; Merchan-Perez and Liberman, 1996); low-threshold, high-SR fibers tend to synapse on the pillar side of the IHC, whereas the high-threshold, low-SR fibers tend to synapse on the modiolar side (Liberman, 1982). This physiological gradient also appears in confocal images from immunostained cochlear whole mounts as complementary gradients in ribbon and GluR-patch size on the pillar vs. modiolar sides of the IHC; large ribbons and small receptor patches tend to be localized to the IHC’s modiolar side compared to small ribbons and large receptor patches on the pillar side (Yin et al., 2014). These gradients appear to be part of the morphological substrate for the low-SR/high-SR gradient in cochlear nerve response (Liberman, 1978).
In normal ears, the density of synapses tends to be greater on the modiolar side of the IHC (Fig. 5A). After noise, loss of synapses also appears greater on the modiolar side (Liberman et al., 2015), consistent with physiological reports of selective loss of low-SR fibers in this noise damage model (Furman et al., 2013). However, synaptic positions along the IHC’s basolateral membrane appear to transiently redistribute along the habenularcuticular and modiolar-pillar axes after noise, particularly within the region of greatest noise-induced synaptopathy, recovering by 1 wk post exposure. Thus, interpreting synaptic position after noise is complicated by dynamic changes that occur in the acute post-exposure time frame. Spatial segregation of high- and low-SR fibers in the OSL as shown in Fig. 5B and C may be useful in assessing which fiber type has degenerated after cochlear insult.
Fig. 5. Gradients in synaptic and afferent fiber morphology.
IHC synapses in confocal z-stacks, acquired in the x-y plane (A) and re-projected into the y-z (B) plane. A Pre- and post-synaptic elements in the IHC area are counted in cochlear whole mounts quadruple-immunostained for CtBP2 (red), GluA2 (green), NaK ATPase (blue), and myosin VIIa (white). B Size gradients in pre- and post-synaptic elements are quantified according to location along habenular-cuticular and modiolar-pillar axes (Liberman et al., 2015). (C) Tracing of peripheral axons from a cross section through the osseous spiral lamina (OSL; D) in a normal cat shows the SR-based gradient from thin (low-SR) to thick (high-SR) fibers (Kawase and Liberman, 1992).
Other dynamic, post-noise changes to synaptic structure have been observed. In the normal cochlea, confocal images document a one-to-one association between pre-synaptic ribbons and postsynaptic glutamate receptor patches (Kujawa and Liberman, 2009), consistent with ultrastructural analyses (Liberman, 1980; Stamataki et al., 2006). After noise, there is a transient increase in the number of ‘orphan’ ribbons, restricted to basal cochlear regions within the noise-damage focus (Fernandez et al., 2015; Liberman et al., 2015). This change in the number of GluA2 puncta could reflect a transient internalization of surface glutamate receptors, as documented previously in response to glutamate agonists in vitro or noise in vivo (Chen et al., 2007). This reversible down regulation of surface AMPA receptors may serve a protective function (Chen et al., 2007, 2009) by modulating synaptic strength.
Despite progress in describing morphological differences between low- and high-SR fibers and their contacts with the IHCs, mechanisms underlying the apparent vulnerability of low-SR neurons remain poorly understood. Neurotransmitter released from the IHC must be maintained at levels low enough to ensure high signal-to-noise ratio and to prevent excitotoxic damage to afferent neurons. Rapid clearance of synaptic glutamate is accomplished by the uptake system of glutamate transporters (Bridges and Esslinger, 2005; Danbolt, 2001; Hakuba et al., 2000; Seal and Amara, 1999) and immunostaining for glutamate transporters is less intense on the low-SR side of the IHC (Furness and Lawton, 2003). Low-SR fibers also have fewer mitochondria which, in the central nervous system, are well documented to be of fundamental importance to Ca++ buffering mechanisms and thus to the control of excitotoxicity (Szydlowska and Tymianski, 2010).
6. Cochlear synaptopathy and relevance to human SNHL
6.1. Synaptopathy in human temporal bones
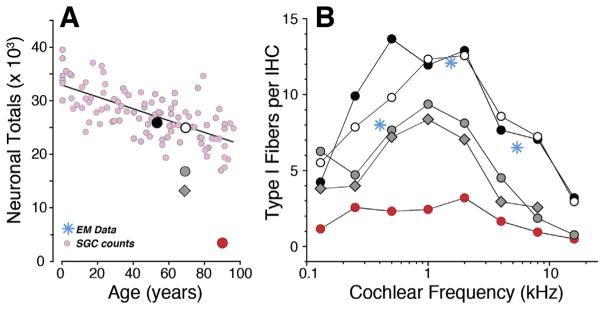
Against this backdrop of animal studies, our working hypothesis is that partial de-afferentation of IHCs is widespread in human ears across a range of acquired SNHL etiologies, with or without overt hearing loss. Using immunostaining for pre- and post-synaptic elements as performed in the animal models, temporal bones from individuals 55–89 years of age with no explicit otopathology revealed dramatic cochlear synaptopathy, with afferent innervation density ranging from 15 synapses per IHC in a 55 yr old to only 2.5 synapses per IHC in an 89 yr old, despite no significant loss of IHCs or OHCs (Fig. 6B). As in normal-aging mice (Sergeyenko et al., 2013), SGC counts decrease throughout the lifespan and throughout the cochlea (Viana et al., 2015; Fig. 4B. In mice, the SGC counts underestimate the degree of IHC de-afferentation, because the SGCs survive for months after the loss of their peripheral synapses with IHCs. Similarly, observations in human temporal bones suggest that the loss of IHC synapses in normal-aging humans also can be significantly greater than the loss of SGCs (Fig. 6A). These data suggest that cochlear synaptopathy may be a major cause of functional impairment in age-related hearing loss in humans.
