Abstract
Introduction
Post-traumatic stress disorder (PTSD) may be associated with physical inactivity, a modifiable lifestyle factor that contributes to risk of cardiovascular and other chronic diseases; however, no study has evaluated the association between PTSD onset and subsequent physical activity (PA) changes.
Method
Analyses were conducted between October 2014 and April 2016, using data from the ongoing Nurses' Health Study II (N=50,327). Trauma exposure and PTSD symptoms were assessed using two previously validated measures, the Brief Trauma Questionnaire and Short Screening Scale for DSM-IV PTSD. Average PA (hours/week) was assessed using self-report measures at six time points across 20 years (1989–2009). Linear mixed models with time-updated PTSD assessed differences in PA trajectories by trauma/PTSD status. Among a subsample of women whose trauma/PTSD onset during follow-up, group differences in PA patterns before and after onset were assessed using linear spline models.
Results
PA decreased more steeply over time among trauma-exposed women reporting four to five (β= −2.5E−3, SE=1.0E−3, p=0.007) or six to seven PTSD symptoms (β= −6.7E−3, SE=1.1E−3, p<0.001) versus women without trauma exposure, adjusting for potential confounders. Among a subsample of women whose trauma/PTSD symptoms onset during follow-up, no differences in PA were observed prior to onset; after onset, women with six to seven PTSD symptoms had a steeper decline (β= −17.1E−3, SE=4.2E−3, p<0.001) in PA over time than trauma-exposed women without PTSD.
Conclusions
Decreases in PA associated with PTSD symptoms may be a pathway through which PTSD influences cardiovascular and other chronic diseases.
Introduction
Post-traumatic stress disorder (PTSD), the sentinel stress-related mental disorder, has been declared “a life sentence” in part based on findings that the disorder is associated with multiple adverse physical health outcomes, particularly cardiometabolic diseases.1–5 Though most PTSD research has focused on men, given initial recognition of the disorder among military veterans,6 lifetime PTSD prevalence among women (10.4%) is twice as high as among men. Further, among women, the disorder is associated with higher functional impairment and is more likely to be chronic.7
A number of biological and behavioral mechanisms have been proposed to explain how PTSD might lead to multiple adverse physical health outcomes. Though many studies have examined PTSD in relation to health behaviors (e.g., cigarette smoking), few have considered physical activity (PA). Insufficient PA is the fourth leading risk factor for mortality8 and a major risk factor for cardiometabolic diseases.9 Moreover, increasing levels of PA has demonstrated efficacy as a disease prevention strategy.10,11 Meta-analyses of RCTs suggest that exercise interventions bestow similar benefits to those of drug interventions in secondary prevention of coronary heart disease and prediabetes, and are more effective than drug treatment among patients with stroke.12
A variety of reasons may explain PTSD's influence on PA. Individuals with PTSD may limit exercise owing to a heightened concern for safety, fear of bodily arousal symptoms, comorbid depression, substance use, insomnia, or lower motivation, self-efficacy, or social support.13 Empirical findings demonstrating that PTSD does in fact influence PA could suggest targeted intervention for individuals with PTSD to prevent long-term adverse health sequelae.
In a recent comprehensive literature review, Hall et al.13 identified 15 studies considering the relationship between PTSD and PA, though PA was identified as a primary outcome in only six of these studies. These studies were limited by cross-sectional designs, male-only samples, small samples (N<90), and lack of control for potential confounders such as depression or consideration for the effect of PTSD symptoms independent of trauma. Findings were mixed, with some studies reporting no association and others reporting a significant negative association between PTSD and PA. In a retrospective study not included in the review, among 50 men and women with PTSD, a reduction in PA was reported following PTSD onset.14 Effects of trauma exposure alone were not differentiated from effects of PTSD. Other work has similarly suggested that patients with affective disorders engage in low levels of PA15; however, recent work has called for more rigorous research on this relationship.16 No prospective study has examined how PTSD influences PA over time, or if the onset of PTSD alters PA patterns.
In the current study, longitudinal data were used to examine change in PA following onset of PTSD symptoms in a large sample of young and middle-aged community-dwelling women exposed to a variety of traumas. Because the role of trauma alone versus PTSD in pathophysiologic processes remains unclear, effects of PTSD symptoms were considered separately from effects of trauma alone. Hypotheses were:
Women who developed PTSD symptoms would show greater decreases in PA over time than women who experienced no trauma, or trauma but no symptoms.
Higher versus lower PTSD symptom severity would be associated with greater reduction in PA in a dose–response manner.
History of depression and anxiety symptoms were adjusted for, as both are commonly comorbid with PTSD17,18 and inversely associated with PA.19,20 Additional covariates possibly related to both PTSD and health behaviors (age, race/ethnicity, childhood adiposity, and region of residence) were selected based on prior work in this cohort2 and other research on PTSD and PA.21
Methods
Study Population
The Nurses' Health Study II is an ongoing cohort study of 116,430 U.S. female registered nurses. The cohort began in 1989 and participants—aged 24–42 years at enrollment—are followed up biennially. In 2008, a supplementary Trauma and PTSD Screening Questionnaire22 was mailed to 60,804 participants who completed a supplemental questionnaire on violence in 2001 and the 2007 biennial questionnaire. To maximize retention, supplemental surveys are only sent to those who return biennial questionnaires. After repeated mailings, 54,224 women returned the questionnaire (89% response rate), which constituted implied consent.
Analysis was limited to 50,327 of these respondents, after excluding women with missing information on trauma or PTSD symptoms (n=3,894),and those with fewer than two measures of PA (n=34) (Figure 1).Women excluded from analyses were similar to those included; however, a smaller proportion had ever been diagnosed with depression (19.7% vs 24.8%). A subsample was also created of women who reported worst trauma exposure or PTSD symptoms occurring during study follow-up, between 1989 and 2009 (n=15,353). Statistical analyses of the data were conducted between October 2014 and April 2016. This study was approved by the Partners Healthcare Human Research Committee.
Figure 1.
