Abstract
4-Hydroxy-2-nonenal (HNE), one of the major α, β-unsaturated aldehydes produced during lipid peroxidation, is a potent messenger in mediating signaling pathways. Lipid peroxidation and HNE production appear to increase with aging. Although the cause and effect relation remains arguable, aging is associated with significant changes in diverse signaling events, characterized by enhanced or diminished responses of specific signaling pathways. In this review we will discuss how HNE may contribute to aging-related alterations of signaling pathways.
Keywords: 4-Hydroxynoneal, HNE, aging, redox signaling, oxidative stress
Graphical abstract
Introduction
4-hydroxy-2-nonenal (HNE) is a major α, β-unsaturated aldehyde derived from the decomposition of peroxidation products of omega-6 polyunsaturated fatty acids; i.e., arachidonic acid and linoleic acid [1–5]. As a consequence of the natural occurrence of lipid peroxidation in aerobic biologic systems, HNE is present, at low concentrations, in almost all cells and tissue fluids under physiological condition. A marked increase of HNE concentration is usually observed during oxidative stress. By forming adducts with proteins, nucleic acids, and lipids, HNE causes dysfunction of targeted biological molecules and is implicated in various diseases, including Alzheimer disease [6–8], cancers [9], chronic obstructive pulmonary disease (COPD) [10], and cardiovascular diseases [11], etc. [12, 13]. Therefore, HNE is recognized as one of the key culprits in cell and tissue damage caused by oxidative stress.
In contrast, numerous studies have shown that at physiological or slightly greater concentrations, HNE acts as a potent mediator that regulates a variety of signaling pathways [14, 15] and cellular processes [12]. The signaling effect of HNE originates from its ability to form adducts with proteins involved in signal transduction and gene expression, including receptors, kinases, phosphatases, and transcription factors [16]. Since it was first proposed by Harman that oxidant (free radical) mediated damage of macromolecules causes aging [17], this idea has remained a major theme in aging research, although it has been strongly questioned in terms of its relative contribution to aging per se and is more likely a participant in age-related diseases. Certainly, increased accumulation of oxidatively modified macromolecules and chronic oxidative/elephophilic state in the elderly [18–22] has been well documented, but accumulation of damaged material may not equate with actual injury. Rather, the involvement of oxidants in aging is far more complex than simple accumulation of oxidized macromolecules [23] and more likely involves disruption of redox signaling and oxidative metabolism [21]. Based on a recent proposal for the dynamic regulation of redox homeostasis and maintenance of oxidant/antioxidant balance [24], we suggest that chronic oxidative stress in aging results in an new antioxidant/electrophile steady state and age-adjusted redox homeostasis that is oxidized compared with that of youth. The signaling mechanisms establishing this new more oxidized steady state poorly adjust to additional stress. Fitting the older paradigm of the free radical theory of Harmann, HNE derived from lipid peroxidation and HNE-modified proteins inevitably increase during aging and are frequently used as a marker of aging-related oxidative stress [22]. But, in regard to the newer proposal that redox signaling and homeostasis are abnormal in aging, the potential role of HNE as a signaling mediator in age-related signaling events has not been given much consideration. Therefore, while the involvement of HNE in age-related pathologies has been discussed by many excellent reviews [6–10, 25–28], here we will discuss the role that HNE may play in altering signaling pathways during aging.
Increased HNE concentration in aging
Under physiologic conditions, HNE is present at very low concentration in plasma, in the range of 0.28–0.68 μM [1, 3, 29], but its concentration in cells, where it is produced, is higher (≤ 5 μM) [5]. Under oxidative stress, HNE concentration can be markedly increased as much as by 100 times [3]. Although it has been well recognized that lipid peroxidation increases with aging [30, 31], data on the change in HNE concentration in aging are limited.
Studies on flies and animals suggest that HNE concentrations, either free HNE or as protein adducts, increase during aging. Zheng et al. measured HNE-adduct accumulation in aging in fruit flies using an ELISA method and found that HNE adduct concentrations remained relatively unchanged during the first half of adult life and then significantly increased by about 2 fold. After reaching a peak, HNE adducts appeared to decrease later in life of flies [32]. The change in HNE in aging in mammals seems more consistent. Using a gas chromatography-mass spectrometry method, Asselin et al. assessed the change of protein-bound HNE in the blood of aging rats (Wistar rats at 7, 15, 22, and 30 weeks of age) and found that the concentration of protein-bound HNE in the blood was significantly increased with aging [33]. In a similar study, Kim et al. showed that HNE adduction to serum proteins was significantly increased by 2–3 fold in old Fischer 344 rats, and free HNE was increased from about 0.3 μM to 0.7 μM (7 months vs 24 months) [34]. The increase of HNE-protein adducts in old animals is supported by others, as observed in the heart of rats (6 months vs 30 months) [35, 36] and bone of mice (6 months vs 25 months) [37].
The change in HNE concentration in aging is less studied in humans. Gil et al. measured HNE and other oxidative stress markers in the blood and plasma of 194 healthy human of ages from 18 to 84 years and found that the concentrations of HNE and other oxidative markers were all increased during aging. Plasma HNE concentration was increased from 68.9 ± 15.0 nmol/L in the young group (up to 30 yr old) to 107.4 ± 27.3 nmol/L in the elderly group (older than 70 yr) [38]. On the other hand, there are many studies on the increased lipid peroxidation with human aging. In these studies, the questionable thiobarbituric acid test, but also the more reliable measurement of protein carbonyls, have been used as indicators of lipid peroxidation. As a principle lipid peroxidation derivative, it is inferred that HNE concentration also increases with aging. Nonetheless, more direct determination of free HNE or its adduct concentration in elderly will help to further understanding the biological effects of HNE in aging.
Aging-related signaling pathways affected by HNE
Aging is characterized by the manifestation of a systematic decline in functions. As a fundamental mechanism underlying most cellular responses and functions, signal transduction also varies in aging. But, whether these changes in signaling are a cause or effect in the aging process remains uncertain. Here we provide several examples to show that HNE may play a potential role in these age-related signaling pathways.
NF-κB signaling
The immune system in the elderly becomes increasingly dysfunctional and the immune response to infectious pathogen and vaccine is blunted [39]. On the other hand, aging is usually accompanied by low-grade chronic inflammation characterized by elevated plasma concentrations of proinflammatory cytokines and acute phase proteins, such as TNFα, IL-6, and C-reactive protein [40–43]. In agreement with this, the expression and activity of the central key player in cytokine regulation and inflammatory response, NF-κB, is increased in cells/tissues from elderly adults [44]. Thus, so-called “inflammaging” is assumed to be the culprit in many age-associated diseases [45, 46].
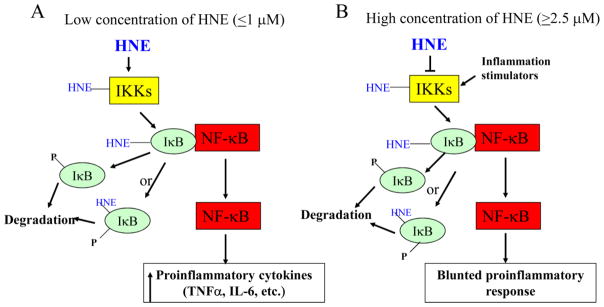
The NF-κB signaling pathway can either be activated or inhibited by HNE, depending on concentration (Fig. 1). At low concentrations, HNE could activate NF-κB signaling pathway via activating IKK. Amma et al. reported that 0.1–1 μM HNE activated IKK and thus increased NF-κB activity via forming adducts with IKK and IκB in human fibroblast cells but, 2.5 μM HNE inhibited IKKs [47]. Similarly at low concentration (1μM), HNE activated IKK and increased NF-κB activity in raw 246.7 cells [48] and vascular smooth muscle cells [49, 50]. In contrast, in studies using HNE concentrations higher than 5 μM (in the range of 5–50 μM), IκBα degradation [51] and phosphorylation [52] were prevented, possibly through inhibition of IKKs [53, 54]. As a result, the basal and inducible NF-κB activity was inhibited [55–59]. Conjugation and inhibition of either IKKα [53] or IKKβ [55] was reported. However, there was controversial report that at high concentration (15 μM) HNE increased IKK phosphorylation and activation, and IkBα degradation, and thus increased NF-κB activation in rat prostate endothelial cells [60]. In summary, the ability of HNE to activate NF-κB signaling at concentration as low as that found in the plasma of elderly suggests a potential role of HNE in the age-related increase of NF-κB activity and proinflammatory cytokines.