Fig. 6. Cochlear de-afferentation in human temporal bones.
Cochlear de-afferentation is seen in human temporal bones as a function of age (A) and cochlear location (B) in cases with no hair cell loss and no explicit otologic disease. The small pink symbols (A) are estimated total SGC counts from archival sections (Makary et al., 2011); the five large symbols (A) are the estimated total peripheral axon counts from the same five cases shown in B. Counts of cochlear nerve terminals per IHC (B) in 5 normal aging temporal bones (55–89 yrs) with no history of otologic disease (Viana et al., 2015). Blue symbols are counts of synapses per IHC from an electron microscopic study of a normal middle-aged human (Nadol, 1988).
6.2. Synaptopathy, low-SR neuropathy and human auditory function
In summary, synapses are lost first as noise dose increases, and synapses are lost first as age progresses. This may be a general finding in other forms of acquired SNHL common in humans, as well. Affected neurons are silenced by the loss of this synaptic connection, even if it takes months to years for the loss to be reflected in SGC loss. Audiometric thresholds are unaffected by diffuse synaptopathy; however, such dramatic disconnection of hair cells and ANFs must have significant perceptual consequences.
Normal response properties of low-SR neurons, in quiet and in noise, have led to speculation regarding functional consequences of their targeted loss. Low-SR neuropathy may be a major contributor to a classic impairment in SNHL, speech-in-noise difficulty (see Kujawa and Liberman, 2015; Plack et al., 2014 for discussion). This notion is not new; low-SR neuropathy has been suggested to contribute to well-documented performance declines with age that include decreased speech understanding in noise and reduced ability to utilize stimulus timing and amplitude modulation cues (Schmiedt et al., 1996). It also may be important in limiting psychophysical performance in “normal hearing” human listeners; that is, those with good threshold sensitivity, and it may help account for performance differences in individuals with similar, elevated audiometric thresholds. In support, deficits in binaural temporal processing, seen as a decrease in the detectability of interaural phase differences in amplitude modulated tones, are highly correlated with changes in ABR responses consistent with the selective loss of low-SR fibers (Bharadwaj et al., 2014, 2015).
Cochlear synaptopathy also may be a key elicitor of what are commonly the most troubling sensory anomalies associated with SNHL, tinnitus and hyperacusis. This may be the result of a compensatory plasticity, wherein the synaptic gain in auditory central circuits is increased when neural signals from the periphery are attenuated (Bauer et al., 2007; Gu et al., 2010; Hickox and Liberman, 2014; Kaltenbach and Afman, 2000; Knipper et al., 2013; Roberts et al., 2010; Schaette and McAlpine, 2011). Results support the long-standing hypothesis that reduced afferent outflow from a damaged cochlea and the associated diminished input to higher auditory centers drives increases in central gain that may, in turn, underlie tinnitus.
Work in this area is in its infancy, and ultimately will be crucial to the translation of these findings to humans, where the histopathology will not be available in life. The TTS animal model of primary neuropathy has provided a powerful platform to characterize synaptopathic/neurodegenerative consequences of noise exposure and to begin to test hypotheses about the special role(s) of low-SR fibers in auditory processing without the confounding variables of hair cell damage and threshold shift. The recording of thresholds and suprathreshold amplitudes of OHC-based DPOAEs and neural-based ABRs in the same ears provides a valuable window into the underlying histopathology in ears with normal thresholds; ABR wave 1 amplitudes recorded in such ears scale closely with the underlying synaptopathy. However, acquired SNHL in humans will encompass a range of threshold losses and underlying damage that may include mixed loss of hair cells and synaptic contacts on surviving IHCs. Metrics robust to such mixed involvements and accompanying threshold elevations will be required. Experiments underway have undertaken assessment of synaptic and functional losses for a range of TTS- and PTS-producing exposures, with and without hair cell loss.
6.3. Monitoring for synaptic injury and treatment efficacy
Pure tone threshold audiometry serves as the standard metric for assaying the effects of noise, ototoxic drugs and other agents on hearing in clinical and occupational settings. Threshold measurement protocols have undergone extensive vetting and standardization. Such measurements also form the basis for population sensitivity norms to which individual sensitivity is compared, and threshold-based estimates of noise risk have informed recommended and enforced occupational exposure standards (e.g. NIOSH, 1998; OSHA, 1983).