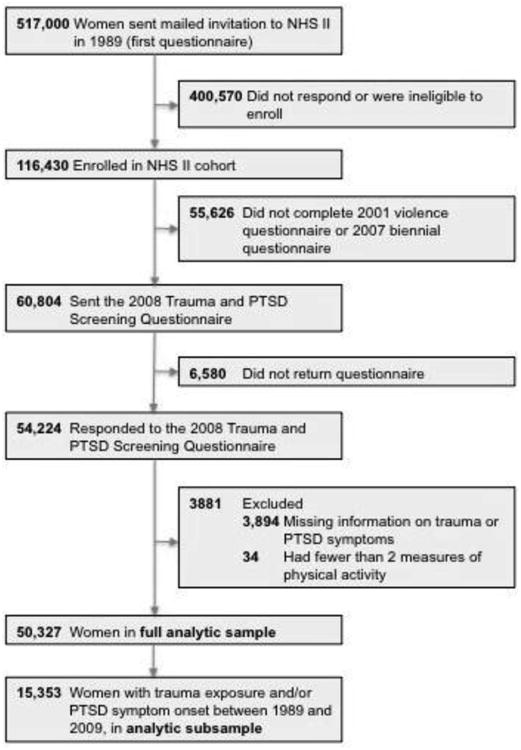
Flowchart of exclusions for deriving the analytic samples.
NHS II, Nurses' Health Study II; PTSD, posttraumatic stress disorder
Measures
A modified version of the reliable and valid Brief Trauma Questionnaire23,24 was used to assess lifetime exposure to any of 15 traumatic events (e.g., physical assault, violent death of a close family member) or “serious traumatic event not already covered.” If participants reported exposure to more than one traumatic event, they identified the event they considered to be their worst trauma, and reported their age at occurrence. PTSD symptoms were queried with respect to the worst (or only) traumatic event using the seven-item Short Screening Scale for DSM-IV PTSD25 (additional details on the Short Screening Scale can be found in Appendix A.) Onset of PTSD was established according to age reported at worst trauma,using a specially designed question sequence developed to improve accuracy of age of onset of reporting of psychiatric disorders.26 Reliability of self-reported age of onset of trauma and PTSD has been found to be excellent in this sample (intraclass correlation, 0.95).27
Trauma and PTSD were coded as time varying. For each year of the study, participants were categorized into one of five groups with increasing severity based on the year at which their worst traumatic event occurred:
no trauma exposure;
trauma exposure but no PTSD symptoms;
trauma exposure with one to three PTSD symptoms (subclinical);
trauma exposure with four or five PTSD symptoms (clinical); and
trauma exposure with six or seven PTSD symptoms (severe).
The trauma/PTSD symptom groups were based on previous research with the Short Screening Scale for DSM-IV PTSD recommending scores of ≥4 and ≥6 as clinical cut offs for PTSD.25,28
Leisure-time PA was assessed at baseline in 1989 and again in 1991, 1997, 2001, 2005, and 2009 with a previously validated questionnaire.29 Participants were asked about the average weekly time doing the following activities during the preceding year: walking or hiking outdoors; jogging; running; bicycling; tennis, squash, or racquet ball; lap swimming; calisthenics, aerobics, aerobic dance, or rowing machine; and performing other vigorous activities (e.g., lawn mowing). In 2001, 2005, and 2009, participants were also asked about time spent doing lower-intensity exercise (e.g., yoga) and weight training.
Following prior work with this sample,9 time/week was summed across all activities to calculate a total number of hours/week of PA. Walking was only included as an activity if pace was brisk, very brisk, or striding (≥3 mph), as prior work has linked brisk walking pace with lower cardiometabolic disease risk.30,31 PA scores were Winsorized at the 99th percentile (25 hours/week) to reduce the impact of extreme outliers and potentially erroneous reports. Given the addition of activity categories in 2001 onward, PA totals at each assessment were standardized (mean, 0; SD=1) to be comparable across time points. Sensitivity analyses—first, including all walking paces (e.g., easy, normal) and non-Winsorized PA scores, and then further adjusting for time-varying history of chronic disease (self-reported stroke, myocardial infarction, diabetes) and physical impairment (inability to walk)—did not change results substantially. These results are included in Appendix B.
In 1989, participants reported their age, race/ethnicity (categorized here as white, black, Asian, Hispanic, or other), and childhood adiposity. Participants selected one of nine pictograms of somatotype (body shape and size) at age 5 years, which was used to estimate childhood adiposity.32,33 Somatotypes 5–9 were collapsed into a single category representing the greatest adiposity (because few respondents endorsed levels >5). Region of residence at age 15 years was assessed in 1993 and categorized as West, Midwest, Northeast, South, or Puerto Rico and non-U.S. Information pertaining to childhood SES was assessed in 2005, with report of both parents' educational attainment at participant's birth (maximum of both parents: high school or less, some college, ≥4 years of college). Lifetime history of depression was based on participant reports of ever being diagnosed with depression by a doctor at or before 2009 (yes/no).Anxiety was assessed in 1993 with the eight-item anxiety subscale from the Crown–Crisp index34 (range, 0–14; higher score indicates more symptoms). A “missing” category was included for all categorical covariates. For covariates other than region of residence (which had approximately 18% missing data), the amount of missing data was <5%.
Statistical Analysis
Linear mixed models were used to estimate the association between time-updated trauma/PTSD symptoms and 20-year PA trajectories in the full sample. Random intercept and random slope models allowed for individual differences in PA at baseline and in change over time. Time was measured as the number of years from study baseline (1989), ranging from 0 to 20. Women with no trauma exposure served as the reference group. Trauma/PTSD status was time updated each year.
A linear spline regression was fitted among the subsample of women whose trauma exposure or onset of PTSD symptoms occurred after baseline in 1989 (i.e., during the study follow-up). In this subsample, PA data before and after trauma exposure or PTSD symptom onset were available, facilitating direct evaluation of changes in PA related to occurrence of trauma and PTSD symptoms. Time was measured as the number of years from trauma/PTSD symptom onset, ranging from –19 (years prior to onset) to +19 (years after onset), with 0 as the inflection point of onset (which differed for each participant). Thus, there were 38 time points. For this analysis, all women were exposed to trauma at some point between 1989 and 2009 (i.e., there was no trauma-unexposed group). Women with trauma exposure but no PTSD symptoms served as the reference group.