Figure 1.
Regulation of NF-κB signaling by HNE. At low concentration (0.1–1 μM) HNE activates NF-κB signaling through conjugating with IKKs (IKKα and IKKβ) and IκB, and increases the expression of proinflammatory cytokines (A). While at high concentration (2.5–50 μM), HNE inhibits IKKs, resulting in the repression of basal and inducible activity of NF-κB signaling (B).
Nrf2 signaling
Nrf2 is a transcription factor involved in the regulation of a large number of antioxidant and detoxification enzymes, and plays a key role in the adaptation response to oxidative stress and maintenance of cellular redox homeostasis. The regulation of Nrf2 signaling involves multiple signaling molecules including KEAP1, p21, p63, PKCs, MAPKs, etc., as discussed in a recent review [61]. Accumulating evidence suggests that the induction of Nrf2 signaling declines in aging, while its basal activity was increased in some animal tissues in aging [61]. Although being extensively studied, the underlying mechanism of aging-related variation of Nrf2 signaling remains elusive.
Studies have found that HNE can activate Nrf2 signaling at concentrations ≤ 0.3 μM [62–64], through activating multiple signaling pathways including atypical protein kinase C iota (aPKCι) [65], phosphatidylinositol 3-kinase (PI3K) [63], and mitogen activated proteine kinases (MAPKs) [66, 67]. This suggests how HNE may be involved in the increase of basal Nrf2 signaling in the elderly but not in the decline of Nrf2 signaling response to stimuli in aging.
AKT/PKB signaling
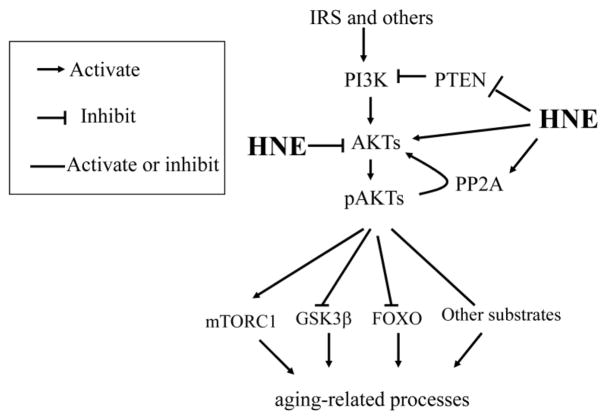
AKT, which is also called protein kinase B (PKB), is a central player in processes downstream of activated growth factor receptor signaling such as insulin and epidermal growth factor receptors, and plays important roles in various processes including cell survival, cell growth, apoptosis, protein synthesis, energy metabolism, and oncogenesis [68, 69]. In the canonical pathway, AKT activation occurs sequentially in the order of binding of growth factors to tyrosine kinase receptors, activation of PI3K, and increased phosphatidylinositol (3,4,5)-trisphosphate (PIP3)-AKT activation. All three highly conserved AKT isoforms (AKT1, AKT2 and AKT3) are activated by the same mechanism. In addition, AKT can also be regulated through a redox-dependent mechanism [70]. In this non-canonical pathway, AKT signaling is regulated through oxidative modification of signaling molecules involved in AKT activation, including PI3K, PTEN, and AKT itself (Fig. 2).
Figure 2.
HNE mediates AKT signaling. HNE could either activate AKT, through inhibiting PTEN and activating PI3K, or inhibiting AKT, through forming an HNE-AKT adduct or activating PP2A, a phosphatase that dephosphorylates pAKT. HNE may interfere with insulin-activated AKT signaling. Thus the final effect of HNE on AKT signaling may vary depending on cell type or other factors. Active AKT regulates the downstream targets involved in aging processes. IRS, insulin receptor substrate; GSK3β, glycogen synthase kinas 3 beta; FOXO, Forkhead box O; PP2A, Protein phosphatase 2.
AKT plays critical roles in aging-related processes through phosphorylating and regulating the downstream substrates including mammalian target of rapamycin (mTOR) (activation), glycogen synthase kinase beta (GSK3β) (inactivation) and Forkhead box O (FOXO) (inactivation) (Fig. 2), as discussed in a recent review [71]. Accumulating evidence suggests that AKT signaling changes with aging and the variation seems tissue dependent [72]. For example, AKT phosphorylation (at Ser473) and activity were significantly declined in the elderly in mice hippocampus (6 months vs 20 months) [73], skeletal muscle of human [74], but increased the old in rat soleus muscles (6 months vs 33 months) [75] and hypothalamus (6 months vs 24 months) [76].
Many studies have investigated the effect of HNE on AKT in diverse cell models and with a wide range of HNE concentration (Table 1). HNE decreased AKT phosphorylation and activity in a wide concentration range from 5–100 μM in MG63 human osteosarcoma cells [77], 3T3-L1 adipocytes [78], human OA chondrocytes [79] and Jurkat cells [80]. In contrast, increased AKT phosphorylation was observed in vascular smooth muscle cells (1μM) [50], PC12 cells (15 μM) [81], human neuroblastoma IMR-32 cells (10 μM) [82], human corneal epithelial cells (30μM) [83] and retinal pigment epithelial (RPE) cells (0.1–5 μM) [84]. In rat slow-twitch skeletal muscle cells however, HNE did not affect AKT phosphorylation and activity [85]. This evidence indicates that the regulation of HNE on AKT activity is cell dependent. However, it remains unclear what underlies the different regulatory effects of HNE on AKT.
Table 1.
Differential regulation of HNE on AKT signaling
| Signaling molecules | HNE concentration (μM) | Effect | Cells | Reference |
|---|---|---|---|---|
| AKT and PP2A | 20 in 10% FBS medium | Decreased AKT phosphorylation and inhibited its activity via activating PP2A | Jurkat cells | [80] |
| PI3K/AKT | 10 in 10% FBS medium | Activated | Human neuroblastoma IMR-32 cells | [82] |
| AKT | 15 in 10% FBS medium | Activated | PC12 cells | [81] |
| PI3K/AKT | 25–100 μM in 0.2% serum free medium | Inhibited | 3T3-L1 adipocytes | [78] |
| PI3K/AKT | 1 μM in 10% FBS medium | Activated | Vascular smooth muscle cells | [50] |
| AKT | 30 μM in 2% FBS medium | Inhibited | Human OA chondrocytes | [79] |
| AKT1 and AKT2 | 100 μM in serum free medium | Increased AKT2 phosphorylation via inhibiting PTEN, but inhibit its activity via forming AKT-HNE adduct. No effect on AKT1 | HepG2 cells | [87] |
| AKT and PTEN | 12.5–100 μM in serum free medium | Increased AKT phosphorylation at Ser473 and thr308, conjugated and decreased PTEN phosphorylation and activity | HepG2 cells and primary rat hepatocyte | [119] |
| AKT | 0.1–5 μM in 10% FBS medium | Increased AKT phosphorylation | Retinal pigment epithelial (RPE) cells | [84] |
| AKT1 | 25–100 μM in serum free medium | Inhibited AKT1 phosphorylation but not AKT2, and inhibited AKT activity by forming carbonyl AKT1 adduct | HepG2 cells | [86] |
| PI3K/AKT | 30 μM in 5% FBS medium | Activated | Human corneal epithelial cells | [83] |
| AKT | 5–50 μM in 10% FBS medium | Inhibited AKT activity | MG63 human osteosarcoma cells | [77] |
| AKT | 50 μM in 10% FBS medium | No effect on AKT phosporylation at Ser473 | Rat slow-twitch skeletal muscle | [85] |
HNE could regulate AKT activity directly through forming adducts. Shearn et al. investigated the regulation of HNE on AKT1 and AKT2 in HePG2 cells and found that both AKT1 and AT2 could form adducts with HNE, and as a result, the activity of AKT1 or AKT2 was inhibited [86][87]. Interestingly total AKT phosphorylation (Ser473) was increased in both cases. There is evidence that the overall effect of HNE on AKT signaling may be an integrated result of activation via canonical pathway (PI3K activation), dephosphorylation by PTEN and PP2A, and HNE conjugation (inhibition) [80, 86, 87] (Fig. 2). The role of AKT3 in this regulation is unknown.