Against this backdrop, the standard of care in clinical and occupational monitoring for hearing damage in noise- and ototoxic drug-induced injury is assessment of exposure-related changes in threshold sensitivity (OSHA, 1983; ASHA, 1994; AAA, 2009; ACOEM et al., 2012). Such a strategy, particularly if it includes extended high-frequency threshold and OAE monitoring, should be valuable as an early warning of hair cell injury and loss as well as impending performance declines due to reduced audibility. If synapse loss in humans precedes threshold elevation and OHC loss after noise or ototoxic drugs, as it does in all animal models evaluated thus far, clinical decision making and occupational health monitoring protocols would require revision to identify earliest injury, with the goal of preserving hearing function. Similarly, should treatments aimed at preserving, protecting or regenerating cochlear synaptic connections materialize, assays of function with sensitivity to the functional integrity of the synaptic machinery will be required. Preliminary studies in humans have suggested several non-invasive assays that may provide important clues to underlying synaptopathy (Liberman et al., 2016; Mehraei et al., 2016), as has been shown directly in the animal models of noise and aging reviewed here.
7. Summary
New insights from animal studies of noise-induced and age-related hearing loss suggest that the most vulnerable elements in the inner ear are the synaptic connections between hair cells and sensory neurons. Subtotal cochlear synaptopathy, and the primary neural degeneration that follows, does not elevate thresholds. Thus, it can be widespread in ears with intact hair cell populations and normal audiograms. It also occurs in ears with sensory cell injury and loss, resulting in a mixed sensory-neural pathology. We hypothesize that de-afferentation of surviving IHCs may be a major contributor to auditory dysfunction in numerous etiologies of acquired SNHL. Thus, the result has profound human health ramifications. These discoveries are recent, and much remains to be clarified. In our laboratories, the synaptopathy has been largely permanent, indeed progressive, in multiple species. There are reports, however, that spontaneous re-innervation can be seen (Puel et al., 1995; Pujol and Puel, 1999; Sun et al., 2001), or that some of the immediate synapse loss may be reversible (Liu et al., 2012; Shi et al., 2013, 2015, 2016; Song et al., 2016). The source(s) of these discrepancies remain to be identified. Work is ongoing to study the phenomenon, to probe its mechanisms, and to assess the efficacy of a possible therapeutic intervention, using cochlear insults that are highly relevant to the human condition.
Acknowledgments
Research reported here was supported by grants from the DOD (W81XWH-15-1-0103) and the NIH (R01 DC0188, R01 DC08577 and P30 DC 05209).
Abbreviations
- ABR
Auditory Brainstem Response
- AMPA
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid
- ANF
Auditory Nerve Fiber
- CAP
Compound Action Potential
- CtBP2
C-terminal Binding Protein 2
- DPOAE
Distortion Product Otoacoustic Emission
- GLAST
Glutamate Aspartate Transporter
- GluR
Glutamate Receptor
- IHC
Inner Hair Cell
- OHC
Outer Hair Cell
- PTS
Permanent Threshold Shift
- SGC
Spiral Ganglion Cell
- SNHL
Sensorineural Hearing Loss
- SPL
Sound Pressure Level
- SR
Spontaneous Rate
- TTS
Temporary Threshold Shift
References
- ACOEM Task Force on Occupational Hearing Loss. Kirchner DB, Evenson E, Dobie RA, Rabinowitz P, Crawford J, Kopke R, Hudson TW. Occupational noise-induced hearing loss: ACOEM Task Force on Occupational Hearing Loss. J Occup Environ Med. 2012;54:106–108. doi: 10.1097/JOM.0b013e318242677d. [DOI] [PubMed] [Google Scholar]
- American Academy of Audiology Position Statement and Clinical Practice Guidelines. Ototoxicity Monitoring. 2009 Oct; Available at: http://www.audiology.org/publications-resources/document-library/ototoxicitymonitoring.
- American Speech-Language-Hearing Association. Audiologic Management of Individuals Receiving Cochleotoxic Drug Therapy [Guidelines] 1994 Available at: www.asha.org/policy.