Each analysis involved two sets of models: Model 1 adjusted for age only, and Model 2 additionally adjusted for race/ethnicity, parental education, childhood adiposity, adolescent region of residence, history of depression, and anxiety. In all models, trauma/PTSD status was lagged by 1 year relative to when PA levels were assessed (e.g.,PTSD status in 1990 was linked with PA in 1991) to reduce the likelihood that PA measurements preceded (and might have altered) PTSD symptoms in a given year.
Results
Table 1 summarizes the demographic characteristics of participants by trauma exposure and PTSD symptom status at study baseline in 1989. At study inception, slightly more than half the sample (n=25,382) had experienced their worst traumatic event. Over follow-up, an additional 15,353 women reported experiencing their worst trauma. By 2008, 19.9% remained trauma unexposed, 46.2% had experienced trauma but no PTSD symptoms, 16.5% reported one to three PTSD symptoms, 10.8% reported four to five symptoms, and 6.6% reported six to seven symptoms.
Table 1. Sample Characteristics at Baseline (1989), Stratified by Trauma/PTSD Symptoms Reported as of Baseline (N=50,327).
| Covariates | No trauma (n=24,945, 49.6%) | Trauma, no symptoms (n=15,423, 30.7%) | Trauma, 1-3 symptoms (n=4,967, 9.9%) | Trauma, 4-5 symptoms (n=3,014, 6.0%) | Trauma, 6-7 symptoms (n=1,978, 3.9%) |
|---|---|---|---|---|---|
| Age, yearsa | 34.3 (4.7) | 35.2 (4.5) | 35.3 (4.5) | 35.2 (4.4) | 35.1 (4.4) |
| Physical activity, hours/weeka | 3.6 (4.7) | 3.6 (4.7) | 3.5 (4.7) | 3.5 (4.5) | 3.7 (4.7) |
| White race | 23,412 (93.9) | 14,403 (93.4) | 4,677 (94.16) | 2,858 (94.8) | 1,871 (94.6) |
| Parental education ≥college | 5,857 (23.5) | 3,354 (21.8) | 1,136 (22.9) | 668 (22.2) | 491 (24.8) |
| Highest age 5 adiposity group | 1,634 (6.6) | 1,090 (7.1) | 328 (6.6) | 228 (7.6) | 167 (8.4) |
| Age 15 region of residence | |||||
| Northeast | 2,431 (9.8) | 1,665 (10.8) | 611 (12.3) | 355 (11.8) | 267 (13.5) |
| Midwest | 9,037 (36.2) | 5,364 (34.8) | 1,765 (35.5) | 1,056 (35.0) | 634 (32.1) |
| West | 5,611 (22.5) | 3,278 (21.3) | 1,038 (20.9) | 637 (21.1) | 417 (21.1) |
| South | 2,761 (11.1) | 1,841 (11.9) | 600 (12.1) | 398 (13.2) | 270 (13.7) |
| Puerto Rico or non-U.S. | 420 (1.7) | 264 (1.7) | 76 (1.5) | 45 (1.5) | 27 (1.4) |
| Missing | 4,685 (18.8) | 3,011 (19.5) | 877 (17.7) | 523 (17.4) | 363 (18.4) |
| Lifetime depression | 5,541 (22.2) | 2,872 (18.6) | 1,472 (29.6) | 1,390 (46.1) | 1,192 (60.3) |
| Anxiety symptoms | 2.08 (1.75) | 2.10 (1.76) | 2.22 (1.79) | 2.48 (2.02) | 2.88 (2.18) |
Mean with SD in parentheses; all other entries are N (%)
PTSD, posttraumatic stress disorder
The PA trajectories by time-varying PTSD status over the 20-year follow-up period in the full sample are presented in Figure 2. Relative to women without trauma, there was a decrease in PA z-score over time among women with trauma exposure and four to five (β= −2.24E−3, SE=0.89E−3; p=0.01) or six to seven PTSD symptoms (β= −5.71E−3, SE=1.08E−3, p<0.001), in age-adjusted linear mixed models. Additional adjustment for race, somatotype, parental education, region of residence, history of depression, and anxiety did not change the results substantially (Appendix Table 1).
Figure 2.
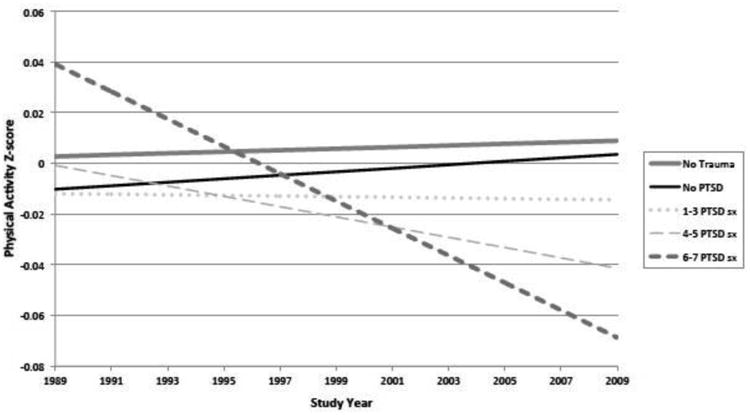
Age-adjusted predicted physical activity trajectory over time by Trauma/PTSD group in the full sample.
PTSD, posttraumatic stress disorder
Figure 3 shows PA trajectories before and after onset of trauma/PTSD symptoms among the subsample of women whose PTSD symptoms onset during follow-up. Prior to trauma/PTSD symptom onset, no significant between-group differences in PA levels were observed. That is, the PA trajectories among those who later developed PTSD symptoms were similar to those of women who were exposed to trauma but did not develop PTSD symptoms. After trauma/PTSD symptom onset, women with six to seven PTSD symptoms had a steeper decrease in PA than trauma-exposed women with no PTSD symptoms, in minimally (β= −16.1E−3, SE=4.0E−3, p<0.001) and fully adjusted models (Appendix Table 2).
Figure 3.