In summary, HNE may affect age-related AKT signaling in a complex manner (Fig. 2). Since most studies on AKT signaling regulation used much higher HNE concentrations than physiological concentration, the exact role of HNE in the change of AKT signaling with aging in vivo needs to be further elucidated. Further studies on how HNE affects the AKT signaling initiated by insulin are expected, as recent report that HNE inhibited the response of AKT to insulin and H2O2 [86].
mTOR signaling
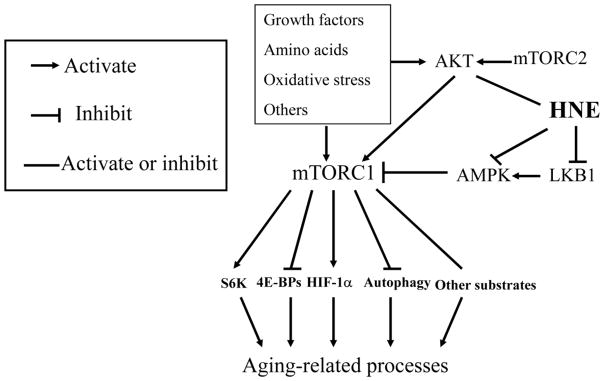
Mechanistic target of rapamycin (mTOR) has been being extensively studied as a key modulator of aging and aging-related diseases, and its role as a key regulatory nexus of modulating anabolic processes versus catabolic processes and involvement in aging-related processes have been well discussed by many [88, 89]. The signaling network of mTOR is described briefly here. mTOR is a serine/threonine protein kinase of the PI3K-related family that functions in two distinct complexes: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) (Fig. 3) [88], and the former is much more extensively studied than the latter. Upstream, mTORC1 can be activated by growth factors such as insulin, and environmental nutrients such as amino acids, and oxidative stress, or repressed by AMP-activated protein kinase (AMPK), a key sensor of cellular energy status. Downstream, mTORC1 phosphorylates and regulates a diverse substrates including ribosomal protein S6 kinases (S6K), NF-κB, hypoxia inducible factor 1 alpha (HIF-1α), etc. (Fig. 3), thus promotes cell growth and proliferation, lipid synthesis, and modulates mitochondria function and autophagy [90–92]. In contrast, mTORC2 is less studied, and available evidence suggests it is regulated by growth factors and primarily involved in cytoskeleton assembly and cell size modulation [93, 94]. As feedback regulation, S6K could inhibit mTORC1 pathway through phosphorylating and inhibiting insulin receptor (IRS)/PI3K/AKT signaling and thus benefit longevity processes. On the other hand, mTORC2 could activate AKT pathway and enhance aging. The actual situation is much more complex as interactions occur among signaling networks.
Figure 3.
HNE is involvement in mTOR signaling. HNE could conjugate and inhibit LKB1, thus inhibiting AMPK activity, and subsequently leading to the activation of mTORC1 and regulation of its downstream targets that are involved in aging processes. HNE could also either activate or inhibit mTORC1 through regulating AKT signaling as illustrated in Fig. 2. S6K, ribosome S6 kinase; 4E-BPs, translation initiation factor 4E-binding proteins; HIF-1α, hypoxia inducible factor 1alpha.
HNE could modulate mTOR-signaling network via acting on several targets. Besides modulating AKT signaling to mTOR as discussed above (Fig. 2), HNE could also regulate mTOR signaling through liver kinase B1 (LKB1)-AMPK-mTORC1 pathway (Table 2, Fig. 3).
Table 2.
Regulation of HNE on mTOR signaling molecules
| Signaling molecules | HNE concentration (μM) | Effect | Cells | Reference |
|---|---|---|---|---|
| LKB1 and AMPK | N/A | Conjugated with LKB1, and inhibited LKB1 and AMPK activity | MCF-7 and RKO cells | [120] |
| LKP1 and AMPK | 40 μM in 10% FBS medium | Conjugated and inhibited LKB1; inhibited AMPK activity | Cardiomyocytes | [95] |
| AMPK | 10–30 μM in 10% FBS medium | Inhibited AMPKα activity via decreasing its phosphorylation at Thr172 | Human retinal pigment epithelium cell line ARPE19 | [100] |
| LKB1 and AMPK | 20 μM in 10% FBS medium | Decreased total and phosphorylated LKB1, and inhibited AMPK activity | Primary Mouse cardiomyocytes | [96] |
| LKB1 | 1–40 μM in 10% FBS medium | Conjugated with LKB1 and inhibited its activity | HEK293T cells | [97] |
| LKB1 | 10 μM in 10% FBS medium | Conjugated with LKB1 and inhibited its activity; inhibited AMPK activity, increased mTOR–p70S6K–RPS6 signaling | Rat ventricular cardiomyocytes | [98] |
| AMPK | 10 and 30 μM in 10% FBS medium | Inhibited AMPK activity | 3T3-L1 adipocyte | [99] |
| LKB1 | N/A | Increase of HNE-LKB1 adducts | Mouse heart tissue | [121] |
LKB1, also called serine/threonine kinase 11, is kinase that phosphorylates and activates AMPK, a central metabolic sensor that regulates lipid, cholesterol and glucose metabolism. Dolinsky et al. first reported that HNE (40 μM) could conjugate with LKB1 and repress its activity, and inhibit its downstream substrate AMPK activity, and thus result in the activation of mTOR/p70S6 kinas pathway in isolated cardiomyocytes [95]. This finding was supported by other studies, which demonstrated that LKB1 was inhibited by HNE in various cell models, including primary mouse cardiomyocytes (20 μM) [96], HEK293T cells (1–40 μM) [97], and rat ventricular cardiomyocytes (10 μM) [98]. In these studies, HNE-LKB1 adducts were detected and total LKB1 decreased, and this was postulated as the mechanism of LKB1 inhibition. HNE inhibition on LKB1 would suppress subsequent AMPK activity, and thus activate mTORC1 signaling. However, this hypothesis is challenged by a recent study, in which LKB1 knockout did not replicate the effect of HNE on mTORC1-S6K-RPS6 signaling [98]. It is suggested that HNE may activate mTORC1 signaling through direct inhibition of AMPK. Although AMPK activity was inhibited by HNE [95, 96, 99, 100], direct evidence of HNE-AMPK1 adducts has not been demonstrated.