- Antoli-Candela F, Jr, Kiang NYS. Unit activity underlying the N1 potential. In: Naunton RF, Fernandez C, editors. Evoked Electrical Activity in the Auditory Nervous System. Academic Press; New York: 1978. pp. 165–191. [Google Scholar]
- Bae WY, Kim LS, Hur DY, Jeong SW, Kim JR. Secondary apoptosis of spiral ganglion cells induced by aminoglycoside: Fas-Fas ligand signaling pathway. Laryngoscope. 2008;118(9):1659–1668. doi: 10.1097/MLG.0b013e31817c1303. [DOI] [PubMed] [Google Scholar]
- Basile AS, Huang JM, Xie C, Webster D, Berlin C, Skolnick P. N-methyl-D-aspartate antagonists limit aminoglycoside antibiotic-induced hearing loss. Nat Med. 1996;2(12):1338–1343. doi: 10.1038/nm1296-1338. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Brozoski TJ, Myers K. Primary afferent dendrite degeneration as a cause of tinnitus. J Neurosci Res. 2007;85(7):1489–1498. doi: 10.1002/jnr.21259. [DOI] [PubMed] [Google Scholar]
- Bharadwaj HM, Verhulst S, Shaheen L, Liberman MC, Shinn-Cunningham BG. Cochlear neuropathy and the coding of supra-threshold sound. Front Syst Neurosci. 2014 Feb 21;8:26. doi: 10.3389/fnsys.2014.00026. http://dx.doi.org/10.3389/fnsys.2014.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj HM, Masud S, Meharie G, Verhulst S, Shinn-Cunningham BG. Individual differences reveal correlates of hidden hearing deficits. J Neurosci. 2015;35(5):2161–2172. doi: 10.1523/JNEUROSCI.3915-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohne BA, Harding GW. Degeneration in the cochlea after noise damage: primary versus secondary events. Am J Otol. 2000;21(4):505–509. [PubMed] [Google Scholar]
- Bourien J, Tang Y, Batrel C, Huet A, Lenoir M, Ladrech S, Desmadryl G, Nouvian R, Puel JL, Wang J. Contribution of auditory nerve fibers to compound action potential of the auditory nerve. J Neurophysiol. 2014 Sep 1;112(5):1025–1039. doi: 10.1152/jn.00738.2013. http://dx.doi.org/10.1152/jn.00738.2013. [DOI] [PubMed] [Google Scholar]
- Bridges RJ, Esslinger CS. The excitatory amino acid transporters: pharmacological insights on substrate and inhibitor specificity of the EAAT subtypes. Pharmacol Ther. 2005;107(3):271–285. doi: 10.1016/j.pharmthera.2005.01.002. (Review) [DOI] [PubMed] [Google Scholar]
- Buchwald JS, Huang C. Far-field acoustic response: origins in the cat. Science. 1975;189:382–384. doi: 10.1126/science.1145206. [DOI] [PubMed] [Google Scholar]
- Chen Z, Kujawa SG, Sewell WF. Auditory sensitivity regulation via rapid changes in expression of surface AMPA receptors. Nat Neurosci. 2007;10(10):1238–1240. doi: 10.1038/nn1974. [DOI] [PubMed] [Google Scholar]
- Chen Z, Peppi M, Kujawa SG, Sewell WF. Regulated expression of surface AMPA receptors reduces excitotoxicity in auditory neurons. J Neurophysiol. 2009;102:1152–1159. doi: 10.1152/jn.00288.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SI, Gao SS, Xia A, Wang R, Salles FT, Raphael PD, Abaya H, Wachtel J, Baek J, Jacobs D, Rasband MN, Oghalai JS. Mechanisms of hearing loss after blast injury to the ear. PLoS One. 2013;8(7):e67618. doi: 10.1371/journal.pone.0067618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costalupes JA, Young ED, Gibson DJ. Effects of continuous noise backgrounds on rate response of auditory nerve fibers in cat. J Neurophysiol. 1984;51:1326–1344. doi: 10.1152/jn.1984.51.6.1326. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. doi: 10.1016/s0301-0082(00)00067-8. (Review) [DOI] [PubMed] [Google Scholar]
- Dodson HC, Mohuiddin A. Response of spiral ganglion neurones to cochlear hair cell destruction in the Guinea pig. N J Neurocytol. 2000;29(7):525–537. doi: 10.1023/a:1007201913730. [DOI] [PubMed] [Google Scholar]
- Duan M, Agerman K, Ernfors P, Canlon B. Complementary roles of neurotrophin 3 and N-methyl-D-aspartate antagonist in the protection of noise and aminoglycoside-induced ototoxicity. Proc Natl Acad Sci U S A. 2000;97(13):7597–7602. doi: 10.1073/pnas.97.13.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder E, Schrott-Fischer A. Quantitative evaluation of myelinated nerve fibres and hair cells in cochleae of humans with age-related high-tone hearing loss. Hear Res. 1995;91(1–2):19–32. doi: 10.1016/0378-5955(95)00158-1. [DOI] [PubMed] [Google Scholar]