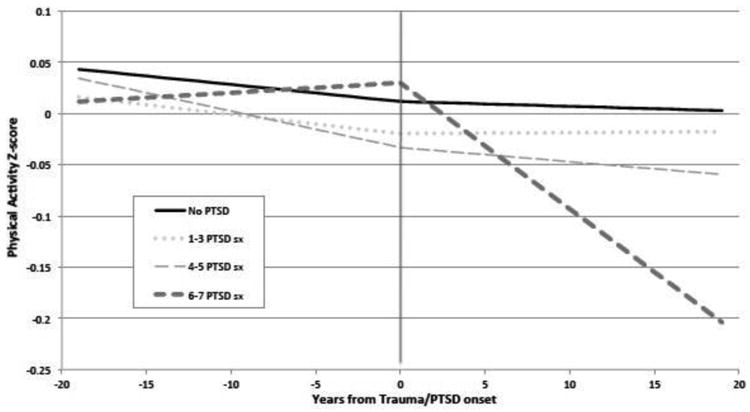
Age-adjusted predicted physical activity trajectory over time by Trauma/PTSD group, before and after trauma/PTSD onset, among women reporting trauma or PTSD onset during following up.
Notes: Includes women with trauma/PTSD onset during follow-up, between 1989-2009 (n=15,353). Physical activity is calculated in hours per week and standardized using Z-score. Predicted values of physical activity are plotted. Zero marks the year of trauma/PTSD onset.
PTSD, posttraumatic stress disorder
Discussion
This study is the first to examine the longitudinal relationship between PTSD symptoms and PA trajectories in women exposed to a wide range of traumatic events occurring in civilian settings. Moreover, this is the first study to examine trends in PA both before and after onset of trauma or PTSD. Overall, higher PTSD symptom levels were associated with sharper declines in PA over time. Among women whose trauma exposure occurred during follow-up, an altered PA trajectory was evident for those women who further developed the highest number of PTSD symptoms (six to seven). Before PTSD symptom onset, PA trajectories were comparable to those of women who did not subsequently develop PTSD symptoms; however, after symptom onset, PA levels decreased at a faster rate. Associations were independent of depression and anxiety, and trauma exposure alone was not associated with changes in PA.
Potential Mechanisms
Symptoms of PTSD may influence PA levels in a number of ways. For example, individuals with PTSD may be less likely to exercise given a heightened concern for safety, which is common in PTSD,35 or fear of trauma-relevant bodily arousal symptoms triggered by exercise, such as shortness of breath, increased heart rate, and perspiration.36 This possibility is supported by recent evidence that PTSD hyperarousal symptoms (as opposed to re-experiencing or avoidance symptoms) show a particularly robust inverse relationship with exercise.35–37 Additionally, PTSD symptoms may undermine motivation to engage in PA and make it more difficult to start and maintain an exercise program. For example, in one study, patients with PTSD reported lack of motivation (24% before vs 71% after PTSD onset) as a justification for reducing activity.14
Having PTSD also increases comorbidity with other conditions or behaviors associated with decreased motivation to exercise, such as fibromyalgia,38 anhedonia or depression,18 tobacco use,39 and alcohol use.40 Sleep disruption may create another barrier to PA among women with PTSD symptoms. Research suggests that approximately 70%–80% of patients diagnosed with PTSD experience nightmares and insomnia,41 and greater sleepiness is negatively correlated with subsequent exercise duration.42 In addition to these behavioral barriers, psychosocial factors that may promote exercise, such as social support and self-efficacy, are lower among those with PTSD symptoms. Future research should further identify and test potential mechanisms, in order to develop and implement effective interventions.
Limitations
This study has several limitations. Although date of PTSD onset was available, PTSD symptoms were assessed at only one time point and in relation to the worst traumatic event, which may have led to some misclassification of timing and severity of PTSD symptoms, and perhaps an underestimation of the impact of the worst event if women had already experienced a somewhat less traumatic event. Furthermore, recall bias could be a concern as trauma exposure and PTSD symptoms were assessed retrospectively, which may have resulted in an underestimation of lifetime PTSD prevalence.43 However, these limitations in assessment would likely have biased results toward the null. PA was assessed via self-report, which has questionable validity among people with severe mental illness44; however, among individuals with PTSD, correlations between self-reported PA and accelerometers are of a similar magnitude to those in the general population.45 Another limitation is generalizability, given the population of predominately white and highly educated female nurses. The women in this study who experienced PTSD symptoms may represent a healthy survivor sample, as they all had successful nursing careers; thus, it is possible results are conservative estimates of the true effect of PTSD on PA. Despite adjustment for several covariates, adjustment for psychotropic medication and substance use could be explored in future research, along with other potential mediators.
This study has numerous strengths, including longitudinal data, a large well-characterized study population, extensive data on potential confounding factors, and repeated measures of PA before and after the onset of PTSD symptoms. This study considered effects in a community-dwelling sample of women with exposure to a range of traumas, and was able to disentangle potential effects of PTSD symptoms versus trauma exposure alone or depression/anxiety.
Conclusions
Studies have consistently shown that regular PA is associated with better long-term mental and physical health, and rigorous evidence of increased cardiometabolic disease risk among individuals with PTSD continues to amass.1–3 Findings suggest that decreasing PA after PTSD onset may be a pathway through which severe PTSD symptoms influence chronic disease risk among women. The presence of PTSD symptoms should raise clinician concerns about the potential development of physical health problems and prompt closer attention to health behaviors, which may be modifiable even in a potentially high-risk population. Though the size of associations in this study was small, determinants of health behaviors such as PA are likely multifactorial and any one variable will likely contribute only modestly to such outcomes. Given robust evidence of an inverse linear dose–response relationship between PA and cardiometabolic disease risk,46–51 even small reductions in PA over time may have clinically meaningful impacts on long-term health.
Despite growing recognition in the clinical community that the adverse impact of PTSD extends well beyond its psychiatric parameters, current clinical management of PTSD rarely addresses these sequelae of PTSD. As noted by Levine and colleagues,52 “Current management of PTSD focuses on the psychiatric parameters of this condition with little emphasis on addressing the comorbid cardiometabolic risk factors that impair overall long-term health outcomes.” At present, apart from cigarette smoking, health behaviors typically fall outside the scope of PTSD treatment and consideration by clinicians. This study highlights the importance of expanding PTSD treatments to address health-promoting behaviors that influence morbidity and mortality. In particular, health promotion interventions targeting PA among women with PTSD are of great interest. In turn, increased PA may further reduce PTSD symptoms, creating a positive health cycle.53,54
Extensive research on tobacco use among combat veterans with PTSD has led to several evidence-based smoking-cessation interventions for that population. With continued research and replication of findings, a similar scientific basis can be established to inform PA interventions for broader PTSD-affected populations, including women. With better prevention, the potential for earlier intervention, and more-effective treatment strategies, patient outcomes may improve significantly.