Other age-related signaling
The aging process is accompanied by variation in multiple signaling pathways that have been less examined. P21, a protein implicated in cell cycle arrest and senescence [101, 102], was increased by HNE at concentration of as low as 1 μM [103, 104] in p53-dependent- [103, 105] or p53-independent manner [106]. The activity of both 20S proteasome and telomerase declines with aging and are implicated in the aging processes. Both 20S proteasome and telomerase were reportedly inhibited by HNE [107, 108]. It is expected that HNE with its many targets will be shown to alter additional signaling and other enzymatic activities associated with aging as work continues in this field.
Summary and future prospective
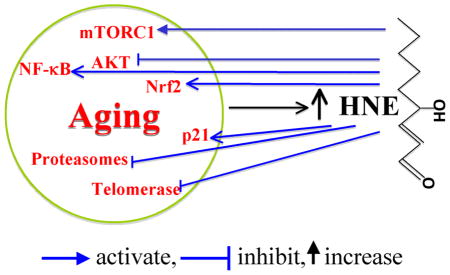
HNE concentration (in free or adduct form) increases with aging. Given its modification of multiple signaling molecules and its meditation of a wide range of signaling pathways, HNE is involved in aging-related signaling pathways through multiple entry points. In this article, we discussed the potential contribution of HNE to NF-κB, AKT, Nrf2, and mTOR, four signaling pathways that are implicated in aging processes (Fig. 4). Although not yet specifically examined, other signaling pathways involved in aging, such as that related to growth factor signaling EGFR [109–114], PDGFR [115–117], and others [118], are also mediated by HNE. It is noted that most of the evidence cited here is based on cell models and further evidence with model organisms would help elucidate the role of HNE in aging.
Figure 4.
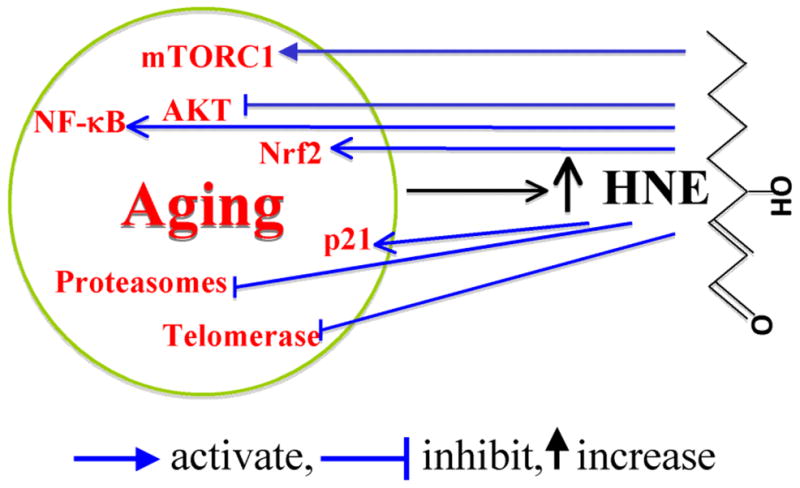
A summary of HNE involvement in aging-related signaling alteration. HNE may contribute to the aging process through activating or inhibiting aging-related signaling. It should be noted that HNE might have a dual effect on some signaling molecules such as AKT, as discussed in the text.
Highlights.
HNE concentration in the plasma and cells/tissues increases with aging
HNE regulates NF-κB signaling in concentration-dependent manner
HNE contributes to aging-related decline in Nrf2 signaling response
HNE causes aging-related variation of AKT signaling via PTEN and others.
HNE alters aging-related mTORC1 signaling through LKB1, AMPK and others.
Acknowledgments
This work was supported by NIH grant ES023864.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic Biol Med. 2009;47:469–484. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 2.Semchyshyn HM. Reactive carbonyl species in vivo: generation and dual biological effects. Scientific World Journal. 2014;2014:417842. doi: 10.1155/2014/417842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 4.Spickett CM. The lipid peroxidation product 4-hydroxy-2-nonenal: Advances in chemistry and analysis. Redox Biol. 2013;1:145–152. doi: 10.1016/j.redox.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaur RJ, Siems W, Bresgen N, Eckl PM. 4-Hydroxy-nonenal-A Bioactive Lipid Peroxidation Product. Biomolecules. 2015;5:2247–2337. doi: 10.3390/biom5042247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lovell MA, Ehmann WD, Mattson MP, Markesbery WR. Elevated 4-hydroxynonenal in ventricular fluid in Alzheimer’s disease. Neurobiology of aging. 1997;18:457–461. doi: 10.1016/s0197-4580(97)00108-5. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda M, Kanou F, Shimada N, Sawabe M, Saito Y, Murayama S, Hashimoto M, Maruyama N, Ishigami A. Elevated levels of 4-hydroxynonenal-histidine Michael adduct in the hippocampi of patients with Alzheimer’s disease. Biomed Res. 2009;30:227–233. doi: 10.2220/biomedres.30.227. [DOI] [PubMed] [Google Scholar]
- 8.Bradley MA, Markesbery WR, Lovell MA. Increased levels of 4-hydroxynonenal and acrolein in the brain in preclinical Alzheimer disease. Free Radic Biol Med. 2010;48:1570–1576. doi: 10.1016/j.freeradbiomed.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong H, Yin H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: focusing on mitochondria. Redox Biol. 2015;4:193–199. doi: 10.1016/j.redox.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu H, Xu JY. The role of 4-hydroxynonenal in assessment of chronic obstructive pulmonary disease severity. Zhonghua Jie He He Hu Xi Za Zhi. 2012;35:758–761. [PubMed] [Google Scholar]
- 11.Leonarduzzi G, Chiarpotto E, Biasi F, Poli G. 4-Hydroxynonenal and cholesterol oxidation products in atherosclerosis. Mol Nutr Food Res. 2005;49:1044–1049. doi: 10.1002/mnfr.200500090. [DOI] [PubMed] [Google Scholar]
- 12.Shoeb M, Ansari NH, Srivastava SK, Ramana KV. 4-Hydroxynonenal in the pathogenesis and progression of human diseases. Curr Med Chem. 2014;21:230–237. doi: 10.2174/09298673113209990181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Csala M, Kardon T, Legeza B, Lizak B, Mandl J, Margittai E, Puskas F, Szaraz P, Szelenyi P, Banhegyi G. On the role of 4-hydroxynonenal in health and disease. Biochim Biophys Acta. 2015;1852:826–838. doi: 10.1016/j.bbadis.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Forman HJ, Fukuto JM, Miller T, Zhang H, Rinna A, Levy S. The chemistry of cell signaling by reactive oxygen and nitrogen species and 4-hydroxynonenal. Archives of biochemistry and biophysics. 2008;477:183–195. doi: 10.1016/j.abb.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaganjac M, Cacev T, Cipak A, Kapitanovic S, Gall Troselj K, Zarkovic N. Even stressed cells are individuals: second messengers of free radicals in pathophysiology of cancer. Croat Med J. 2012;53:304–309. doi: 10.3325/cmj.2012.53.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ullery JC, Marnett LJ. Protein modification by oxidized phospholipids and hydrolytically released lipid electrophiles: Investigating cellular responses. Biochim Biophys Acta. 2012;1818:2424–2435. doi: 10.1016/j.bbamem.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harman D. Aging: a theory based on free radical and radiation chemistry. Journal of gerontology. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 18.Miura Y, Endo T. Survival responses to oxidative stress and aging. Geriatrics & gerontology international. 2010;10(Suppl 1):S1–9. doi: 10.1111/j.1447-0594.2010.00597.x. [DOI] [PubMed] [Google Scholar]