- Fernandez KA, Jeffers PW, Lall K, Liberman MC, Kujawa SG. Aging after noise exposure: acceleration of cochlear synaptopathy in “recovered” ears. J Neurosci. 2015;35(19):7509–7520. doi: 10.1523/JNEUROSCI.5138-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Tessarollo L, Coppola E, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog Brain Res. 2004;146:265–278. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- Furman AC, Kujawa SG, Liberman MC. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol. 2013;110(3):577–586. doi: 10.1152/jn.00164.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness DN, Lawton DM. Comparative distribution of glutamate transporters and receptors in relation to afferent innervation density in the mammalian cochlea. J Neurosci. 2003;23:11296–11304. doi: 10.1523/JNEUROSCI.23-36-11296.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates GA, Schmid P, Kujawa SG, Nam B, D’Agostino R. Longitudinal threshold changes in older men with audiometric notches. Hear Res. 2000;141(1–2):220–228. doi: 10.1016/s0378-5955(99)00223-3. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Transmitter release at the hair cell ribbon synapse. Nat Neurosci. 2002;5(2):147–154. doi: 10.1038/nn796. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S. Hearing loss and aging: new research findings and clinical implications. J Rehabil Res Dev. 2005;42:9–24. doi: 10.1682/jrrd.2005.01.0006. [DOI] [PubMed] [Google Scholar]
- Grose JH, Mamo SK. Processing of temporal fine structure as a function of age. Ear Hear. 2010;31:755–760. doi: 10.1097/AUD.0b013e3181e627e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JW, Halpin CF, Nam EC, Levine RA, Melcher JR. Tinnitus, diminished sound-level tolerance, and elevated auditory activity in humans with clinically normal hearing sensitivity. J Neurophysiol. 2010;104(6):3361–3370. doi: 10.1152/jn.00226.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakuba N, Koga K, Gyo K, Usami SI, Tanaka K. Exacerbation of noise-induced hearing loss in mice lacking the glutamate transporter GLAST. J Neurosci. 2000;20(23):8750–8753. doi: 10.1523/JNEUROSCI.20-23-08750.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin C, Thornton A, Hasso M. Low-frequency sensorineural loss: clinical evaluation and implications for hearing aid fitting. Ear Hear. 1994;15:71–81. [PubMed] [Google Scholar]
- Hickox AE, Liberman MC. Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J Neurophysiol. 2014;111(3):552–564. doi: 10.1152/jn.00184.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinojosa R, Lerner SA. Cochlear neural degeneration without hair cell loss in two patients with aminoglycoside ototoxicity. J Infect Dis. 1987;156(3):449–455. doi: 10.1093/infdis/156.3.449. [DOI] [PubMed] [Google Scholar]
- Johnsson LG. Sequence of degeneration of Corti’s organ and its first-order neurons. Ann Otol Rhinol Laryngol. 1974;83(3):294–303. doi: 10.1177/000348947408300303. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Afman CE. Hyperactivity in the dorsal cochlear nucleus after intense sound exposure and its resemblance to tone-evoked activity: a physiological model for tinnitus. Hear Res. 2000;140(1–2):165–172. doi: 10.1016/s0378-5955(99)00197-5. [DOI] [PubMed] [Google Scholar]
- Kawase T, Liberman MC. Spatial organization of the auditory nerve according to spontaneous discharge rate. J Comp Neurol. 1992;319(2):312–318. doi: 10.1002/cne.903190210. [DOI] [PubMed] [Google Scholar]
- Knipper M, Van Dijk P, Nunes I, Rüttiger L, Zimmermann U. Advances in the neurobiology of hearing disorders: recent developments regarding the basis of tinnitus and hyperacusis. Prog Neurobiol. 2013;111:17–33. doi: 10.1016/j.pneurobio.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci. 2006;26(7):2115–2123. doi: 10.1523/JNEUROSCI.4985-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29(45):14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Synaptopathy in the noise-exposed and aging cochlea: primary neural degeneration in acquired sensorineural hearing loss. Hear Res. 2015;330(Pt B):191–199. doi: 10.1016/j.heares.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Micucci S, Liberman MC. Noise-induced primary neural degeneration: effects of spectrum, duration, intensity and survival. Midwinter Meeting of the Association for Research in Otolaryngology. 2011:56. [Google Scholar]
- Lawner BE, Harding GW, Bohne BA. Time course of nerve-fiber regeneration in the noise-damaged mammalian cochlea. Int J Dev Neurosci. 1997;15(4–5):601–617. doi: 10.1016/s0736-5748(96)00115-3. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Auditory-nerve response from cats raised in a low-noise chamber. J Acoust Soc Am. 1978;63(2):442–455. doi: 10.1121/1.381736. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Morphological differences among radial afferent fibers in the cat cochlea: an electron microscopic study of serial sections. Hear Res. 1980;3(1):45–63. doi: 10.1016/0378-5955(80)90007-6. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Single-neuron labeling in the cat auditory nerve. Science. 1982;216(4551):1239–1241. doi: 10.1126/science.7079757. [DOI] [PubMed] [Google Scholar]