Acknowledgments
This study was supported by NIH grants R01MH101269-01A1 (to KCK, LDK, AW), T32MH017119 (to PG), K01HL130650 (to JAS), and UM1CA176726 (for NHS II infrastructure), as well as the Yerby Postdoctoral Fellowship Program (to PG).The sponsors had no role in the study design.
Appendix A – The Short Screening Scale for DSM-IV Ptsd
With respect to the worst (or only) traumatic event, Breslau's 7-item Short Screening Scale for DSM-IV PTSD1 was used to assess whether the participant: (1) avoided places/people/activities associated with the trauma; (2) lost interest in important/enjoyable activities; (3) felt isolated/distant from others; (4) found it hard to have love/affection for others; (5) had a sense of foreshortened future; (6) had sleep difficulties; or (7) became jumpy/easily startled. This scale has been demonstrated to have strong psychometric properties in several validation studies.1-4
Symptom cut-points for the current study were selected based on prior work demonstrating that in relation to worst trauma, a cut-point of 6 or more symptoms has the highest positive predictive values (87.1%) and a high negative predictive value (95%) for clinically relevant cases, while a score of 4 or more on this scale defines positive cases of PTSD with a sensitivity of 85%, specificity of 93%, positive predictive value of 68%, and negative predictive value of 98%.1
References
Breslau N, Peterson EL, Kessler RC, Schultz LR. Short screening scale for DSM-IV posttraumatic stress disorder. Am J Psychiatry. 1999;156(6):908-911. https://doi.org/10.1176/ajp.156.6.908.
Bohnert KM, Breslau N. Assessing the performance of the short screening scale for posttraumatic stress disorder in a large nationally-representative survey.Int J Methods Psychiatr Res.2011;20(1):e1-e5. https://doi.org/10.1002/mpr.331.
Freedy JR, Steenkamp MM, Magruder KM, et al. Post-traumatic stress disorder screening test performance in civilian primary care. Fam Pract. 2010;27(6):615-624. https://doi.org/10.1093/fampra/cmq049.
Kimerling R, Ouimette P, Prins A, et al. Brief report: Utility of a short screening scale for DSM-IV PTSD in primary care. J Gen Intern Med. 2006;21(1):65-67. https://doi.org/10.1111/j.1525-1497.2005.00292.x.
Appendix B - Sensitivity Analyses
Physical Activity Trajectories by Time-Varying PTSD Status Over the 20-Year Follow-up Period in the Full Sample
In analyses including walking at a ‘normal’ or ‘easy’ pace (<3 mph) and with exercise not winsorized at 25 hours/week, original results were slightly attenuated, but showed the same pattern. Relative to women without trauma, physical activity z-score decreased over time among women with trauma exposure and 4-5 (β=-1.85E-3, SE=0.91E-3; p=0.04) or 6-7 PTSD symptoms (β=-3.44E-3, SE=1.10E-3, p<0.01), in age-adjusted linear mixed models. Further adjustment for covariates did not meaningfully change results. Results were also similar in models adjusted for chronic disease history and physical impairment (4-5 symptoms, β=-3.0E-3, SE=0.97E-3, p<0.01; 6-7 PTSD symptoms, β=-5.26E-3, SE=1.17E-3, p<0.0001).
Physical Activity Trajectories Before and After Onset of Trauma/PTSD Symptoms Among the Subsample of Women Whose PTSD Symptoms Onset During Follow-up
In analyses including walking at a “normal” or “easy” pace (<3mph) and with exercise not winsorized at 25 hours/week, results were similar, with slightly greater effect sizes. After trauma/PTSD symptom onset, women with 6-7 PTSD symptoms had a steeper decrease in physical activity than trauma-exposed women with no PTSD symptoms, in minimally (β=-21.1E-3, SE=5.7E-3, p<0.01) and fully adjusted models.
Appendix Table 1. Linear Mixed Models of Yearly Change in Physical Activity z-Scores by PTSD Symptoms, 1989-2009 (Full Sample, N=50,327).
| Trauma/PTSD category | Model 1 a | Model 2 b | ||
|---|---|---|---|---|
| b value (SE) | p-value | b value (SE) | p-value | |
| No trauma | Ref | Ref | Ref | Ref |
| 0 PTSD sx | 0.33 ×10-3 (0.54 ×10-3) | 0.55 | 0.35 ×10-3 (0.57 ×10-3) | 0.57 |
| 1-3 PTSD sx | -0.36 ×10-3 (0.75 ×10-3) | 0.63 | -0.72 ×10-3 (0.77 ×10-3) | 0.35 |
| 4-5 PTSD sx | -2.24 ×10-3 (0.89 ×10-3) | 0.01* | -2.49 ×10-3 (0.95 ×10-3) | 0.007** |
| 6-7 PTSD sx | -5.71 ×10-3 (1.08 ×10-3) | <0.001*** | -6.71 ×10-3 (1.13 ×10-3) | <0.001*** |
Note: Boldface indicates statistical significance
p<0.05;
p<0.01;
p<0.001.
Adjusts for age at baseline.