- 19.Sykiotis GP, Bohmann D. Stress-activated cap’n’collar transcription factors in aging and human disease. Sci Signal. 2010;3:re3. doi: 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Sohal RS, Orr WC. The redox stress hypothesis of aging. Free Radic Biol Med. 2012;52:539–555. doi: 10.1016/j.freeradbiomed.2011.10.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimniak P. Relationship of electrophilic stress to aging. Free Radic Biol Med. 2011;51:1087–1105. doi: 10.1016/j.freeradbiomed.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez VI, Bokov A, Van Remmen H, Mele J, Ran Q, Ikeno Y, Richardson A. Is the oxidative stress theory of aging dead? Biochim Biophys Acta. 2009;1790:1005–1014. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ursini F, Maiorino M, Forman HJ. Redox homeostasis: The Golden Mean of healthy living. Redox Biol. 2016;8:205–215. doi: 10.1016/j.redox.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perluigi M, Coccia R, Butterfield DA. 4-Hydroxy-2-nonenal, a reactive product of lipid peroxidation, and neurodegenerative diseases: a toxic combination illuminated by redox proteomics studies. Antioxid Redox Signal. 2012;17:1590–1609. doi: 10.1089/ars.2011.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed TT. Lipid peroxidation and neurodegenerative disease. Free Radic Biol Med. 2011;51:1302–1319. doi: 10.1016/j.freeradbiomed.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 27.Jaganjac M, Tirosh O, Cohen G, Sasson S, Zarkovic N. Reactive aldehydes--second messengers of free radicals in diabetes mellitus. Free Radic Res. 2013;47(Suppl 1): 39–48. doi: 10.3109/10715762.2013.789136. [DOI] [PubMed] [Google Scholar]
- 28.Ansari NH, Wang L, Srivastava SK. Role of lipid aldehydes in cataractogenesis: 4-hydroxynonenal-induced cataract. Biochem Mol Med. 1996;58:25–30. doi: 10.1006/bmme.1996.0028. [DOI] [PubMed] [Google Scholar]
- 29.Spies-Martin D, Sommerburg O, Langhans CD, Leichsenring M. Measurement of 4-hydroxynonenal in small volume blood plasma samples: modification of a gas chromatographic-mass spectrometric method for clinical settings. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;774:231–239. doi: 10.1016/s1570-0232(02)00242-8. [DOI] [PubMed] [Google Scholar]
- 30.Pandey KB, Rizvi SI. Markers of oxidative stress in erythrocytes and plasma during aging in humans. Oxid Med Cell Longev. 2010;3:2–12. doi: 10.4161/oxim.3.1.10476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacob KD, Noren Hooten N, Trzeciak AR, Evans MK. Markers of oxidant stress that are clinically relevant in aging and age-related disease. Mech Ageing Dev. 2013;134:139–157. doi: 10.1016/j.mad.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng J, Mutcherson R, 2nd, Helfand SL. Calorie restriction delays lipid oxidative damage in Drosophila melanogaster. Aging Cell. 2005;4:209–216. doi: 10.1111/j.1474-9726.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 33.Asselin C, Bouchard B, Tardif JC, Des Rosiers C. Circulating 4-hydroxynonenal-protein thioether adducts assessed by gas chromatography-mass spectrometry are increased with disease progression and aging in spontaneously hypertensive rats. Free Radic Biol Med. 2006;41:97–105. doi: 10.1016/j.freeradbiomed.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Kim CH, Zou Y, Kim DH, Kim ND, Yu BP, Chung HY. Proteomic analysis of nitrated and 4-hydroxy-2-nonenal-modified serum proteins during aging. J Gerontol A Biol Sci Med Sci. 2006;61:332–338. doi: 10.1093/gerona/61.4.332. [DOI] [PubMed] [Google Scholar]
- 35.Asano S, Rice KM, Kakarla S, Katta A, Desai DH, Walker EM, Wehner P, Blough ER. Aging influences multiple indices of oxidative stress in the heart of the Fischer 344/NNia x Brown Norway/BiNia rat. Redox report : communications in free radical research. 2007;12:167–180. doi: 10.1179/135100007X200254. [DOI] [PubMed] [Google Scholar]
- 36.Kakarla SK, Fannin JC, Keshavarzian S, Katta A, Paturi S, Nalabotu SK, Wu M, Rice KM, Manzoor K, Walker EM, Jr, Blough ER. Chronic acetaminophen attenuates age-associated increases in cardiac ROS and apoptosis in the Fischer Brown Norway rat. Basic Res Cardiol. 2010;105:535–544. doi: 10.1007/s00395-010-0094-3. [DOI] [PubMed] [Google Scholar]
- 37.Almeida M, Ambrogini E, Han L, Manolagas SC, Jilka RL. Increased lipid oxidation causes oxidative stress, increased peroxisome proliferator-activated receptor-gamma expression, and diminished pro-osteogenic Wnt signaling in the skeleton. J Biol Chem. 2009;284:27438–27448. doi: 10.1074/jbc.M109.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gil L, Siems W, Mazurek B, Gross J, Schroeder P, Voss P, Grune T. Age-associated analysis of oxidative stress parameters in human plasma and erythrocytes. Free Radic Res. 2006;40:495–505. doi: 10.1080/10715760600592962. [DOI] [PubMed] [Google Scholar]
- 39.Pawelec G. Immunosenescence comes of age. Symposium on Aging Research in Immunology: The Impact of Genomics. EMBO Rep. 2007;8:220–223. doi: 10.1038/sj.embor.7400922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, Nevitt M, Harris TB. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 41.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franchini A, Ottaviani E. IL-6 immunoreactivity changes during aging in the polychaete Ophryotrocha labronica (Polychaeta: Dorvilleidae) Tissue Cell. 2007;39:27–34. doi: 10.1016/j.tice.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Friedman EM, Hayney M, Love GD, Singer BH, Ryff CD. Plasma interleukin-6 and soluble IL-6 receptors are associated with psychological well-being in aging women. Health Psychol. 2007;26:305–313. doi: 10.1037/0278-6133.26.3.305. [DOI] [PubMed] [Google Scholar]
- 44.Tilstra JS, Clauson CL, Niedernhofer LJ, Robbins PD. NF-kappaB in Aging and Disease. Aging Dis. 2011;2:449–465. [PMC free article] [PubMed] [Google Scholar]
- 45.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 46.Cevenini E, Monti D, Franceschi C. Inflamm-ageing. Curr Opin Clin Nutr Metab Care. 2013;16:14–20. doi: 10.1097/MCO.0b013e32835ada13. [DOI] [PubMed] [Google Scholar]
- 47.Amma H, Naruse K, Ishiguro N, Sokabe M. Involvement of reactive oxygen species in cyclic stretch-induced NF-kappaB activation in human fibroblast cells. Br J Pharmacol. 2005;145:364–373. doi: 10.1038/sj.bjp.0706182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramana KV, Fadl AA, Tammali R, Reddy AB, Chopra AK, Srivastava SK. Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264.7 murine macrophages. J Biol Chem. 2006;281:33019–33029. doi: 10.1074/jbc.M603819200. [DOI] [PubMed] [Google Scholar]
- 49.Ruef J, Moser M, Bode C, Kubler W, Runge MS. 4-hydroxynonenal induces apoptosis, NF-kappaB-activation and formation of 8-isoprostane in vascular smooth muscle cells. Basic Res Cardiol. 2001;96:143–150. doi: 10.1007/s003950170064. [DOI] [PubMed] [Google Scholar]
- 50.Lee SJ, Seo KW, Yun MR, Bae SS, Lee WS, Hong KW, Kim CD. 4-Hydroxynonenal enhances MMP-2 production in vascular smooth muscle cells via mitochondrial ROS-mediated activation of the Akt/NF-kappaB signaling pathways. Free Radic Biol Med. 2008;45:1487–1492. doi: 10.1016/j.freeradbiomed.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 51.Hattori Y, Hattori S, Kasai K. 4-hydroxynonenal prevents NO production in vascular smooth muscle cells by inhibiting nuclear factor-kappaB-dependent transcriptional activation of inducible NO synthase. Arterioscler Thromb Vasc Biol. 2001;21:1179–1183. doi: 10.1161/hq0701.092135. [DOI] [PubMed] [Google Scholar]