- Liberman LD, Wang H, Liberman MC. Opposing gradients of ribbon size and AMPA receptor expression underlie sensitivity differences among cochlearnerve/hair cell synapses. J Neurosci. 2011;31(3):801–808. doi: 10.1523/JNEUROSCI.3389-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman LD, Suzuki J, Liberman MC. Dynamics of cochlear synaptopathy after acoustic overexposure. J Assoc Res Otolaryngol. 2015;16(2):205–219. doi: 10.1007/s10162-015-0510-3. [added] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Epstein MJ, Cleveland SS, Wang H, Maison SF. Toward a differential diagnosis of hidden hearing loss in humans. PLoS One. 2016 Sep 12;11(9):e0162726. doi: 10.1371/journal.pone.0162726. http://dx.doi.org/10.1371/journal.pone.0162726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wang H, Shi L, Almuklass A, He T, Aiken S, et al. Silent damage of noise on cochlear afferent innervation in Guinea pigs and the impact on temporal processing. PLoS One. 2012;7:e49550. doi: 10.1371/journal.pone.0049550. http://dx.doi.org/10.1371/journal.pone.0049550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Salvi R, Ding D. Insensitivity of the audiogram to carboplatin induced inner hair cell loss in chinchillas. Hear Res. 2013;302:113–120. doi: 10.1016/j.heares.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makary CA, Shin J, Kujawa SG, Liberman MC, Merchant SN. Age-related primary cochlear neuronal degeneration in human temporal bones. J Assoc Res Otolaryngol. 2011;12(6):711–717. doi: 10.1007/s10162-011-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Jiang H, Salvi RJ. Time course of efferent fiber and spiral ganglion cell degeneration following complete hair cell loss in the chinchilla. Brain Res. 2004;997(1):40–51. doi: 10.1016/j.brainres.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Mehraei G, Hickox AE, Bharadwaj HM, Goldberg H, Verhulst S, Liberman MC, Shinn-Cunningham BG. Auditory brainstem response latency in noise as a marker of cochlear synaptopathy. J Neurosci. 2016;36(13):3755–3764. doi: 10.1523/JNEUROSCI.4460-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchan-Perez A, Liberman MC. Ultrastructural differences among afferent synapses on cochlear hair cells: correlations with spontaneous discharge rate. J Comp Neurol. 1996;371(2):208–221. doi: 10.1002/(SICI)1096-9861(19960722)371:2<208::AID-CNE2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Miller JM, Chi DH, O’Keeffe LJ, Kruszka P, Raphael Y, Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int J Dev Neurosci. 1997;15(4–5):631–643. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- Moore BCJ. Dead regions in the cochlea: conceptual foundations, diagnosis, and clinical applications. Ear Hear. 2004;25(2):98–116. doi: 10.1097/01.aud.0000120359.49711.d7. [DOI] [PubMed] [Google Scholar]
- Moser T, Neef A, Khimich D. Mechanisms underlying the temporal precision of sound encoding at the inner hair cell ribbon synapse. J Physiol. 2006;576(1):55–62. doi: 10.1113/jphysiol.2006.114835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadol JB., Jr Application of electron microscopy to human otopathology. Ultrastructural findings in neural presbycusis, Meniere’s disease and Usher’s syndrome. Acta Otolaryngol. 1988;105(5–6):411–419. doi: 10.3109/00016488809119494. [DOI] [PubMed] [Google Scholar]
- National Institute for Occupational Safety and Health. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health Publication No. 98–126. NIOSH; Cincinnati: 1998. Criteria for a Recommended Standard: Occupational Noise Exposure, revised criteria 1998. [Google Scholar]
- Nouvian R, Beutner D, Parsons TD, Moser T. Structure and function of the hair cell ribbon synapse. J Membr Biol. 2006;209:153–165. doi: 10.1007/s00232-005-0854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi N, Duscha S, Boukari H, Meyer M, Xie J, Wei G, Schrepfer T, Roschitzki B, Boettger EC, Schacht J. XBP1 mitigates aminoglycoside-induced endoplasmic reticulum stress and neuronal cell death. Cell Death Dis. 2015;6:e1763. doi: 10.1038/cddis.2015.108. http://dx.doi.org/10.1038/cddis.2015.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSHA. Occupational Noise Exposure; Hearing Conservation Amendment. 1983;46:9738–9785. Final Rule. 29 C.F.R. 1910.95. Federal Register 48. [Google Scholar]
- Plack CJ, Barker D, Prendergast G. Perceptual consequences of “hidden” hearing loss. Trends hear. 2014 Sep;9:18. doi: 10.1177/2331216514550621. http://dx.doi.org/10.1177/2331216514550621 pii: 2331216514550621 (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel JL. Chemical synaptic transmission in the cochlea. Prog Neurobiol. 1995;47:449–476. doi: 10.1016/0301-0082(95)00028-3. [DOI] [PubMed] [Google Scholar]
- Puel JL, Safieddine S, D’Aldin C, Eybalin M, Pujol R. Synaptic regeneration and functional recovery after excitotoxic injury in the Guinea pig cochlea. C R Acad Sci Paris Ser III. 1995;318:67–75. [PubMed] [Google Scholar]
- Puel JL, Ruel J, d’Aldin CG, Pujol R. Excitotoxicity and repair of cochlear synapses after noise-trauma induced hearing loss. NeuroReport. 1998;9:2109–2114. doi: 10.1097/00001756-199806220-00037. [DOI] [PubMed] [Google Scholar]