Adjusts for age, race, parental education, adiposity, region of residence, depression, and anxiety
PTSD, posttraumatic stress disorder
Appendix Table 2. Linear Spline Mixed Models of Yearly Change in Physical Activity z-Scores, 1989-2009, Before and After Trauma/PTSD Symptom Onset (Subsample, N=15,353).
| Trauma/PTSD category | Model 1a | Model 2b | ||
|---|---|---|---|---|
| b value (SE) | p-value | b value (SE) | p-value | |
| Before onset | ||||
| 0 PTSD sx | Ref | Ref | Ref | Ref |
| 1-3 PTSD sx | -0.04 ×10-3 (1.43 ×10-3) | 0.98 | 0.48 ×10-3 (1.49 ×10-3) | 0.75 |
| 4-5 PTSD sx | -1.71 ×10-3 (1.60 ×10-3) | 0.28 | -1.64 ×10-3 (1.66 ×10-3) | 0.32 |
| 6-7 PTSD sx | 3.11 ×10-3 (2.04 ×10-3) | 0.13 | 3.25 ×10-3 (2.15 ×10-3) | 0.13 |
| After onset | ||||
| 0 PTSD sx | Ref | Ref | Ref | Ref |
| 1-3 PTSD sx | 0.16 ×10-3 (2.76 ×10-3) | 0.95 | 0.87 ×10-3 (2.89 ×10-3) | 0.76 |
| 4-5 PTSD sx | 0.42 ×10-3 (3.15 ×10-3) | 0.90 | 1.05 ×10-3 (3.28 ×10-3) | 0.75 |
| 6-7 PTSD sx | -16.14 ×10-3 (4.01 ×10-3) | <0.001 | -17.12 ×10-3 (4.21 ×10-3) | <0.001 |
Note: Boldface indicates statistical significance (p<0.001).
Adjusts for age at baseline.
Adjusts for age, race, parental education, adiposity, region of residence, depression, and anxiety
PTSD, posttraumatic stress disorder
Footnotes
The research presented in this paper is that of the authors and does not reflect the official policy of NIH.
Dr. Winning had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study design and concept: Winning, Koenen, Kubzansky; Acquisition, analysis, or interpretation of data: Winning, Gilsanz, Koenen, Chen, Roberts, Sumner, Rimm, Glymour, Kubzansky; Drafting of the manuscript: Winning Critical revision of the manuscript for important intellectual content: Winning, Gilsanz, Koenen, Chen, Roberts, Sumner, Rimm, Glymour, Kubzansky; Statistical analysis: Winning, Chen; Obtained funding: Koenen, Kubzansky; Administrative, technical, or material support: Gilsanz; Study supervision: Koenen, Kubzansky.
No financial disclosures were reported by the authors of this paper.
References
- 1.Sumner JA, Kubzansky LD, Elkind MS, et al. Trauma Exposure and Posttraumatic Stress Disorder Symptoms Predict Onset of Cardiovascular Events in Women. Circulation. 2015;132(4):251–259. doi: 10.1161/CIRCULATIONAHA.114.014492. https://doi.org/10.1161/CIRCULATIONAHA.114.014492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kubzansky LD, Bordelois P, Jun HJ, et al. The weight of traumatic stress: a prospective study of posttraumatic stress disorder symptoms and weight status in women. JAMA Psychiatry. 2014;71(1):44–51. doi: 10.1001/jamapsychiatry.2013.2798. https://doi.org/10.1001/jamapsychiatry.2013.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts AL, Agnew-Blais JC, Spiegelman D, et al. Posttraumatic stress disorder and incidence of type 2 diabetes mellitus in a sample of women: a 22-year longitudinal study. JAMA Psychiatry. 2015;72(3):203–210. doi: 10.1001/jamapsychiatry.2014.2632. https://doi.org/10.1001/jamapsychiatry.2014.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenbaum S, Stubbs B, Ward PB, Steel Z, Lederman O, Vancampfort D. The prevalence and risk of metabolic syndrome and its components among people with posttraumatic stress disorder: a systematic review and meta-analysis. Metabolism. 2015;64(8):926–933. doi: 10.1016/j.metabol.2015.04.009. https://doi.org/10.1016/j.metabol.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Vancampfort D, Rosenbaum S, Ward PB, et al. Type 2 Diabetes Among People With Posttraumatic Stress Disorder: Systematic Review and Meta-Analysis. Psychosom Med. 2016;78(4):465–473. doi: 10.1097/PSY.0000000000000297. https://doi.org/10.1097/PSY.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 6.Van der Kolk BA. The body keeps the score: brain, mind, and body in the healing of trauma. New York, NY: Penguin Books; 2014. [Google Scholar]
- 7.Olff M, Langeland W, Draijer N, Gersons BP. Gender differences in posttraumatic stress disorder. Psychol Bull. 2007;133(2):183–204. doi: 10.1037/0033-2909.133.2.183. https://doi.org/10.1037/0033-2909.133.2.183. [DOI] [PubMed] [Google Scholar]
- 8.WHO. Global health risks mortality and burden of disease attributable to selected major risks. 2009 http://site.ebrary.com/id/10363978.
- 9.Chomistek AK, Chiuve SE, Eliassen AH, Mukamal KJ, Willett WC, Rimm EB. Healthy lifestyle in the primordial prevention of cardiovascular disease among young women. J Am Coll Cardiol. 2015;65(1):43–51. doi: 10.1016/j.jacc.2014.10.024. https://doi.org/10.1016/j.jacc.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart AL, Hays RD, Wells KB, Rogers WH, Spritzer KL, Greenfield S. Long-term functioning and well-being outcomes associated with physical activity and exercise in patients with chronic conditions in the Medical Outcomes Study. J Clin Epidemiol. 1994;47(7):719–730. doi: 10.1016/0895-4356(94)90169-4. https://doi.org/10.1016/0895-4356(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 11.Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33(12):e147–167. doi: 10.2337/dc10-9990. https://doi.org/10.2337/dc10-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naci H, Ioannidis JP. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ. 2013;347:f5577. doi: 10.1136/bmj.f5577. https://doi.org/10.1136/bmj.f5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall KS, Hoerster KD, Yancy WS., Jr Post-traumatic stress disorder, physical activity, and eating behaviors. Epidemiol Rev. 2015;37(1):103–115. doi: 10.1093/epirev/mxu011. https://doi.org/10.1093/epirev/mxu011. [DOI] [PubMed] [Google Scholar]