- 52.Dou X, Li S, Wang Z, Gu D, Shen C, Yao T, Song Z. Inhibition of NF-kappaB activation by 4-hydroxynonenal contributes to liver injury in a mouse model of alcoholic liver disease. Am J Pathol. 2012;181:1702–1710. doi: 10.1016/j.ajpath.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaillancourt F, Morquette B, Shi Q, Fahmi H, Lavigne P, Di Battista JA, Fernandes JC, Benderdour M. Differential regulation of cyclooxygenase-2 and inducible nitric oxide synthase by 4-hydroxynonenal in human osteoarthritic chondrocytes through ATF-2/CREB-1 transactivation and concomitant inhibition of NF-kappaB signaling cascade. J Cell Biochem. 2007;100:1217–1231. doi: 10.1002/jcb.21110. [DOI] [PubMed] [Google Scholar]
- 54.Ji C, Kozak KR, Marnett LJ. IkappaB kinase, a molecular target for inhibition by 4-hydroxy-2-nonenal. J Biol Chem. 2001;276:18223–18228. doi: 10.1074/jbc.M101266200. [DOI] [PubMed] [Google Scholar]
- 55.Page S, Fischer C, Baumgartner B, Haas M, Kreusel U, Loidl G, Hayn M, Ziegler-Heitbrock HW, Neumeier D, Brand K. 4-Hydroxynonenal prevents NF-kappaB activation and tumor necrosis factor expression by inhibiting IkappaB phosphorylation and subsequent proteolysis. J Biol Chem. 1999;274:11611–11618. doi: 10.1074/jbc.274.17.11611. [DOI] [PubMed] [Google Scholar]
- 56.Camandola S, Poli G, Mattson MP. The lipid peroxidation product 4-hydroxy-2,3-nonenal inhibits constitutive and inducible activity of nuclear factor kappa B in neurons. Brain Res Mol Brain Res. 2000;85:53–60. doi: 10.1016/s0169-328x(00)00234-5. [DOI] [PubMed] [Google Scholar]
- 57.Donath B, Fischer C, Page S, Prebeck S, Jilg N, Weber M, da Costa C, Neumeier D, Miethke T, Brand K. Chlamydia pneumoniae activates IKK/I kappa B-mediated signaling, which is inhibited by 4-HNE and following primary exposure. Atherosclerosis. 2002;165:79–88. doi: 10.1016/s0021-9150(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 58.Kaarniranta K, Ryhanen T, Karjalainen HM, Lammi MJ, Suuronen T, Huhtala A, Kontkanen M, Terasvirta M, Uusitalo H, Salminen A. Geldanamycin increases 4-hydroxynonenal (HNE)-induced cell death in human retinal pigment epithelial cells. Neurosci Lett. 2005;382:185–190. doi: 10.1016/j.neulet.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 59.Marantos C, Mukaro V, Ferrante J, Hii C, Ferrante A. Inhibition of the lipopolysaccharide-induced stimulation of the members of the MAPK family in human monocytes/macrophages by 4-hydroxynonenal, a product of oxidized omega-6 fatty acids. Am J Pathol. 2008;173:1057–1066. doi: 10.2353/ajpath.2008.071150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park S, Sung B, Jang EJ, Kim DH, Park CH, Choi YJ, Ha YM, Kim MK, Kim ND, Yu BP, Chung HY. Inhibitory action of salicylideneamino-2-thiophenol on NF-kappaB signaling cascade and cyclooxygenase-2 in HNE-treated endothelial cells. Arch Pharm Res. 2013;36:880–889. doi: 10.1007/s12272-013-0116-4. [DOI] [PubMed] [Google Scholar]
- 61.Zhang H, Davies KJ, Forman HJ. Oxidative stress response and Nrf2 signaling in aging. Free Radic Biol Med. 2015;88:314–336. doi: 10.1016/j.freeradbiomed.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang H, Court N, Forman HJ. Submicromolar concentrations of 4-hydroxynonenal induce glutamate cysteine ligase expression in HBE1 cells. Redox report : communications in free radical research. 2007;12:101–106. doi: 10.1179/135100007X162266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang H, Forman HJ. Signaling pathways involved in phase II gene induction by alpha, beta-unsaturated aldehydes. Toxicol Ind Health. 2009;25:269–278. doi: 10.1177/0748233709102209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang H, Liu H, Dickinson DA, Liu RM, Postlethwait EM, Laperche Y, Forman HJ. gamma-Glutamyl transpeptidase is induced by 4-hydroxynonenal via EpRE/Nrf2 signaling in rat epithelial type II cells. Free Radic Biol Med. 2006;40:1281–1292. doi: 10.1016/j.freeradbiomed.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Numazawa S, Ishikawa M, Yoshida A, Tanaka S, Yoshida T. Atypical protein kinase C mediates activation of NF-E2-related factor 2 in response to oxidative stress. Am J Physiol Cell Physiol. 2003;285:C334–342. doi: 10.1152/ajpcell.00043.2003. [DOI] [PubMed] [Google Scholar]
- 66.Iles KE, Dickinson DA, Wigley AF, Welty NE, Blank V, Forman HJ. HNE increases HO-1 through activation of the ERK pathway in pulmonary epithelial cells. Free Radic Biol Med. 2005;39:355–364. doi: 10.1016/j.freeradbiomed.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang H, Liu H, Iles KE, Liu RM, Postlethwait EM, Laperche Y, Forman HJ. 4-Hydroxynonenal induces rat gamma-glutamyl transpeptidase through mitogen-activated protein kinase-mediated electrophile response element/nuclear factor erythroid 2-related factor 2 signaling. Am J Respir Cell Mol Biol. 2006;34:174–181. doi: 10.1165/rcmb.2005-0280OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 69.Franke TF. PI3K/Akt: getting it right matters. Oncogene. 2008;27:6473–6488. doi: 10.1038/onc.2008.313. [DOI] [PubMed] [Google Scholar]
- 70.Nakanishi A, Wada Y, Kitagishi Y, Matsuda S. Link between PI3K/AKT/PTEN Pathway and NOX Proteinin Diseases. Aging Dis. 2014;5:203–211. doi: 10.14336/AD.2014.0500203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.CON. PI3-kinase/Akt/mTOR signaling: impaired on/off switches in aging, cognitive decline and Alzheimer’s disease. Exp Gerontol. 2013;48:647–653. doi: 10.1016/j.exger.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 72.Zhang H, Liu H, Davies KJ, Sioutas C, Finch CE, Morgan TE, Forman HJ. Nrf2-regulated phase II enzymes are induced by chronic ambient nanoparticle exposure in young mice with age-related impairments. Free Radic Biol Med. 2012;52:2038–2046. doi: 10.1016/j.freeradbiomed.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang F, Chu X, Yin M, Liu X, Yuan H, Niu Y, Fu L. mTOR and autophagy in normal brain aging and caloric restriction ameliorating age-related cognition deficits. Behav Brain Res. 2014;264:82–90. doi: 10.1016/j.bbr.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 74.Wu M, Falasca M, Blough ER. Akt/protein kinase B in skeletal muscle physiology and pathology. J Cell Physiol. 2011;226:29–36. doi: 10.1002/jcp.22353. [DOI] [PubMed] [Google Scholar]