- Pujol R, Puel JL. Excitotoxicity, synaptic repair, and functional recovery in the mammalian cochlea: a review of recent findings. Ann N Y Acad Sci. 1999;884:249–254. doi: 10.1111/j.1749-6632.1999.tb08646.x. [DOI] [PubMed] [Google Scholar]
- Ramekers D, Versnel H, Grolman W, Klis SF. Neurotrophins and their role in the cochlea. Hear Res. 2012;288:19–33. doi: 10.1016/j.heares.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Reijntjes DOJ, Pyott SJ. The afferent signaling complex: regulation of type I spiral ganglion neuron responses in the auditory periphery. Hear Res. 2016;336:1–16. doi: 10.1016/j.heares.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA. Ringing ears: the neuroscience of tinnitus. J Neurosci. 2010;30(45):14972–14979. doi: 10.1523/JNEUROSCI.4028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. Functional significance of dendritic swelling after loud sounds in the Guinea pig cochlea. Hear Res. 1983;9:263–278. doi: 10.1016/0378-5955(83)90031-x. [DOI] [PubMed] [Google Scholar]
- Ruan Q, Ao H, He J, Chen Z, Yu Z, Zhang R, Wang J, Yin S. Topographic and quantitative evaluation of gentamicin-induced damage to peripheral innervation of mouse cochleae. Neurotoxicology. 2014;40:86–96. doi: 10.1016/j.neuro.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Ruel J, Wang J, Pujol R, Hameg A, Dib M, Puel JL. Neuroprotective effect of riluzole in acute noise-induced hearing loss. Neuroreport. 2005;16(10):1087–1090. doi: 10.1097/00001756-200507130-00011. [DOI] [PubMed] [Google Scholar]
- Ruel J, Wang J, Rebillard G, Eybalin M, Lloyd R, Pujol R, Puel JL. Physiology, pharmacology and plasticity at the inner hair cell synaptic complex. Hear Res. 2007;227(1–2):19–27. doi: 10.1016/j.heares.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Ruggles D, Bharadwaj H, Shinn-Cunningham BG. Normal hearing is not enough to guarantee robust encoding of suprathreshold features important in everyday communication. Proc Natl Acad Sci U S A. 2011;108(37):15516–15521. doi: 10.1073/pnas.1108912108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybalko N, Chumak T, Bureš Z, Popelář J, Šuta D, Syka J. Development of the acoustic startle response in rats and its change after early acoustic trauma. Behav Brain Res. 2015;286:212–221. doi: 10.1016/j.bbr.2015.02.046. [DOI] [PubMed] [Google Scholar]
- Schaette R, McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci. 2011;31(38):13452–13457. doi: 10.1523/JNEUROSCI.2156-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalk TB, Sachs MB. Nonlinearities in auditory-nerve fiber responses to bandlimited noise. J Acoust Soc Am. 1980;67(3):903–913. doi: 10.1121/1.383970. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA, Mills JH, Boettcher FA. Age-related loss of activity of auditory-nerve fibers. J Neurophysiol. 1996;76(4):2799–2803. doi: 10.1152/jn.1996.76.4.2799. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF, Gacek MR. Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol. 1993;102:1–16. doi: 10.1177/00034894931020S101. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF, Woellner RC. An experimental and clinical study of deafness from lesions of the cochlear nerve. J Laryngol Otol. 1955;69(2):75–97. doi: 10.1017/s0022215100050465. [DOI] [PubMed] [Google Scholar]
- Seal RP, Amara SG. Excitatory amino acid transporters: a family in flux. Ann Rev Pharmacol Toxicol. 1999;39:431–456. doi: 10.1146/annurev.pharmtox.39.1.431. [DOI] [PubMed] [Google Scholar]
- Sedo-Cabezon L, Boadas-Vaello P, Soler-Martin C, Llorens J. Vestibular damage in chronic ototoxicity: a mini-review. NeuroToxicology. 2014;43:21–27. doi: 10.1016/j.neuro.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC, Kujawa SG. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci. 2013;33(34):13686–13694. doi: 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Liu L, He T, Guo X, Yu Z, Yin S, Wang J. Ribbon synapse plasticity in the cochleae of Guinea pigs after noise-induced silent damage. PLoS One. 2013;8:e81566. doi: 10.1371/journal.pone.0081566. http://dx.doi.org/10.1371/journal.pone.0081566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Liu K, Wang H, Zhang Y, Hong Z, Wang M, Wang X, Jiang X, Yang S. Noise induced reversible changes of cochlear ribbon synapses contribute to temporary hearing loss in mice. Acta Otolaryngol. 2015;135:1093–1102. doi: 10.3109/00016489.2015.1061699. [DOI] [PubMed] [Google Scholar]
- Shi L, Chang Y, Li X, Aiken SJ, Liu L, Wang J. Coding deficits in noise-induced hidden hearing loss may stem from incomplete repair of ribbon synapses in the cochlea. Front Neurosci. 2016:25. doi: 10.3389/fnins.2016.00231. http://dx.doi.org/10.3389/fnins.2016.00231. [DOI] [PMC free article] [PubMed]