- 14.de Assis MA, de Mello MF, Scorza FA, et al. Evaluation of physical activity habits in patients with posttraumatic stress disorder. Clinics (Sao Paulo) 2008;63(4):473–478. doi: 10.1590/S1807-59322008000400010. https://doi.org/10.1590/S1807-59322008000400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vancampfort D, Stubbs B. Physical activity and metabolic disease among people with affective disorders: Prevention, management and implementation. J Affect Disord. doi: 10.1016/j.jad.2016.07.042. In press. Online July 19, 2016. https://doi.org/10.1016/j.jad.2016.07.042. [DOI] [PubMed]
- 16.Vancampfort D, Stubbs B, Richards J, et al. Physical fitness in people with posttraumatic stress disorder: a systematic review. Disabil Rehabil. doi: 10.1080/09638288.2016.1226412. In press. Online September 15, 2016, https://doi.org/10.1080/09638288.2016.1226412. [DOI] [PubMed]
- 17.Ginzburg K, Ein-Dor T, Solomon Z. Comorbidity of posttraumatic stress disorder, anxiety and depression: a 20-year longitudinal study of war veterans. J Affect Disord. 2010;123(1-3):249–257. doi: 10.1016/j.jad.2009.08.006. https://doi.org/10.1016/j.jad.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Breslau N, Davis GC, Peterson EL, Schultz L. Psychiatric sequelae of posttraumatic stress disorder in women. Arch Gen Psychiatry. 1997;54(1):81–87. doi: 10.1001/archpsyc.1997.01830130087016. https://doi.org/10.1001/archpsyc.1997.01830130087016. [DOI] [PubMed] [Google Scholar]
- 19.Scarapicchia TM, Sabiston CM, O'Loughlin E, Brunet J, Chaiton M, O'Loughlin JL. Physical activity motivation mediates the association between depression symptoms and moderate-to-vigorous physical activity. Prev Med. 2014;66:45–48. doi: 10.1016/j.ypmed.2014.05.017. https://doi.org/10.1016/j.ypmed.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Azevedo Da Silva M, Singh-Manoux A, Brunner EJ, et al. Bidirectional association between physical activity and symptoms of anxiety and depression: the Whitehall II study. Eur J Epidemiol. 2012;27(7):537–546. doi: 10.1007/s10654-012-9692-8. https://doi.org/10.1007/s10654-012-9692-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zen AL, Whooley MA, Zhao S, Cohen BE. Post-traumatic stress disorder is associated with poor health behaviors: findings from the heart and soul study. Health Psychol. 2012;31(2):194–201. doi: 10.1037/a0025989. https://doi.org/10.1037/a0025989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan CA, 3rd, Hazlett G, Wang S, Richardson EG, Jr, Schnurr P, Southwick SM. Symptoms of dissociation in humans experiencing acute, uncontrollable stress: a prospective investigation. Am J Psychiatry. 2001;158(8):1239–1247. doi: 10.1176/appi.ajp.158.8.1239. https://doi.org/10.1176/appi.ajp.158.8.1239. [DOI] [PubMed] [Google Scholar]
- 23.Schnurr PP, Lunney CA, Sengupta A, Spiro A., 3rd A longitudinal study of retirement in older male veterans. J Consult Clin Psychol. 2005;73(3):561–566. doi: 10.1037/0022-006X.73.3.561. https://doi.org/10.1037/0022-006X.73.3.561. [DOI] [PubMed] [Google Scholar]
- 24.Schnurr PP, Spiro A, Vielhauer MJ, Findler MN, Hamblen JL. Trauma in the Lives of Older Men: Findings from the Normative Aging Study. Journal of Clinical Geropsychology. 2002;8(3):175–187. https://doi.org/10.1023/A:1015992110544. [Google Scholar]
- 25.Breslau N, Peterson EL, Kessler RC, Schultz LR. Short screening scale for DSM-IV posttraumatic stress disorder. Am J Psychiatry. 1999;156(6):908–911. doi: 10.1176/ajp.156.6.908. https://doi.org/10.1176/ajp.156.6.908. [DOI] [PubMed] [Google Scholar]
- 26.Knauper B, Cannell CF, Schwarz N, Bruce ML, Kessler RC. Improving accuracy of major depression age-of-onset reports in the U.S. National Comorbidity Survey. Int J Methods Psychiatr Res. 1999;8(1):39–48. https://doi.org/10.1002/mpr.55. [Google Scholar]
- 27.Lee YC, Agnew-Blais J, Malspeis S, et al. Post-Traumatic Stress Disorder and Risk for Incident Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2016;68(3):292–298. doi: 10.1002/acr.22683. https://doi.org/10.1002/acr.22683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimerling R, Ouimette P, Prins A, et al. Brief report: Utility of a short screening scale for DSM-IV PTSD in primary care. J Gen Intern Med. 2006;21(1):65–67. doi: 10.1111/j.1525-1497.2005.00292.x. https://doi.org/10.1111/j.1525-1497.2005.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–999. doi: 10.1093/ije/23.5.991. https://doi.org/10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 30.Hu FB, Stampfer MJ, Colditz GA, et al. Physical activity and risk of stroke in women. JAMA. 2000;283(22):2961–2967. doi: 10.1001/jama.283.22.2961. https://doi.org/10.1001/jama.283.22.2961. [DOI] [PubMed] [Google Scholar]
- 31.Hu FB, Sigal RJ, Rich-Edwards JW, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA. 1999;282(15):1433–1439. doi: 10.1001/jama.282.15.1433. https://doi.org/10.1001/jama.282.15.1433. [DOI] [PubMed] [Google Scholar]
- 32.Magnusson C, Baron J, Persson I, et al. Body size in different periods of life and breast cancer risk in post-menopausal women. Int J Cancer. 1998;76(1):29–34. doi: 10.1002/(sici)1097-0215(19980330)76:1<29::aid-ijc6>3.0.co;2-#. https://doi.org/10.1002/(SICI)1097-0215(19980330)76:1<29∷AID-IJC6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 33.Must A, Willett WC, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. Am J Epidemiol. 1993;138(1):56–64. doi: 10.1093/oxfordjournals.aje.a116777. [DOI] [PubMed] [Google Scholar]
- 34.Crown S, Crisp AH. A short clinical diagnostic self-rating scale for psychoneurotic patients. The Middlesex Hospital Questionnaire (M.H.Q.) Br J Psychiatry. 1966;112(490):917–923. doi: 10.1192/bjp.112.490.917. https://doi.org/10.1192/bjp.112.490.917. [DOI] [PubMed] [Google Scholar]