- 75.Wu M, Katta A, Gadde MK, Liu H, Kakarla SK, Fannin J, Paturi S, Arvapalli RK, Rice KM, Wang Y, Blough ER. Aging-associated dysfunction of Akt/protein kinase B: S-nitrosylation and acetaminophen intervention. PLoS One. 2009;4:e6430. doi: 10.1371/journal.pone.0006430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.da Janner DR, Jacob MH, Jahn MP, Kucharski LC, Ribeiro MF. Dehydroepiandrosterone effects on Akt signaling modulation in central nervous system of young and aged healthy rats. J Steroid Biochem Mol Biol. 2010;122:142–148. doi: 10.1016/j.jsbmb.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 77.Ji GR, Yu NC, Xue X, Li ZG. 4-Hydroxy-2-nonenal induces apoptosis by inhibiting AKT signaling in human osteosarcoma cells. Scientific World Journal. 2014;2014:873525. doi: 10.1155/2014/873525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Demozay D, Mas JC, Rocchi S, Van Obberghen E. FALDH reverses the deleterious action of oxidative stress induced by lipid peroxidation product 4-hydroxynonenal on insulin signaling in 3T3-L1 adipocytes. Diabetes. 2008;57:1216–1226. doi: 10.2337/db07-0389. [DOI] [PubMed] [Google Scholar]
- 79.Vaillancourt F, Fahmi H, Shi Q, Lavigne P, Ranger P, Fernandes JC, Benderdour M. 4-Hydroxynonenal induces apoptosis in human osteoarthritic chondrocytes: the protective role of glutathione-S-transferase. Arthritis Res Ther. 2008;10:R107. doi: 10.1186/ar2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu W, Akhand AA, Takeda K, Kawamoto Y, Itoigawa M, Kato M, Suzuki H, Ishikawa N, Nakashima I. Protein phosphatase 2A-linked and -unlinked caspase-dependent pathways for downregulation of Akt kinase triggered by 4-hydroxynonenal. Cell death and differentiation. 2003;10:772–781. doi: 10.1038/sj.cdd.4401238. [DOI] [PubMed] [Google Scholar]
- 81.Chen ZH, Saito Y, Yoshida Y, Sekine A, Noguchi N, Niki E. 4-Hydroxynonenal induces adaptive response and enhances PC12 cell tolerance primarily through induction of thioredoxin reductase 1 via activation of Nrf2. J Biol Chem. 2005;280:41921–41927. doi: 10.1074/jbc.M508556200. [DOI] [PubMed] [Google Scholar]
- 82.Dozza B, Smith MA, Perry G, Tabaton M, Strocchi P. Regulation of glycogen synthase kinase-3beta by products of lipid peroxidation in human neuroblastoma cells. J Neurochem. 2004;89:1224–1232. doi: 10.1111/j.1471-4159.2004.02413.x. [DOI] [PubMed] [Google Scholar]
- 83.Zheng R, Po I, Mishin V, Black AT, Heck DE, Laskin DL, Sinko PJ, Gerecke DR, Gordon MK, Laskin JD. The generation of 4-hydroxynonenal, an electrophilic lipid peroxidation end product, in rabbit cornea organ cultures treated with UVB light and nitrogen mustard. Toxicol Appl Pharmacol. 2013;272:345–355. doi: 10.1016/j.taap.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vatsyayan R, Chaudhary P, Sharma A, Sharma R, Rao Lelsani PC, Awasthi S, Awasthi YC. Role of 4-hydroxynonenal in epidermal growth factor receptor-mediated signaling in retinal pigment epithelial cells. Exp Eye Res. 2011;92:147–154. doi: 10.1016/j.exer.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prasannarong M, Santos FR, Hooshmand P, Giovannini FJ, Henriksen EJ. The lipid peroxidation end-product and oxidant 4-hydroxynonenal induces insulin resistance in rat slow-twitch skeletal muscle. Arch Physiol Biochem. 2014;120:22–28. doi: 10.3109/13813455.2013.834937. [DOI] [PubMed] [Google Scholar]
- 86.Shearn CT, Reigan P, Petersen DR. Inhibition of hydrogen peroxide signaling by 4-hydroxynonenal due to differential regulation of Akt1 and Akt2 contributes to decreases in cell survival and proliferation in hepatocellular carcinoma cells. Free Radic Biol Med. 2012;53:1–11. doi: 10.1016/j.freeradbiomed.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shearn CT, Fritz KS, Reigan P, Petersen DR. Modification of Akt2 by 4-hydroxynonenal inhibits insulin-dependent Akt signaling in HepG2 cells. Biochemistry. 2011;50:3984–3996. doi: 10.1021/bi200029w. [DOI] [PubMed] [Google Scholar]
- 88.Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochim Biophys Acta. 2009;1790:1067–1074. doi: 10.1016/j.bbagen.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda) 2006;21:362–369. doi: 10.1152/physiol.00024.2006. [DOI] [PubMed] [Google Scholar]
- 91.Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. The Journal of clinical investigation. 2015;125:25–32. doi: 10.1172/JCI73939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Perluigi M, Di Domenico F, Butterfield DA. mTOR signaling in aging and neurodegeneration: At the crossroad between metabolism dysfunction and impairment of autophagy. Neurobiol Dis. 2015;84:39–49. doi: 10.1016/j.nbd.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 93.Frias MA, Thoreen CC, Jaffe JD, Schroder W, Sculley T, Carr SA, Sabatini DM. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16:1865–1870. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 94.Graber TE, McCamphill PK, Sossin WS. A recollection of mTOR signaling in learning and memory. Learn Mem. 2013;20:518–530. doi: 10.1101/lm.027664.112. [DOI] [PubMed] [Google Scholar]
- 95.Dolinsky VW, Chan AY, Robillard Frayne I, Light PE, Des Rosiers C, Dyck JR. Resveratrol prevents the prohypertrophic effects of oxidative stress on LKB1. Circulation. 2009;119:1643–1652. doi: 10.1161/CIRCULATIONAHA.108.787440. [DOI] [PubMed] [Google Scholar]
- 96.Ma H, Guo R, Yu L, Zhang Y, Ren J. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: role of autophagy paradox and toxic aldehyde. Eur Heart J. 2011;32:1025–1038. doi: 10.1093/eurheartj/ehq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Calamaras TD, Lee C, Lan F, Ido Y, Siwik DA, Colucci WS. Post-translational modification of serine/threonine kinase LKB1 via Adduction of the Reactive Lipid Species 4-Hydroxy-trans-2-nonenal (HNE) at lysine residue 97 directly inhibits kinase activity. J Biol Chem. 2012;287:42400–42406. doi: 10.1074/jbc.M112.385831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Calamaras TD, Lee C, Lan F, Ido Y, Siwik DA, Colucci WS. The lipid peroxidation product 4-hydroxy-trans-2-nonenal causes protein synthesis in cardiac myocytes via activated mTORC1-p70S6K-RPS6 signaling. Free Radic Biol Med. 2015;82:137–146. doi: 10.1016/j.freeradbiomed.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang X, Wang Z, Li J, Gu D, Li S, Shen C, Song Z. Increased 4-hydroxynonenal formation contributes to obesity-related lipolytic activation in adipocytes. PLoS One. 2013;8:e70663. doi: 10.1371/journal.pone.0070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qin S, Rodrigues GA. Differential roles of AMPKalpha1 and AMPKalpha2 in regulating 4-HNE-induced RPE cell death and permeability. Exp Eye Res. 2010;91:818–824. doi: 10.1016/j.exer.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 101.Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 102.Baker DJ, Weaver RL, van Deursen JM. p21 both attenuates and drives senescence and aging in BubR1 progeroid mice. Cell Rep. 2013;3:1164–1174. doi: 10.1016/j.celrep.