- Singer W, Zuccotti A, Jaumann M, Lee SC, Panford-Walsh R, Xiong H, Zimmermann U, Franz C, Geisler HS, Köpschall I, Rohbock K, Varakina K, Verpoorten S, Reinbothe T, Schimmang T, Rüttiger L, Knipper M. Noise-induced inner hair cell ribbon loss disturbs central arc mobilization: a novel molecular paradigm for understanding tinnitus. Mol Neurobiol. 2013;47(1):261–279. doi: 10.1007/s12035-012-8372-8. [DOI] [PubMed] [Google Scholar]
- Smith PF. Are vestibular hair cells excited to death by aminoglycoside antibiotics? J Vestib Res. 1999;10:1–5. [PubMed] [Google Scholar]
- Sone M, Schachern PA, Paparella MM. Loss of spiral ganglion cells as a primary manifestation of aminoglycoside ototoxicity. Hear Res. 1998;115:217–223. doi: 10.1016/s0378-5955(97)00191-3. [DOI] [PubMed] [Google Scholar]
- Song Q, Shen P, Li X, Shi L, Liu L, Wang J, et al. Coding deficits in hidden hearing loss induced by noise: the nature and impacts. Front Neurosci. 2016;10:231. doi: 10.1038/srep25200. http://dx.doi.org/10.3389/fnins.2016.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoendlin H. Primary structural changes in the organ of Corti after acoustic overstimulation. Acta Otolaryngol. 1971;71:166–176. doi: 10.3109/00016487109125346. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Innervation densities of the cochlea. Acta Otolaryngol. 1972;73:235–248. doi: 10.3109/00016487209138937. [DOI] [PubMed] [Google Scholar]
- Stamataki S, Francis HW, Lehar M, May BJ, Ryugo DK. Synaptic alterations at inner hair cells precede spiral ganglion cell loss in aging C57BL/6J mice. Hear Res. 2006;221:104–118. doi: 10.1016/j.heares.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Stankovic K, Rio C, Xia A, Sugawara M, Adams JC, Liberman MC, Corfas G. Survival of adult spiral ganglion neurons requires erbB receptor signaling in the inner ear. J Neurosci. 2004;24:8651–8661. doi: 10.1523/JNEUROSCI.0733-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara M, Corfas G, Liberman MC. Influence of supporting cells on neuronal degeneration after hair cell loss. J Assoc Res Otolaryngol. 2005;6(2):136–147. doi: 10.1007/s10162-004-5050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Hashino E, Ding DL, Salvi RJ. Reversible and irreversible damage to cochlear afferent neurons by kainic acid excitotoxicity. J Comp Neurol. 2001;430(2):172–181. doi: 10.1002/1096-9861(20010205)430:2<172::aid-cne1023>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Ushio M, Yamasoba T. Time course of apoptotic cell death in Guinea pig cochlea following intratympanic gentamicin application. Acta Otolaryngol. 2008;128(7):724–731. doi: 10.1080/00016480701714244. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Corfas G, Liberman MC. Round-window delivery of neurotrophin 3 regenerates cochlear synapses after acoustic overexposure. Sci Rep. 2016 Apr 25;6:24907. doi: 10.1038/srep24907. http://dx.doi.org/10.1038/srep24907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47:122, e129. doi: 10.1016/j.ceca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Takeno S, Wake M, Mount RJ, Harrison RV. Degeneration of spiral ganglion cells in the chinchilla after inner hair cell loss induced by carboplatin. Audiol Neurootol. 1998;3(5):281–290. doi: 10.1159/000013800. [DOI] [PubMed] [Google Scholar]
- Tsuji J, Liberman MC. Intracellular labeling of auditory nerve fibers in Guinea pig: central and peripheral projections. J Comp Neurol. 1997;381:188–202. [PubMed] [Google Scholar]
- Viana LM, O’Malley JT, Burgess BJ, Jones DD, Oliveira CA, Santos F, Merchant SN, Liberman MC. Cochlear neuropathy in human presbycusis: confocal analysis of hidden hearing loss in post-mortem tissue. Hear Res. 2015;327:78–88. doi: 10.1016/j.heares.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan G, Gómez-Casati ME, Gigliello AR, Liberman MC, Corfas G. Neurotrophin-3 regulates ribbon synapse density in the cochlea and induces synapse regeneration after acoustic trauma. Elife. 2014:3. doi: 10.7554/eLife.03564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ren C. Effects of repeated “benign” noise exposures in young CBA mice: shedding light on age-related hearing loss. J Assoc Res Otolaryngol. 2012;13(4):505–515. doi: 10.1007/s10162-012-0329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol. 2002;3(3):248–268. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster DB, Webster M. Cochlear nerve projections following organ of corti destruction. Otolaryngol. 1978;86(2):342–353. doi: 10.1177/019459987808600228. [DOI] [PubMed] [Google Scholar]
- Winter IM, Robertson D, Yates GK. Diversity of characteristic frequency rate-intensity functions in Guinea pig auditory nerve fibres. Hear Res. 1990;45:191–202. doi: 10.1016/0378-5955(90)90120-e. [DOI] [PubMed] [Google Scholar]
- Yin Y, Liberman LD, Maison SF, Liberman MC. Olivocochlear innervation maintains the normal modiolar-pillar and habenular-cuticular gradients in cochlear synaptic morphology. J Assoc Res Otolaryngol. 2014;15(4):571–583. doi: 10.1007/s10162-014-0462-z. [DOI] [PMC free article] [PubMed] [Google Scholar]