- 35.Rutter LA, Weatherill RP, Krill SC, Orazem R, Taft CT. Posttraumatic stress disorder symptoms, depressive symptoms, exercise, and health in college students. Psychol Trauma. 2013;5(1):56–61. https://doi.org/10.1037/a0021996. [Google Scholar]
- 36.Vancampfort D, Richards J, Stubbs B, et al. Physical Activity in People With Posttraumatic Stress Disorder: A Systematic Review of Correlates. J Phys Act Health. 2016;13(8):910–918. doi: 10.1123/jpah.2015-0436. https://doi.org/10.1123/jpah.2015-0436. [DOI] [PubMed] [Google Scholar]
- 37.Vujanovic AA, Farris SG, Harte CB, Smits JA, Zvolensky MJ. Smoking Status and Exercise in relation to PTSD Symptoms: A Test among Trauma-Exposed Adults. Ment Health Phys Act. 2013;6(2):132–138. doi: 10.1016/j.mhpa.2012.12.001. https://doi.org/10.1016/j.mhpa.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Afari N, Ahumada SM, Wright LJ, et al. Psychological trauma and functional somatic syndromes: a systematic review and meta-analysis. Psychosom Med. 2014;76(1):2–11. doi: 10.1097/PSY.0000000000000010. https://doi.org/10.1097/PSY.0000000000000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. JAMA. 2000;284(20):2606–2610. doi: 10.1001/jama.284.20.2606. https://doi.org/10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 40.Mills KL, Teesson M, Ross J, Peters L. Trauma, PTSD, and substance use disorders: findings from the Australian National Survey of Mental Health and Well-Being. Am J Psychiatry. 2006;163(4):652–658. doi: 10.1176/ajp.2006.163.4.652. https://doi.org/10.1176/ajp.2006.163.4.652. [DOI] [PubMed] [Google Scholar]
- 41.Leskin GA, Woodward SH, Young HE, Sheikh JI. Effects of comorbid diagnoses on sleep disturbance in PTSD. J Psychiatr Res. 2002;36(6):449–452. doi: 10.1016/s0022-3956(02)00025-0. https://doi.org/10.1016/S0022-3956(02)00025-0. [DOI] [PubMed] [Google Scholar]
- 42.Baron KG, Reid KJ, Zee PC. Exercise to improve sleep in insomnia: exploration of the bidirectional effects. J Clin Sleep Med. 2013;9(8):819–824. doi: 10.5664/jcsm.2930. https://doi.org/10.5664/jcsm.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moffitt TE, Caspi A, Taylor A, et al. How common are common mental disorders? Evidence that lifetime prevalence rates are doubled by prospective versus retrospective ascertainment. Psychol Med. 2010;40(6):899–909. doi: 10.1017/S0033291709991036. https://doi.org/10.1017/S0033291709991036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soundy A, Roskell C, Stubbs B, Vancampfort D. Selection, use and psychometric properties of physical activity measures to assess individuals with severe mental illness: a narrative synthesis. Arch Psychiatr Nurs. 2014;28(2):135–151. doi: 10.1016/j.apnu.2013.12.002. https://doi.org/10.1016/j.apnu.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 45.Rosenbaum S, Tiedemann A, Sherrington C, van der Ploeg HP. Assessing physical activity in people with posttraumatic stress disorder: feasibility and concurrent validity of the International Physical Activity Questionnaire--short form and actigraph accelerometers. BMC Res Notes. 2014;7:576. doi: 10.1186/1756-0500-7-576. https://doi.org/10.1186/1756-0500-7-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sattelmair J, Pertman J, Ding EL, Kohl HW, 3rd, Haskell W, Lee IM. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation. 2011;124(7):789–795. doi: 10.1161/CIRCULATIONAHA.110.010710. https://doi.org/10.1161/CIRCULATIONAHA.110.010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kohl HW., 3rd Physical activity and cardiovascular disease: evidence for a dose response. Med Sci Sports Exerc. 2001;33(6 Suppl):S472–483. doi: 10.1097/00005768-200106001-00017. https://doi.org/10.1097/00005768-200106001-00017. [DOI] [PubMed] [Google Scholar]
- 48.Oja P. Dose response between total volume of physical activity and health and fitness. Med Sci Sports Exerc. 2001;33(6 Suppl):S428–437. doi: 10.1097/00005768-200106001-00011. https://doi.org/10.1097/00005768-200106001-00011. [DOI] [PubMed] [Google Scholar]
- 49.Lee IM, Skerrett PJ. Physical activity and all-cause mortality: what is the dose-response relation? Med Sci Sports Exerc. 2001;33(6 Suppl):S459–471. doi: 10.1097/00005768-200106001-00016. https://doi.org/10.1097/00005768-200106001-00016. [DOI] [PubMed] [Google Scholar]
- 50.Haennel RG, Lemire F. Physical activity to prevent cardiovascular disease. How much is enough? Can Fam Physician. 2002;48:65–71. [PMC free article] [PubMed] [Google Scholar]
- 51.Ross R, Janssen I. Physical activity, total and regional obesity: dose-response considerations. Med Sci Sports Exerc. 2001;33(6 Suppl):S521–527. doi: 10.1097/00005768-200106001-00023. https://doi.org/10.1097/00005768-200106001-00023. [DOI] [PubMed] [Google Scholar]
- 52.Levine AB, Levine LM, Levine TB. Posttraumatic stress disorder and cardiometabolic disease. Cardiology. 2014;127(1):1–19. doi: 10.1159/000354910. https://doi.org/10.1159/000354910. [DOI] [PubMed] [Google Scholar]
- 53.Rosenbaum S, Vancampfort D, Steel Z, Newby J, Ward PB, Stubbs B. Physical activity in the treatment of Post-traumatic stress disorder: A systematic review and meta-analysis. Psychiatry Res. 2015;230(2):130–136. doi: 10.1016/j.psychres.2015.10.017. https://doi.org/10.1016/j.psychres.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 54.Chiuve SE, McCullough ML, Sacks FM, Rimm EB. Healthy lifestyle factors in the primary prevention of coronary heart disease among men: benefits among users and nonusers of lipid-lowering and antihypertensive medications. Circulation. 2006;114(2):160–167. doi: 10.1161/CIRCULATIONAHA.106.621417. https://doi.org/10.1161/CIRCULATIONAHA.106.621417. [DOI] [PubMed] [Google Scholar]