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Laurora S, Tamagno E, Briatore F, Bardini P, Pizzimenti S, Toaldo C, Reffo P, Costelli P, Dianzani MU, Danni O, Barrera G. 4-Hydroxynonenal modulation of p53 family gene expression in the SK-N-BE neuroblastoma cell line. Free Radic Biol Med. 2005;38:215–225. doi: 10.1016/j.freeradbiomed.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 104.Cerbone A, Toaldo C, Laurora S, Briatore F, Pizzimenti S, Dianzani MU, Ferretti C, Barrera G. 4-Hydroxynonenal and PPARgamma ligands affect proliferation, differentiation, and apoptosis in colon cancer cells. Free Radic Biol Med. 2007;42:1661–1670. doi: 10.1016/j.freeradbiomed.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 105.Sharma A, Sharma R, Chaudhary P, Vatsyayan R, Pearce V, Jeyabal PV, Zimniak P, Awasthi S, Awasthi YC. 4-Hydroxynonenal induces p53-mediated apoptosis in retinal pigment epithelial cells. Archives of biochemistry and biophysics. 2008;480:85–94. doi: 10.1016/j.abb.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chaudhary P, Sharma R, Sahu M, Vishwanatha JK, Awasthi S, Awasthi YC. 4-Hydroxynonenal induces G2/M phase cell cycle arrest by activation of the ataxia telangiectasia mutated and Rad3-related protein (ATR)/checkpoint kinase 1 (Chk1) signaling pathway. J Biol Chem. 2013;288:20532–20546. doi: 10.1074/jbc.M113.467662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hyun DH, Lee MH, Halliwell B, Jenner P. Proteasomal dysfunction induced by 4-hydroxy-2,3-trans-nonenal, an end-product of lipid peroxidation: a mechanism contributing to neurodegeneration? J Neurochem. 2002;83:360–370. doi: 10.1046/j.1471-4159.2002.01125.x. [DOI] [PubMed] [Google Scholar]
- 108.Pizzimenti S, Menegatti E, Berardi D, Toaldo C, Pettazzoni P, Minelli R, Giglioni B, Cerbone A, Dianzani MU, Ferretti C, Barrera G. 4-hydroxynonenal, a lipid peroxidation product of dietary polyunsaturated fatty acids, has anticarcinogenic properties in colon carcinoma cell lines through the inhibition of telomerase activity. J Nutr Biochem. 2010;21:818–826. doi: 10.1016/j.jnutbio.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 109.Suc I, Meilhac O, Lajoie-Mazenc I, Vandaele J, Jurgens G, Salvayre R, Negre-Salvayre A. Activation of EGF receptor by oxidized LDL. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1998;12:665–671. doi: 10.1096/fasebj.12.9.665. [DOI] [PubMed] [Google Scholar]
- 110.Liu W, Akhand AA, Kato M, Yokoyama I, Miyata T, Kurokawa K, Uchida K, Nakashima I. 4-hydroxynonenal triggers an epidermal growth factor receptor-linked signal pathway for growth inhibition. J Cell Sci. 1999;112(Pt 14):2409–2417. doi: 10.1242/jcs.112.14.2409. [DOI] [PubMed] [Google Scholar]
- 111.Vacaresse N, Vieira O, Robbesyn F, Jurgens G, Salvayre R, Negre-Salvayre A. Phenolic antioxidants trolox and caffeic acid modulate the oxidized LDL-induced EGF-receptor activation. Br J Pharmacol. 2001;132:1777–1788. doi: 10.1038/sj.bjp.0703981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Negre-Salvayre A, Vieira O, Escargueil-Blanc I, Salvayre R. Oxidized LDL and 4-hydroxynonenal modulate tyrosine kinase receptor activity. Molecular aspects of medicine. 2003;24:251–261. doi: 10.1016/s0098-2997(03)00020-7. [DOI] [PubMed] [Google Scholar]
- 113.Robbesyn F, Auge N, Vindis C, Cantero AV, Barbaras R, Negre-Salvayre A, Salvayre R. High-density lipoproteins prevent the oxidized low-density lipoprotein-induced epidermal [corrected] growth factor receptor activation and subsequent matrix metalloproteinase-2 upregulation. Arterioscler Thromb Vasc Biol. 2005;25:1206–1212. doi: 10.1161/01.ATV.0000164805.73558.80. [DOI] [PubMed] [Google Scholar]
- 114.Uno K, Kato K, Kusaka G, Asano N, Iijima K, Shimosegawa T. The balance between 4-hydroxynonenal and intrinsic glutathione/glutathione S-transferase A4 system may be critical for the epidermal growth factor receptor phosphorylation of human esophageal squamous cell carcinomas. Mol Carcinog. 2011;50:781–790. doi: 10.1002/mc.20699. [DOI] [PubMed] [Google Scholar]
- 115.Escargueil-Blanc I, Salvayre R, Vacaresse N, Jurgens G, Darblade B, Arnal JF, Parthasarathy S, Negre-Salvayre A. Mildly oxidized LDL induces activation of platelet-derived growth factor beta-receptor pathway. Circulation. 2001;104:1814–1821. doi: 10.1161/hc4001.097179. [DOI] [PubMed] [Google Scholar]
- 116.Vindis C, Escargueil-Blanc I, Uchida K, Elbaz M, Salvayre R, Negre-Salvayre A. Lipid oxidation products and oxidized low-density lipoproteins impair platelet-derived growth factor receptor activity in smooth muscle cells: implication in atherosclerosis. Redox report : communications in free radical research. 2007;12:96–100. doi: 10.1179/135100007X162248. [DOI] [PubMed] [Google Scholar]
- 117.Robino G, Parola M, Marra F, Caligiuri A, De Franco RM, Zamara E, Bellomo G, Gentilini P, Pinzani M, Dianzani MU. Interaction between 4-hydroxy-2,3-alkenals and the platelet-derived growth factor-beta receptor. Reduced tyrosine phosphorylation and downstream signaling in hepatic stellate cells. J Biol Chem. 2000;275:40561–40567. doi: 10.1074/jbc.M007694200. [DOI] [PubMed] [Google Scholar]
- 118.Larroque-Cardoso P, Mucher E, Grazide MH, Josse G, Schmitt AM, Nadal-Wolbold F, Zarkovic K, Salvayre R, Negre-Salvayre A. 4-Hydroxynonenal impairs transforming growth factor-beta1-induced elastin synthesis via epidermal growth factor receptor activation in human and murine fibroblasts. Free Radic Biol Med. 2014;71:427–436. doi: 10.1016/j.freeradbiomed.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 119.Shearn CT, Smathers RL, Stewart BJ, Fritz KS, Galligan JJ, Hail N, Jr, Petersen DR. Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) inhibition by 4-hydroxynonenal leads to increased Akt activation in hepatocytes. Mol Pharmacol. 2011;79:941–952. doi: 10.1124/mol.110.069534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wagner TM, Mullally JE, Fitzpatrick FA. Reactive lipid species from cyclooxygenase-2 inactivate tumor suppressor LKB1/STK11: cyclopentenone prostaglandins and 4-hydroxy-2-nonenal covalently modify and inhibit the AMP-kinase kinase that modulates cellular energy homeostasis and protein translation. J Biol Chem. 2006;281:2598–2604. doi: 10.1074/jbc.M509723200. [DOI] [PubMed] [Google Scholar]
- 121.Zhang Z, Wang S, Zhou S, Yan X, Wang Y, Chen J, Mellen N, Kong M, Gu J, Tan Y, Zheng Y, Cai L. Sulforaphane prevents the development of cardiomyopathy in type 2 diabetic mice probably by reversing oxidative stress-induced inhibition of LKB1/AMPK pathway. J Mol Cell Cardiol. 2014;77:42–52. doi: 10.1016/j.yjmcc.2014.09.022. [DOI] [PubMed] [Google Scholar]